Abstract
The presence of serum bactericidal antibodies is a proven correlate of protection against systemic infection with the important human pathogen Neisseria meningitidis. We have identified three serogroup C N. meningitidis (MenC) isolates recovered from patients with invasive meningococcal disease that resist killing by bactericidal antibodies induced by the MenC conjugate vaccine. None of the patients had received the vaccine, which has been successfully introduced in countries in North America and Europe. The increased resistance was not caused by changes either in lipopolysaccharide sialylation or acetylation of the α2-9–linked polysialic acid capsule. Instead, the resistance of the isolates resulted from the presence of an insertion sequence, IS1301, in the intergenic region (IGR) between the sia and ctr operons, which are necessary for capsule biosynthesis and export, respectively. The insertion sequence led to an increase in the transcript levels of surrounding genes and the amount of capsule expressed by the strains. The increased amount of capsule was associated with down-regulation of the alternative pathway of complement activation, providing a generic mechanism by which the bacterium protects itself against bactericidal antibodies. The strains with IS1301 in the IGR avoided complement-mediated lysis in the presence of bactericidal antibodies directed at the outer membrane protein, PorA, or raised against whole cells.
Bacteria have a remarkable capacity to respond to new environments and adapt to novel selective pressures. This versatility reflects their genetic diversity, which is generated by high mutation rates, the acquisition of novel sequences by horizontal transfer, and mobile elements and repeated sequences in their genomes (1). Consequently, populations of bacteria often harbor variants that may have a selective advantage in a new or changing environment. The diversity of bacteria and their ability to generate novel variants can promote their virulence and limit the success of prophylactic and therapeutic interventions.
Neisseria meningitidis is a Gram-negative diplococcus that remains a leading cause of bacterial meningitis and septicaemia. The complement system is critical for immunity against this important pathogen, as indicated by the exquisite sensitivity of individuals with complement deficiencies to meningococcal infection (2). This is particularly true for people lacking components (C5 to C9, inclusive) of the membrane attack complex (MAC), which is necessary for bacterial lysis (3). Furthermore, the most validated correlate of protection against meningococcal infection is the presence in serum of bactericidal antibodies (4–6). These antibodies initiate the classical pathway (CP) of complement activation, ultimately leading to the insertion of the MAC into the outer membrane of N. meningitidis and bacteriolysis.
The N. meningitidis serogroup C conjugate (MCC) vaccine has been recently introduced into countries across Europe and North America (7, 8), primarily in response to the rising incidence of serogroup C N. meningitidis (MenC) disease caused by strains belonging to sequence type 11 (ST-11) (9). ST-11 strains have been responsible for meningococcal infection across the world, causing endemic disease usually as MenC isolates and occasional outbreaks as serogroup W135 isolates (10–12). The MCC vaccine consists of the α2-9–linked polysialic capsular polysaccharide coupled to a carrier protein (13), and protection is mediated by bactericidal antibodies directed against the capsule (4, 6). The level of these antibodies in individuals is measured by the serum bactericidal antibody (SBA) assay, and an SBA titer of ≥8 is a proven correlate of protection against MenC disease (4, 14).
The MCC vaccine has been a major success and has resulted in dramatic decreases in the incidence of MenC disease to <5% of prevaccine levels, with an estimated vaccine efficacy between 95–98% (7, 8, 15). So far, concerns (16) that the MCC vaccine would lead to the emergence of ST-11 strains expressing other capsular types or that other strains of N. meningitidis would fill the ecological niche vacated by MenC have proven largely unfounded, although continuing surveillance is still underway (8).
In this study, we describe the identification and characterization of three MenC strains with enhanced resistance against bactericidal antibodies elicited by the MCC vaccine. We found that an insertion sequence, IS1301, in the 134-bp intergenic region (IGR) between the sia and ctr operons (involved in capsule biosynthesis and export, respectively) is responsible for the resistance. Previously, IS1301 has only been described as causing gene inactivation in N. meningitidis (17). Insertion of this element in the IGR results in increased transcript levels in both operons, leading to an increase in the amount of capsular polysaccharide and impairment of the alternative pathway (AP) of complement activation. This single genetic change provides the meningococcus with a generic mechanism to evade killing by bactericidal antibodies, and is of broad significance given the widespread distribution of IS1301 in N. meningitidis and the bacterium's competence for DNA uptake.
RESULTS
Identification of MenC strains that avoid bactericidal antibodies elicited by the MCC vaccine
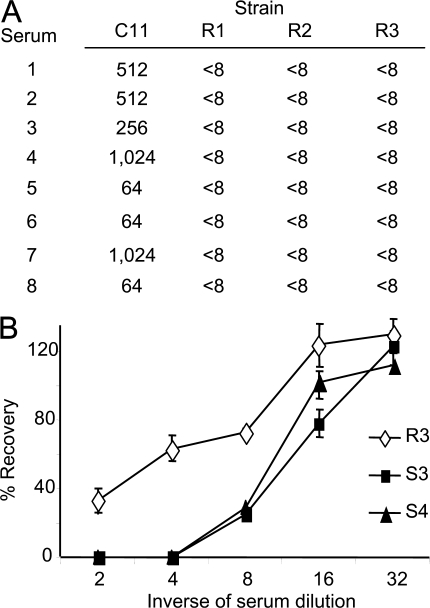
To identify meningococcal strains that are resistant to SBAs raised by the MCC vaccine, we used sera from three vaccinees to screen 109 MenC isolates from patients with proven meningococcal disease received by the Spanish National Reference laboratory between 2000 and 2003. The three vaccinees had been immunized with an MCC vaccine and had high level SBA titers against C11, a MenC strain routinely used for assessment of vaccine responses (Fig. 1 A) (18, 19). Three strains, designated R1, R2, and R3, were identified, against which the SBA titers of sera from all three vaccinees was <8. The strains were tested with sera from five more vaccinees with identical results. SBA titers against MenC are routinely measured with rabbit complement (6, 14); similar results were obtained when assays were performed with human complement (Table S1, available at http://www.jem.org/cgi/content/full/jem.20072577/DC1). Isolate R1 was recovered from a 37-yr-old female with fatal meningococcal septicaemia in Madrid in 2000, whereas R2 was obtained from blood cultures taken from a 40-yr-old female from Léon in 2003. R3 was isolated from blood cultures from a 19-yr-old female in Valladolid in the same year. None of the patients had received a MenC vaccine, and the patients infected with strains R2 and R3 made uneventful recoveries. The R strains were phenotypically C:2a:P1.5 and ST-11, subgroup ET-15 (ST-11/ET-15) by multilocus sequencing typing and fumC sequencing (20). Five fully sensitive C:2a:P1.5, ST-11/ET-15 isolates (designated S1–S5) were selected from the collection at the reference laboratory as controls. We also examined the recovery of one of the R strains after incubation in immune sera, which includes human complement; R3 was relatively more resistant against killing in human immune sera than the control strains, S3 and S4 (Fig. 1 B).
Figure 1.
Identification of strains with increased resistance against bactericidal antibodies elicited by the MenC conjugate vaccine. (A) SBA titers using sera from eight vaccinees against the R strains and the control strain, C11. All sera had SBA titers of <8 with the R strains. (B) The percent survival of strains S3, S4, and R3 after 1 h of incubation in dilutions of human immune serum. Assays were performed in triplicate, and the error bars show the SEM. The survival of R3 was significantly increased compared with other strains. P = 0.0002 and 0.0001 at a 1:2 and 1:4 serum dilution, respectively, with the Student's t test.
Strains are not resistant because of changes in LPS sialylation or acetylation of capsular polysaccharide
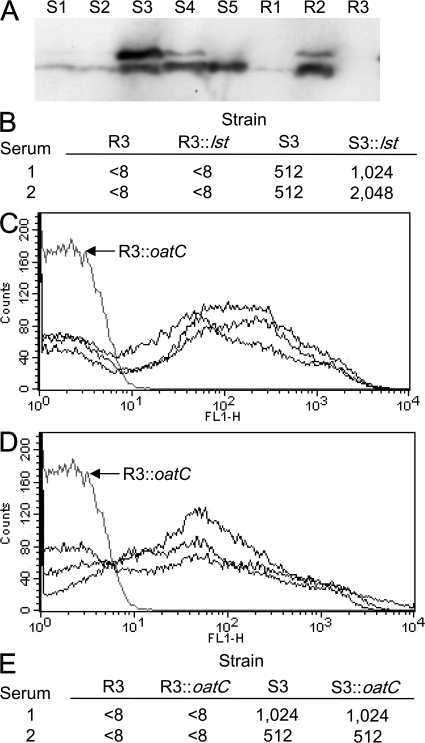
To understand the basis of the increased resistance of the R strains against immune sera, we first examined the degree of LPS sialylation of strains, as this modification can contribute to the avoidance of complement-mediated lysis (3, 21). There were no consistent differences in the degree of LPS sialylation between the R and S strains, as determined by Western blot analysis using mAb 3F11, which binds to unsialylated LPS (Fig. 2 A) (22). To exclude the possibility that LPS sialylation was responsible for the resistance of strains, we inactivated lst (which encodes the LPS-specific sialyl transferase) in strain R3. This had no impact on the survival of this strain in the presence of immune sera, demonstrating that its resistance is independent of LPS sialylation (Fig. 2 B).
Figure 2.
Resistance of strains is not due to changes in LPS sialylation or O-acetylation of capsular polysaccharide. (A) The extent of LPS sialylation of the R and S strains. Samples were separated by SDS-PAGE and transferred to membranes that were then incubated with the mAb 3F11, which detects unsialylated LPS. The strains are indicated above each lane. (B) SBA titers of two vaccinees' sera against R3, S3, and the corresponding lst mutants, which are unable to sialylate their LPS; a lack of LPS sialylation did not affect the survival of the R or S strains. Both the S (C) and R (D) strains express an O-acetylated polysaccharide capsule. FACS analysis of strains was performed with the mAb 1125. The gray line shows results obtained with the oatC-negative mutant, R3∷oatC. (E) The sensitivity of S3 and R3 to antibodies in sera from two vaccinees is unaffected by acetylation. Identical SBA titers were obtained with wild-type strains and their corresponding oatC mutants.
We next examined whether alterations in the acetylation status of the capsule affected the resistance of strains. Capsule O-acetylation is mediated by a transferase encoded by oatC, which is located in the capsule biosynthesis locus (cps) (23). The oatC open reading frame contains two homopolymeric tracts that can mediate ON:OFF switching of acetylation after alteration in the tract length during DNA replication (23). The nucleotide sequence of the oatC gene in the R and S strains indicated that the gene is predicted to be expressed in all strains (unpublished data). The capsules of all strains were acetylated, as demonstrated by FACS analysis using the mAb 1125 (Fig. 2, C and D). To define the role of O-acetylation in the behavior of the R strains, we generated an oatC mutant, R3∷oatC, by insertional inactivation. Loss of oatC did not alter the survival of this strain in the presence of immune sera (Fig. 2 E), excluding acetylation as the cause of the difference between the R and S strains.
Insertion of IS1301 in the capsule biosynthesis locus is responsible for increased resistance
To investigate the role of the capsule itself, we generated capsule-negative derivatives of strains R3 and S3 by inactivating siaD, which encodes the capsule-specific polysialyl transferase (24). Although the SBA titer of the vaccinees' sera (which contain antibodies against subcapsular antigens as well as against capsular polysaccharide) against strain S3 was largely unchanged after loss of the capsule, R3 became markedly more susceptible to killing (Fig. 3 A), with the SBA titer increasing from <8 for R3 to ≥512 for R3ΔsiaD. This demonstrates that expression of the capsule is necessary for the enhanced resistance of R3, suggesting that the R strains might harbor changes in the cps that mediate their enhanced survival in the presence of immune sera. To test this hypothesis, we attempted to transfer the resistance from an R strain with a marker in the cps into a sensitive strain. Genomic DNA from strain R3∷oatC (Fig. 3 B) was used to transform S3, and kanamycin-resistant transformants were analyzed for their sensitivity to bactericidal antibodies. As expected (depending on whether recombination transferred in sequences responsible for increased resistance; Fig. 3 B), we identified transformants that had become resistant to killing (S3∷T1) to antibodies elicited by the MCC vaccine, as well as those that remained fully sensitive (S3∷T2 and S3∷T3).
Figure 3.
Insertion of IS1301 between the sia and ctr operons leads to enhanced resistance. (A) The increased resistance of R3 requires expression of the polysaccharide capsule. The siaD mutant of R3 is sensitive to killing mediated by sera from two vaccinees, compared with the parental strain. (B) Transfer of resistance from strain R3 to S3 using genomic DNA from R3∷oatC as the donor. This was performed to test the hypothesis that R3 (DNA shown as a continuous red line) has a region (indicated with a black square) in the cps that confers resistance and is different from the S strains (DNA shown as a blue dotted line). Transformants have enhanced resistance (S3∷T1) or are fully sensitive (S3∷T2 and S3∷T3) transformants after transformation of S3, as would be expected depending on the site of recombination. (C) Overlapping PCR products spanning the cps were amplified from the transformants. The strain used as the target is indicated above each lane, and primers are given underneath; the sizes of a kb ladder are shown. White lines indicate that intervening lanes have been spliced out. (D) Analysis of PCR products obtained with primers NG732 and NG733 from the R and S strains. The R strains gave a product of around 1.1 kb, ∼800 bp larger than that obtained from the sensitive strains. (E) Location of the IS1301 in the 134-bp siaA-ctrA intergenic region (thick black line). which contains a divergent promoter. Transfer of an IGR containing IS1301 (amplified with primers NG679 and NG687) to S3 (resulting in S3∷R) is sufficient to confer resistance against killing mediated by bactericidal antibodies in vaccinees' sera. The location of the kan resistance marker in strains R3∷IS1301kan and S3∷R is indicated with a triangle.
To identify regions of the cps that might confer the increased resistance, we amplified overlapping fragments of the cps from the transformants S3∷T1, S3∷T2, and S∷T3. One region of the cps (amplified with primers NG638 and NG639) yielded a significantly larger product from the transformant with enhanced resistance (S3∷T1) compared with the fully sensitive transformants (S3∷T2 and S3∷T3; Fig. 3 C). Further PCRs were performed to define the precise location of this polymorphic region; using primers NG732 and NG733 (Fig. 3), an ∼1.1-kb fragment was amplified from S3∷T1, whereas an ∼300-bp product was obtained from S3∷T2 and S3∷T3 (not depicted). We also examined the sizes of fragments obtained with these primers from the isolates R1–R3 and S1–S5, and 27 more fully sensitive, ST-11 strains from the Spanish collection. The R strains all gave the larger ∼1.1-kb product, whereas the ∼300-bp product was amplified from all sensitive strains, demonstrating a direct correlation between the size of this region of the cps and the ability of strains to withstand SBAs elicited by immunization with the MCC vaccine (Fig. 3 D and not depicted).
Sequence analysis demonstrated that the polymorphism in the cps locus resulted from the presence of an insertion sequence, IS1301, in the 134-bp IGR between siaA and ctrA in all R strains and S3∷T1. siaA and ctrA are the first genes in monocistronic operons involved in the biosynthesis and export of capsular polysaccharide, respectively. The operons are divergently transcribed from promoters in the IGR (25–27). IS1301 is an 844-bp mobile element that is present in multiple copies in the genomes of some meningococcal strains, including ST-11 isolates belonging to ET-15 (17, 28). The insertion sequence was located at the identical site in the same orientation in all three R strains, 54 bp upstream of the ATG start codon of siaA. To establish whether IS1301 in the siaA-ctrA IGR is responsible for the resistance of the R strains, we transferred this element from R3 into the sensitive strain, S3. First, a marker encoding kanamycin resistance was introduced into nucleotide 472 of IS1301 in R3, generating R3∷IS1301kan; the marker had no effect on the SBA of pooled immune sera against R3 (Fig. 3 E). Then, we transferred the only modified IGR into S3, generating the isogenic strain S3∷R, and this was sufficient to transfer increased resistance against bactericidal antibodies to strain S3 (Fig. 3 E).
Insertion of IS1301 in the IGR increases capsule expression but does not affect its structure
We next determined whether the insertion of IS1301 affected the antigenic properties of the capsular polysaccharide. Initially immune sera were raised against the isogenic strains, S3 and S3∷R, and against R3. As expected, sera raised against S3 mediated killing of the homologous strain S3 (SBA titer 1,024) but not S3∷R or R3 (Table I). High dilutions of sera raised against S3∷R and R3 were also able to elicit complement-mediated lysis of S3, consistent with the capsular polysaccharide from the R strains retaining its immunogenicity. However, even high concentrations of the same sera failed to mediate killing of S3∷R or R3 (Table I), suggesting that the strains' increased resistance was not caused by an alteration in the nature of antigens expressed by the strains.
Table I.
SBA titers of sera raised against different strains in assays against homologous or heterologous strains
| SBA titer against
|
|||
|---|---|---|---|
| Sera raised versus | S3 | S3∷R | R3 |
| S3 | 1,024 | <8 | <8 |
| SR | 1,024 | <8 | <8 |
| R3 | 1,024 | <8 | <8 |
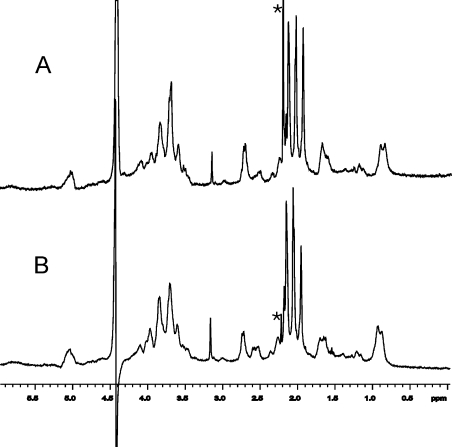
To establish whether there are qualitative changes in the capsule from the R strains, we assessed the composition of the capsular polysaccharide of strains by 1H NMR spectroscopy (29). The one-dimensional NMR spectrum of the serogroup C polysaccharide is sensitive to O-acetylation at C7 or C8, and the local sequence of O-acetylated monosaccharide residues (29). The spectrum of purified capsule from S3 (Fig. 4 A) was similar to that obtained for R3 and S3∷R (Fig. 4 B and not depicted), and this was confirmed by two-dimensional total correlated spectroscopy (not depicted). This demonstrated that the capsular polysaccharides of the strains had identical linkages between the sialic acid monomers and the same distribution of O-acetylation. Therefore, insertion of IS1301 in the IGR did not result in a detectable change in the composition of the capsule.
Figure 4.
1H NMR spectra of purified capsular polysaccharide. No significant difference was identified between the capsules from strains S3 (A) and R3 (B). The asterisk denotes the internal standard (acetone), and the prominent and distorted peaks at ∼4.8 parts per million (ppm) in both spectra are caused by partially deuterated water.
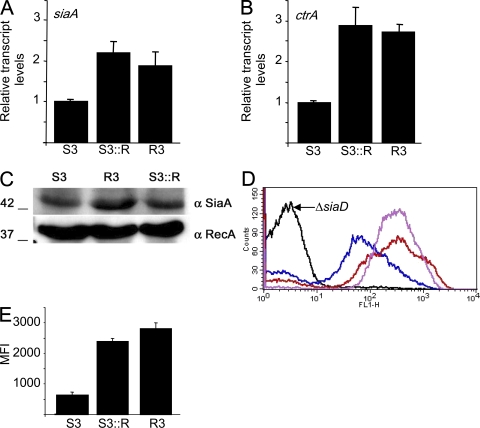
We next examined whether insertion of IS1301 in the IGR affected the amount of capsule expressed by influencing the expression of neighboring genes. We performed quantitative real-time RT-PCR (qrtRT-PCR) on siaA and ctrA, and compared the results with the transcript levels of a housekeeping gene, gdh (30). Although the transcript levels of gdh were not significantly different between the sensitive strains and those with enhanced resistance (not depicted), R3 and S3∷R had a two- to threefold increase in mRNA of both siaA and ctrA compared with S3 (P < 0.005 for siaA and ctrA in R3 and S3∷R vs. S3 using the Student's t test; Fig. 5, A and B, respectively). We then determined whether the increase in the transcription of genes in the cps resulted in an increase in the amount of the SiaA protein that is encoded by the first gene of the sia operon. Western blot analysis with an antipeptide antibody demonstrated that R3 and S3∷R produced higher levels of SiaA than strain S3 (Fig. 5 C). We also determined the amount of capsular polysaccharide on the surface of the bacteria. We performed FACS analysis using polyclonal mouse sera raised against the MCC vaccine. Examination of a strain lacking capsule R3ΔsiaD demonstrated that there was no nonspecific binding of the antisera to N. meningitidis (Fig. 5 D). There was a significant increase in capsule expression by strains R3 and S3∷R when compared with S3 (P < 0.005 for both strains with increased resistance vs. S3 using the Student's t test; Fig. 5, D and E); the mean fluorescence index (MFI) of the strains with enhanced resistance was approximately three times higher when compared with the sensitive S3 strain.
Figure 5.
Insertion of IS1301 increases the levels of siaA and ctrA transcripts and the amount of polysaccharide capsule expressed by bacteria. The relative amounts of siaA (A) and ctrA (B) transcripts from S3, R3, and S3∷R measured by qrtRT-PCR. Error bars show the SEM of assays performed in triplicate. P < 0.005 for differences in siaA and ctrA mRNA levels in R3 and S3∷R versus S3 using the Student's t test. (C) Western blot analysis of SiaA and RecA in whole-cell lysates. The strains are indicated above each lane, and the sizes of the proteins (in kD) are shown. (D) Expression of the polysialic capsule detected by FACS. Bacteria were incubated with polyclonal antisera raised against the MCC vaccine (black, capsule-negative strain R3ΔsiaD; blue, S3; red, S3∷R; purple, R3). (E) Relative amount of capsule detected by FACS. Results are shown as the MFI (calculated as the geometric mean multiplied by the percentage of positive cells). Error bars show the SEM. P < 0.005 for both strains with increased resistance versus S3 using the Student's t test.
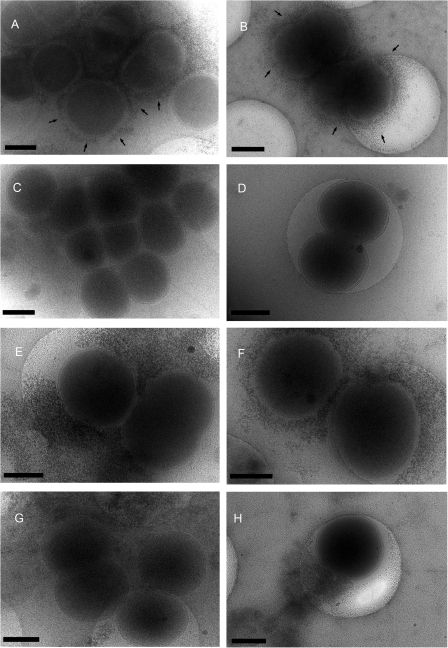
Furthermore, we examined the strains by cryo–transmission electron microscopy (cryo-TEM) after labeling live bacteria with the electron-dense, cationic stain ferritin, which binds to negatively charged bacterial capsules (31). After incubation with ferritin, bacteria were fixed by rapid freezing and viewed by TEM without sectioning. With this method, capsular polysaccharide was clearly visualized around strain S3 (Fig. 6, A and B); no staining was detected around the siaD mutant (Fig. 6, C and D), demonstrating the specificity of staining. In contrast, there was much more capsule surrounding R3 and S3∷R than observed around S3 (Fig. 6, E and F; and G and H, respectively), with capsular material extending well beyond the bacterial surface occasionally as aggregates of extracellular material. The differences between the strains were consistent on three independent occasions.
Figure 6.
Ferritin staining of capsular polysaccharide detected by cryo-TEM. Capsular material was detected around the surface of the sensitive strain S3 (A and B, arrows) but not the corresponding siaD mutant (C and D). Strains R3 and S3∷R (E and F; and G and H, respectively) expressed larger amounts of capsule, which formed large extracellular aggregates in places. Bars, 400 nm.
Activation of the alternative complement pathway is reduced on the strains with enhanced resistance
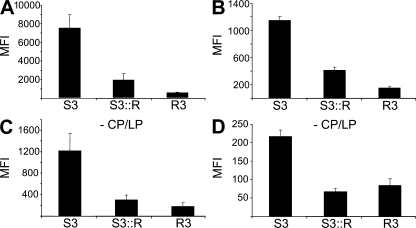
An increase in the expression of capsule, the antigen recognized by SBAs elicited by the MCC vaccine, could enhance the sensitivity of strains to immune sera rather than render them more resistant. Therefore, to further understand the underlying reason for the resistance of the strains, we examined the deposition of complement factors on bacteria after incubation in immune sera. Both R3 and S3∷R had significantly less C3 and MAC on their surfaces compared with the fully sensitive strain S3 (Fig. 7, A and B, respectively), consistent with the results of SBA titers and serum assays (Fig. 1). Sialic acid–containing capsules can inhibit the amplification of the complement cascade by interfering with the AP of complement activation (32–34); the AP serves to amplify the amount of complement activation on pathogen surfaces (35). Therefore, we examined whether there were differences in the extent of the AP activity on the surface of the strains after incubation in immune sera by blocking the CP and lectin pathway (LP) with magnesium and EGTA (36, 37). As expected, this resulted in significantly lower overall levels of complement factors deposited on the bacteria. However, there was still significantly less C3 and MAC present on R3 and S3∷R than S3 even in the absence of the CP and LP (P < 0.01; Fig. 7, C and D, respectively), demonstrating that strains with enhanced resistance had reduced activity of the AP on their surface.
Figure 7.
Enhanced activity of the AP on sensitive strains. Binding of C3 (A and C) and MAC (B and D) to bacteria detected by FACS analysis after incubation in immune human serum pooled from 10 donors. Results are shown as the MFI (calculated as geometric mean multiplied by the percentage of gated cells), and the error bars show the SEM. In B and D, strains were incubated with serum in the presence of magnesium and EGTA to block the CP and LP (-CP/LP). Error bars show the SEM of assays performed in triplicate. There was significantly less binding of complement factors to R3 and S3∷R compared with S3. P < 0.01 in all assays with the Student's t test.
If the increased amount of capsular polysaccharide inhibits the AP, this could provide a generic mechanism that protects the bacterium against bactericidal antibodies irrespective of the target antigen. We therefore tested the sensitivity of strains against an mAb against PorA (P1.5), an immunogenic outer membrane protein that elicits bactericidal antibodies (38). Although the mAb mediated lysis of the S strains with titers of 128 or 256, the titer against the R strains was <8; these findings were confirmed in assays using a human complement source (Table S1) and are consistent with the lack of killing in the presence of antibodies raised against whole cells (Table I).
DISCUSSION
We have identified MenC strains that have increased resistance against complement-mediated bacterial lysis, the most critical aspect of immunity against N. meningitidis. The strains belong to the ST-11 clone, which has spread across the world since first emerging in Canada (10, 12). The strains contain the identical genetic change in the cps, with IS1301 inserted at the same site in the IGR between operons necessary for sialic acid biosynthesis and capsule export. None of the sensitive strains had this genetic change, and transfer of IS1301 into the IGR of an S strain was sufficient to render it resistant to SBAs directed against the capsule, the outer membrane protein, PorA, and against whole cells.
There are numerous instances of bacteria avoiding immune killing through antigenic variation or by reducing expression of antigens on their surface. These mechanisms have been described for PorA, the major immunogen in outer membrane vesicle vaccines (39). A single amino acid change in this protein can affect the activity of bactericidal antibodies (40, 41), whereas changes in the length of homopolymeric tracts in the porA promoter or coding sequences can affect transcription or translation, respectively (42).
In contrast, our data demonstrate that insertion of IS1301 in the sia-ctr IGR actually leads to an increase in the amount of the target antigen on the bacterial surface. However, the capsule is not only the target of the immune response but is also an important means by which bacteria are protected against complement-mediated killing. The reduced level of C3 bound to the strains with increased resistance in the absence of the CP and LP is in keeping with their enhanced resistance resulting from inhibition of the AP associated with increased capsule expression (32–34). The function of the AP is to amplify complement activation initiated by the CP and LP, or the spontaneous low level generation of C3b from C3 in the circulation (35). Therefore, inhibition of the AP reduces complement activation regardless of the initial stimulus. However, other mechanisms could also contribute, including elevated turnover and shedding of capsular antigen by the R strains (directing SBAs away from the bacterial surface). Furthermore, the enhanced capsule could also directly inhibit insertion of the MAC (a large multiprotein complex of around 1 mD) into the outer membrane, thereby preventing bacterial lysis (35, 43). Masking of subcapsular antigens might promote resistance against killing mediated by α-PorA mAbs and antibodies raised against whole cells.
This is the first time that IS1301 has been shown to increase gene expression in the meningococcus, although it has been described before in Actinobacillus actinomycetemcomitans (44). Instead, this element has been associated with reversible inactivation of genes, including porA, siaA, and oatC (23, 45, 46). ST-11/ET-15 isolates, which contain between 15 and 20 copies of IS1301 (17), have caused outbreaks of meningococcal disease with high case fatality rates (47, 48). Although it is not known whether these strains are inherently more virulent, IS1301 could have a significant impact on the pathogenesis of ET-15 infections by up-regulating expression of specific virulence determinants, including capsule. The mechanisms by which IS1301 increases mRNA levels of both siaA and ctrA are likely to be complex, as these genes are divergently transcribed from overlapping promoters (26, 27). Our preliminary data indicate that IS1301 harbors promoter elements (unpublished data), although this does not exclude the possibility that the insertion displaces negatively acting regulators, as described previously (44).
The cryo-TEM technique we used (49) permitted visualization of bacteria without sectioning or contrast enhancement; ferritin was used to stain bacteria through its charge-based attraction to anionic capsules (31). The specificity of this method was demonstrated by the lack of staining of the capsule-negative mutant (Fig. 6). Strains R3 and S3∷R produced more capsule compared with S3, consistent with FACS results (Fig. 5). Rather than being evenly distributed over the entire surface of the bacterium, the increased capsule in the R strains occasionally formed irregular extracellular aggregates, which might serve to divert bactericidal antibodies and complement factors away from the bacterial outer membrane. However, this appearance may be an artifact of TEM, and further studies are underway to measure the rates of capsule shedding by the strains.
Serogroups of N. meningitidis that express sialic acid–containing capsules present a significant threat to human health (13). There are no broadly effective, licensed vaccines against serogroup B N. meningitidis, which remains the leading cause of disease in North America and Europe. Serogroup W135 strains have caused large outbreaks of meningococcal disease in sub-Saharan Africa and the global spread of infection from the Hajj pilgrimage in 2000 (11), whereas serogroup Y infections have emerged as a major problem in North America (50). The sia-ctr IGR is present in all meningococcal serogroups that produce sialic acid capsules (23). Therefore, strains belonging to these serogroups could become capsule hyperproducers after acquisition of IS1301 in the IGR by transformation.
Typing methods for the meningococcus do not routinely assess sensitivity to bactericidal antibodies, as this is a laborious task that is difficult to standardize (51). However, this information is vital for understanding the potential range of efficacy of novel vaccines, virulence mechanisms used by strains, and the molecular basis of the emergence of pathogenic clones. Clearly, the presence of IS1301 in the sia-ctr IGR can be detected by PCR and could be used to screen for strains with increased resistance, which, as hyperproducers of capsular polysaccharide, could be valuable for vaccine manufacturing. Furthermore, this genetic change might explain some of the different behaviors of isolates in serum assays, as well as the variations obtained between laboratories.
From a collection of 109 strains from Spain, we identified three isolates with IS1301 in the siaA-ctrA IGR. Although this insertion confers a marked survival advantage to strains in the presence of immune sera, there are several potential reasons why this change is not more widespread among isolates. First, it is possible that these strains may be effectively cleared by other immune mechanisms, such as opsonphagocytosis (52). Second, the insertion sequence may have only recently inserted into the IGR, and this change might not have yet had the opportunity to spread among disease isolates. Finally, although increased expression of the capsule might be beneficial for survival in serum, there may be trade-offs in which the insertion of IS1301 in the IGR is detrimental for the bacterium at other stages of its lifecycle. Sialic acid is synthesized from N-acetyl mannosamine and phosphoenol pyruvate (53). The latter is part of intermediary carbon metabolism, and channeling of phosphoenol pyruvate into capsule biosynthesis may lead to a reduced growth in certain environments; we have been unable to demonstrate a growth defect for the R strains in complete or defined media. Furthermore, the presence of capsule is known to inhibit pilus-mediated adhesion to human epithelial cells (54, 55), and a minor impairment of this critical step during colonization may provide sufficient selective pressure to prevent the widespread emergence of these strains with increased resistance against complement-mediated killing.
Many mechanisms can generate genetic diversity among meningococcal isolates. In contrast to other changes, insertion of IS1301 in the siaA-ctrA IGR is a single genetic change that can provide strains with a generic mechanism for avoiding complement-mediated lysis, regardless of the nature of the target antigen. This is indicated by the increased resistance of the strains against killing mediated by the α-Por mAb, mouse sera raised against whole bacteria, and human sera from vaccinees. However, it is not clear whether strains with this change will compromise the efficacy of meningococcal vaccines; polysaccharide vaccines have been available against MenC for >30 yr with no reports of emerging resistant strains to date. Despite this, studies are needed to examine the distribution and spread of strains with IS1301 in the siaA-ctrA IGR during meningococcal carriage and disease, and before and after the implementation of immunization programs.
MATERIALS AND METHODS
Bacterial strains and growth.
N. meningitidis isolates used in this study are shown in Table II, and were characterized by their expression of surface antigens, fumC sequencing, and multilocus sequencing typing (20). Meningococcal strains were grown on brain heart infusion (BHI) medium with 5% Levanthal's supplement. Escherichia coli was propagated on Luria-Bertani media with antibiotics as required.
Table II.
Isolates used in this study
| Strain | Serogroup | Type | ST | Immunotype |
|---|---|---|---|---|
| C11 | C | 16:P1.7 | 345 | 1 |
| S1 (13213) | C | 2a:P1.5 | 11 | 2, 5 |
| S2 (14624) | C | 2a:P1.5 | 11 | 2, 5 |
| S3 (14709) | C | 2a:P1.5 | 11 | n.k. |
| S4 (14715) | C | 2a:P1.5 | 11 | 2, 5 |
| S5 (14840) | C | 2a:P1.5 | 11 | 2, 5 |
| R1 (14654) | C | 2a:P1.5 | 11 | 2, 5 |
| R2 (16015) | C | 2a:P1.5 | 11 | n.k. |
| R3 (15967) | C | 2a:P1.5 | 11 | n.k. |
Reference laboratory numbers are shown in parentheses. n.k., not known.
Molecular methods.
Genomic and plasmid DNA were extracted by standard methods, and PCRs were performed with Taq polymerase (Sigma-Aldrich). The oligonucleotides used in this study are shown in Table S2 (available at http://www.jem.org/cgi/content/full/jem.20072577/DC1). For RNA isolation, bacteria were grown in a 10-ml volume in liquid BHI media with gentle agitation to midlogarithmic phase with an OD A600 of ∼0.6. Cells were harvested by centrifugation at 4,000 g, and RNA was extracted with the RNeasy mini kit (QIAGEN). Genomic DNA was removed by treatment with DNase (QIAGEN). 500 ng RNA was reverse transcribed to cDNA using the SuperScript III First-Strand Synthesis kit (Invitrogen). Transcript levels were measured by qrtRT-PCR) using SensiMix with SYBR green detection (Quantace) on a thermal cycler (Rotor-Gene 3000; Corbett Research). Results for the transcription of siaA and ctrA were normalized with levels for the housekeeping gene gdh. The following primers were used to monitor transcript levels: NG770 and NG771 to generate a 221-bp siaA product; NG772 and NG773 to generate a 194-bp ctrA product; and NG886 and NG887 to generate a 235-bp gdh product. Data were analyzed using the comparative quantitation method by Rotor-Gene software (version 6.0; Corbett Research). Controls included reactions with no template and samples of RNA that had not been treated with RT. qrtRT-PCR was performed in triplicate on cDNA samples derived from three independent cultures.
Strains lacking lst and siaD have been described previously (56). For inactivation of oatC, a 2.2-kb fragment, including this gene, was amplified using primers NG541 and NG542. The product was ligated into pCR 2.1 Topo (Invitrogen), and the gene encoding kanamycin resistance was removed from the vector by SphI digestion and self-ligation. The resulting plasmid was subjected to in vitro mutagenesis with EZ-Tn5 (EPICENTRE), and the transposon insertion sites were mapped by sequencing. One plasmid, containing Tn5 at nucleotide 1,084 of the oatC open reading frame, was used for transformation. A similar strategy was used to mark IS1301 in the siaA-ctrA IGR before transfer into S3; a 1.6-kb fragment, including the IGR with IS1301 and part of siaA and ctrA, was amplified from strain R3 with primers NG679 and NG687, ligated into pCR 2.1 Topo, and the kanamycin-sensitive derivative was subjected to in vitro mutagenesis. Primers NG732 and NG733 were used to screen isolates for the presence of IS1301. Transformants were verified by PCR and Southern analysis.
Western and FACS analyses.
For LPS analysis, whole-cell extracts were prepared in loading buffer, treated with proteinase K (QIAGEN), and subjected to Tricine SDS-PAGE analysis. LPS was subjected to Western analysis using mAb 3F11, which detects unsialylated LPS (provided by M. Apicella, University of Iowa, Iowa City, Iowa), as described previously (56). Antipeptide antibodies were generated by immunizing rabbits with a SiaA peptide (DVGTRQNNRHMGKSI) on three occasions, and the sera was affinity purified and used at a final dilution of 1:500. The anti-RecA antibody (BioAcademia) was used at a 1:10,000 concentration. The secondary antibody was horseradish peroxidase–conjugated goat anti–rabbit Ig antibodies (1:10,000 concentration; Dako).
For FACS analysis, strains were grown to midlogarithmic phase in liquid media as in the previous section, collected by centrifugation, fixed in 3% paraformaldehyde for at least 1 h, and washed three times with PBS. Next, bacteria (2 × 107 cells) were incubated with a primary antibody at the following dilutions: mAb 1125 (provided by H. Claus, University of Würzburg, Würzburg, Germany) at a 1:500 dilution; and anticapsular sera at a 1:10 dilution in PBS for 30 min at 37°C. The cells were then washed twice, resuspended in PBS/0.1% Tween 20 (PBS-T) containing an FITC-conjugated donkey anti–mouse polyclonal antibody (1:200 dilution; Jackson ImmunoResearch Laboratories), and incubated for 30 min on ice. After washing with PBS-T, fluorescence was measured using an analyzer (FACSCalibur; Becton Dickson), recording at least 104 events. The gate was set at ∼2% of cells after incubation with PBS without a primary antibody. Results were calculated as the MFI (calculated as geometric mean multiplied by the percentage of positive cells).
To assess the binding of complement factors, bacteria were grown and fixed to midlogarithmic phase in liquid culture, and 2 × 107 cells were incubated with pooled immune sera from 10 vaccinees (1:5 dilution) for 30 min at 37°C, washed twice, and resuspended in PBS-T with FITC-conjugated polyclonal goat anti–human C3 (1:300 dilution; MP Biomedicals), or a polyclonal rabbit anti–human C5b-9 (1:200 dilution; EMD), which was detected with goat anti–rabbit IgG Alexa Fluor 488 (1:200; Invitrogen). The activity of the CP and LP was inhibited in sera in the presence of 2 mM MgCl2 and 2 mM EGTA (37).
SBA and serum assays.
SBA titers were measured according to standard protocols from the Centers for Disease Control (57). In brief, N. meningitidis was grown on solid media overnight and subcultured on plates for 4–5 h. Bacteria were harvested and resuspended in SBA buffer, and 104 bacteria were incubated for 1 h with serial dilutions of heat-inactivated (56°C for 30 min) vaccinees' serum in the presence of baby rabbit complement (Pelfreeze). The antiserosubtyping P1.5 mAb (hybridoma 4023; National Institute for Biological Standards and Control) was used in SBA assays to detect killing mediated against PorA. Control assays were performed without serum or without complement. The proportion of bacteria surviving was determined by plating to solid media. Assays were performed on four independent occasions, and results were given as the inverse of the highest dilution of serum that gave >50% killing of the input. SBA titers were also determined with human complement obtained from a patient with agammaglobulinaemia before he had received replacement therapy.
For serum assays, bacteria were grown in liquid media to midlogarithmic phase in liquid culture, 104 CFU was incubated with serial dilutions of pooled human immune serum (from 10 donors) for 1 h, and the proportion of bacteria surviving was determined (56). Control assays included sera inactivated by heating at 56°C for 30 min; significant differences were examined with the Student's t test.
Electron microscopy.
Bacteria were grown overnight on solid media, harvested, and resuspended in PBS prewarmed to 37°C. An aliquot of the suspension was mixed with prewarmed, cationized ferritin (F7879; Sigma-Aldrich) to a final concentration of 0.5 mg/ml. Plasma-cleaned hydrophilic Quantifoil TEM grids (S143-8; Agar Scientific Ltd.) were floated on the sample and incubated for 30 min at 37°C in the presence of 5% CO2. Excess fluid was removed by wicking, and the samples were vitrified by plunging into liquid ethane (CPC; Leica) before being transferred into a liquid N2–cooled specimen holder (model 626; Gatan). A TEM (JEM-2100; JEOL) operating at 200 keV was used to record images on a 4,048 × 4,048–pixel charge-coupled device camera (Gatan). The cumulative electron dose was maintained at <55 electrons/Å2. The microscope was calibrated against a carbon waffle (Grating Replica, Waffle, 2160 lines/mm; Ted Pella Inc.).
Capsule purification and NMR analysis.
Capsules were purified as described previously (18). In brief, bacteria were grown in 200 ml of BHI media overnight, and cetavlon (Sigma-Aldrich) was added to a final concentration of 1% to lyse the bacteria and precipitate the polysaccharide. The precipitates were collected by centrifugation and resuspended in distilled water, and CaCl2 was added to separate the polysaccharide from the detergent. Nucleic acids in the solution were removed by ethanol precipitation (25% vol/vol). The capsular polysaccharide in the supernatant was precipitated by ethanol at a final concentration of 80% (vol/vol); traces of cetavlon and CaCl2 were removed by washing with 100% ethanol. NMR spectra (500MHz at 60°C in D2O) were recorded on a spectrometer (INOVA 500; Varian) using pulse sequences provided by the manufacturer (29, 58).
Immunization.
Groups of 8-wk-old BABL/c mice were immunized with 0.1 ml of MCC vaccine (Wyeth Vaccines) by the subcutaneous route on days 0, 14, and 21. Serum was obtained from immunized animals on day 28. The same schedule was used to generate immune sera from animals that were given 107 CFU of S3, R3, or S3∷R by the intraperitoneal route in 250 μl BHI on three occasions. Bacterial strains were grown overnight on solid media and harvested to PBS, and the number of CFU was determined by measuring the OD A260 of a lysate of cells in 0.1 M NaOH/1% SDS (56). All procedures were approved by a Home Office license.
Online supplemental material.
Results of further SBAs are provided (Table S1), as well as primers and isolates used in this study (Table S2). Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20072577/DC1.
Supplementary Material
Acknowledgments
We thank the staff from the Hospitals Severo Ochoa and Universitario Río Hortega an Complejo, and the Hospitalario de San Millán y San Pedro, who provided strains. We thank Luisa Arreaza, Elisabeth Kugelberg, David Holden, Samantha Sampson, Chris Jones, and Xavier Lemercinier for helpful discussions.
We are grateful to Meningitis UK, the Medical Research Council, and the Wellcome Trust for supporting the work in C.M. Tang's laboratory. M.J. Uria received scholarships from the Beca de Formación en Investigación/Fondo de Investigación Sanitaria and the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica, and R.M. Exley is a Leverhulme Trust Research Fellow.
The authors have no conflicting financial interests.
Abbreviations used: AP, alternative pathway; BHI, brain heart infusion; CP, classical pathway; cryo-TEM, cryo–transmission electron microscopy; IGR, intergenic region; LP, lectin pathway; MAC, membrane attack complex; MCC, N. meningitidis serogroup C conjugate; MenC, serogroup C N. meningitidis; MFI, mean fluorescence index; qrtRT-PCR, quantitative real-time RT-PCR; SBA, serum bactericidal antibody; ST-11, sequence type 11.
References
- 1.Ochman, H., J.G. Lawrence, and E.A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature. 405:299–304. [DOI] [PubMed] [Google Scholar]
- 2.Figueroa, J.E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider, M.C., R.M. Exley, S. Ram, R.B. Sim, and C.M. Tang. 2007. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 15:233–240. [DOI] [PubMed] [Google Scholar]
- 4.Goldschneider, I., E.C. Gotschlich, and M.S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zollinger, W. 1997. New and improved vaccines against meningococcal vaccines. In New Generation Vaccines. Second edition. M.M. Levine, G.C. Woodrow, J.B. Kaper, and G.S. Cobon, editors. Marcel Dekker, Inc., New York. 469–488.
- 6.Richmond, P., R. Borrow, E. Miller, S. Clark, F. Sadler, A. Fox, N. Begg, R. Morris, and K. Cartwright. 1999. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J. Infect. Dis. 179:1569–1572. [DOI] [PubMed] [Google Scholar]
- 7.De Wals, P. 2004. Meningococcal C vaccines: the Canadian experience. Pediatr. Infect. Dis. J. 23:S280–S284. [PubMed] [Google Scholar]
- 8.Trotter, C.L., and M.E. Ramsay. 2007. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol. Rev. 31:101–107. [DOI] [PubMed] [Google Scholar]
- 9.Miller, E., D. Salisbury, and M. Ramsay. 2001. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 20(Suppl. 1):S58–S67. [DOI] [PubMed] [Google Scholar]
- 10.Whalen, C.M., J.C. Hockin, A. Ryan, and F. Ashton. 1995. The changing epidemiology of invasive meningococcal disease in Canada, 1985 through 1992. Emergence of a virulent clone of Neisseria meningitidis. JAMA. 273:390–394. [PubMed] [Google Scholar]
- 11.Taha, M.K., M. Achtman, J.M. Alonso, B. Greenwood, M. Ramsay, A. Fox, S. Gray, and E. Kaczmarski. 2000. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet. 356:2159. [DOI] [PubMed] [Google Scholar]
- 12.Traore, Y., B.M. Njanpop-Lafourcade, K.L. Adjogble, M. Lourd, S. Yaro, B. Nacro, A. Drabo, I. Parent du Chatelet, J.E. Mueller, M.K. Taha, et al. 2006. The rise and fall of epidemic Neisseria meningitidis serogroup W135 meningitis in Burkina Faso, 2002-2005. Clin. Infect. Dis. 43:817–822. [DOI] [PubMed] [Google Scholar]
- 13.Jodar, L., I.M. Feavers, D. Salisbury, and D.M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet. 359:1499–1508. [DOI] [PubMed] [Google Scholar]
- 14.Goldschneider, I., E.C. Gotschlich, and M.S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trotter, C.L., N.J. Andrews, E.B. Kaczmarski, E. Miller, and M.E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 364:365–367. [DOI] [PubMed] [Google Scholar]
- 16.Maiden, M.C., and B.G. Spratt. 1999. Meningococcal conjugate vaccines: new opportunities and new challenges. Lancet. 354:615–616. [DOI] [PubMed] [Google Scholar]
- 17.Elias, J., and U. Vogel. 2007. IS1301 fingerprint analysis of Neisseria meningitidis strains belonging to the ET-15 clone. J. Clin. Microbiol. 45:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotschlich, E.C., T.Y. Liu, and M.S. Artenstein. 1969. Human immunity to the meningococcus. III. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J. Exp. Med. 129:1349–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maslanka, S.E., L.L. Gheesling, D.E. Libutti, K.B. Donaldson, H.S. Harakeh, J.K. Dykes, F.F. Arhin, S.J. Devi, C.E. Frasch, J.C. Huang, et al. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin. Diagn. Lab. Immunol. 4:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiden, M.C., J.A. Bygraves, E. Feil, G. Morelli, J.E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D.A. Caugant, et al. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA. 95:3140–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel, U., and M. Frosch. 1999. Mechanisms of neisserial serum resistance. Mol. Microbiol. 32:1133–1139. [DOI] [PubMed] [Google Scholar]
- 22.Mandrell, R.E., R. McLaughlin, Y. Aba Kwaik, A. Lesse, R. Yamasaki, B. Gibson, S.M. Spinola, and M.A. Apicella. 1992. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect. Immun. 60:1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claus, H., R. Borrow, M. Achtman, G. Morelli, C. Kantelberg, E. Longworth, M. Frosch, and U. Vogel. 2004. Genetics of capsule O-acetylation in serogroup C, W-135 and Y meningococci. Mol. Microbiol. 51:227–239. [DOI] [PubMed] [Google Scholar]
- 24.Edwards, U., A. Muller, S. Hammerschmidt, R. Gerardy-Schahn, and M. Frosch. 1994. Molecular analysis of the biosynthesis pathway of the alpha-2,8 polysialic acid capsule by Neisseria meningitidis serogroup B. Mol. Microbiol. 14:141–149. [DOI] [PubMed] [Google Scholar]
- 25.Frosch, M., D. Muller, K. Bousset, and A. Muller. 1992. Conserved outer membrane protein of Neisseria meningitidis involved in capsule expression. Infect. Immun. 60:798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swartley, J.S., J.H. Ahn, L.J. Liu, C.M. Kahler, and D.S. Stephens. 1996. Expression of sialic acid and polysialic acid in serogroup B Neisseria meningitidis: divergent transcription of biosynthesis and transport operons through a common promoter region. J. Bacteriol. 178:4052–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Loewenich, F.D., E. Wintermeyer, M. Dumig, and M. Frosch. 2001. Analysis of transcriptional control mechanisms of capsule expression in Neisseria meningitidis. Int. J. Med. Microbiol. 291:361–369. [DOI] [PubMed] [Google Scholar]
- 28.Hilse, R., J. Stoevesandt, D.A. Caugant, H. Claus, M. Frosch, and U. Vogel. 2000. Distribution of the meningococcal insertion sequence IS1301 in clonal lineages of Neisseria meningitidis. Epidemiol. Infect. 124:337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemercinier, X., and C. Jones. 1996. Full 1H NMR assignment and detailed O-acetylation patterns of capsular polysaccharides from Neisseria meningitidis used in vaccine production. Carbohydr. Res. 296:83–96. [DOI] [PubMed] [Google Scholar]
- 30.Martin, P., T. van de Ven, N. Mouchel, A.C. Jeffries, D.W. Hood, and E.R. Moxon. 2003. Experimentally revised repertoire of putative contingency loci in Neisseria meningitidis strain MC58: evidence for a novel mechanism of phase variation. Mol. Microbiol. 50:245–257. [DOI] [PubMed] [Google Scholar]
- 31.Anderson, K.L. 1998. Cationized ferritin as a stain for electron microscopic observation of bacterial ultrastructure. Biotech. Histochem. 73:278–288. [DOI] [PubMed] [Google Scholar]
- 32.Jarvis, G.A., and N.A. Vedros. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 55:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marques, M.B., D.L. Kasper, M.K. Pangburn, and M.R. Wessels. 1992. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect. Immun. 60:3986–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pluschke, G., J. Mayden, M. Achtman, and R.P. Levine. 1983. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect. Immun. 42:907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walport, M.J. 2001. Complement. First of two parts. N. Engl. J. Med. 344:1058–1066. [DOI] [PubMed] [Google Scholar]
- 36.Drogari-Apiranthitou, M., E.J. Kuijper, N. Dekker, and J. Dankert. 2002. Complement activation and formation of the membrane attack complex on serogroup B Neisseria meningitidis in the presence or absence of serum bactericidal activity. Infect. Immun. 70:3752–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jongerius, I., J. Kohl, M.K. Pandey, M. Ruyken, K.P. van Kessel, J.A. van Strijp, and S.H. Rooijakkers. 2007. Staphylococcal complement evasion by various convertase-blocking molecules. J. Exp. Med. 204:2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine. 23:2222–2227. [DOI] [PubMed] [Google Scholar]
- 39.Bjune, G., E.A. Hoiby, J.K. Gronnesby, O. Arnesen, J.H. Fredriksen, A. Halstensen, E. Holten, A.K. Lindbak, H. Nokleby, E. Rosenqvist, et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 338:1093–1096. [DOI] [PubMed] [Google Scholar]
- 40.Suker, J., I.M. Feavers, M. Achtman, G. Morelli, J.F. Wang, and M.C. Maiden. 1994. The porA gene in serogroup A meningococci: evolutionary stability and mechanism of genetic variation. Mol. Microbiol. 12:253–265. [DOI] [PubMed] [Google Scholar]
- 41.Martin, S.L., R. Borrow, P. van der Ley, M. Dawson, A.J. Fox, and K.A. Cartwright. 2000. Effect of sequence variation in meningococcal PorA outer membrane protein on the effectiveness of a hexavalent PorA outer membrane vesicle vaccine. Vaccine. 18:2476–2481. [DOI] [PubMed] [Google Scholar]
- 42.van der Ende, A., C.T. Hopman, and J. Dankert. 2000. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis. Infect. Immun. 68:6685–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joiner, K.A., M.A. Schmetz, M.E. Sanders, T.G. Murray, C.H. Hammer, R. Dourmashkin, and M.M. Frank. 1985. Multimeric complement component C9 is necessary for killing of Escherichia coli J5 by terminal attack complex C5b-9. Proc. Natl. Acad. Sci. USA. 82:4808–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell, C., L. Gao, and D.R. Demuth. 2003. Positive and negative cis-acting regulatory sequences control expression of leukotoxin in Actinobacillus actinomycetemcomitans 652. Infect. Immun. 71:5640–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammerschmidt, S., R. Hilse, J.P. van Putten, R. Gerardy-Schahn, A. Unkmeir, and M. Frosch. 1996. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 15:192–198. [PMC free article] [PubMed] [Google Scholar]
- 46.Newcombe, J., K. Cartwright, S. Dyer, and J. McFadden. 1998. Naturally occurring insertional inactivation of the porA gene of Neisseria meningitidis by integration of IS1301. Mol. Microbiol. 30:453–454. [DOI] [PubMed] [Google Scholar]
- 47.Tribe, D.E., A.M. Zaia, J.M. Griffith, P.M. Robinson, H.Y. Li, K.N. Taylor, and G.G. Hogg. 2002. Increase in meningococcal disease associated with the emergence of a novel ST-11 variant of serogroup C Neisseria meningitidis in Victoria, Australia, 1999-2000. Epidemiol. Infect. 128:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsang, R.S., C.M. Tsai, P. Zhu, L. Ringuette, M. Lorange, and D.K. Law. 2004. Phenotypic and genetic characterization of a unique variant of serogroup C ET-15 meningococci (with the antigenic formula C:2a:P1.7,1) causing invasive meningococcal disease in Quebec, Canada. J. Clin. Microbiol. 42:1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiu, W. 1986. Electron microscopy of frozen, hydrated biological specimens. Annu. Rev. Biophys. Biophys. Chem. 15:237–257. [DOI] [PubMed] [Google Scholar]
- 50.McEllistrem, M.C., J.A. Kolano, M.A. Pass, D.A. Caugant, A.B. Mendelsohn, A.G. Fonseca Pacheco, K.A. Shutt, J. Razeq, and L.H. Harrison. 2004. Correlating epidemiologic trends with the genotypes causing meningococcal disease, Maryland. Emerg. Infect. Dis. 10:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jolley, K.A., C. Brehony, and M.C. Maiden. 2007. Molecular typing of meningococci: recommendations for target choice and nomenclature. FEMS Microbiol. Rev. 31:89–96. [DOI] [PubMed] [Google Scholar]
- 52.Welsch, J.A., and D. Granoff. 2004. Naturally acquired passive protective activity against Neisseria meningitidis Group C in the absence of serum bactericidal activity. Infect. Immun. 72:5903–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganguli, S., G. Zapata, T. Wallis, C. Reid, G. Boulnois, W.F. Vann, and I.S. Roberts. 1994. Molecular cloning and analysis of genes for sialic acid synthesis in Neisseria meningitidis group B and purification of the meningococcal CMP-NeuNAc synthetase enzyme. J. Bacteriol. 176:4583–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stephens, D.S., P.A. Spellman, and J.S. Swartley. 1993. Effect of the (alpha 2-8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J. Infect. Dis. 167:475–479. [DOI] [PubMed] [Google Scholar]
- 55.Virji, M., K. Makepeace, I.R. Peak, D.J. Ferguson, M.P. Jennings, and E.R. Moxon. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18:741–754. [DOI] [PubMed] [Google Scholar]
- 56.Exley, R.M., J. Shaw, E. Mowe, Y.H. Sun, N.P. West, M. Williamson, M. Botto, H. Smith, and C.M. Tang. 2005. Available carbon source influences the resistance of Neisseria meningitidis against complement. J. Exp. Med. 201:1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maslanka, S.E., J.W. Tappero, B.D. Plikaytis, R.S. Brumberg, J.K. Dykes, L.L. Gheesling, K.B. Donaldson, A. Schuchat, J. Pullman, M. Jones, et al. 1998. Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from Montana immunized with a licensed polysaccharide vaccine. Infect. Immun. 66:2453–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones, C., and X. Lemercinier. 2002. Use and validation of NMR assays for the identity and O-acetyl content of capsular polysaccharides from Neisseria meningitidis used in vaccine manufacture. J. Pharm. Biomed. Anal. 30:1233–1247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.