Abstract
To define the roles of endothelial-intrinsic nuclear factor κB (NF-κB) activity in host defense and multiple organ injury in response to sepsis, we generated double transgenic (TG) mice (EC-rtTA/I-κBαmt) that conditionally overexpress a degradation-resistant form of the NF-κB inhibitor I-κBα (I-κBαmt) selectively on vascular endothelium. The EC-rtTA/I-κBαmt mice had no basal, but a relatively high level of doxycycline-inducible, I-κBαmt expression. I-κBαmt expression was detected in endothelial cells, but not in fibroblasts, macrophages, and whole blood cells, confirming that transgene expression was restricted to the endothelium. When subjected to endotoxemia, EC-rtTA/I-κBαmt mice showed endothelial-selective blockade of NF-κB activation, repressed expression of multiple endothelial adhesion molecules, reduced neutrophil infiltration into multiple organs, decreased endothelial permeability, ameliorated multiple organ injury, reduced systemic hypotension, and abrogated intravascular coagulation. When subjected to cecal ligation and puncture–induced sepsis, the TG mice had less severe multiple organ injury and improved survival compared with wild-type (WT) mice. WT and EC-rtTA/I-κBαmt mice had comparable capacity to clear three different pathogenic bacteria. Our data demonstrate that endothelial NF-κB activity is an essential mediator of septic multiple organ inflammation and injury but plays little role in the host defense response to eradicate invading pathogenic bacteria.
Sepsis and septic multiple organ dysfunction and injury (MOD/I) are leading causes of death among critically ill patients. During sepsis, bacterial pathogens trigger the release of hundreds of inflammatory mediators, including cytokines, chemokines, adhesion molecules, reactive oxygen species, and reactive nitrogen species (1–4). Although these molecules are important for host defense response against invading bacterial pathogens, excessive production of these mediators cause systemic inflammation and collateral tissue damage that lead to septic sequelaes, including coagulation, endothelial injury, microvascular leakage, and MOD/I.
Bacterial pathogens initiate systemic inflammation by activating cytokine networks and by inducing the expression of proinflammatory genes, a process that is principally mediated by activating an inducible transcription factor, such as NF-κB. NF-κB activation is a driving force in the initiation and progression of systemic inflammation and septic pathology (4, 5). NF-κB is activated by a variety of bacteria and their products known to cause sepsis syndrome. NF-κB activation appears to be a central common pathway, through which a variety of pathogens cause septic syndrome (4). NF-κB regulates the expression of hundreds of proinflammatory genes, whose products mediate or contribute to the development of septic shock and septic MOD/I (4–6). Patients with septic shock showed an increased NF-κB activity, which correlates with the severity of the disease and predicts clinical outcome (7–9). Animal studies have demonstrated that inhibition of NF-κB activation decreases multiple inflammatory gene expression (4, 10), reverses systemic hypotension (4, 11), corrects myocardial dysfunction (4, 12), diminishes intravascular coagulation (4, 7), reduces tissue neutrophil influx and microvascular endothelial permeability (4, 13), prevents multiple organ injury, and reduces endotoxemic lethality (4, 14) in endotoxemic or septic animals. Mice deficient in NF-κB–dependent genes are resistant to septic shock and sepsis-related death (4).
Despite all of the evidence, the contribution of endothelial NF-κB to the development of a septic phenotype remains unclear. The pathological process underlying sepsis and septic MOD/I involves complex cell–cell and mediator–mediator interactions. Each cell type and its NF-κB system may play distinct roles in this process. The role of endothelial NF-κB in the pathology of sepsis and septic MOD/I has not been well defined. Additionally, disruption of the NF-κB signaling pathway or abolition of NF-κB–dependent genes impairs the host defense capacity to eliminate invading bacteria and leads to a worsened outcome in a bacterial infection model of sepsis (4, 15–17), indicating that NF-κB is also protective. The detrimental versus beneficial effects of NF-κB activation at the organ level may depend on multiple factors, including the cell type in which NF-κB activity is predominantly localized. Thus, elucidation of the specific contribution of each cellular NF-κB to the overall detrimental or beneficial effect of NF-κB activation at the organ level will help to better explain the complicated pathogenic roles of NF-κB in sepsis, septic MOD/I, and other inflammatory conditions.
In this study, we sought to define the roles of endothelial-intrinsic NF-κB activity in multiple organ inflammation and injury (detrimental) and in bacterial clearance (beneficial) using both LPS and cecal ligation and puncture (CLP) models of sepsis. We generated double transgenic (TG) mice that conditionally overexpress a degradation-resistant form of I-κBα (I-κBαmt), a superior inhibitor of NF-κB, selectively on endothelium. Studies on these mice showed that endothelial-selective blockade of NF-κB activation ameliorated multiple organ inflammation and injury in both LPS and CLP models of sepsis but had no effects on bacterial clearance capacity. Our results demonstrate that endothelial NF-κB is an essential mediator of septic multiple organ inflammation and injury but plays little role in the host defense response to eliminate bacterial pathogens.
RESULTS
Generating TG mice that conditionally overexpress I-κBαmt selectively on endothelium
TG mice overexpressing the mutant I-κBα selectively on endothelium have been generated (18). Studies on those mice demonstrated an important role of NF-κB in embryonic development of endothelial lineage (18). Adult mice of this strain have structural and functional defects in endothelial cells (ECs), as indicated by loss of endothelial tight junction, increased sensitivity to LPS-induced endothelial permeability, and enhanced susceptibility to tumor metastasis (18). A similar mouse strain with endothelium-restricted overexpression of I-κBαΔN, an NF-κB super repressor, has also been generated (19). Although the basal structure and function of endothelium were not examined, endothelial NF-κB activity required for embryonic development of endothelial lineage is likely to be suppressed during embryonic development in this mouse strain (19). To avoid the embryonic side effects and examine the effects of endothelial-selective blockade of the NF-κB pathway on septic response under a physiological setting, we generated TG mice that conditionally overexpress I-κBαmt selectively on endothelium using a tetracycline-regulated gene expression system (20). Our TG mice do not express the I-κBαmt gene until it is induced by feeding adult mice with doxycycline (Dox), and have normal NF-κB activity that is critically required for embryonic development and for the development of normal endothelial lineage cells.
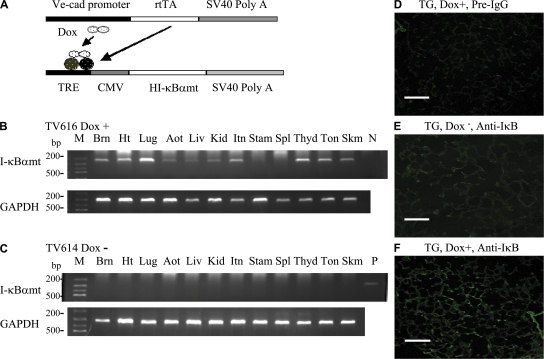
We achieved endothelium-restricted rtTA expression using the vascular endothelial (VE)–cadherin-5 promoter, which has been shown to drive endothelial-specific transgene expression in widespread organs in TG mice (21). I-κBαmt expression was controlled by a TreCMV fusion promoter, whose activation requires the binding of rtTA and Dox (Fig. 1 A).
Figure 1.
Generation of double TG EC-rtTA/I-κBαmt mice. (A) Schematic representation of VeCadrtTA and TreI-κBαmt transgenes. Transactivator (rtTA) expression is controlled by the endothelial-specific promoter VE–cadherin-5 (Ve-cad). Human I-κBαmt gene expression is controlled by a TRE-CMV fusion promoter, whose activation requires the binding of rtTA and Dox. (B and C) RT-PCR photograph showing Dox-induced I-κBαmt mRNA expression in EC-rtTA/I-κBαmt TG mice. Mouse TV616 was fed with Dox for 4 d, and mouse TV614, a transgene-positive littermate of TV616, was not fed with Dox. RT-PCR analysis detected I-κBαmt mRNA expression in 11 out of the 12 organs from mouse TV616 (B) but detected no I-κBαmt expression in any organ from mouse TV614 (C). GAPDH serves as internal control. Aot, aorta; Brn, brain; Ht, heart; Itn, intestine; Kid, kidney; Liv, liver; Lug, lungs; M, DNA marker; N, negative control; P, positive control; Skm, skeletal muscle; Spl, spleen; Stam, stomach; Thyd, thyroid; Ton, tongue. (D–F) Immunofluorescence staining for Dox-induced I-κBαmt protein expression in lung sections of EC-rtTA/I-κBαmt mice. (D) Dox+ mice, preimmune IgG, no staining. (E) Dox− mice, anti–human I-κBα, weak staining (endogenous mouse I-κBα). (F) Dox+ mice, anti–human I-κBα, stronger staining (Dox-induced I-κBαmt protein). Bars, 100 μm.
One inherent weakness of the tetracycline-regulated gene expression system is so-called “leakiness” (basal transgene expression in the absence of Dox). To resolve the “leaky” problem, we generated seven independent EC-rtTA and six independent TreI-κBαmt single TG mouse lines. We intercrossed each of the EC-rtTA lines with each of the TreI-κBαmt lines, which gave rise to 42 double TG mouse lines that carried both the rtTA and I-κBαmt transgenes (EC-rtTA/I-κBαmt mice). We performed RT-PCR analysis of basal and Dox-induced I-κBαmt mRNA expression using transgene-specific primers and RNAs from 12 organs of each EC-rtTA/I-κBαmt mouse line. Those analyses revealed that 8 out of the 42 EC-rtTA/I-κBαmt mouse lines had high basal and low Dox-induced I-κBαmt mRNA expression (leakiness), 31 out of the 42 lines had no basal but little Dox-induced I-κBαmt mRNA expression (no expression), and 3 out of the 42 lines had no basal and relatively high level of Dox-induced I-κBαmt mRNA expression. Among the 3 good lines, line 2/36 was the best and was further studied. Fig. 1, B and C shows I-κBαmt mRNA expression in 12 organs of mouse line 2/36. After 35 cycles of PCR amplification, no I-κBαmt mRNA was detected in any organ from a mouse that was not fed with Dox (Fig. 1 C). I-κBαmt mRNA was detected in all organs examined, except spleen, from a mouse that was fed with Dox (Fig. 1 B). Further analysis of the spleen sample showed an I-κBαmt mRNA expression, albeit at a low level (unpublished data). The Dox-inducible I-κBαmt mRNA abundance varied among organs in a pattern consistent with the enrichment of ECs in these organs (Fig. 1 B).
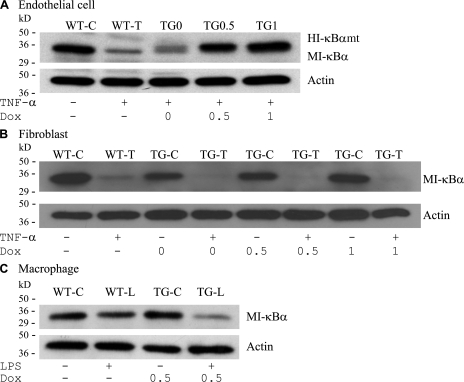
We performed immunofluorescence staining of lung section from mouse line 2/36 using anti–human I-κBα antibody, which has a low level of cross-reactivity with mouse I-κBα. Immunofluorescence staining showed Dox-induced I-κBαmt protein expression on lung sections from mice fed with Dox (Fig. 1, D–F). To ascertain if Dox-induced I-κBαmt protein expression is restricted to ECs, we isolated vascular ECs and fibroblasts from the lungs and macrophages from the peritoneal cavity of line 2/36 mice (TG) and their transgene-negative littermates (WT). These cells exhibited morphological characteristics of ECs, fibroblasts, and macrophages, respectively. The EC phenotype was further confirmed by staining for endothelial-specific markers, platelet/EC adhesion molecule 1, and intercellular adhesion molecule (ICAM) 1. Macrophages and fibroblasts were confirmed by staining with anti-Mac3 (macrophage marker) and anti-procollagen (fibroblast marker) antibodies. Western blotting using an antibody reactive to human and mouse I-κBα detected a single I-κBα band (endogenous mouse I-κBα) in the cytoplasmic protein of WT ECs, but two I-κBα bands in the cytoplasmic protein of TG ECs (Fig. 2 A, TG0). The top band (Fig. 2 A, HI-κBαmt) represents transgene product, and the bottom band (Fig. 2 A, MI-κBα) represents endogenous mouse I-κBα. The HI-κBαmt band (top) was weak in control ECs (Fig. 2 A, TG0) but increased in Dox-treated ECs (Fig. 2 A, TG0.5 and TG1). The endogenous MI-κBα band (bottom) diminished after TNF-α treatment (Fig. 2 A, WT-T), indicating degradation of endogenous I-κBα, whereas the Dox-induced HI-κBαmt band (top) was not affected by TNF-α treatment (Fig. 2 A, TG0.5 and TG1), indicating degradation resistance of the TG HI-κBαmt protein. Western blotting detected a single I-κBα band in cytoplasmic proteins of both WT and TG fibroblasts from the same lungs where ECs were isolated (Fig. 2 B). The I-κBα band intensity was not affected by treatment with the same concentrations of Dox (Fig. 2 B) but was diminished by TNF-α treatment (Fig. 2 B), representing endogenous MI-κBα. Similarly, both WT and TG macrophages expressed a single mouse I-κBα protein, which was significantly degraded by TNF-α treatment (Fig. 2 C), confirming that TG macrophages do not express TG I-κBαmt.
Figure 2.
Endothelial-selective I-κBαmt protein expression in EC-rtTA/I-κBαmt mice. (A) TG, but not WT, ECs express Dox-induced I-κBαmt protein. WT ECs were untreated (WT-C) or stimulated with TNF-α (WT-T) for 15 min. TG ECs were untreated (TG0) or treated with 0.5 μg/ml (TG0.5) or 1 μg/ml (TG1) Dox for 48 h to induce I-κBαmt expression, and stimulated with TNF-α for 15 min. Western blotting using anti–I-κBα antibody detected a single band on cytoplasmic protein from WT ECs, and two bands on cytoplasmic protein from TG ECs, one representing endogenous mouse I-κBα (MI-κBα) and the other representing the transgene product human I-κBαmt (HI-κBαmt). The HI-κBαmt band was weak in cells without Dox incubation (TG0), but its intensity increased with increasing doses of Dox (TG0.5 and TG1). The MI-κBα band was diminished by TNF-α treatment, indicating degradation, whereas the HI-κBαmt band was not affected by TNF-α stimulation, indicating degradation resistance. (B) TG fibroblasts express no Dox-induced I-κBαmt protein. Fibroblasts were isolated from the same lungs where ECs were isolated, cultured, and treated with Dox and TNF-α in an identical way as in ECs. Western blotting detected a single MI-κBα band, which was diminished by TNF-α treatment, but detected no HI-κBαmt band. TG-C, untreated TG cells; TG-T, TNF-α–treated TG cells. (C) TG macrophages express no Dox-induced I-κBαmt protein. TG macrophages were treated with Dox to induce I-κBαmt expression. Cells were unstimulated (WT-C and TG-C) or stimulated with LPS (WT-L and TG-L) for 30 min. Western blotting detected a single MI-κBα band, which was diminished by LPS treatment, but detected no Dox-induced HI-κBαmt band. Membranes for I-κBα were reblotted with actin antibody (Actin).
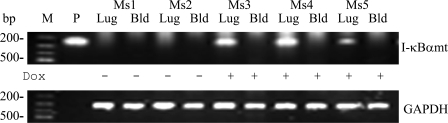
The VE–cadherin-5 promoter is reported to drive the Cre recombinase gene expression in a subset of hematopoietic cells in TG mice (22). We have therefore examined whether hematopoietic lineage cells express the I-κBαmt transgene in our TG EC mice by performing RT-PCR analysis of I-κBαmt mRNA expression in whole blood cells and lungs (as a positive control) from line 2/36 TG mice. As illustrated in Fig. 3, I-κBαmt mRNA was not detected in either lungs or blood cells of the two TG mice that were not fed with Dox. The I-κBαmt band was detected in lungs but not in whole blood cells from the three TG mice that were fed with Dox for 4 d (Fig. 3). Thus, hematopoietic cells of our TG mice are unlikely to express I-κBαmt mRNA. Collectively, our results demonstrate that our EC-rtTA/I-κBαmt mice overexpress Dox-inducible I-κBαmt selectively on endothelium.
Figure 3.
I-κBαmt mRNA expression in whole blood cells of EC-rtTA/I-κBαmt mice. Mice 1 and 2 (Ms1 and Ms2) were not fed with Dox, and mice 3–5 (Ms3, Ms4, and Ms5) were fed with Dox for 4 d. RT-PCR detected no I-κBαmt expression in whole blood cells and lungs of Ms1 and Ms2 (without Dox), and detected a strong I-κBαmt band in lungs but not in whole blood cells of Ms3, Ms4, and Ms5 (with Dox). GAPDH serves as internal control. Bld, whole blood cells; Lug, lungs; M, DNA marker; P, positive control.
EC-rtTA/I-κBαmt mice selectively block NF-κB activation in ECs
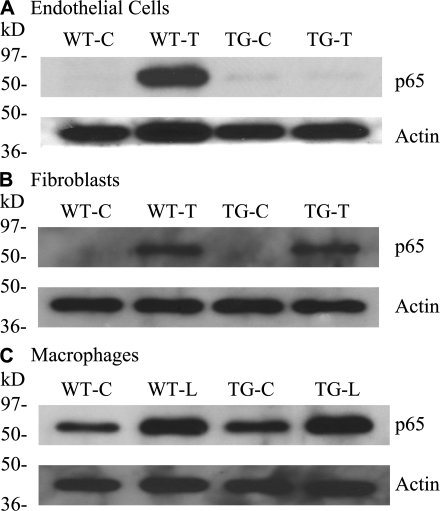
To examine the functionality of the overexpressed I-κBαmt protein and to confirm that NF-κB blockade is endothelial restricted, TNF-α– or LPS-induced nuclear translocation of p65 was compared between WT and TG lung ECs and fibroblasts, and peritoneal macrophages. To avoid the effects of Dox, all cells were treated with Dox. Compared with control cells, TNF-α or LPS stimulation markedly increased p65 nuclear accumulation in all cell types (Fig. 4, A–C, WT-T and WT-L), which was blocked in TG ECs (Fig. 4 A, TG-T) but not in fibroblasts (Fig. 4 B, TG-T) and macrophages (Fig. 4 C, TG-L). Thus, our TG mice selectively blocked TNF-α– or LPS-induced NF-κB activation in vascular ECs.
Figure 4.
Endothelial-selective blockade of NF-κB activation in EC-rtTA/I-κBαmt mice. We used p65 nuclear translocation as an indicator of NF-κB activation. All cells were treated with Dox for 48 h to avoid the Dox effect. Control cells (WT-C and TG-C) were untreated. Treated ECs and fibroblasts were stimulated with TNF-α (WT-T and TG-T) for 15 min, and macrophages were stimulated with LPS (WT-L and TG-L) for 30 min. Western blotting detected a strong p65 band in nuclear proteins from all three WT cells stimulated with TNF-α or LPS (WT-T and WT-L), indicating NF-κB activation. The TNF-α– or LPS-induced p65 band was abrogated in TG ECs (A, TG-T) but not in TG fibroblasts and TG macrophages (B and C, TG-T and TG-L), indicating EC-selective blockade of NF-κB activation. Membranes for p65 were reblotted with actin antibody (Actin).
Reduced expression of endothelial adhesion molecules in EC-rtTA/I-κBαmt mice
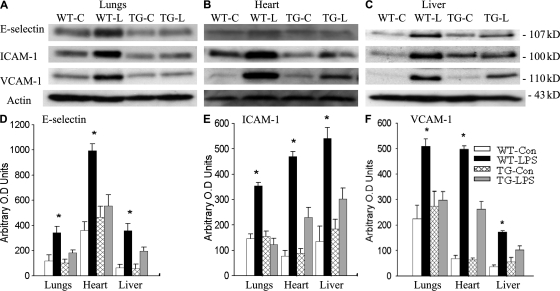
To study the consequence of endothelial-selective blockade of NF-κB activation, we examined tissue levels of E-selectin, ICAM-1, and vascular adhesion molecule (VCAM) 1 expressions in the lungs, heart, and liver of WT and TG mice subjected to endotoxemia. Fig. 5 (A–C) depicts Western blot photographs showing tissue levels of E-selectin, ICAM-1, and VCAM-1 protein expression in three organs of WT-Con, WT-LPS, TG-Con, and TG-LPS mice. Western blot bands were quantified using densitometry and summarized in Fig. 5 (D–F). Compared with WT-con and TG-con, tissue E-selectin, ICAM-1, and VCAM-1 protein levels increased significantly in all three organs of WT-LPS mice (Fig. 5). The LPS-induced expression of the three adhesion molecules was significantly reduced in the three organs of TG-LPS mice (Fig. 5).
Figure 5.
Repressed expression of adhesion molecules in EC-rtTA/I-κBαmt mice. (A–C) Western blot photographs showing levels of E-selectin, ICAM-1, and VCAM-1 protein expression in the lungs (A), heart (B), and liver (C) of WT-Con (WT-C), WT-LPS (WT-L), TG-Con (TG-C), and TG-LPS (TG-L) mice. Membrane for E-selectin, ICAM-1, or VCAM-1 blotting was reblotted with actin antibody (Actin). (D–F) Densitometry quantification of E-selectin, ICAM-1, and VCAM-1 bands. Tissue levels of the three adhesion molecules were similar between WT-Con and TG-Con mice. Compared with WT-LPS mice, the LPS-induced E-selectin, ICAM-1, and VCAM-1 protein expression was significantly reduced in all three organs of TG-LPS mice. The means ± SEM of five animals are shown. *, P < 0.05 compared with the other three groups.
Reduced neutrophil infiltration and ameliorated multiple organ injury in endotoxemic EC-rtTA/I-κBαmt mice
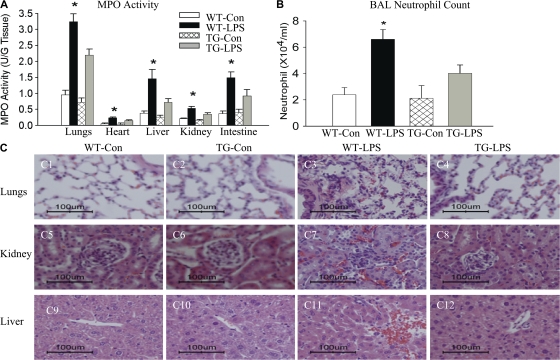
Using tissue myeloperoxidase (MPO) activity as a marker, we examined neutrophil infiltration into the lungs, heart, liver, kidney, and intestine. Compared with WT-Con and TG-Con mice, WT-LPS mice showed a two- to threefold increase in tissue MPO activity in all five organs examined (Fig. 6 A). Tissue MPO activity in the five organs of TG-LPS mice was significantly lower than that in WT-LPS mice (Fig. 6 A). Consistent with changes in MPO activity, WT-LPS mice showed a marked increase in bronchoalveolar lavage fluid neutrophil count, which reduced significantly in TG-LPS mice (Fig. 6 B). PMN infiltration into organs was further demonstrated by histological examination (Fig. 6 C; and see Discussion). These results demonstrate that endothelial-selective NF-κB blockade reduces neutrophil infiltration into multiple organs.
Figure 6.
Reduced neutrophil infiltration and alleviated organ injury in endotoxemic EC-rtTA/I-κBαmt mice. (A) MPO activity in the lungs, heart, liver, kidney, and intestine of WT and TG mice treated with saline or LPS for 4 h. Compared with WT-Con and TG-Con mice, WT-LPS mice showed a marked increase in MPO activity in all five organs, which was significantly reduced in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (B) Neutrophil counts in BAL fluids. Compared with WT-Con and TG-Con mice, WT-LPS mice had a marked increase in BAL fluid neutrophil count, which was reversed in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (C) Histopathological evaluation of PMN infiltration and organ injury. Paraffin-embedded sections were prepared from the lungs, kidney, and liver of WT and TG mice treated with saline or LPS for 5 h, and subjected to hematoxylin and eosin staining. Lungs of WT-Con and TG-Con mice show thin alveolar wall and normal cellularity (C1 and C2). The lung of a WT-LPS mouse shows intense cellular infiltration, alveolar septal wall thickness, interstitial edema, and alveolar congestion (C3). Kidneys of WT-Con and TG-Con mice show normal histological structures of cortex with glomerulus (C5 and C6). The kidney of a WT-LPS mouse shows increased cellular infiltration, glomerular and sinusoidal congestion, and signs of tubular swelling and cell injury (C7). Livers of WT-Con and TG-Con mice show a normal histological appearance of the central vein surrounded by hepatocytes and sinusoids (C9 and C10). The liver of a WT-LPS mouse shows a congested central vein, hemorrhage, and signs of hepatic cell injury and necrosis (C11). The pathological changes caused by LPS were significantly alleviated in TG-LPS mice in all three organs (C4, C8, and C12). Bars, 100 μm.
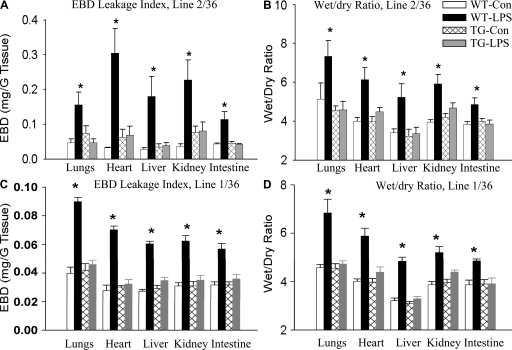
We assessed organ injury by measuring endothelial Evans blue dye (EBD) leakage index and by determining tissue water accumulation using tissue wet/dry ratio as an indicator. As illustrated in Fig. 7 (A and B), endothelial leakage index and tissue wet/dry ratio in all five organs were similar between WT-Con and TG-Con mice. WT-LPS mice showed a marked increase in EBD leakage index and tissue wet/dry ratio in all five organs (Fig. 7, A and B). The increases in EBD leakage index and tissue wet/dry ratio were reversed in all five organs of TG-LPS mice in mouse line 2/36 (Fig. 7, A and B). To ascertain that protection from endotoxemic organ injury in TG mice is a result of NF-κB inhibition but not a result of other changes in TG mice, we also examined the protective effect of endothelial-selective I-κBαmt overexpresion on LPS-induced multiple organ injury in mouse line 1/36, our second-best EC TG line. Likewise, the LPS-induced increases in EBD leakage index and tissue wet/dry ratio were reversed in all five organs of line 1/36 TG-LPS mice (Fig. 7, C and D).
Figure 7.
Reduced endothelial permeability and organ edema in endotoxemic EC-rtTA/I-κBαmt mice. (A) EBD leakage index in the lungs, heart, liver, kidney, and intestine of WT and line 2/36 TG mice 5 h after LPS injection. Compared with WT-Con and TG-Con mice, WT-LPS mice had a marked increase in EBD leakage index in all five organs, which was reversed in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (B) Tissue wet/dry ratio in the lungs, heart, liver, kidney, and intestine of WT and line 2/36 TG mice 5 h after LPS injection. Compared with WT-Con and TG-Con mice, WT-LPS mice showed a significantly increased tissue wet/dry ratio in all five organs, which was reversed in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (C) EBD leakage index in the lungs, heart, liver, kidney, and intestine of WT and line 1/36 TG mice 5 h after LPS injection. WT-LPS mice had a marked increase in EBD leakage index in all five organs, which was reversed in TG-LPS mice. The means ± SEM of six animals are shown. *, P < 0.05 compared with the other three groups. (D) Tissue wet/dry ratio in the lungs, heart, liver, kidney, and intestine of WT and line 1/36 TG mice 5 h after LPS injection. WT-LPS mice showed a significantly increased tissue wet/dry ratio in all five organs, which was reversed in TG-LPS mice. The means ± SEM of six animals are shown. *, P < 0.05 compared with the other three groups.
Multiple organ injury and inflammatory cell infiltration were further evaluated by histopathological examination of lung, liver, and kidney sections from WT and line 2/36 TG mice, which revealed multiple organ inflammation and injury in WT-LPS mice. Intense PMN infiltration, interstitial edema, and signs of endothelial and epithelial damage were observed in lung sections (Fig. 6 C, C1–C4). Increased PMN infiltration, glomerular and sinusoidal congestion, and signs of renal tubular and hepatic injury were observed in kidney (Fig. 6 C, C5–C8) and liver (Fig. 6 C, C9–C12) sections. The histopathological changes caused by LPS were significantly alleviated in TG-LPS mice (Fig. 6 C, C4, C8, and C12). Collectively, these results demonstrate that endothelial-selective NF-κB blockade effectively prevents endotoxemic multiple organ injury.
Alleviated multiple organ injury and improved survival in septic EC-rtTA/I-κBαmt mice
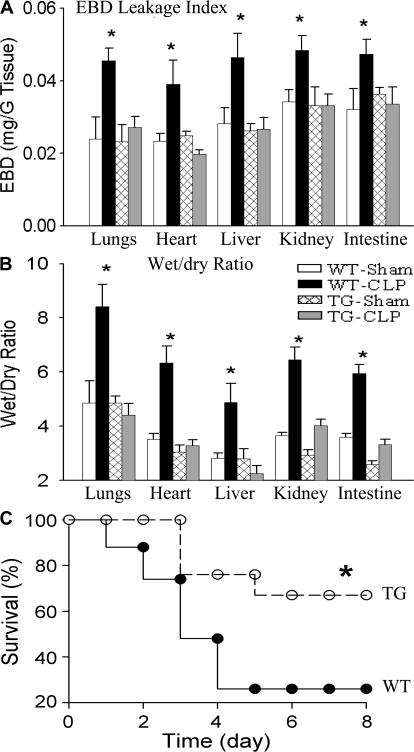
WT-sham and TG-sham mice showed similar endothelial EBD leakage index and tissue wet/dry ratio in the lungs, heart, liver, kidney, and intestine (Fig. 8, A and B). Compared with WT-sham and TG-sham mice, WT-CLP mice exhibited significantly increased endothelial EBD leakage index and tissue wet/dry ratio (Fig. 8, A and B). The increases in EBD leakage index and tissue wet/dry ratio were prevented in all five organs of TG-CLP mice (Fig. 8, A and B), indicating that endothelial-selective NF-κB inhibition prevents septic multiple organ injury. Ameliorated organ injury has resulted in a reduced mortality. As illustrated in Fig. 8 C, the 2-wk survival rate was 26% in WT-CLP mice and 67% in TG-CLP mice. Thus, we have demonstrated that endothelial-selective blockade of NF-κB activation inhibits multiple organ inflammation, prevents multiple organ injury, and improves survival in mice subjected to endotoxemia or sepsis.
Figure 8.
Reduced organ injury and improved survival in septic EC-rtTA/I-κBαmt mice. (A) EBD leakage index in the lungs, heart, liver, kidney, and intestine of WT and line 2/36 TG mice 24 h after sham or CLP operation. Compared with WT-sham and TG-sham mice, WT-CLP mice showed a marked increase in EBD leakage index in all five organs, which was reversed in TG-CLP mice. The means ± SEM of eight animals are shown. *, P < 0.05 compared with the other three groups. (B) Tissue wet/dry ratio in the lungs, heart, liver, kidney, and intestine of WT and line 2/36 TG mice. Compared with WT-sham and TG-sham mice, WT-CLP mice showed a significantly increased tissue wet/dry ratio, which was reversed in TG-CLP mice. The means ± SEM of eight animals are shown. *, P < 0.05 compared with the other three groups. (C) Survival of WT-CLP and TG-CLP mice. Animals were subjected to CLP and followed for 14 d (no further mortality after 8 d). Compared with WT mice, TG mice showed a significantly improved survival. *, P < 0.0003 compared with WT mice using the log-rank test (21 mice per group).
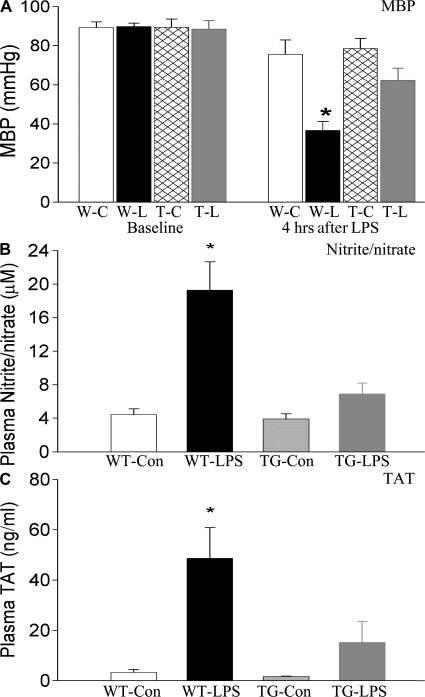
Reduced systemic hypotension and coagulation in endotoxemic EC-rtTA/I-κBαmt mice
Systemic hypotension and coagulation are two major factors contributing to septic MOD/I. We have, therefore, examined the effects of endothelial-selective NF-κB inhibition on systemic hypotension and coagulation in endotoxemic mice. Baseline systemic mean arterial blood pressure (MBP) was identical among WT-Con, TG-Con, WT-LPS, and TG-LPS groups of mice. At 4 h after LPS challenge, MBP dropped by ∼50% in WT-LPS mice, which was virtually reversed in TG-LPS groups of mice (Fig. 9 A). In parallel with a drop in MBP, WT-LPS mice showed a marked elevation in the plasma level of nitrite/nitrate, the stable end products of nitric oxide, which was significantly lower in that of TG-LPS mice (Fig. 9 B). Compared with WT-Con and TG-Con mice, WT-LPS mice had a significantly elevated plasma level of thrombin–antithrombin (TAT) complex, an indicator of intravascular coagulation (Fig. 9 C). Compared with WT-LPS mice, TG-LPS mice showed a significantly lower plasma level of TAT (Fig. 9 C).
Figure 9.
Reduced systemic hypotension and coagulation in endotoxemic EC-rtTA/I-κBαmt mice. (A) Systemic MBP was monitored before (baseline) and at 4 h after LPS challenge. Baseline MBP was identical among the four groups of mice. Compared with WT-Con (W-C) and TG-Con (T-C) mice, WT-LPS (W-L) mice showed a significant drop in MBP at 4 h after LPS, which was reduced in TG-LPS (T-L) mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (B) Plasma concentration of nitrite/nitrate in WT and EC-rtTA/I-κBαmt mice. Plasma levels of nitrite/nitrate were low in WT-Con and TG-Con mice, markedly elevated in WT-LPS mice, and significantly reduced in TG-LPS mice, as compared with WT-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups. (C) Plasma levels of TAT complex in WT and EC-rtTA/I-κBαmt mice. The plasma level of TAT, an indicator of coagulation, was very low in WT-Con and TG-Con mice but markedly elevated in WT-LPS mice, and reduced significantly in TG-LPS mice. The means ± SEM of seven animals are shown. *, P < 0.05 compared with the other three groups.
Blockade of endothelial NF-κB had no effects on bacterial clearance capacity
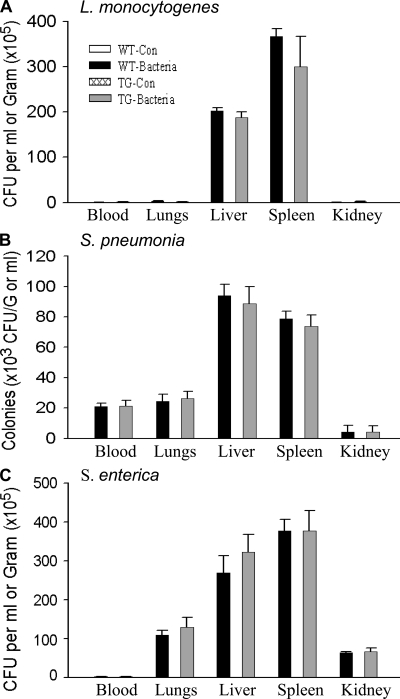
To examine the role of endothelial NF-κB in host defense response to eliminate bacterial pathogens, mice were infected with the intracellular bacterium Listeria monocytogenes, the extracellular gram-positive bacterium Streptococcus pneumoniae, and the intracellular gram-negative bacterium Salmonella enterica. Bacterial colonies that formed in blood and tissue homogenate cultures were counted and compared between WT and TG mice. Little bacteria grew in blood and tissue homogenates of WT-Con and TG-Con mice (Fig. 10, A–C). Consistent with these organs being the main sites of Listeria infection, L. monocytogenes grew in liver and spleen homogenates of infected mice. The numbers of Listeria colonies formed were identical or similar between WT and TG mice (Fig. 10 A). S. pneumoniae and S. enterica grew in the lungs, liver, kidney, and spleen homogenates of infected mice (Fig. 10, B and C). The number of colonies formed varied among organs but were identical between WT and TG mice in all organs examined (Fig. 10, B and C). These results illustrate that WT and EC-rtTA/I-κBαmt mice have identical capacities to eliminate invading bacteria, suggesting that endothelial NF-κB does not play an important role in mediating host defense response against bacterial pathogens.
Figure 10.
EC-rtTA/I-κBαmt mice preserve bacterial clearance capacity. WT and TG mice were injected with saline or pathogenic bacteria. Bacterial colonies that formed in blood and tissue homogenate cultures were counted and expressed as CFU per milliliter of blood or per gram of tissue. (A) Bacterial CFU in blood and tissue homogenates of the lungs, liver, spleen, and kidney collected 24 h after i.v. injection of 108 CFU of L. monocytogenes. No bacteria grew in the blood and any tissue homogenate from WT-Con and TG-Con mice. WT-Bacteria and TG-Bacteria mice showed comparable CFU in blood and organ homogenates. The means ± SEM of six animals are shown. (B) Bacterial CFU in blood and tissue homogenates of the lungs, liver, spleen, and kidney collected 12 h after i.v. injection of 107 CFU of S. pneumoniae. No bacteria grew in the blood and any tissue homogenate from WT-Con and TG-Con mice. WT-Bacteria and TG-Bacteria mice showed comparable CFU in blood and organ homogenates. The means ± SEM of six animals are shown. (C) Bacterial CFU in blood and tissue homogenates of the lungs, liver, spleen, and kidney collected 12 h after i.v. injection of 108 CFU of S. enterica. No bacteria grew in the blood and tissue homogenates from WT-Con and TG-Con mice. WT-Bacteria and TG-Bacteria mice showed comparable CFU in blood and organ homogenates. The means ± SEM of six animals are shown.
DISCUSSION
The major finding of this study is that endothelial NF-κB plays distinct roles in the inflammatory/injurious and host defense responses. Endothelial NF-κB activity is critically required for the inflammatory and injurious responses that lead to septic multiple organ inflammation and injury. Compared with WT mice, TG mice that have the endothelial NF-κB pathway blocked showed decreased expression of multiple adhesion molecules, reduced neutrophil infiltration into multiple organs, reduced systemic hypotension, alleviated endothelial injury, and ameliorated multiple organ injury when subjected to endotoxemia. Those TG mice also exhibited alleviated multiple organ injury and improved survival in the CLP model of sepsis. Thus, selective blockade of the endothelial NF-κB pathway is sufficient to reduce multiple organ inflammation, prevent multiple organ injury, and improve survival, indicating that endothelial NF-κB plays a critical role in septic multiple organ inflammation and injury. In supporting this contention, Henke et al. have recently reported that endothelial-specific NF-κB suppression attenuates hypertension-induced renal damage (19).
In contrast, endothelial NF-κB does not appear to play an important role in host defense response to eradicate invading bacterial pathogens. When infected with three different classes of pathogenic bacteria, S. pneumoniae, L. monocytogenes, and S. enterica, WT and TG mice with endothelial-selective NF-κB inhibition exhibited identical bacterial clearance capability. Because clearance of the three pathogenic bacteria involves a different host defense mechanism (23), the lack of effect of blocking endothelial NF-κB on bacterial clearance indicates that endothelial NF-κB does not play an important role in host defense response to eliminate those pathogenic bacteria.
NF-κB activation is a key component of host immune response (4). NF-κB p50 knockout mice showed severely defective clearance of S. pneumoniae and L. monocytogenes (15). The lack of effect of endothelial-selective NF-κB inhibition on bacterial clearance is somewhat surprising. It would be interesting to know which cellular NF-κB system plays a major role in the host defense response against bacterial pathogens. TG mice whose hepatocyte NF-κB pathway is selectively inhibited displayed a severely impaired capacity to clear L. monocytogenes (16), indicating a requirement of hepatocyte NF-κB activity for the host immune response against L. monocytogenes infection. However, the same immune response does not appear to involve the endothelial NF-κB system, because blocking endothelial NF-κB had no effect on the clearance of this bacterium, as we demonstrated in this study. These results suggest that the involvement of a particular cellular NF-κB system in the host defense response may be pathogen dependent. This question warrants further investigation.
Investigation into the role of NF-κB activation in sepsis and other inflammatory conditions has been hampered by the fact that NF-κB knockout mice have multifocal defects in immune responses and are embryonically lethal (15, 24–27). Conventional TG mice overexpressing I-κBαmt selectively on endothelium have structural and functional defects in endothelium, as indicated by loss of endothelial tight junction, increased sensitivity to LPS-induced endothelial permeability, and enhanced susceptibility to tumor metastasis (18). To overcome those problems, we took a conditional TG approach. We generated TG mice that conditionally overexpress I-κBαmt selectively on endothelium using a tetracycline-regulated gene expression system (20), which has been widely used to define various gene functions in TG animals (12, 16, 28–31). Our TG mice do not express I-κBαmt until induced by feeding adult mice with Dox, and have normal NF-κB activity that is critically required for embryonic development, avoiding embryonic side effects seen in NF-κB knockout or conventional TG mice. NF-κB inhibition in our TG mice is transient and restricted to endothelium, which would have minimal effects on immune cell differentiation, development, and function, allowing us to study septic or other inflammatory responses under a physiological setting.
I-κBαmt bears two mutations at serine 32 and 36 residues, which prevent its phosphorylation and subsequent degradation (32), a key step leading to the activation of the classical NF-κB pathway. As a specific and effective NF-κB inhibitor, I-κBαmt has been widely used to define the role NF-κB in various pathological processes (12, 16, 33, 34). The overexpressed I-κBαmt protein is degradation resistant and constantly binds to any form of NF-κB dimer, resulting in a more effective blockade of the NF-κB pathway and resolving the problem of functional redundancy between the NF-κB family of proteins seen in single NF-κB knockout mice.
Endothelial dysfunction, inflammation, and injury are increasingly recognized as an important pathogenic mechanism of many pathological conditions, including sepsis (35), hypertension (36), and atherosclerosis (37). Endothelial phenotypic changes are dictated by alteration in the expression of endothelial-specific genes. The ability to selectively manipulate endothelial gene expression in vivo will allow investigators to clearly define the role of individual endothelial-specific gene in the alteration of endothelial phenotypes. Therefore, there has been great interest in endothelial-selective gene knockout or TG animal models. Conventional gene knockout and TG animals have limitations such as developmental defects and embryonic lethality (18, 24–27), which limit their application and relevance to the adult disease state. In this context, the conditional TG approach is clearly advantageous. Although the Tet-regulated gene expression system (20) has been successfully applied to various fields (28–31, 33, 34), its application to endothelial-selective gene expression has been less successful. Coupling the tie2 promoter to the Tet-off system, Sarao and Dumont were able to direct the reporter gene (LacZ) expression only in embryonic endothelium (38). Using an extended Tie promoter coupled to the Tet-on system, Teng et al. were able to drive LacZ expression on the endothelium of adult mice, although those mice have leakiness (39). In the current study, we took a different approach by using the VE–cadherin-5 promoter (21) to drive endothelial-selective rtTA expression and by generating multiple independent rtTA and TreI-κBαmt mouse lines. Intercrossing between those independent rtTA and TreI-κBαmt lines produced 42 double TG mouse lines, which allowed us to select the best dual TG lines. Immunofluorescence staining and cell-culture experiments confirmed that our TG mice express Dox-inducible I-κBαmt protein in ECs but not in fibroblasts and macrophages. The time-consuming and labor-intensive screening and selection processes were proven to be necessary, as 73.8% of the 42 double TG lines had little Dox-inducible I-κBαmt expression, and 19% of them had a leaky problem, indicating the technical difficulty of applying the Tet-on system to endothelial-specific gene expression. To our knowledge, this is the first study that has successfully achieved endothelial-specific expression of a real mammalian gene in TG mice using the Tet-on system. This mouse model allows us to study endotoxemic or septic responses under a physiological setting and provides a useful tool for studying in vivo endothelial biology in any disease model in which endothelial dysfunction, inflammation, or injury plays a role. Our EC-rtTA mice can be intercrossed with any mouse strain carrying a Tre responder gene and would be a valuable tool for conditional gene manipulation in endothelium.
In summary, by using the Tet-on system, we have created double TG mice that conditionally express I-κBαmt selectively on vascular endothelium. Our EC-rtTA/I-κBαmt mice express no basal but a relatively high level of Dox-inducible I-κBαmt on ECs, but not on fibroblasts, macrophages, and whole blood cells. When subjected to endotoxemia or sepsis, EC-rtTA/I-κBαmt mice showed endothelial-selective blockade of NF-κB activation, repressed expression of multiple endothelial adhesion molecules, reduced neutrophil infiltration into multiple organs, decreased endothelial permeability, ameliorated multiple organ injury, and improved survival. EC-rtTA/I-κBαmt mice exhibited significantly reduced systemic hypotension under the condition of endotoxemia. Compared with WT mice, EC-rtTA/I-κBαmt mice had an identical capacity to clear three major pathogenic bacteria. These results demonstrate that endothelial NF-κB plays divergent roles in the inflammatory/injurious and host defense responses against bacterial infection. Endothelial NF-κB activity is critically required for the inflammatory and injurious responses that lead to septic multiple organ inflammation and injury, but it plays little role in the host defense response to eradicate invading pathogenic bacteria.
MATERIALS AND METHODS
Generation of VE-rtTA/I-κBαmt mice.
All animal protocols and experiments were approved by the institute's institutional animal care and use committee, and complied with National Institutes of Health guidelines for the care and use of laboratory animals.
VeCadrtTA and TreI-κBαmt transgene constructs are illustrated in Fig. 1 A. The 2.5-kb mouse VE–cadherin-5 promoter was generated by PCR amplification and inserted into a Tet-on plasmid DNA (Clontech Laboratories, Inc.), replacing the CMV promoter. TreI-κBαmt was constructed by inserting the full-length of human I-κBαmt cDNA (supplied by U. Siebenlist, National Institute of Allergy and Infectious Diseases, Bethesda, MD) into the TRE plasmid DNA (Clontech Laboratories, Inc.). Authenticities of PCR products and DNA constructs were confirmed by DNA sequencing.
The transgenes were linealized, purified, and injected into fertilized FVB mouse eggs at the Albert Einstein College of Medicine Transgenic Core Facility. Potential founders of VE-rtTA and TreI-κBαmt mice were identified by Southern blot analysis of genomic DNA from tail biopsies. All founders were bred to homozygous and developed to mouse lines. RT-PCR analysis was performed using RNAs from the organs of VE-rtTA mice to verify rtTA mRNA expression. Dual TG EC-rtTA/I-κBαmt mice that carry both the VE-rtTA and TreI-κBαmt transgenes were created by intercrossing the VE-rtTA and TreI-κBαmt mouse lines.
EC-rtTA/I-κBαmt mice were fed without (for control) or with 1.5 mg/ml Dox in the drinking water for 4 d. The dose and duration of Dox were determined in separate time-course and dose–response studies. Total RNAs were extracted from the brain, heart, lungs, aorta, liver, spleen, kidney, stomach, intestine, thyroid, tongue, skeletal muscle, and whole blood cells; digested with RNase-free DNase; and reverse transcribed into cDNAs. The cDNAs (50 ng) were PCR amplified using transgene-specific primers and conditions as follow: 1 cycle of 95°C for 4 min; 35 cycles of 95°C for 45 s, 60°C for 40 s, and 72°C for 45 s; and 1 cycle of 72°C for 10 min.
Animal groups.
We studied four groups of mice: transgene-negative control (WT-Con), transgene-negative LPS (WT-LPS), EC-rtTA/I-κBαmt control (TG-Con) and EC-rtTA/I-κBαmt LPS (TG-LPS) mice.
Western blotting.
Cytoplasmic, nuclear, and membrane proteins were extracted from cultured cells or various organs of the mice. Equal amounts of protein were separated on 7.5–10% SDS-PAGE, electroblotted onto a polyvinylidene fluoride membrane. Western blotting was performed as we previously described (40), using antibodies against I-κBα, p65, E-selectin, ICAM-1, VCAM-1, and actin.
Histology and immunofluorescence staining.
For histological examination, 5-μm paraffin-embedded sections were prepared from the lungs, kidney, and liver of WT and TG mice fed with Dox, stained with hematoxylin and eosin, and examined under a light microscope (Optithot; Nikon) with a digital camera (DXM1200; Nikon). For immunofluorescence staining, 8-μm cryosections were prepared from lungs, fixed with paraformaldehyde, permeabilized with Triton X-100 in PBS, and blocked with blocking reagents. Cellular localization of I-κBαmt protein was detected using anti–human I-κBα antibody, which a has low level of cross-reactivity with mouse I-κBα (Santa Cruz Biotechnology, Inc.), and FITC-conjugated secondary antibody.
Cell isolation and culture.
Vascular ECs and fibroblasts were isolated from the lungs of WT and TG mice according to the protocols previously described (41, 42). Peritoneal macrophages were isolated by peritoneal lavage of the four groups of mice 4 d after intraperitoneal injection of 0.5 ml of thioglycolate medium. ECs were cultured in EC culture medium containing 20% FCS, DMEM, 2 mM l-glutamine, 2 mM sodium pyruvate, 20 mM Hepes, 1% nonessential amino acids, 100 μg/ml streptomycin, 100 U/ml penicillin, 100 μg/ml heparin, and 100 μg/ml EC growth supplement. Fibroblasts were cultured in DME containing 10% FCS. Macrophages were cultured in DMEM containing 10% FCS. EC phenotype was confirmed by positive staining of the endothelial-specific markers platelet/EC adhesion molecule 1 and ICAM-1. Macrophages and fibroblasts were confirmed by staining with Mac3 (macrophages) and procollagen (fibroblasts) antibodies. Subconfluent cells were plated out and incubated with Dox for 48 h. Some ECs and fibroblasts were stimulated with 100 ng/ml TNF-α for 15 min, and macrophages were stimulated with 100 ng/ml LPS for 30 min before protein extraction.
Measurement of tissue MPO activity.
We used tissue MPO activity as an indicator of tissue neutrophil infiltration. The lungs, heart, kidney, liver, and intestine were collected from WT-Con, WT-LPS, TG-Con, and TG-LPS mice at 4 h after LPS. Tissue MPO activity was determined, as we previously described (13), and was normalized to tissue weight.
Bronchoaleolar lavage (BAL) and neutrophil counting.
BAL was performed by three intratracheal instillations of 0.5 ml of warmed PBS, followed by gentle aspiration. Total fluids from the three collections were pooled and centrifuged to pellet cells. Cells were resuspended in PBS and centrifuged to cytospin slides, which were stained. Cells were differentially counted.
Assessment of endothelial permeability and organ injury.
Microvascular endothelial permeability was assessed using EBD leakage index as a marker (43, 44). Mice in the WT-Con and TG-Con or in WT-LPS and TG-LPS groups were injected with saline or 10 mg/kg LPS i.p. At 3.5 h after LPS, 20 mg/kg EBD was injected i.v. At 5 h after LPS, mice were injected with 200 U heparin i.v. After blood withdrawal, the organ vasculature was flushed free of blood by gentle infusion of 10 ml of prewarmed PBS through the left ventricle. The lungs, heart, liver, kidney, and intestine were then excised, and weighed before and after being dried at 70°C for 16 h. Dry tissues were homogenized in formamide, incubated at 60°C for 16 h, and centrifuged, and supernatant absorbances at 620 and 740 nm were recorded. Tissue heme pigment contamination was corrected using A740 readings. Tissue EBD content (mg EBD/g fresh tissue) was calculated by comparing tissue supernatant A620 readings with an EBD standard curve. Organ edema was assessed by determining organ wet/dry ratio, which was calculated by dividing the wet weight of each organ by its dry weight.
CLP model of sepsis.
Mice in the WT-sham and TG-sham groups were subjected to sham, and mice in the WT-CLP and TG-CLP groups were subjected to CLP operation, as previously described (45) using an 18-gauge needle. Mice were injected with 20 mg/kg EBD i.v. at 22 h and heparinized at 24 h after operation. Microvascular endothelial permeability in the lungs, heart, liver, kidney, and intestine were assessed as described in the previous section. In separate experiments, WT and TG mice were subjected to CLP, and survival was monitored for up to 14 d.
Monitoring systemic arterial blood pressure.
Mice in each group were anesthetized with 300 mg/kg tribromoethanol i.p., intubated, and ventilated with a mouse ventilator, as we previously described (12). The carotid artery was cannulated with a mouse arterial catheter, which was connected to a pressure transducer connected to a chart recorder. Systemic arterial blood pressure was recorded before and at 4 h after LPS challenge, and compared. Plasma levels of nitrite/nitrate and thrombin/antithrombin were measured using commercial kits.
Bacterial clearance.
Mice in control and bacteria groups were injected with saline or one of the following bacteria: intracellular bacterium (L. monocytogenes), extracellular gram-positive bacterium (S. pneumoniae), and intracellular gram-negative bacterium (S. enterica). To eliminate the effects of Dox, which was used to induce I-κBαmt expression in TG mice, all four groups of mice were fed with an identical dose of Dox for 4 d before i.v. injection of bacteria, which was prepared in Dox-containing media.
12 h after S. enterica (108 CFU/mouse) or S. pneumoniae (107 CFU/mouse) injection, or 24 h after L. monocytogenes (108 CFU/mouse) injection, mice were killed by exsanguination. The blood, lungs, liver, spleen, and kidney were collected. Organ homogenates were prepared in a series of 10-fold dilutions aseptically, spread onto culture plates, and incubated in culture media and conditions, as recommended by the American Type Culture Collection. Bacterial colonies formed were counted and expressed as colony-forming units per gram of tissue or per milliliter of blood.
Statistical analysis.
Data were expressed as mean ± SEM. Multiple group comparisons were made using analysis of variance or the Kruskal-Wallis rank test. Comparisons between two groups were analyzed using a Bonferroni-corrected t test or a Mann-Whitney U test. The null hypothesis was rejected at the 5% level.
Acknowledgments
We would like to thank Dr. J.W. Pollard and the staff at the Albert Einstein College of Medicine Transgenic and Gene Targeting Facility for help in generating TG mice, Dr. U. Siebenlist for providing human I-κBαmt cDNA, and Drs. S.M. Goyert and P. Wang for helpful discussions.
This study was supported by the National Institute of General Medical Science (grant 063907 to S.F. Liu) and in part by the Faculty Award Program of the Feinstein Institute for Medical Research.
The authors have no conflicting financial interests.
Abbreviations used: BAL, bronchoaleolar lavage; CLP, cecal ligation and puncture; Dox, doxycycline; EBD, Evans blue dye; EC, endothelial cell; ICAM, intercellular adhesion molecule; MBP, mean arterial blood pressure; MOD/I, multiple organ dysfunction and injury; MPO, myeloperoxidase; TAT, thrombin–antithrombin; TG, transgenic; VCAM, vascular adhesion molecule; VE, vascular endothelial.
X. Ye and J. Ding contributed equally to this work.
References
- 1.Martin, G.S., D.M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 2.Riedemann, N.C., R.F. Guo, and P.A. Ward. 2003. The enigma of sepsis. J. Clin. Invest. 112:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature. 420:885–891. [DOI] [PubMed] [Google Scholar]
- 4.Liu, S.F., and A.B. Malik. 2006. NF-κB activation as a pathologic mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L622–L645. [DOI] [PubMed] [Google Scholar]
- 5.Brown, M.A., and W.K. Jones. 2004. NF-kappaB action in sepsis: the innate immune system and the heart. Front. Biosci. 9:1201–1217. [DOI] [PubMed] [Google Scholar]
- 6.Pahl, H.L. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. [DOI] [PubMed] [Google Scholar]
- 7.Bohrer, H., F. Qiu, T. Zimmermann, Y. Zhang, T. Jllmer, D. Mannel, B.W. Bottiger, D.M. Stern, R. Waldherr, H.D. Saeger, et al. 1997. Role of NFkappaB in the mortality of sepsis. J. Clin. Invest. 100:972–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnalich, F., E. Garcia-Palomero, J. Lopez, M. Jimenez, R. Madero, J. Renart, J.J. Vazquez, and C. Montiel. 2000. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect. Immun. 68:1942–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paterson, R.L., H.F. Galley, J.K. Dhillon, and N.R. Webster. 2000. Increased nuclear factor kappa B activation in critically ill patients who die. Crit. Care Med. 28:1047–1051. [DOI] [PubMed] [Google Scholar]
- 10.Liu, S.F., X. Ye, and A.B. Malik. 1999. Inhibition of NF-κB activation by pyrrolidine dithiocarbamate prevents in vivo expression proinflammatory genes. Circulation. 100:1330–1337. [DOI] [PubMed] [Google Scholar]
- 11.Liu, S.F., X. Ye, and A.B. Malik. 1997. In vivo inhibition of NF-κB activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J. Immunol. 159:3976–3983. [PubMed] [Google Scholar]
- 12.Haudek, S.B., E. Spencer, D.D. Bryant, D.J. White, D. Maass, J.W. Horton, Z.J. Chen, and B.P. Giroir. 2001. Overexpression of cardiac I-kappaBalpha prevents endotoxin-induced myocardial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 280:H962–H968. [DOI] [PubMed] [Google Scholar]
- 13.Liu, S.F., X. Ye, and A.B. Malik. 1999. Pyrrolidine dithiocarbamate prevents I-κB degradation and reduces microvascular injury induced by lipopolysaccharide in multiple organs. Mol. Pharmacol. 55:658–667. [PubMed] [Google Scholar]
- 14.Sheehan, M., H.R. Wong, P.W. Hake, V. Malhotra, M. O'Connor, and B. Zingarelli. 2002. Parthenolide, an inhibitor of the nuclear factor-kappaB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol. Pharmacol. 61:953–963. [DOI] [PubMed] [Google Scholar]
- 15.Sha, W.C., H.C. Liou, E.I. Tuomanen, and D. Baltimore. 1995. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 80:321–330. [DOI] [PubMed] [Google Scholar]
- 16.Lavon, I., I. Goldberg, S. Amit, L. Landsman, S. Jung, B.Z. Tsuberi, I. Barshack, J. Kopolovic, E. Galun, H. Bujard, and Y. Ben-Neriah. 2000. High susceptibility to bacterial infection, but no liver dysfunction, in mice compromised for hepatocyte NF-kappaB activation. Nat. Med. 6:573–577. [DOI] [PubMed] [Google Scholar]
- 17.Ashare, A., L.S. Powers, N.S. Butler, K.C. Doerschug, M.M. Monick, and G.W. Hunninghake. 2005. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L633–L640. [DOI] [PubMed] [Google Scholar]
- 18.Kisseleva, T., S. Li, M. Vorontchikhina, N. Feirt, J. Kitajewski, and C. Schindler. 2006. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J. Clin. Invest. 116:2955–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henke, N., R. Schmidt-Ullrich, R. Dechend, J.K. Park, F. Qadri, M. Wellner, M. Obst, V. Gross, R. Dietz, F.C. Luft, et al. 2007. Vascular endothelial cell-specific NF-kappaB suppression attenuates hypertension-induced renal damage. Circ. Res. 101:268–276. [DOI] [PubMed] [Google Scholar]
- 20.Kistner, A., M. Gossen, F. Zimmermann, J. Jerecic, C. Ullmer, H. Lubbert, and H. Bujard. 1996. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. USA. 93:10933–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gory, S., M. Vernet, M. Laurent, E. Dejana, J. Dalmon, and P. Huber. 1999. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 93:184–192. [PubMed] [Google Scholar]
- 22.Alva, J.A., A.C. Zovein, A. Monvoisin, T. Murphy, A. Salazar, N.L. Harvey, P. Carmeliet, and M.L. Iruela-Arispe. 2006. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 235:759–767. [DOI] [PubMed] [Google Scholar]
- 23.Bancroft, G.J., R.D. Schreiber, and E.R. Unanue. 1991. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol. Rev. 124:5–24. [DOI] [PubMed] [Google Scholar]
- 24.Franzoso, G., L. Carlson, L. Poljak, E.W. Shores, S. Epstein, A. Leonardi, A. Grinberg, T. Tran, T. Scharton-Kersten, M. Anver, et al. 1998. Mice deficient in nuclear factor (NF)-κB/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J. Exp. Med. 187:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beg, A.A., W.C. Sha, R.T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 376:167–170. [DOI] [PubMed] [Google Scholar]
- 26.Weih, F., D. Carrasco, S.K. Durham, D.S. Barton, C.A. Rizzo, R.P. Ryseck, S.A. Lira, and R. Bravo. 1995. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 80:331–340. [DOI] [PubMed] [Google Scholar]
- 27.Kontgen, F., R.J. Grumont, A. Strasser, D. Metcalf, R. Li, D. Tarlinton, and S. Gerondakis. 1995. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 9:1965–1977. [DOI] [PubMed] [Google Scholar]
- 28.Fishman, G.I. 1998. Timing is everything in life: conditional transgene expression in the cardiovascular system. Circ. Res. 82:837–844. [DOI] [PubMed] [Google Scholar]
- 29.Furth, P.A., L. St Onge, H. Boger, P. Gruss, M. Gossen, A. Kistner, H. Bujard, and L. Hennighausen. 1994. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc. Natl. Acad. Sci. USA. 91:9302–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray, P., W. Tang, P. Wang, R. Homer, C. Kuhn III, R.A. Flavell, and J.A. Elias. 1997. Regulated overexpression of interleukin 11 in the lung. Use to dissociate development-dependent and –independent phenotypes. J. Clin. Invest. 100:2501–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tichelaar, J.W., W. Lu, and J.A. Whitsett. 2000. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 275:11858–11864. [DOI] [PubMed] [Google Scholar]
- 32.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 267:1485–1488. [DOI] [PubMed] [Google Scholar]
- 33.Poynter, M.E., C.G. Irvin, and Y.M.W. Janssen-Heininger. 2003. Prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J. Immunol. 170:6257–6265. [DOI] [PubMed] [Google Scholar]
- 34.Eldor, R., A. Yeffet, K. Baum, V. Doviner, D. Amar, Y. Ben-Neriah, G. Christofori, A. Peled, J.C. Carel, C. Boitard, et al. 2006. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc. Natl. Acad. Sci. USA. 103:5072–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aird, W.C. 2003. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 101:3765–3777. [DOI] [PubMed] [Google Scholar]
- 36.Taddei, S., A. Virdis, L. Ghiadoni, D. Versari, and A. Salvetti. 2006. Endothelium, aging, and hypertension. Curr. Hypertens. Rep. 8:84–89. [DOI] [PubMed] [Google Scholar]
- 37.Deanfield, J.E., J.P. Halcox, and T.J. Rabelink. 2007. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 38.Sarao, R., and D.J. Dumont. 1998. Conditional transgene expression in endothelial cells. Transgenic Res. 7:421–427. [DOI] [PubMed] [Google Scholar]
- 39.Teng, P.I., M.R. Dichiara, L.G. Dich Kömüves, K. Abe, T. Quertermous, and J.N. Topper. 2002. Inducible and selective transgene expression in murine vascular endothelium. Physiol. Genomics. 11:99–107. [DOI] [PubMed] [Google Scholar]
- 40.Ye, X., and S.F. Liu. 2002. Lipopolysaccharide down-regulates promoter selective transcription factor 1 binding activity by promoting Sp1 protein dephosphorylation and degradation. J. Biol. Chem. 277:31863–31870. [DOI] [PubMed] [Google Scholar]
- 41.Dong, Q.G., S. Bernasconi, S. Lostaglio, R.W. De Calmanovici, I. Martin-Padura, F. Breviario, C. Garlanda, S. Ramponi, A. Mantovani, and A. Vecchi. 1997. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler. Thromb. Vasc. Biol. 17:1599–1604. [DOI] [PubMed] [Google Scholar]
- 42.Phan, S.H., J. Varani, and D. Smith. 1985. Rat lung fibroblast collagen metabolism in bleomycin-induced pulmonary fibrosis. J. Clin. Invest. 76:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green, T.P., D.E. Johnson, R.P. Marchessault, and C.W. Gatto. 1988. Transvascular flux and tissue accrual of Evans blue: effects of endotoxin and histamine. J. Lab. Clin. Med. 111:173–183. [PubMed] [Google Scholar]
- 44.Parker, J.C., and M.I. Townsley. 2004. Evaluation of lung injury in rats and mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L231–L246. [DOI] [PubMed] [Google Scholar]
- 45.Wang, H., H. Liao, M. Ochani, M. Justiniani, X. Lin, L. Yang, Y. Al-Abed, H. Wang, C. Metz, E.J. Miller, et al. 2004. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10:1216–1221. [DOI] [PubMed] [Google Scholar]