Abstract
The high rate of mortality in patients with sepsis results from an inappropriately amplified systemic inflammatory response to infection. Toll-like receptors (TLRs) are important for the activation of innate immunity against microbial pathogens. We demonstrate a critical role of TLR9 in the dysregulated immune response and death associated with sepsis. Compared with wild-type (WT) mice, TLR9−/− mice exhibited lower serum inflammatory cytokine levels, higher bacterial clearance, and greater survival after experimental peritonitis induced by cecal ligation and puncture (CLP). Protection of TLR9−/− mice after CLP was associated with a greater number of peritoneal dendritic cells (DCs) and granulocytes than in WT controls. Adoptive transfer of TLR9−/− DCs was sufficient to protect WT mice from CLP and increased the influx of peritoneal granulocytes. Subsequent experiments with a depleting antibody revealed that granulocytes were required for survival in TLR9−/− mice. Remarkably, a single injection of an inhibitory CpG sequence that blocks TLR9 protected WT mice, even when administered as late as 12 h after CLP. Our findings demonstrate that the detrimental immune response to bacterial sepsis occurs via TLR9 stimulation. TLR9 blockade is a potential strategy for the treatment of human sepsis.
Sepsis remains the leading cause of death in critically ill patients, with mortality rates ranging between 30 and 70% (1). The syndrome of sepsis develops when the initially appropriate host response to infection becomes excessive, resulting in widespread inflammation and multiorgan failure (2). Several strategies to treat human sepsis have targeted proinflammatory mediators. Unfortunately, most of these therapies have not proven to be efficacious in large multicenter clinical trials (3). Blockade of TNF, IL-1β, bradykinin, platelet-activating factor, elastase, nitric oxide, and LPS has not affected outcome (4). In addition, it was recently demonstrated that corticosteroid therapy for septic shock failed to alter survival in a randomized trial (5). Consequently, the current treatment of sepsis is largely supportive, and definitive therapies have remained elusive.
Septic peritonitis has a particularly high mortality and is characterized by a massive infiltration of neutrophils into the peritoneum, where they act as a first line of defense against microbial pathogens. When innate defenses become overwhelmed, bacteria escape the peritoneum and disseminate throughout the host, inducing an exaggerated inflammatory response (2). Immune recognition of bacteria occurs via pattern-recognition receptors that detect conserved microbial components or products (6). The best-characterized pattern-recognition receptors are the Toll-like receptors (TLRs). There are at least 10 TLRs in mammals. TLR4 functions as the signal-transducing receptor for LPS, and TLR2 detects a broad range of Gram-positive bacterial products, including lipotechoic acid and peptidoglycan. The ligands for TLR9 are unmethylated CpG motifs present in bacterial DNA (6). The importance of TLRs in the pathogenesis of sepsis has been demonstrated in mice deficient in the signaling protein myeloid differentiation factor 88 (MyD88) (7). MyD88 has been identified as a central adaptor protein for the signal transduction of all TLRs except for TLR3 (8). Although MyD88−/− mice have attenuated responses to polymicrobial infection, the contribution of individual TLRs seems less critical. In a mouse model of septic peritonitis, it was shown that mice with single or combined deficiency in TLR2 and TLR4 had similar survival as WT mice (7). These surprising findings raised the possibility that individual TLRs are dispensable for the progression of bacterial sepsis and instead support an alternative hypothesis that stimulation of multiple TLRs is needed for an overwhelming inflammatory response. In this report, however, we show that eliminating TLR9 stimulation is sufficient to avert the unrestrained immune response in polymicrobial sepsis.
RESULTS AND DISCUSSION
TLR9−/− mice are resistant to polymicrobial sepsis
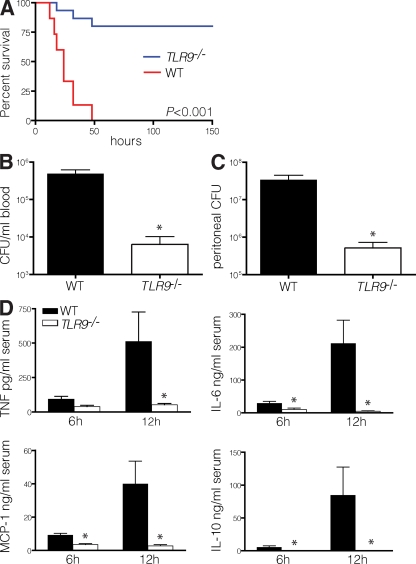
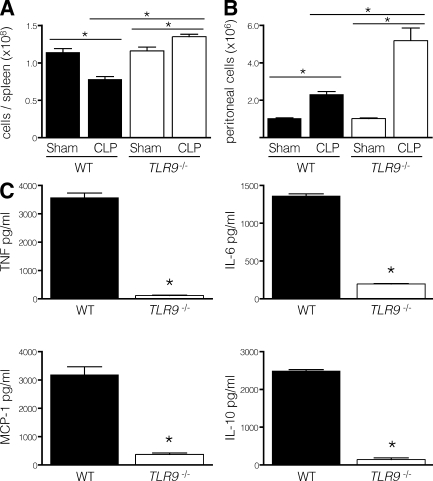
To determine the relative importance of TLR9 stimulation in polymicrobial sepsis, we performed cecal ligation and puncture (CLP) in TLR9−/− mice. Strikingly, nearly all TLR9−/− mice survived, whereas WT mice died at a median of 24 h (Fig. 1 A). The increased survival of TLR9−/− mice was associated with enhanced clearance of bacteria from the blood (Fig. 1 B) and peritoneal cavity (Fig. 1 C), as well as a dramatic decrease in serum inflammatory cytokines (Fig. 1 D). The reduction in cytokine levels was most evident at 12 h and ranged between 15- and 300-fold, depending on the particular cytokine. To understand the basis of protection, we analyzed the cellular composition of the spleen and peritoneum. Compared with animals undergoing sham laparotomy, WT mice that underwent CLP had fewer splenocytes, which was consistent with previous data demonstrating apoptosis of splenic lymphocytes during sepsis (9). In contrast, we found that TLR9−/− mice had a greater number of total splenocytes after CLP (Fig. 2 A). The absence of lymphodepletion in TLR9−/− mice likely contributed to their protection because inhibiting apoptosis improves survival in sepsis (10). In the peritoneum, the number of lymphocytes increased markedly after CLP in TLR9−/− animals and to a lesser extent in WT mice (Fig. 2 B). Consistent with our findings of reduced serum cytokine levels in TLR9−/− mice (Fig. 1 D), the peritoneal cells of TLR9−/− mice isolated 12 h after CLP and cultured without restimulation made fewer cytokines than peritoneal cells from WT mice (Fig. 2 C).
Figure 1.
TLR9−/− mice have less inflammation and increased survival after CLP. (A) The survival rates of WT B6 and TLR9−/− mice were monitored for 6 d after CLP (15 mice per group, with data pooled from three experiments, each of which had similar statistical significance). Dilutions of blood (B) or peritoneal lavage fluid (C) obtained from TLR9−/− or WT mice 12 h after CLP were cultured on BHI agar plates, and the number of bacterial colonies was counted (five mice per group). The colony count indicated is of 1 ml of blood and 1 ml of peritoneal fluid. (D) Serum cytokine levels were determined 6 and 12 h after CLP by using a cytometric bead array (five mice per group). Data shown are means of values ± SEM obtained from individual mice and are representative of at least two independent experiments. *, P < 0.05.
Figure 2.
TLR9−/− mice have increased lymphocyte infiltration and decreased local inflammatory cytokine production after CLP. 12 h after CLP or sham laparotomy, the numbers of viable splenocytes (A) and infiltrating peritoneal lymphocytes (B) were counted on a hemocytometer (BrightLine; Hausser Scientific) after Trypan blue staining (three to five mice per group). Peritoneal cells are expressed per milliliter of peritoneal fluid. (C) Peritoneal cells pooled from three to five mice 12 h after CLP were cultured without restimulation for 24 h, and the supernatant cytokine levels were determined with a cytometric bead array. Peritoneal cells from mice that underwent sham laparotomy secreted a minimal amount of cytokines (not depicted). Data shown are means of values ± SEM obtained from individual mice (except for cells pooled in C) and are representative of at least two independent experiments. *, P < 0.05.
TLR9−/− DCs are protective during sepsis
A variety of cells express TLR9, most notably those of myeloid lineage. In mice, TLR9 is primarily expressed on DCs, B cells, and, to a lesser extent, macrophages (11, 12). DCs play a pivotal role in sepsis, and DC recruitment to sites of inflammation in response to chemotactic stimuli is necessary for an optimal immune response (13, 14). During sepsis in mice and humans, the number of DCs is reduced (2, 15). In addition, depletion of the remaining DCs in an inducible model of DC ablation demonstrated that DCs are protective during sepsis (16). Furthermore, intrapulmonary transfer of DCs to mice 2 wk after sublethal CLP has been shown to reverse the sepsis-induced susceptibility to Aspergillus infection (17).
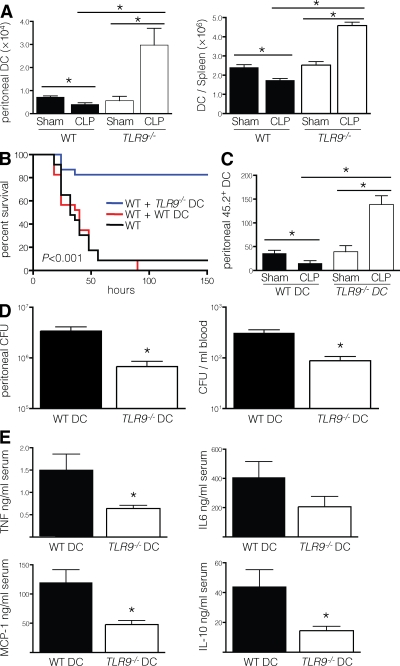
We therefore determined whether DCs were altered in TLR9−/− mice during sepsis. As expected, DCs were reduced in the spleen and peritoneum of WT mice after CLP. However, the reverse was seen in TLR9−/− mice, as sepsis actually increased the number of DCs in both the spleen and peritoneum (Fig. 3 A). We then examined the maturation, function, and subset composition of DCs 12 h after CLP. There was no difference in DC maturation between WT and TLR9−/− mice after sham laparotomy, as measured by CD40, CD80, and CD86 expression. However, CLP induced a similar increase in the maturation of DCs from WT and TLR9−/− mice (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20080162/DC1). DCs from TLR9−/− mice that underwent CLP induced greater alloproliferation (Fig. S1 B). There was no difference in the composition of myeloid (CD11b+CD8α−), lymphoid (CD11b−CD8α+), and plasmacytoid (mPDCA-1+) DCs from the spleen (Fig. S1 C) or peritoneum (not depicted) between WT and TLR9−/− mice after CLP.
Figure 3.
TLR9−/− DCs reduce the sepsis-induced lethality of WT mice. (A) 12 h after CLP or sham laparotomy, the number of conventional DCs (CD11chighMHCII+) were determined by flow cytometry in the spleen and peritoneal fluid of WT or TLR9−/− mice (three to five mice per group). (B) The survival rates of WT mice receiving WT or TLR9−/− DCs were monitored for 6 d after CLP (23 mice per group, with data pooled from two experiments, each of which had similar statistical significance). (C) 107 Flt3L-expanded WT or TLR9−/− DCs (CD45.2+) were injected i.v. into CD45.1 WT recipients (three to five mice per group). 12 h after transfer, the mice underwent CLP or sham laparotomy, and 12 h later the number of CD45.2+ DCs were enumerated from the peritoneal cells by flow cytometry. (D) Dilutions of blood or peritoneal lavage fluid obtained from WT mice pretreated with WT or TLR9−/− DCs 12 h after CLP were cultured on BHI agar plates, and the number of bacterial colonies was counted (five mice per group). (E) Serum cytokine levels were determined 12 h after CLP by cytometric bead array (five mice per group). Data shown are means of values ± SEM obtained from individual mice and are representative of at least two independent experiments. *, P < 0.05.
To ascertain whether TLR9−/− DCs were protective, we transferred them into WT mice before CLP. Although adoptive transfer of WT DCs did not affect survival, the transfer of TLR9−/− DCs significantly decreased mortality (Fig. 3 B). This coincided with greater accumulation of transferred TLR9−/− DCs than WT DCs in the peritoneum of WT mice after CLP (Fig. 3 C). The reduced mortality after adoptive transfer of TLR9−/− DCs was also associated with a decrease in the burden of blood and peritoneal bacteria (Fig. 3 D), as well as lower serum levels of inflammatory cytokines (Fig. 3 E). Thus, the adoptive transfer of TLR9−/− DCs was sufficient to reduce the extent of bacterial infection and the associated inflammatory response in WT mice.
TLR9−/− mice have a greater influx of granulocytes into the peritoneum
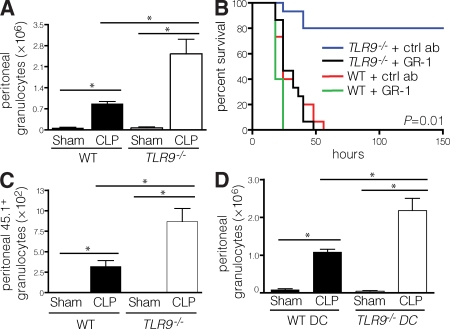
Early clearance of bacteria is mediated by granulocytes and is critical for the prevention of an overwhelming immune response (18). Because TLR9−/− mice had enhanced bacterial clearance, we assessed their peritoneal granulocyte content. CLP was associated with a robust recruitment of granulocytes to the peritoneum in TLR9−/− mice and to a lesser extent in WT mice (Fig. 4 A). To determine if granulocytes were required for the enhanced survival observed in TLR9−/− mice, we performed CLP in WT and TLR9−/− animals after antibody-mediated depletion of granulocytes. Indeed, depletion of granulocytes abrogated the survival advantage of TLR9−/− mice (Fig. 4 B).
Figure 4.
Peritoneal granulocytes are increased by CLP and are necessary for protection. (A) 12 h after CLP or sham laparotomy, the number of peritoneal granulocytes in WT or TLR9−/− mice (three to five mice per group) was determined by flow cytometry. (B) WT or TLR9−/− mice received 500 μg anti-GR1 antibody or isotype control 24 and 2 h before CLP, and the survival rates were monitored for 6 d after CLP (15 mice per group, with data pooled from three experiments, each of which had similar statistical significance). (C) 2 × 106 CD45.1 splenic granulocytes were injected i.v. into WT or TLR9−/− (CD45.2) recipients (three to five mice per group). 12 h after transfer, the mice underwent CLP or sham laparotomy, and 12 h later the number of CD45.1+ granulocytes were enumerated from the peritoneal cells by flow cytometry. (D) 12 h after CLP or sham laparotomy, the number of granulocytes (Ly6G+) was determined by flow cytometry in the peritoneal fluid of WT mice pretreated with 107 Flt3L-expanded WT or TLR9−/− DCs (three to five mice per group). Treatment with anti-GR1 antibody achieved >97% depletion of granulocytes at the time of and 24 h after CLP (not depicted). Data shown are means of values ± SEM obtained from individual mice and are representative of at least two independent experiments. *, P < 0.05.
Activated DCs have been shown to promote granulocyte chemotaxis in vivo by the secretion of chemokines (19). TLR9−/− mice demonstrated accumulation of peritoneal DCs during CLP (Fig. 3 A), and transfer of TLR9−/− DCs was sufficient to protect WT animals from CLP (Fig. 3 B). We therefore sought to determine if preferential granulocyte recruitment occurs in TLR9−/− animals and if DCs from TLR9−/− mice increased granulocyte trafficking to the peritoneum. After adoptive transfer of congenic WT granulocytes into WT and TLR9−/− mice before CLP, a greater number of WT granulocytes accumulated in the peritoneum of TLR9−/− versus WT recipients after CLP (Fig. 4 C). Furthermore, there was no significant difference in the number of adoptively transferred granulocytes in the blood or spleen between WT and TLR9−/− mice after CLP (unpublished data). Adoptive transfer of TLR9−/− DCs enhanced granulocyte recruitment, as treatment of WT mice with TLR9−/− DCs before CLP led to a significantly increased number of peritoneal granulocytes than adoptive transfer of WT DCs (Fig. 4 D).
Inhibitory CpG (iCpG) reduces the mortality of polymicrobial sepsis
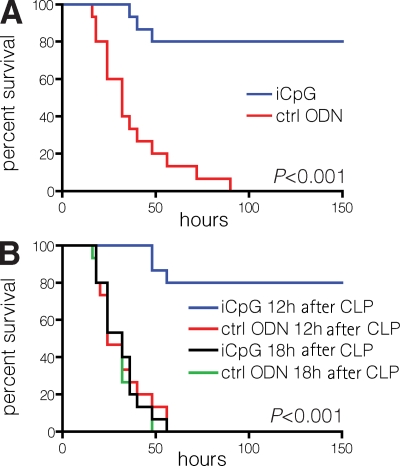
Because TLR9−/− mice survived septic peritonitis, we postulated that TLR9 blockade in WT mice may protect them from sepsis. A single 100-μg dose of an iCpG sequence (20) administered immediately before CLP significantly improved the survival of WT mice (Fig. 5 A). It is possible that multiple or higher iCpG doses would achieve even greater survival in WT mice subjected to CLP. Using lower doses, we found that 50 μg achieved 30% survival in 10 mice (P = 0.1), whereas a 5-μg dose did not yield any survivors (unpublished data). In vitro, iCpG also inhibited the production of inflammatory cytokines when WT splenocytes were cultured directly with heat-inactivated contents from mouse cecum (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20080162/DC1).
Figure 5.
TLR9 inhibition protects WT mice from CLP. (A) Immediately before CLP, WT mice received 100 μg iCpG or control oligodeoxynucleotide (ODN), and survival rates were monitored for 6 d. (B) 12 and 18 h after CLP, WT mice received 100 μg iCpG or control ODN, and survival rates were monitored for 6 d. Survival curves of mice treated with iCpG at 3 and 6 h were similar to the one shown for the 12-h iCpG group (not depicted). Data shown include 15 mice per group, pooled from three experiments, each of which had similar statistical significance.
Because there is often a delay in the diagnosis of sepsis in humans, we determined the window of time during which iCpG could offer protection. Dramatically, we found that iCpG protected against mortality up to 12 h after CLP (Fig. 5 B). Thus, continued TLR9 stimulation was necessary for progression to death, because WT mice could be rescued at 12 h despite already being burdened by high bacterial counts and widespread inflammation (Fig. 1).
TLR9−/− mice have been shown to be highly susceptible to Gram-negative bacterial pneumonia (21), Gram-positive pneumonia (22), and infection with the intracellular parasite Toxoplasma gondii (23). In contrast, we have found TLR9−/− mice to be markedly resistant to septic peritonitis. One potential explanation for this apparent discrepancy may be the diversity and magnitude of TLR stimulation between infection with a single pathogen and polymicrobial peritonitis. TLR stimulation may be protective up to a certain threshold, beyond which it becomes detrimental. It is conceivable that during infection by a single pathogen there is a restricted degree of TLR stimulation that initiates protective innate immunity, whereas in polymicrobial peritonitis there is widespread TLR stimulation that leads to an exaggerated inflammatory response. Nevertheless, in certain settings, stimulation of individual TLRs can be lethal. For instance, administration of bacterial DNA or LPS after sensitization with d-galactosamine is rapidly lethal (24, 25). In CLP, a more relevant model of clinical sepsis (26), the diverse pathogens residing in the enteric tract stimulate multiple TLRs, but inhibition of TLR9, as we have shown, and not TLR2 or TLR4 (7), affects the outcome. In polymicrobial infection, it has generally been assumed that the stimulation of multiple TLRs collectively promotes the pathological hyperinflammatory response. Our findings, however, suggest a hierarchy of TLR responses, with TLR9 having a major role in the immunopathogenesis of polymicrobial sepsis. This is exemplified by the near abrogation of serum inflammatory cytokines in TLR9−/− mice, a finding also observed in MyD88−/− mice, which are largely devoid of TLR signaling (7).
Collectively, our data demonstrate that the absence of TLR9 signaling during peritoneal sepsis promotes the local influx of DCs, which is associated with an enhanced granulocyte response that is necessary for survival. The dependence on granulocytes may be independent of TLR9 or DC involvement. Nevertheless, we speculate that DCs mediate the enhanced granulocyte recruitment into the peritoneum, because adoptive transfer of TLR9−/− DCs into WT recipients enhanced granulocyte recruitment (Fig. 4 D). DCs are known to produce high levels of chemokines, and their ability to do so has been shown to enhance the recruitment and activation of granulocytes (19). Finally, the availability of inhibitors to human TLR9 provides the opportunity to translate our findings to the treatment of human sepsis.
MATERIALS AND METHODS
Animal procedures.
6–10-wk-old WT CD45.1 and CD45.2 C57BL/6 (B6) mice were purchased from the Jackson Laboratory. TLR9−/− mice on a B6 background (obtained from S. Akira, Osaka University, Osaka, Japan) were bred in our laboratory. For CLP, the surgeon was blinded to the experimental groups, and a midline laparotomy incision was performed in an aseptic fashion and the cecum was ligated distal to the ileocecal valve, taking care not to disrupt bowel continuity. The ligated cecum was punctured once with an 18-gauge needle, as previously described (27). Mice subjected to sham laparotomy underwent the same procedure without the CLP. Mice were monitored every 8 h for 6 d to determine survival. Granulocyte depletion was accomplished with an i.p. injection of 500 μg anti-GR1 antibody (RB6.8C5; Monoclonal Core Facility, Sloan-Kettering Institute) 24 and 2 h before CLP. In vivo blockade of TLR9 was accomplished with a subcutaneous injection of 100 μg iCpG or control DNA sequence (InvivoGen) (28). The animals were maintained in a pathogen-free animal housing facility at the Memorial Sloan-Kettering Cancer Center. All procedures were approved by the Institutional Animal Care and Use Committee.
Cell isolation and adoptive transfer.
Peritoneal cells were isolated by injecting 5 ml PBS i.p. and retrieving the fluid 1 min later. The data depicted in the figures indicate the cell or bacterial count per milliliter of the retrieved peritoneal fluid. Spleens were mechanically disrupted, digested in 0.05% type IV collagenase (Sigma-Aldrich), and rendered free of erythrocytes by treatment with a hypotonic solution of ammonium chloride. In vivo expansion of DCs was accomplished with daily i.p. injections of 10 μg of recombinant human fms-like tyrosine kinase 3 ligand (Flt3L; Amgen) for 10 d. Expanded splenic DCs were purified with anti-CD11c immunomagnetic beads (Miltenyi Biotec). Splenic granulocytes were purified from CD45.1 mice with an anti-Ly6G (1A8) biotinylated antibody and streptavidin microbeads (Milteny Biotec). 107 expanded WT or TLR9−/− CD45.2 splenic DCs or 2 × 106 splenic CD45.1 granulocytes were injected into the lateral tail vein of CD45.1 or CD45.2 WT and TLR9−/− mice, respectively. Adoptively transferred cells were also labeled with 50 μM CFSE (Invitrogen) to facilitate their proper identification.
Flow cytometry.
Flow cytometry was performed on a FACSAria (BD Biosciences). Fc receptors were blocked with 1 μg anti-FcγRIII/II antibody (2.4G2; Monoclonal Core Facility, Sloan-Kettering Institute) per 106 cells. Conventional DCs were defined as CD11chighMHCII+ and granulocytes were defined as Ly6G+ (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20080162/DC1). Cells were stained with fluorescent-conjugated CD11c (HL-3), MHCII (AF6-120.1, I-Ab), CD45.1 (A20), CD45.2 (104), and Ly6G (1A8) antibodies (BD Biosciences). Dead cells were excluded with 7-amino-actinomycin D (BD Biosciences).
Measurement of cytokines and CFUs.
For cytokine measurement, peritoneal cells were cultured at a concentration of 106 cells/ml in media for 24 h. Blood was obtained by direct cardiac puncture. Supernatant and serum cytokine levels were determined using a cytometric bead array (BD Biosciences). Aerobic bacterial CFUs were determined by plating serial dilutions of blood and peritoneal fluid on brain–heart infusion (BHI) agar plates.
Statistics.
Statistical significance was determined by the Student's t test and the log-rank test using statistical software (Prism 4.0; GraphPad Software, Inc.). P < 0.05 was deemed significant.
Online supplemental material.
Fig. S1 shows DC maturation, function, and subset composition in WT and TLR9−/− mice after CLP. Fig. S2 shows that iCpG blocks cytokine secretion by WT splenocytes cultured with cecal contents in vitro. Fig. S3 shows representative gating for flow cytometry used in these experiments. Supplemental materials and methods provides information about antibodies used and the mixed leukocyte reaction performed. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080162/DC1.
Acknowledgments
The authors would like to acknowledge Dr. Eric Pamer for helpful discussions and advice.
This work was supported by National Institutes of Health grants AI70658 and DK068346.
The authors have no conflicting financial interests.
References
- 1.Riedemann, N.C., R.F. Guo, and P.A. Ward. 2003. The enigma of sepsis. J. Clin. Invest. 112:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138–150. [DOI] [PubMed] [Google Scholar]
- 3.Riedemann, N.C., R.F. Guo, and P.A. Ward. 2003. Novel strategies for the treatment of sepsis. Nat. Med. 9:517–524. [DOI] [PubMed] [Google Scholar]
- 4.Russell, J.A. 2006. Management of sepsis. N. Engl. J. Med. 355:1699–1713. [DOI] [PubMed] [Google Scholar]
- 5.Sprung, C.L., D. Annane, D. Keh, R. Moreno, M. Singer, K. Freivogel, Y.G. Weiss, J. Benbenishty, A. Kalenka, H. Forst, et al. 2008. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 358:111–124. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135–145. [DOI] [PubMed] [Google Scholar]
- 7.Weighardt, H., S. Kaiser-Moore, R.M. Vabulas, C.J. Kirschning, H. Wagner, and B. Holzmann. 2002. Cutting edge: myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J. Immunol. 169:2823–2827. [DOI] [PubMed] [Google Scholar]
- 8.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 11:115–122. [DOI] [PubMed] [Google Scholar]
- 9.Qin, S., H. Wang, R. Yuan, H. Li, M. Ochani, K. Ochani, M. Rosas-Ballina, C.J. Czura, J.M. Huston, E. Miller, et al. 2006. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 203:1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss, R.S., K.C. Chang, P.E. Swanson, K.W. Tinsley, J.J. Hui, P. Klender, S. Xanthoudakis, S. Roy, C. Black, E. Grimm, et al. 2000. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 1:496–501. [DOI] [PubMed] [Google Scholar]
- 11.Ishii, K.J., and S. Akira. 2006. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 27:525–532. [DOI] [PubMed] [Google Scholar]
- 12.Sester, D.P., K.J. Stacey, M.J. Sweet, S.J. Beasley, S.L. Cronau, and D.A. Hume. 1999. The actions of bacterial DNA on murine macrophages. J. Leukoc. Biol. 66:542–548. [DOI] [PubMed] [Google Scholar]
- 13.Del Prete, A., W. Vermi, E. Dander, K. Otero, L. Barberis, W. Luini, S. Bernasconi, M. Sironi, A. Santoro, C. Garlanda, et al. 2004. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. EMBO J. 23:3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunn, M.D., S. Kyuwa, C. Tam, T. Kakiuchi, A. Matsuzawa, L.T. Williams, and H. Nakano. 1999. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinsley, K.W., M.H. Grayson, P.E. Swanson, A.M. Drewry, K.C. Chang, I.E. Karl, and R.S. Hotchkiss. 2003. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J. Immunol. 171:909–914. [DOI] [PubMed] [Google Scholar]
- 16.Scumpia, P.O., P.F. McAuliffe, K.A. O'Malley, R. Ungaro, T. Uchida, T. Matsumoto, D.G. Remick, M.J. Clare-Salzler, L.L. Moldawer, and P.A. Efron. 2005. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J. Immunol. 175:3282–3286. [DOI] [PubMed] [Google Scholar]
- 17.Benjamim, C.F., S.K. Lundy, N.W. Lukacs, C.M. Hogaboam, and S.L. Kunkel. 2005. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood. 105:3588–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan, C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173–182. [DOI] [PubMed] [Google Scholar]
- 19.Guo, Z., M. Zhang, H. Tang, and X. Cao. 2005. Fas signal links innate and adaptive immunity by promoting dendritic-cell secretion of CC and CXC chemokines. Blood. 106:2033–2041. [DOI] [PubMed] [Google Scholar]
- 20.Krieg, A.M., T. Wu, R. Weeratna, S.M. Efler, L. Love-Homan, L. Yang, A.K. Yi, D. Short, and H.L. Davis. 1998. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc. Natl. Acad. Sci. USA. 95:12631–12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhan, U., N.W. Lukacs, J.J. Osterholzer, M.W. Newstead, X. Zeng, T.A. Moore, T.R. McMillan, A.M. Krieg, S. Akira, and T.J. Standiford. 2007. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J. Immunol. 179:3937–3946. [DOI] [PubMed] [Google Scholar]
- 22.Albiger, B., S. Dahlberg, A. Sandgren, F. Wartha, K. Beiter, H. Katsuragi, S. Akira, S. Normark, and B. Henriques-Normark. 2007. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell. Microbiol. 9:633–644. [DOI] [PubMed] [Google Scholar]
- 23.Minns, L.A., L.C. Menard, D.M. Foureau, S. Darche, C. Ronet, D.W. Mielcarz, D. Buzoni-Gatel, and L.H. Kasper. 2006. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J. Immunol. 176:7589–7597. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann, V., M.A. Freudenberg, and C. Galanos. 1987. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine–treated mice. J. Exp. Med. 165:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rittirsch, D., L.M. Hoesel, and P.A. Ward. 2007. The disconnect between animal models of sepsis and human sepsis. J. Leukoc. Biol. 81:137–143. [DOI] [PubMed] [Google Scholar]
- 27.Echtenacher, B., W. Falk, D.N. Mannel, and P.H. Krammer. 1990. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J. Immunol. 145:3762–3766. [PubMed] [Google Scholar]
- 28.Cerullo, V., M.P. Seiler, V. Mane, N. Brunetti-Pierri, C. Clarke, T.K. Bertin, J.R. Rodgers, and B. Lee. 2007. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 15:378–385. [DOI] [PubMed] [Google Scholar]