Abstract
T cell–dependent immune responses develop soon after birth, whereas it takes 2 yr for humans to develop T cell–independent responses. We used this dissociation to analyze the repertoire diversification of IgM+IgD+CD27+ B cells (also known as “IgM memory” B cells), comparing these cells with switched B cells in children <2 yr of age, with the aim of determining whether these two subsets are developmentally related. We show that the repertoire of IgM+IgD+CD27+ B cells in the spleen and blood displays no sign of antigen-driven activation and expansion on H-CDR3 spectratyping, despite the many antigenic challenges provided by childhood vaccinations. This repertoire differed markedly from those of switched B cells and splenic germinal center B cells, even at the early stage of differentiation associated with μ heavy chain expression. These data provide evidence for the developmental diversification of IgM+IgD+CD27+ B cells, at least in very young children, outside of T cell–dependent and –independent immune responses.
In humans, peripheral blood B cells expressing the CD27 marker and mutated Ig receptors are usually considered to be memory B cells generated in germinal centers (GCs) during immune responses to T-dependent (TD) antigens. In adults, approximately half of these CD27+ B cells are isotype-switched cells carrying surface IgG or IgA, the other half being IgM+IgD+ cells, often referred to as “IgM memory” (1, 2). CD27 is not present on naive B cells, but other minor CD27− populations have been recently described. These populations include transitional cells, and, more surprisingly, IgG+ and IgA+ B cells (3–8). These isotype-switched CD27− cells expressing a mutated Ig have been shown to be bona fide memory cells and can be discriminated from CD27− naive cells by the absence of ATP-binding cassette B1 transporter (ABCB1) activity (6–8). Strikingly, CD27 surface expression was also recently reported for both CD19+CD10+ and CD19+CD34+ Ig-negative B cell precursors in the bone marrow (9, 10). Thus, the relationship between CD27 expression and memory B cell phenotype appears to be far from unequivocal.
There is still considerable debate concerning the exact function and phenotype of IgM+IgD+CD27+ B cells in humans (11). It has been suggested that these cells are not memory cells, but are instead splenic marginal zone (SMZ) B cells involved in T cell–independent (TI) responses, and that, unlike the equivalent B cell population in mice, these cells not only recirculate, but also diversify their Ig receptors by hypermutation in a GC-independent pathway (12, 13). Evidence supporting this hypothesis is provided by the consistent observation of such an IgM+IgD+CD27+ subset with mutated Ig genes in patients harboring genetic defects in the T-B collaborative response (deficiencies in CD40L, CD40, and ICOS), although this subset generally accounts for a proportion of cells only one-third of that found in normal individuals (12–16). Further evidence is provided by the similarity in surface phenotype between circulating IgM+IgD+CD27+ B cells and SMZ B cells and the sharing of B cell clones between these blood and spleen subsets (12). The dependence of these cells on the splenic microenvironment, as highlighted by their reduced numbers in splenectomized patients, and the correlation between this specific subset and protective immunity against pneumococcal infections, are also consistent with this hypothesis (17–19).
An alternative hypothesis is based on a presumed obligate link between hypermutation and the GC reaction, and uses as an argument the lack of detectable expression of activation-induced cytidine deaminase (AID), an absolute prerequisite for the induction of Ig gene mutation, in the human SMZ on immunocytochemistry (20). According to this hypothesis, IgM+IgD+CD27+ cells are derived from GC-activated B cells that fail to undergo isotype switching (21). Abortive GC development, like that described in transgenic mouse models of TI responses (22), would then account for their presence (and mutation frequency) in hyper-IgM patients.
In humans, GC reactions and memory B cell generation begin soon after birth, but TI antigens raise no immune response in children under the age of 2 yr (23, 24). Blood IgM+IgD+CD27+ cells are present and mutated in infants <2 yr old, which led us to suggest, consistent with our general proposition, that these cells would be generated and diversified at these early stages, along a developmental program outside the framework of an immune response (12). We made use of this dissociation between development and TI response to analyze the repertoire of the two main CD27+ IgD+ and IgD− subsets, in the blood and spleen of children under the age of 2 yr. We compared these subsets with the aim of determining whether they belonged to identical or different lineage pathways. As expected, switched and GC B cells were found to express a very restricted repertoire. In contrast, the Ig repertoire of IgM+IgD+CD27+ B cells appeared to be highly diverse and similar to that of naive B cells, but with a shift in CDR3 sizes suggesting a different selection process. The IgM+IgD+CD27+ subset thus has a mutated Ig repertoire, but displays no sign of antigen-driven clonal expansion, which is the hallmark of an immune response, despite the numerous vaccinations to which young children are usually subjected.
RESULTS
Analysis of the repertoire of blood B cell subsets reveals major differences between IgM+IgD+CD27+ and switched B cells in children under the age of 2 yr
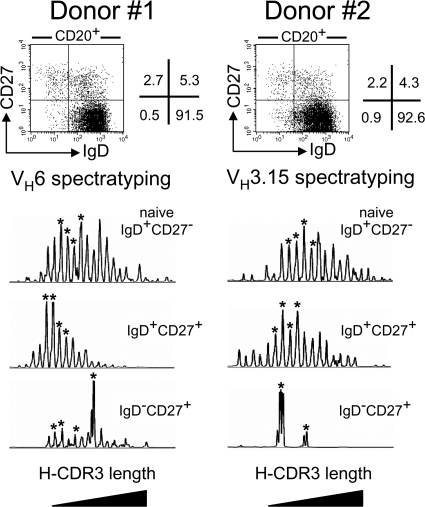
The different B cell subsets in blood samples from young donors were analyzed by H-CDR3 spectratyping (25–27). With this approach, the repertoire of specific rearranged VH genes can be assessed by evaluating the global distribution of CDR3 lengths, and its diversity can be estimated by determining the number of non redundant sequences corresponding to a defined CDR3 size. IgM+IgD+CD27+ cells, switched CD27+ cells and naive B cells were sorted from the blood samples of two 11-mo-old children. The possible contamination of switched cells with IgD−CD27++ plasma cells producing large amounts of Ig transcripts was excluded by selecting the different B cell fractions on the basis of CD20 marker expression, this marker being absent from plasma cells (28, 29). H-CDR3 spectratyping of the VH6 and VH3.15 μ or γ transcripts in these different subsets was performed with a seminested strategy (see Supplemental materials and methods, available at http://www.jem.org/cgi/content/full/jem.20071555/DC1). The VH6- and VH3.15-CDR3 spectratypes for naive cells from both donors (Fig. 1) displayed a wide, Gaussian-type distribution, indicative of a highly diverse repertoire. A regular distribution of H-CDR3 sizes was also observed for IgM+IgD+CD27+ B cells from both donors, with a marked shift toward shorter CDR3 lengths. The mean difference in CDR3 size between IgD+CD27+ and naive cells was 11 bp for VH6 transcripts (donor 1) and 8.6 bp for VH3.15 transcripts (donor 2) based on mean peak height values. Unlike IgM+IgD+CD27+ or naive B cells, switched CD27+ cells expressed a very restricted repertoire. The spectratyping of VH6-γ transcripts from donor 1 gave an irregular profile, with one major peak and several minor ones. For donor 2, the VH3.15 repertoire of γ transcripts was even more restricted, with only two peaks detectable.
Figure 1.
H-CDR3 spectratypes of the VH6 or VH3.15 transcripts expressed by the blood B cell subsets of two 11-mo-old children. Naive IgD+CD27−, IgD+CD27+, and switched IgD−CD27+ B cells were sorted from the blood samples of donors 1 and 2. Total RNA from each cell fraction was reverse transcribed and VH6 or VH3.15 μ or γ transcripts were amplified by PCR, using a seminested strategy (see Materials and methods). The PCR products were labeled by a run-off reaction with specific fluorescent VH-FR3 primers, and subjected to electrophoresis on an automated sequencer. The resulting size distribution of the peaks directly reflects the size distribution of H-CDR3 for the given transcripts. Peaks identified by an asterisk were further sequenced to evaluate intrapeak clonal diversity.
The clonal diversity represented by defined CDR3 lengths was evaluated further, by sequencing several peaks (identified in Fig. 1) and counting the number of different rearrangements (Table I). For the VH6 spectratyping of switched cells (donor 1), we selected four peaks for sequencing, including the major one. Clonal diversity, defined as the proportion of different VHDJH junctions among all the sequences obtained, was 27%, and ranged from 38% for the smallest peak to a single rearrangement for the major peak (Table S1, available at http://www.jem.org/cgi/content/full/jem.20071555/DC1). For donor 2, as expected from the VH3-15 spectratype profile of switched B cells, no clonal diversity was detected for the two peaks observed, with each peak corresponding to a single clone. This finding contrasts sharply with that for IgM+IgD+CD27+ cells. Indeed, for VH6 and VH3.15 spectratyping, overall clonal diversity was 88 and 63%, respectively, and was as high as that for naive B cells (80 and 62%, respectively). Moreover, if peaks were considered individually, numerous clones were present in even the two largest peaks observed in the VH6 and VH3.15 spectratypings of the μ transcripts expressed by IgM+IgD+CD27+ cells (Table S1), and clonal diversity was, again, comparable to that of individual peaks for naive B cells. Thus, unlike switched B cells, IgD+CD27+ cells appear to have a highly diverse repertoire, with no signs of antigen-driven clonal expansion and/or activation.
Table I.
VH repertoire analysis of the peripheral B cell subsets of two 11-mo-old children
| Donor
|
B cell subset
|
Transcripts
|
Number of peaks analyzeda |
Total number of sequences |
Number of different VDJ junctions |
Clonal diversityb |
P valuec
|
|---|---|---|---|---|---|---|---|
| % | |||||||
| D1 | IgD+CD27+ | VH6 μ | 4 | 104 | 92 | 88 | |
| Naive | VH6 μ | 4 | 56 | 45 | 80 | ||
| Switched | VH6 γ | 4 | 67 | 18 | 27 | 10−15 | |
| D2 | IgD+CD27+ | VH3.15 μ | 4 | 82 | 52 | 63 | |
| Naive | VH3.15 μ | 4 | 84 | 52 | 62 | ||
| Switched | VH3.15 γ | 2 | 40 | 2 | 5 | 4 × 10−9 |
The peaks that were analyzed are identified by an asterisk in Fig. 1.
Number of different VDJ among all the sequences.
Statistical significance (see Materials and methods) of differences in clonal diversity between IgD+CD27+ and switched cells from the same donor.
Analysis of the repertoire of different splenic B cell subsets in an 8-mo-old child: the IgM+IgD+CD27+ VH repertoire is polyclonal, whereas GC and switched B cells display a restricted VH repertoire
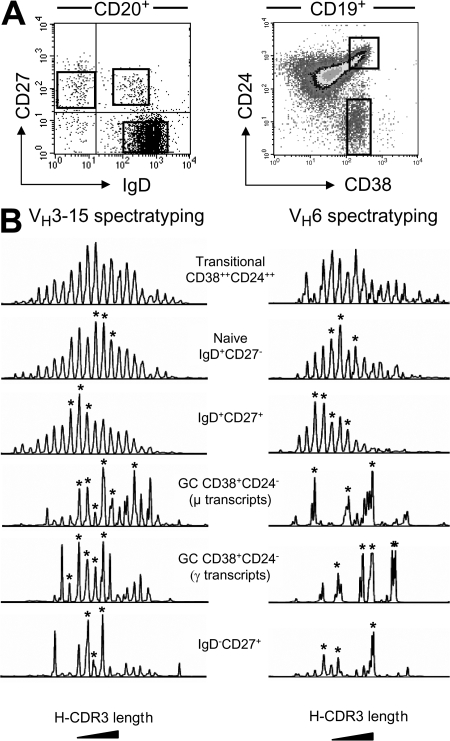
The VH repertoires of different splenic B cell subsets were compared by sorting fractions from a splenic sample taken from an 8-mo-old donor (donor 3), using two different types of labeling. Indeed, the proportion of GC B cells was surprisingly high, accounting for 80% of the IgD−CD27+ population. We made use of this situation to isolate GC B cells, based on CD19, CD24, and CD38 labeling (GCs are CD19+CD38+CD24−), and transitional B cells, which are easy to identify with these markers (CD19+CD38++CD24++) (4, 30). The other three fractions were purified, as previously des-cribed, based on the expression of CD20, IgD, and CD27 (Fig. 2 A). The IgD−CD27+ fraction thus corresponded to a mixture of GC B cells (IgD−-CD27intCD38+) and post-GC switched cells (IgD−CD27+CD38−).
Figure 2.
H-CDR3 spectratypes of the VH3.15 and VH6 transcripts expressed by the splenic B cell subsets of an 8-mo-old child. (A) Two different marker combinations were used to isolate splenic GC (CD19+CD24−CD38+) and transitional B cells (CD19+CD38++CD24++) or naive IgD+CD27+, IgD+CD27+ and switched IgD−CD27+ B cells. (B) Spectratyping was performed on VH3.15 and VH6 μ and/or γ transcripts from each cell fraction, as described in the legend of Fig. 1. Peaks identified by an asterisk were further sequenced to evaluate intrapeak clonal diversity.
We performed H-CDR3 spectratyping on VH3.15 μ or γ transcripts from these different cell fractions (Fig. 2 B). Transitional and naive cells displayed a Gaussian distribution, indicative of a highly diverse repertoire. A similar regular distribution of H-CDR3 sizes was observed for the VH3.15-μ transcripts of IgD+CD27+ cells, with peaks separated by three bases distributed around the mean with decreasing intensity. As for blood samples, mean CDR3 length was shorter for IgD+CD27+ cells than for naive cells, with a difference in mean CDR3 size of 4 bp between the 2 subsets.
Unlike IgD+CD27+ cells, splenic IgD−CD27+ cells displayed an irregular distribution of CDR3 sizes with a few dominant peaks, suggesting a more restricted repertoire and specific clonal expansions, consistent with both proliferation and selection in the GC during TD responses. For GC CD38+CD24− B cells, VH3.15 spectratyping was performed for the μ and γ transcripts expressed before and after isotype switch. Both profiles appeared irregular, with progressive changes between the μ and γ spectratypes, and between the GC and IgD−CD27+ spectratypes.
The repertoire diversity of each B cell fraction was determined by sequencing the peaks identified in Fig. 2 B (results summarized in Table II). We selected the two largest peaks and one minor peak from the VH3.15 spectratype of the γ transcripts expressed by IgD−CD27+ cells. Overall clonal diversity was 48%, ranging from 68% for the smallest peak to 32 and 45% for the two largest peaks, respectively (Table S2, available at http://www.jem.org/cgi/content/full/jem.20071555/DC1). For GC CD38+CD24− cells, we sequenced six and five peaks present in the VH3.15 spectratypes of μ and γ transcripts, respectively (Fig. 2 B, Table II, and Table S2). The percentage of different VDJ junctions was ∼50% for both types of transcript and, not unexpectedly, was similar to that for splenic IgD−CD27+ B cells, of which they constitute the major fraction. Intrapeak diversity was evaluated for IgD+CD27+ and naive B cells by sequencing three peaks in the corresponding VH3.15 spectratypes (Fig. 2 and Table II). Overall clonal diversity was 90% for naive cells and 97% for IgD+CD27+ cells, in which clonal diversity ranged from 94 to 100% in individual peaks, and was therefore high, even in the largest peak (Table S2).
Table II.
VH3.15 repertoire analysis of the splenic B cell subsets of an 8-mo-old child
| Donor
|
B cell subset
|
Transcripts
|
Number of peaks analyzeda |
Total number of sequences |
Number of different VDJ junctions |
Clonal diversityb |
P valuec
|
|---|---|---|---|---|---|---|---|
| % | |||||||
| D3 | IgD+CD27+ | VH3.15 μ | 3 | 58 | 56 | 97 | |
| Naives | VH3.15 μ | 3 | 71 | 64 | 90 | ||
| IgD−CD27+ (switched + GC) |
VH3.15 γ | 3 | 73 | 35 | 48 | 3 × 10−9 | |
| CD38+CD24− (GC) |
VH3.15 μ | 6 | 133 | 71 | 53 | 4 × 10−9 | |
| VH3.15 γ | 5 | 98 | 51 | 52 | 10−8 |
The peaks that were analyzed are identified by an asterisk in Fig. 2.
Number of different VDJ among all sequences.
Statistical significance (see Materials and methods) of differences in clonal diversity between IgD+CD27+ and IgD−CD27+ or GC cells from the same donor.
H-CDR3 spectratyping of μ or γ transcripts of the same splenic B cell fractions was performed for a second gene, VH6 (Fig. 2 B). Again, a regular distribution of H-CDR3 sizes was observed for IgD+CD27+ cells, with a shift of 6 bp compared with naive cells. In contrast, GC CD38+CD24− and IgD−CD27+ cells displayed markedly irregular profiles indicative of restricted repertoires. As expected from the profiles of IgD−CD27+ and GC B cells, the clonal diversity evaluated by the sequencing of selected peaks was very low. Overall clonal diversity was of 24% for IgD−CD27+ cells and 14.5 and 6.5% for GC μ and γ transcripts, respectively, with only 1 or 2 clones in 6 out of 10 peaks analyzed (Table III and Table S3, available at http://www.jem.org/cgi/content/full/jem.20071555/DC1). In contrast, the clonal diversity was 85% for IgD+CD27+ cells, thus being even higher than the one determined for naive B cells (64%).
Table III.
VH6 repertoire analysis of the splenic B cell subsets of an 8-mo-old child
| Donor
|
B cell subset
|
Transcripts
|
Number of peaks analyzeda |
Total number of sequences |
Number of different VDJ junctions |
Clonal diversityb |
P valuec
|
|---|---|---|---|---|---|---|---|
| % | |||||||
| D3 | IgD+CD27+ | VH6 μ | 4 | 68 | 58 | 85 | |
| Naives | VH6 μ | 3 | 56 | 36 | 64 | ||
| IgD−CD27+ (switched + GC) |
VH6 γ | 3 | 50 | 12 | 24 | 7 × 10−11 | |
| CD38+CD24− (GC) |
VH6 μ | 3 | 55 | 8 | 14.5 | 2 × 10−14 | |
| VH6 γ | 4 | 76 | 5 | 6.5 | 10−20 |
The peaks that were analyzed are identified by an asterisk in Fig. 2.
Number of different VDJ among all sequences.
Statistical significance (see Materials and methods) of differences in clonal diversity between IgD+CD27+ and IgD−CD27+ or GC cells from the same donor.
These observations were extended to two older individuals and two additional genes, by studying either the VH3.30/33 or the VH5.51 repertoire of splenic B cell subsets of a 22- and a 23-mo-old child (D4 and D7; Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20071555/DC1). Again, we observed a Gaussian distribution for the naive and the IgD+CD27+ B cells and a more restricted pattern for the IgD−CD27+ cells, which are in their majority GC B cells at this age (75 and 85% of the IgD−CD27+ B cells in D4 and D7, respectively). For donor 7, the VH5.51 repertoire diversity was evaluated for each B cell subset by sequencing several peaks identified in Fig. S1. The overall clonal diversity was of 45% for IgD−CD27+, and was thus significantly lower than the one observed for IgD+CD27+ (91%) and for naive B cells (90%; Table S4).
Altogether, splenic IgD+CD27+ B cells had a highly diverse Ig repertoire, similar to that observed for naive B cells, with no signs of clonal expansion and/or activation. In contrast, the GC cells and the splenic IgD−CD27+ cells, the latter being a mixture of bona fide switched memory cells and GC cells, expressed a restricted repertoire, although it was generally less restricted than that displayed by switched memory cells in the blood.
Blood and splenic IgM+IgD+CD27+ cells are already diversified in infants
The mutation frequencies of Ig genes were determined for the blood and/or splenic IgD+CD27+ and IgD−CD27+ subsets of four donors (D1–D4, described in Materials and methods). We analyzed somatic mutations in the JH4-JH5 introns flanking rearranged VHDJH4 sequences amplified from genomic DNA. The JH4 segment is used in 50% of rearranged VH genes (31), so this approach facilitates the sampling of unselected sequences, even if only limited numbers of cells are available (32). Consistent with our previous results for children under the age of 2 yr (12), the blood IgD+CD27+ cells of both 11-mo-old donors (D1 and D2) were already diversified (1.2 and 1.9 mutations per 100 base pairs, with 75 and 83% of the sequences being mutated, Table IV). The blood IgD+CD27+ cells of the youngest donor (D3, 8 mo) also displayed mutations, but with a lower range and frequency, (a 0.54% mutation frequency with 60% of mutated sequences). Similar observations were made for the splenic IgD+CD27+ cells from both the 8-mo-old donor and the 22-mo-old donor, with about half the JH4-JH5 intronic sequences being mutated at a frequency of 0.32 and 0.51%, respectively. Such a large proportion of germline sequences was not observed in the various IgD−CD27+ cell fractions analyzed (Table IV).
Table IV.
Somatic mutations in JH4-JH5 introns flanking VDJH4 rearrangements from splenic and blood B cell subsets
| Donors | Age (months) |
Tissue | CD20-positive B cell subsets |
Number of sequences
|
Mutations
|
||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
Total
|
Mutated
|
Range
|
Number
|
Frequency/ total sequences |
Frequency/ mutated sequences |
| % | % | ||||||||
| D3 | 8 | spleen | IgD+CD27+ | 21 | 10 (48%) |
0–6 | 23 | 0.32 | 0.67 |
| IgD−CD27+ | 20 | 19 (95%) |
0–14 | 105 | 1.54 | 1.55 | |||
| blood | IgD+CD27+ | 20 | 12 (60%) |
0–7 | 37 | 0.54 | 0.90 | ||
| IgD−CD27+ | 18 | 13 (72%) |
0–16 | 103 | 1.67 | 2.32 | |||
| D1 | 11 | blood | IgD+CD27+ | 20 | 15 (75%) |
0–17 | 79 | 1.16 | 1.54 |
| IgD−CD27+ | 20 | 18 (90%) |
0–22 | 117 | 1.71 | 1.90 | |||
| D2 | 11 | blood | IgD+CD27+ | 18 | 15 (83%) |
0–16 | 116 | 1.89 | 2.27 |
| IgD−CD27+ | 17 | 16 (94%) |
0–21 | 127 | 2.19 | 2.32 | |||
| D4 | 22 | spleen | IgD+CD27+ | 25 | 12 (48%) |
0–14 | 40 | 0.51 | 1.09 |
| IgD−CD27+ | 14 | 13 (93%) |
0–23 | 91 | 1.90 | 2.05 | |||
For donor D3 (8 mo old), we also analyzed somatic mutations in VH3.15 transcripts from sorted splenic IgD+CD27+ cells, GC B cells, and IgD−CD27+ cells, which included both GC and post-GC switched cells, as previously described (Table V). We analyzed both μ and γ transcripts for GC B cells. The GC μ transcripts had a lower frequency of mutations than the γ transcripts of either GC or IgD−CD27+ B cells (1.4 vs. 2.4 and 2.9%, respectively), with a lower mutation range. The VH3.15 μ transcripts of IgD+CD27+ and GC cells displayed a similar mutation frequency per mutated sequence, but there was nevertheless a striking difference in the proportion of mutated sequences in each subset, with 30% of μ transcripts expressed by IgD+CD27+ cells being mutated versus 90% in GC B cells. In contrast, VH3.15 γ transcripts from GC or IgD−CD27+ B cells presented a similarly high frequency of mutated sequences.
Table V.
Mutation frequencies in VH3.15 transcripts from different splenic subsets of donor 3
| B cell subset | Transcripts | Number of sequences
|
Mutations
|
||||
|---|---|---|---|---|---|---|---|
|
|
|
Total
|
Mutated
|
Range
|
Number
|
Frequency/total sequences |
Frequency/mutated sequences |
| % | % | ||||||
| CD20+IgD+CD27+ | μ | 60 | 18 (30%) |
0–13 | 77 | 0.52 | 1.75 |
| CD20+IgD−CD27+ | γ | 20 | 19 (95%) |
0–23 | 137 | 2.86 | 3 |
| CD19+CD38+CD24− (GC) |
μ | 31 | 28 (90%) |
0–9 | 108 | 1.42 | 1.58 |
| γ | 38 | 38 (100%) |
1–21 | 224 | 2.41 | 2.41 | |
Blood and splenic IgM+IgD+CD27+ cells are thus clearly mutated in children under the age of 2 yr. In several samples, this subset contrasted with switched B cells in having a large proportion of cells that, although being CD27 positive, harbored unmutated Ig sequences. No such dissociation was observed for this subpopulation in samples from normal adults and older children (12).
AID expression is detected in the splenic IgM+IgD+CD27+ subset of children under the age of 2 yr, but not in older individuals
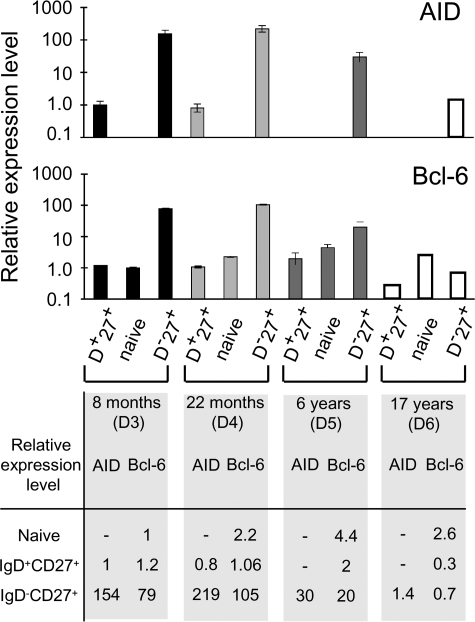
AID is an absolute requisite for Ig gene hypermutation (33, 34). We therefore determined its relative expression by real-time quantitative PCR in the various splenic subsets. IgD+CD27+, IgD−CD27+, and naive IgD+CD27− cells were sorted from splenic samples from 4 patients aged 8 mo (donor 3), 22 mo (donor 4), 6 yr (donor 5), and 17 yr (donor 6). As previously mentioned, the percentage of CD38+ cells in the IgD−CD27+ fraction of the 2 youngest donors was very high (83 and 75%, respectively), which is consistent with the presence of numerous GCs in the splenic sections of these patients (unpublished data). In older patients, this percentage was much lower, falling to 20 and 2% in donors 5 and 6, respectively, in whom GCs were rare in splenic sections. However, it should be noted that the spleen of the 17-yr-old donor was removed after trauma, and therefore without prior immunization. In contrast, the other three donors underwent splenectomy for spherocytosis, and were vaccinated before surgery. We detected no significant AID expression in the naive cells of the four donors (Fig. 3). In contrast, AID was detected in the splenic IgD+CD27+ cells of the two youngest donors, although to a much lower level than in IgD−CD27+ cells (a 150–270-fold difference). AID was not detectable in the IgD+CD27+ cells of the older donors (6 and 17 yr). AID expression was markedly decreased in the IgD−CD27+ fraction of the older donors, in agreement with their lower GC B cell content, reaching at 17 yr an expression level equivalent to the one observed in the IgD+CD27+ B cells of the 8-mo-old donor. We quantified Bcl-6 expression for each fraction. Whereas Bcl-6 is expressed at a low level in many cell types, its expression is markedly increased in centroblasts, thus constituting a reliable marker of contamination with GC B cells (35). In the two AID-positive IgD+CD27+ subsets, Bcl-6 expression levels were close to those of the corresponding naive B cells. Together with the high purity of the sorted fractions, this suggests that AID expression in IgD+CD27+ B cells is unlikely to be accounted for by simple centroblast contamination. Moreover, no AID expression was detected in the naive CD27− subset that includes transitional B cells (15 and 20% for the two youngest donors, estimated by FACS analysis) indicating that, in contrast to recent data reported in mice, human transitional B cells do not express AID, at least above the detection threshold of our experiment (36, 37). Moreover, no somatic mutations were detected in JH4-JH5 intronic sequences from the purified CD24highCD38high transitional subset from the 8-mo-old donor (unpublished data).
Figure 3.
Detectable AID expression in splenic IgD+CD27+ cells from very young children. Relative AID and Bcl-6 expression levels were determined for various splenic B cell subsets from different donors. AID and Bcl-6 sequences were amplified by real-time quantitative PCR from cDNA from IgD+CD27+, IgD+CD27− (naive), and IgD−CD27+ cells purified by two consecutive cell sortings. The results shown were obtained from two independent PCRs (except for D6), each performed in triplicate. The relative expression of AID or Bcl-6 in each subset was calculated by the comparative method, normalizing to 1 the expression of AID in the IgD+CD27+ fraction and of Bcl-6 in the naive subset of the 8-mo-old donor (D3). PCRs with a threshold cycle (Ct) >35 were considered NS.
IgD+CD27+CD1chigh cells are already present in the SMZ of an 8-mo-old child
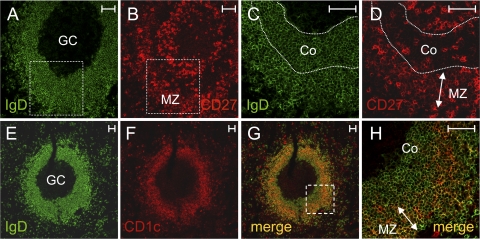
It has been reported that the SMZ of infants and young children is populated by naive B cells, CD27+ MZ B cells first being observed in spleen 2 yr after birth (38). However, a distinct IgM+IgD+CD27+ population was systematically detected in all the spleen and blood samples of young children that we have studied (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20071555/DC1). The spleen sample from the 8-mo-old child was analyzed further by immunofluorescence: double staining of IgD and either the CD27 or the CD1c marker was performed on serial spleen sections, framed by sections stained for both CD20 and CD3 (not depicted) to visualize the T and B cell zones. Representative confocal images are shown in Fig. 4. The sections contained numerous secondary B cell follicles with full-blown IgD-negative GCs surrounded by a broad IgD-positive zone (Fig. 4, A and E). Sections double-stained with IgD and CD27 (Fig. 4, A–D) revealed two distinct areas within this broad IgD+ ring surrounding the GC, an inner zone of mostly CD27− cells corresponding to the corona and populated by naive B cells, and a CD27low outer zone with some scattered CD27high cells. Although they expressed CD27 only weakly, these CD27+ ring structures were found recurrently around B cell follicles and corresponded to the MZ. Thus, in contrast to what is seen in older individuals (39), the corona and MZ could not be distinguished by the presence of IgDhigh and IgDlow B cells in this very young child, both zones being similarly stained with IgD. The CD27high cells scattered in the MZ did not stain for IgD and most probably corresponded to CD3+ T cells, which appeared with a similar arrangement in the adjacent section (not depicted). Similar CD27low staining was observed by immunohistochemistry with tyramide amplification on paraffin-embedded sections of a splenic specimen from another 8-mo-old child (Steiniger, B., personal communication). The difference between our findings and previous studies probably results from differences in the nature of the samples analyzed. Our samples were obtained after surgery, rather than through autopsy, in which autolysis may interfere with the detection of a weak CD27 signal (Steiniger, B., personal communication).
Figure 4.
IgD+CD27+CD1chigh cells are already present in the SMZ of an 8-mo-old child. Serial splenic cryosections were double-labeled either with anti-IgD (green) and anti-CD27 (red) antibodies (A–D) or with anti-IgD (green) and anti-CD1c (red) antibodies (E–H), and then examined under a confocal microscope. CD27low cells (B and D) were present in the MZ corresponding to the outer zone of the IgD-positive ring surrounding the GC (A and C). Boxes with dotted lines in A and B indicate the zone magnified in C and D. A higher level of CD1c expression was observed in the MZ (F), resulting in a yellow appearance in the merged images (G and H), caused by the coexpression of IgD and CD1c at similar intensities. H shows higher magnification of the zone delimited by the box with dotted lines in G. Co, corona. Bars, 50 μm.
On sections labeled with anti-IgD and -CD1c antibodies (Fig. 4, E–H), GCs mostly remained unstained, whereas the corona around GCs stained for CD1c. However, the most strongly CD1c-positive B cells were located in the outer area, corresponding to the CD27low MZ. This is clear in the merged images of Fig. 4 (G and H), in which the corona appeared green, whereas the MZ was orange-yellow in appearance because of the coexpression of IgD (green) and CD1c (red), with similar intensities. On sections double-stained for IgM and CD27, the CD27low ring was also IgM+ (not depicted). Thus, IgM+IgD+CD27lowCD1chigh B cells are already present with a localization corresponding to the MZ in an 8-mo-old child.
DISCUSSION
Both CD27+ B cell subsets—switched and IgM+IgD+ cells—develop and expand in parallel after birth, whereas only TD responses appear to be functional. GCs can be seen in the spleens of infants as young as 7 wk of age, and they consist of B cells and follicular dendritic cells with immunophenotypes essentially similar to those in adults (40). In contrast, although a clearly demarcated MZ can be observed from the age of 4 mo, this zone has been shown to lack CD27 expression (38, 40). We readdressed this latter issue, because in all splenic samples of young children (8, 19, 22, 23, and 25 mo), an IgD+CD27+ B cell subset was clearly detectable by flow cytometry, accounting for 8–18% of the B cell compartment (Fig. S2). Using immunocytochemistry, we show that IgD+CD27low cells are already present in the SMZ of an 8-mo-old child. The nature of sample collection (i.e., surgical removal vs. autopsy) may account for the difference in CD27 detection between this study and previous works. In contrast to the situation in specimens from older children or adults with IgDlow cells in the MZ (39), the IgD and IgM markers were expressed at similar intensities on MZ B cells, consistent with previous reports (40). However, these cells displayed a high level of CD1c expression, similar to that described for older individuals, and which appears to constitute a reliable marker for discrimination from switched memory B cells (12). B cells displaying an IgM+IgD+CD27lowCD1chigh phenotype are thus present in the SMZ before the age of 2 yr, and the lack of TI immune responses thus cannot be attributed to their absence at that stage, but instead probably result from the immaturity of the cells mediating the response: macrophages, dendritic cells, or the MZ B cells themselves.
We made use of the unique context encountered in the first 2 yr of life, in which only TD responses are effective, to compare the immune repertoire of IgM+IgD+CD27+, switched memory, GC, and naive B cells in the blood and spleen of very young children (one 8-mo-old child and two 11-mo-old children). The repertoire was analyzed by H-CDR3 spectratyping, which is used to study the complexity of the heavy chain variable (VH) repertoire based on the global distribution of CDR3 size for a given rearranged VH gene, and clonal diversity, based on the number of nonredundant rearranged sequences harboring a CDR3 of a specific length (25–27, 41). All the children studied had had the standard childhood vaccinations eliciting TD responses in the weeks or months before sample collection (see Materials and methods). We show that, despite these numerous antigenic challenges, the overall diversity of the repertoire of IgM+IgD+CD27+ cells in blood and spleen was similar to that in naive B cells, with no sign of antigen activation or clonal expansion. Switched CD27+ B cells from blood had a highly restricted repertoire, with a small number of expanded clones. This subset was more polyclonal in the spleen, but remained much more restricted than that of IgM+IgD+CD27+ B cells.
B cells proliferating within GCs undergo repeated cycles of hypermutation, with antigen-selected clones eventually leaving the GC to become memory B cells (42). One possible scenario for the generation of IgM+IgD+CD27+ cells involves an early branching point in GC differentiation, before the onset of isotype switching (43, 44), accounting for the lower mutation frequency generally observed for these cells than for switched B cells from the same individual (12, 45). Surprisingly, the major fraction of splenic IgD−CD27+ B cells from these young children appeared to consist of GC B cells (75–80%). We thus sorted GC B cells from the spleen sample of an 8-mo-old child, and analyzed their repertoire and mutation frequency at the level of both γ and μ transcripts, with the latter one corresponding to an earlier step in the GC reaction. μ transcripts from splenic IgM+IgD+CD27+ and GC cells had similar levels of mutation if only mutated sequences were considered. However, whereas most of the sequences in GC cells bore mutations, only 30% of the IgM+IgD+CD27+ splenic B cells were mutated. Moreover, whether they expressed μ or γ transcripts, GC CD38+CD24− cells had a repertoire strikingly different from that of IgD+CD27+ cells, with approximately half of the amplified VH sequences being redundant, whereas IgM+IgD+CD27+ B cells were comparable to naive B cells with few redundant sequences.
Later in life, encountering external TI antigens in the mature environment of the SMZ is assumed to trigger the effector function of IgM+IgD+CD27+ cells that is, differentiation into plasma cells, at the IgM stage or after isotype switch, and the secretion of mutated antibodies (46–48). Indeed, we have observed a significant expansion of defined VH clones in the blood IgM+IgD+CD27+ cells of 6- and 8-yr-old children after antipneumococcal vaccination (unpublished data) (12), indicating that, unlike in very young children, clonal expansion takes place and can easily be detected in the mature, functional IgM+IgD+CD27+ compartment.
The next obvious question concerns when and where these cells diversify their repertoire. We have previously shown that diversification begins after birth, with a progressive increase in mutation frequencies in blood IgM+IgD+CD27+ cells during the first few years of life (12). We confirm here that these IgM+IgD+CD27+ cells are mutated in the spleen of a child of 8 mo, with a range and frequency of mutations lower than those in the corresponding switched cells. This, together with the high proportion of unmutated Ig sequences in a subset harboring the CD27+ surface marker, suggests that these cells are still undergoing diversification at this stage and that this diversification may be a much slower and more progressive process than that occurring in the space of a few weeks in GCs.
Somatic hypermutation is strictly dependent on the enzyme AID (33, 34). We therefore analyzed the relative expression of AID by quantitative PCR in the different splenic subsets taken from children of various ages. Consistent with the results reported by Willenbrock et al. (20), who performed immunocytochemistry on the MZ of adult splenic tissues, we detected no significant AID expression in the splenic IgM+IgD+CD27+ fraction of older donors (6 and 17 yr old). In contrast, AID expression was detected, at a low level, in children under the age of 2 yr. The similar expression of Bcl-6 in splenic IgM+IgD+CD27+ cells of these young children compared with naive B cells tends to indicate that this AID expression is not caused by a contamination by GC B cells. This finding would moreover be compatible with hypermutation in a small number of cells from the IgM+IgD+CD27+ fraction, and with the slow progressive accumulation of mutations in these cells, but may also reflect residual activation triggered elsewhere, leaving the question of the site of diversification open at this stage.
Blood IgM+IgD+CD27+ cells have been reported to harbor shorter H-CDR3 than naive cells in adults, and similar data have been obtained for MZ B cells in rodents (45, 47, 49–52). Our findings extend this observation to the blood and splenic IgM+IgD+CD27+ cells of infants, demonstrating the existence of an intrinsic bias that emerges even in the absence of TI responses. This difference in repertoire suggests that these IgM+IgD+CD27+ cells were selected in a manner different from that in naive cells and raises questions about the nature of the selection occurring during their development. It has been shown that developing B cells in humans express large numbers of self-reactive antibodies, most of which are removed at two checkpoints—one at the immature B cell stage in bone marrow, the other at the transition between new emigrants and mature B cells in the periphery (53). Tsuiji et al. recently provided evidence of a third checkpoint in self-reactivity during IgM+IgD+CD27+ B cell development, this selection apparently being implemented before the onset of somatic hypermutation (50). Polyreactive and autoreactive antibodies generally have longer H-CDR3 (53), and their elimination from IgM+IgD+CD27+ B cells may contribute to the shorter H-CDR3 regions observed. Positive selection may also be applied to IgM+IgD+CD27+ cells during their development, through a tonic signal mediated by the BCR complex, a weak stimulation through the Ig antigen-binding site, or a superantigen-like signal through the binding of framework residues in the VH regions, with any of these signals possibly acting in conjunction with Toll-like receptor–mediated accessory stimulation (54–56). In rabbits, specific gut bacteria shape the development and diversification of the preimmune repertoire through a superantigen-like effect (57, 58), but no evidences have been reported so far for a role of superantigens in the development of the B cell repertoire in humans. B cells expressing auto-/polyreactive antibodies of low avidity have been shown to be selected in the mouse MZ B cell compartment, which is consistent with the signal strength model (59). It therefore remains possible that defined self- or commensal antigens recognized with very low affinity may provide IgM+IgD+CD27+ B cells with a positive signal, selecting for different H-CDR3 lengths. Alternatively, a shorter CDR3 size might affect the nature of a tonic signal delivered during the development of these cells, and as a consequence drive them toward a MZ B cell fate (55).
Collectively, our results support the proposition that IgM+IgD+CD27+ B cells develop and mutate during the first years of life without being engaged in a TD or -independent immune response. Cerny et al. (60) have shown that, in a transgenic mouse model, sorted MZ B cells can be recruited for T cell–dependent responses, forming GCs that subsequently undergo somatic mutation and affinity maturation. Although we cannot formally exclude the possibility that IgM+IgD+CD27+ cells also participate in TD responses later in life, our results are not consistent with such a role in children under the age of 2 yr.
MATERIALS AND METHODS
Donors and patients.
Approval for this research was granted by the Comité de protection des personnes Ile-de-France II. Fresh spleen samples were obtained from patients undergoing splenectomy caused by spherocytosis, a nonimmunological disease. Spleen samples and heparin-treated venous blood samples were retrieved after informed consent from the patients or their parents. Donors 1 and 2 were healthy 11-mo-old twins vaccinated with Pentacoq and Prevnar at 2, 3, and 4 mo, and then 10 d before blood sampling. A splenic sample was collected for the following donors: D3 (8 mo), D4 (22 mo), D5 (6 yr), D6 (17 yr), and D7 (23 mo), which was previously included as C2 (12). Donor 3, who underwent splenectomy at 8 mo, had received doses of Prevnar at 2, 4, and 5 mo, Infanrix at 2 and 5 mo, and Engerix at 6 mo. Donor 6 had his spleen removed because of traumatic splenic rupture.
Flow cytometry and cell sorting.
The following antibodies were used: biotin anti-IgM (clone G20-127), biotin anti-IgD (clone IA6-2), APC anti-CD38 (clone HIT2), and PE anti-CD24 (clone ML5) were purchased from BD Biosciences; PE-Cy5 or FITC anti-CD19 (clone J4.119), PE-Cy5 anti-CD20 (clone B9E9), and PE anti-CD27 (clone1A4-CD27) were obtained from Beckman Coulter; and FITC-conjugated goat F (ab')2 anti-IgD was purchased from Invitrogen. Biotinylated antibodies were detected with streptavidin PE-Cy7 (BD Biosciences). B cells from peripheral blood were enriched with human B cells by negative selection on a Ficoll gradient with the RosetteSep B cell enrichment cocktail (StemCell Technologies). Splenic B cells were obtained by Ficoll density centrifugation, followed by enrichment to >98% purity with the B cell negative isolation kit (Dynal). Enriched B cells were stained with antibodies and analyzed on a FACSCalibur with CellQuest software, or sorted with a FACSVantage machine (BD Biosciences). For the cell sorting of splenic or blood IgD+CD27+, IgD−CD27+, and naive IgD+CD27− cells, purified B cells were stained with CD27-PE, IgD-FITC, and CD20-PC5. Splenic transitional and GC B cells were sorted after CD19-PC5, CD38-APC, and CD24-PE staining, and gates were set to collect CD38highCD24highCD19+ and CD38+CD24−CD19+ cells.
Analysis of the mutation frequencies of VH3.15 transcripts, JH4-JH5 introns flanking rearranged VHDJH4.
Starting from sorted B cell subsets, VH3.15-FR1- μ or γ transcripts (donor D3) were amplified, as for H-CDR3 spectratyping. For donors 1 through 4, total genomic DNA was extracted from 2,500–3,000 IgD+CD27+ and IgD−CD27+ cells by proteinase K digestion. The JH4-JH5 intronic region was amplified with Phusion DNA polymerase (Finnzymes), using a mixture of six FR3 primers designed to amplify all VH gene sequences (FR3#1-6 mixed in a 5:7:1:1:1:1 ratio) and a primer binding 5′ to the JH5 exon (sequences are available in the Supplemental materials and methods). PCR conditions were as follows: 50 cycles of 98°C for 10 s, 60°C for 20 s, and 72°C for 20 s. The resulting JH4-JH5 and VH3.15FR1-μ/γ PCR products were gel purified and cloned with the Zero Blunt PCR Cloning kit (Invitrogen). Sequences were run in an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). Mutation frequencies were calculated by comparing the sequences obtained with germline intronic JH4-JH5 sequences over 341 bp, starting at the 3′ border of JH4, or the germline VH3.15 sequence, over 244 bp.
Analysis of AID expression in splenic subsets.
Splenic CD19-positive IgD+CD27+, IgD−CD27+, and IgD+CD27− cells from 4 patients were purified by 2 successive rounds of cell sorting, to achieve >99% purity. Total RNA was isolated from each fraction with the RNeasy Micro kit (QIAGEN) and reverse transcribed by random priming with the ProSTAR First-Strand RT-PCR synthesis kit (Stratagene). Real-time quantitative PCR (qPCR) for the human AID, Bcl-6, and β-2 microglobulin (β2m) transcripts was performed with specific TaqMan gene expression assays designed by Applied Biosystems (AID, Hs00757808_m1; Bcl-6, Hs00277037_m1; β2-m, Hs99999907_m1). Two independent PCRs were performed in triplicate. The results were analyzed according to the comparative method, normalizing to 1 the expression of AID in the splenic IgD+CD27+ fraction of the 8-mo-old donor. PCRs with a threshold cycle (Ct) >35 were considered NS.
Immunofluorescence on splenic sections.
Immediately after surgical splenectomy, small pieces of splenic tissue were excised and embedded in OCT, snap-frozen in liquid nitrogen, and stored at -80°C. Serial cryosections (8 μm) were cut, fixed in cold (−20°C) acetone for 10 min, rehydrated in wash buffer (TBS, pH 7.6), and incubated in blocking buffer (0.5% BSA, and 5% goat serum in PBS) for 1 h at room temperature. Then, sections were incubated for 30 min at room temperature, with the indicated primary antibodies diluted in blocking buffer. The following primary antibodies were used at the indicated dilutions: rabbit anti–human IgD (1:1,000), rabbit anti–human CD3, and mouse anti–human CD20 (clone L26; both ready-to-use) were obtained from DAKO; mouse anti–human CD27 (clone 137B4; 1:50) was purchased from Novocastra Laboratories; purified (0.5 mg/ml) mouse anti-CD1c (clone F10/21A3; 1:500) was obtained from B. Moody (Harvard Medical School, Boston, MA). Incubations in which the primary antibodies were omitted were performed as controls. Sections were washed and incubated (30 min at room temperature) with the following secondary antibodies: Alexa Fluor 488–conjugated goat anti–rabbit IgG (H+L; 1:300) and Alexa Fluor 555–conjugated goat anti–mouse IgG (H+L; 1:300; Invitrogen). Because of its weak expression, CD27 was detected by incubation with a biotinylated anti–mouse IgG (H+L; 1:300) antibody from Vector Laboratories, followed by Cy3-conjugated streptavidin (1:500; Jackson ImmunoResearch Laboratories). Sections were then washed and mounted in Fluoromount-G (SouthernBiotech), and images were acquired by confocal microscopy with a LSM 5 Pascal attached to an Axiovert 200 microscope and analyzed using the LSM 5 Image Browser (all from Carl Zeiss, Inc.). Fluorescent dyes were selectively excited, and the fluorescence of single channels was measured, to avoid overlapping emission.
Statistical analysis.
Comparisons between proportions (the percentage of clonal diversity being defined as the number of different VDJ among all sequences) were performed using a χ2 with one degree of freedom as implemented in the FREQ procedure of the SAS software v9.1 (SAS Institute).
Online supplemental material.
Fig. S1 shows H-CDR3 spectratypes of VH3.30/33 and VH5.51 transcripts expressed by splenic B cell subsets of donors D4 and D7. Fig. S2 shows the proportion of IgD+CD27+ and IgD−CD27+ B cells in the spleen and/or blood of infants. Table S1 shows the clonal diversity of each peak sequenced in the VH3.15 and VH6 spectratypes of the different blood B cell subsets of donors D1 and D2. Table S2 and S3 shows the clonal diversity of each peak sequenced in the VH3.15 and VH6 spectratypes of the different splenic B cell subsets of donors D3. Table S4 shows the VH5.51 repertoire analysis of splenic B cell subsets of donor D7 and includes the corresponding intrapeak clonal diversity analysis. The H-CDR3 spectratyping procedure and sequences of primers are described in the Supplemental materials and methods. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20071555/DC1.
Supplementary Material
Acknowledgments
We thank Frederic Delbos for quantitative PCR analysis, Jérome Mégret for his contribution to cell-sorting experiments, and Floriane De Rosa for her excellent technical assistance. We thank Dr. Alexandre Alcais for the statistical analysis, Meriem Garfa for assistance with confocal microscopy, and Dr. Branch Moody for providing the anti-CD1c antibody.
This work was supported by the Fondation Princesse Grace de Monaco and the Ligue Nationale Contre le Cancer (Equipe labellisée). M. Mamani-Matsuda was supported by successive fellowships from the Fondation de France, The Fondation Singer-Polignac, and the Région Ile-de-France. C.-A. Reynaud is Directeur de Recherche at the Centre National de la Recherche Scientifique.
The authors have no conflicting financial interests.
Abbreviations used: AID, activation-induced cytidine deaminase; GC, germinal center; MZ, marginal zone; SMZ, splenic MZ; TD, T-dependent; TI, T cell–independent.
References
- 1.Klein, U., R. Kuppers, and K. Rajewsky. 1997. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood. 89:1288–1298. [PubMed] [Google Scholar]
- 2.Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsetti, R., M.M. Rosado, and H. Wardmann. 2004. Peripheral development of B cells in mouse and man. Immunol. Rev. 197:179–191. [DOI] [PubMed] [Google Scholar]
- 4.Cuss, A.K., D.T. Avery, J.L. Cannons, L.J. Yu, K.E. Nichols, P.J. Shaw, and S.G. Tangye. 2006. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J. Immunol. 176:1506–1516. [DOI] [PubMed] [Google Scholar]
- 5.Sims, G.P., R. Ettinger, Y. Shirota, C.H. Yarboro, G.G. Illei, and P.E. Lipsky. 2005. Identification and characterization of circulating human transitional B cells. Blood. 105:4390–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei, C., J. Anolik, A. Cappione, B. Zheng, A. Pugh-Bernard, J. Brooks, E.H. Lee, E.C. Milner, and I. Sanz. 2007. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol. 178:6624–6633. [DOI] [PubMed] [Google Scholar]
- 7.Wirths, S., and A. Lanzavecchia. 2005. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur. J. Immunol. 35:3433–3441. [DOI] [PubMed] [Google Scholar]
- 8.Fecteau, J.F., G. Cote, and S. Neron. 2006. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J. Immunol. 177:3728–3736. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson, A., A. de Milito, F. Mowafi, G. Winberg, O. Bjork, E.Z. Wolpert, and F. Chiodi. 2005. Expression of CD27-CD70 on early B cell progenitors in the bone marrow: implication for diagnosis and therapy of childhood ALL. Exp. Hematol. 33:1500–1507. [DOI] [PubMed] [Google Scholar]
- 10.Vaskova, M., E. Mejstrikova, T. Kalina, P. Martinkova, M. Omelka, J. Trka, J. Stary, and O. Hrusak. 2005. Transfer of genomics information to flow cytometry: expression of CD27 and CD44 discriminates subtypes of acute lymphoblastic leukemia. Leukemia. 19:876–878. [DOI] [PubMed] [Google Scholar]
- 11.Tangye, S.G., and K.L. Good. 2007. Human IgM+CD27+ B cells: memory B cells or “memory” B cells? J. Immunol. 179:13–19. [DOI] [PubMed] [Google Scholar]
- 12.Weller, S., M.C. Braun, B.K. Tan, A. Rosenwald, C. Cordier, M.E. Conley, A. Plebani, D.S. Kumararatne, D. Bonnet, O. Tournilhac, et al. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 104:3647–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weller, S., A. Faili, C. Garcia, M.C. Braun, F.F. Le Deist, G.G. de Saint Basile, O. Hermine, A. Fischer, C.A. Reynaud, and J.C. Weill. 2001. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl. Acad. Sci. USA. 98:1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agematsu, K., H. Nagumo, K. Shinozaki, S. Hokibara, K. Yasui, K. Terada, N. Kawamura, T. Toba, S. Nonoyama, H.D. Ochs, and A. Komiyama. 1998. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. J. Clin. Invest. 102:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brezinschek, H.P., T. Dorner, N.L. Monson, R.I. Brezinschek, and P.E. Lipsky. 2000. The influence of CD40-CD154 interactions on the expressed human V(H) repertoire: analysis of V(H) genes expressed by individual B cells of a patient with X-linked hyper-IgM syndrome. Int. Immunol. 12:767–775. [DOI] [PubMed] [Google Scholar]
- 16.Warnatz, K., L. Bossaller, U. Salzer, A. Skrabl-Baumgartner, W. Schwinger, M. van der Burg, J.J. van Dongen, M. Orlowska-Volk, R. Knoth, A. Durandy, et al. 2006. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 107:3045–3052. [DOI] [PubMed] [Google Scholar]
- 17.Di Sabatino, A., M.M. Rosado, R. Ciccocioppo, P. Cazzola, R. Morera, G.R. Corazza, and R. Carsetti. 2005. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am. J. Gastroenterol. 100:1788–1795. [DOI] [PubMed] [Google Scholar]
- 18.Kruetzmann, S., M.M. Rosado, H. Weber, U. Germing, O. Tournilhac, H.H. Peter, R. Berner, A. Peters, T. Boehm, A. Plebani, et al. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takizawa, M., K. Sugane, and K. Agematsu. 2006. Role of tonsillar IgD+CD27+ memory B cells in humoral immunity against pneumococcal infection. Hum. Immunol. 67:966–975. [DOI] [PubMed] [Google Scholar]
- 20.Willenbrock, K., B. Jungnickel, M.L. Hansmann, and R. Kuppers. 2005. Human splenic marginal zone B cells lack expression of activation-induced cytidine deaminase. Eur. J. Immunol. 35:3002–3007. [DOI] [PubMed] [Google Scholar]
- 21.Ma, C.S., S. Pittaluga, D.T. Avery, N.J. Hare, I. Maric, A.D. Klion, K.E. Nichols, and S.G. Tangye. 2006. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J. Clin. Invest. 116:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toellner, K.M., W.E. Jenkinson, D.R. Taylor, M. Khan, D.M. Sze, D.M. Sansom, C.G. Vinuesa, and I.C. MacLennan. 2002. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J. Exp. Med. 195:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer, K., M. Zemlin, M. Hummel, S. Pfeiffer, J. Karstaedt, G. Steinhauser, X. Xiao, H. Versmold, and C. Berek. 2002. Diversification of Ig heavy chain genes in human preterm neonates prematurely exposed to environmental antigens. J. Immunol. 169:1349–1356. [DOI] [PubMed] [Google Scholar]
- 24.Zandvoort, A., and W. Timens. 2002. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin. Exp. Immunol. 130:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pannetier, C., J. Even, and P. Kourilsky. 1995. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol. Today. 16:176–181. [DOI] [PubMed] [Google Scholar]
- 26.Holtmeier, W., A. Hennemann, and W.F. Caspary. 2000. IgA and IgM V(H) repertoires in human colon: evidence for clonally expanded B cells that are widely disseminated. Gastroenterology. 119:1253–1266. [DOI] [PubMed] [Google Scholar]
- 27.Itoh, K., V. Patki, R.A. Furie, E.K. Chartash, R.I. Jain, L. Lane, S.E. Asnis, and N. Chiorazzi. 2000. Clonal expansion is a characteristic feature of the B-cell repertoire of patients with rheumatoid arthritis. Arthritis Res. 2:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada, H., M.M. Kawano, N. Huang, Y. Harada, K. Iwato, O. Tanabe, H. Tanaka, A. Sakai, H. Asaoku, and A. Kuramoto. 1993. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood. 81:2658–2663. [PubMed] [Google Scholar]
- 29.Harada, Y., M.M. Kawano, N. Huang, M.S. Mahmoud, I.A. Lisukov, K. Mihara, T. Tsujimoto, and A. Kuramoto. 1996. Identification of early plasma cells in peripheral blood and their clinical significance. Br. J. Haematol. 92:184–191. [DOI] [PubMed] [Google Scholar]
- 30.Galibert, L., N. Burdin, B. de Saint-Vis, P. Garrone, C. Van Kooten, J. Banchereau, and F. Rousset. 1996. CD40 and B cell antigen receptor dual triggering of resting B lymphocytes turns on a partial germinal center phenotype. J. Exp. Med. 183:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada, M., R. Wasserman, B.A. Reichard, S. Shane, A.J. Caton, and G. Rovera. 1991. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J. Exp. Med. 173:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy, Y., N. Gupta, F. Le Deist, C. Garcia, A. Fischer, J.C. Weill, and C.A. Reynaud. 1998. Defect in IgV gene somatic hypermutation in common variable immuno-deficiency syndrome. Proc. Natl. Acad. Sci. USA. 95:13135–13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102:553–563. [DOI] [PubMed] [Google Scholar]
- 34.Revy, P., T. Muto, Y. Levy, F. Geissmann, A. Plebani, O. Sanal, N. Catalan, M. Forveille, R. Dufourcq-Labelouse, A. Gennery, et al. 2000. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell. 102:565–575. [DOI] [PubMed] [Google Scholar]
- 35.Ye, B.H., G. Cattoretti, Q. Shen, J. Zhang, N. Hawe, R. de Waard, C. Leung, M. Nouri-Shirazi, A. Orazi, R.S. Chaganti, et al. 1997. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat. Genet. 16:161–170. [DOI] [PubMed] [Google Scholar]
- 36.Han, J.H., S. Akira, K. Calame, B. Beutler, E. Selsing, and T. Imanishi-Kari. 2007. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 27:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueda, Y., D. Liao, K. Yang, A. Patel, and G. Kelsoe. 2007. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J. Immunol. 178:3593–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zandvoort, A., M.E. Lodewijk, N.K. de Boer, P.M. Dammers, F.G. Kroese, and W. Timens. 2001. CD27 expression in the human splenic marginal zone: the infant marginal zone is populated by naive B cells. Tissue Antigens. 58:234–242. [DOI] [PubMed] [Google Scholar]
- 39.Spencer, J., M.E. Perry, and D.K. Dunn-Walters. 1998. Human marginal-zone B cells. Immunol. Today. 19:421–426. [DOI] [PubMed] [Google Scholar]
- 40.Timens, W., A. Boes, T. Rozeboom-Uiterwijk, and S. Poppema. 1989. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J. Immunol. 143:3200–3206. [PubMed] [Google Scholar]
- 41.Lim, A., B. Lemercier, X. Wertz, S.L. Pottier, F. Huetz, and P. Kourilsky. 2008. Many human peripheral VH5-expressing IgM+ B cells display a unique heavy-chain rearrangement. Int. Immunol. 20:105–116. [DOI] [PubMed] [Google Scholar]
- 42.McHeyzer-Williams, L.J., L.P. Malherbe, and M.G. McHeyzer-Williams. 2006. Checkpoints in memory B-cell evolution. Immunol. Rev. 211:255–268. [DOI] [PubMed] [Google Scholar]
- 43.Liu, Y.J., F. Malisan, O. de Bouteiller, C. Guret, S. Lebecque, J. Banchereau, F.C. Mills, E.E. Max, and H. Martinez-Valdez. 1996. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 4:241–250. [DOI] [PubMed] [Google Scholar]
- 44.White, H., and D. Gray. 2000. Analysis of immunoglobulin (Ig) isotype diversity and IgM/D memory in the response to phenyl-oxazolone. J. Exp. Med. 191:2209–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian, C., G.K. Luskin, K.M. Dischert, J.N. Higginbotham, B.E. Shepherd, and J.E. Crowe Jr. 2007. Evidence for preferential Ig gene usage and differential TdT and exonuclease activities in human naive and memory B cells. Mol. Immunol. 44:2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucas, A.H., K.D. Moulton, V.R. Tang, and D.C. Reason. 2001. Combinatorial library cloning of human antibodies to Streptococcus pneumoniae capsular polysaccharides: variable region primary structures and evidence for somatic mutation of Fab fragments specific for capsular serotypes 6B, 14, and 23F. Infect. Immun. 69:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin, F., and J.F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323–335. [DOI] [PubMed] [Google Scholar]
- 48.Shi, Y., K. Agematsu, H.D. Ochs, and K. Sugane. 2003. Functional analysis of human memory B-cell subpopulations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin. Immunol. 108:128–137. [DOI] [PubMed] [Google Scholar]
- 49.Rosner, K., D.B. Winter, R.E. Tarone, G.L. Skovgaard, V.A. Bohr, and P.J. Gearhart. 2001. Third complementarity-determining region of mutated VH immunoglobulin genes contains shorter V, D, J, P, and N components than non-mutated genes. Immunology. 103:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsuiji, M., S. Yurasov, K. Velinzon, S. Thomas, M.C. Nussenzweig, and H. Wardemann. 2006. A checkpoint for autoreactivity in human IgM+ memory B cell development. J. Exp. Med. 203:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dammers, P.M., A. Visser, E.R. Popa, P. Nieuwenhuis, and F.G. Kroese. 2000. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J. Immunol. 165:6156–6169. [DOI] [PubMed] [Google Scholar]
- 52.Schelonka, R.L., J. Tanner, Y. Zhuang, G.L. Gartland, M. Zemlin, and H.W. Schroeder Jr. 2007. Categorical selection of the antibody repertoire in splenic B cells. Eur. J. Immunol. 37:1010–1021. [DOI] [PubMed] [Google Scholar]
- 53.Wardemann, H., S. Yurasov, A. Schaefer, J.W. Young, E. Meffre, and M.C. Nussenzweig. 2003. Predominant autoantibody production by early human B cell precursors. Science. 301:1374–1377. [DOI] [PubMed] [Google Scholar]
- 54.Pillai, S., A. Cariappa, and S.T. Moran. 2004. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunol. Rev. 197:206–218. [DOI] [PubMed] [Google Scholar]
- 55.Pillai, S., A. Cariappa, and S.T. Moran. 2005. Marginal zone B cells. Annu. Rev. Immunol. 23:161–196. [DOI] [PubMed] [Google Scholar]
- 56.Weill, J.C., and C.A. Reynaud. 2005. Do developing B cells need antigen? J. Exp. Med. 201:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanning, D.K., K.J. Rhee, and K.L. Knight. 2005. Intestinal bacteria and development of the B-lymphocyte repertoire. Trends Immunol. 26:419–425. [DOI] [PubMed] [Google Scholar]
- 58.Rhee, K.J., P.J. Jasper, P. Sethupathi, M. Shanmugam, D. Lanning, and K.L. Knight. 2005. Positive selection of the peripheral B cell repertoire in gut-associated lymphoid tissues. J. Exp. Med. 201:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wen, L., J. Brill-Dashoff, S.A. Shinton, M. Asano, R.R. Hardy, and K. Hayakawa. 2005. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 23:297–308. [DOI] [PubMed] [Google Scholar]
- 60.Song, H., and J. Cerny. 2003. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J. Exp. Med. 198:1923–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.