Abstract
Objective
Acute pyelonephritis is one of the most frequent medical complications of pregnancy, as well as a common cause of antepartum hospitalization. Interferon (IFN)-γ inducible protein, CXCL10/IP-10, is a member of the CXC chemokine family with pro-inflammatory and anti-angiogenic properties. The purpose of this study was to determine whether maternal serum concentrations of CXCL10/IP-10 change in patients with acute pyelonephritis during pregnancy.
Study Design
This cross-sectional study was conducted to determine the difference in maternal serum concentrations of CXCL10/IP-10 in pregnant women with acute pyelonephritis (N=41) and normal pregnant women (N=89). Pyelonephritis was defined in the presence of a positive urine culture, fever and maternal clinical signs; blood cultures were performed in 36 cases. Maternal serum concentrations of CXCL10/IP-10 were measured by a sensitive immunoassay. Non-parametric statistics were used for analysis.
Results
(1) The median serum concentration of CXCL10/IP-10 in pregnant patients with pyelonephritis was significantly higher than in normal pregnant women (median 318.5 pg/mL, range: 78.8–2459.2 vs. median: 116.1 pg/mL, range:40.7–1314.3, respectively; p < 0.001); (2) maternal median serum concentrations of CXCL10/IP-10 did not differ significantly among patients with acute pyelonephritis with and without bacteremia (positive blood cultures: median: 362.6 pg/mL, range: 100.2–2459.2 vs. negative blood cultures: median 298.9 pg/mL, range: 108.5–1148.7, respectively; p = 0.3).
Conclusions
Pyelonephritis in pregnant women is associated with increased maternal serum concentration of the chemokine CXCL10/IP-10.
Keywords: CXCL10, IP, 10, serum, chemokines, chemotactic chemokine, pregnancy, maternal infection, urinary infection, respiratory distress syndrome
INTRODUCTION
Acute pyelonephritis is one of the most frequent medical complications in pregnancy [1], and a common cause of antepartum hospitalization [2], with most of the cases occurring during the second trimester [3]. Complications following an event of acute pyelonephritis during pregnancy include anemia [3–5], transient renal dysfunction [3], preterm labor [1,6], sepsis [3,7], and septic shock [7–11]. Moreover, pregnant women with pyelonephritis are at increased risk to develop acute respiratory distress syndrome (ARDS) [12–14].
The human interferon-inducible protein 10 (IP-10 or CXCL10) is a chemokine of the CXC family [15]. A unique feature of some members of this type of chemokine is that they have pro-inflammatory properties and act as modulators of angiogenesis in conditions such as wound healing, ischemia, and neoplasia. These dual properties are related to the shared expression of specific chemokines receptors by leukocytes and endothelial cells [16–27].
IP-10 is inducible by pro-inflammatory stimuli such as interferon-γ (IFN-γ) [28–39], tumor necrosis factor-α (TNF-α) [37,40–46] viruses, and microbial products [33,37,47–52], either directly or through NF-kB [48,49,53–56]. It has been proposed that this chemokine is also involved in the recruitment and potentiation of T-helper 1 (Th1) responses [57–59].
The objective of this study was to determine whether the maternal serum concentrations of IP-10 are different in normal pregnancies and pregnant women with acute pyelonephritis.
METHODS
Study design
This cross-sectional study included normal pregnant women (N=89) and pregnant patients with acute pyelonephritis (N=41). Patients were considered to have a normal pregnancy if they met the following criteria: 1) no medical, obstetrical or surgical complications; 2) absence of labor at the time of venipuncture, and 3) delivery of a normal term (≥37 weeks) infant whose birth weight was between the 10th to 90th percentile for gestational age [60]. Acute pyelonephritis was diagnosed in the presence of fever (temperature ≥ 38ºC), clinical signs or symptoms of upper urinary infection (e.g., flank pain, costovertebral angle tenderness), pyuria, and a positive urine culture. Blood cultures were performed in 36 patients with pyelonephritis.
Patients were enrolled at the Detroit Medical Center/Hutzel Women’s Hospital, MI, USA. All provided written informed consent for the collection of clinical data, and biological materials under protocols were approved by the Institutional Review Boards of both Wayne State University and the National Institute of Child Health and Human Development of the National Institute of Health (NIH/DHHS). Many of these samples have been employed to study the biology of inflammation (i.e. complement, flow cytometry), hemostasis, angiogenesis regulation, and growth factor concentrations in normal pregnant women and those with complications. Samples from these patients have been used for other studies of the biology of pyelonephritis and inflammation.
IP-10 (CXCL10) determinations
A specific and sensitive enzyme-linked immunoassay was used to determine concentrations of CXCL10/IP-10 in human maternal serum. Immunoassays for CXCL10/IP-10 were obtained from R&D Systems (Minneapolis, MN, USA). Briefly, maternal serum samples were incubated in duplicate wells of the microtiter plates, pre-coated with monoclonal antibodies specific for CXCL10/IP-10. During this incubation step, any IP-10 present in the standards or maternal serum is bound by the immobilized antibodies. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for CXCL10/IP-10 was added to the wells. Following a wash to remove excess and unbound materials, a substrate solution was added to the wells and color developed in proportion to the amount of CXCL10/IP-10 bound in the initial step. The color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentrations of IP-10 in serum samples were determined by interpolation from individual standard curves composed of recombinant human CXCL10/IP-10. The calculated inter and intra assay coefficients of variation (CVs) for CXCL10/IP-10 immunoassays in our laboratory were 7.99% and 4.12%. The lower limit of detection (sensitivity) was calculated to be 5.01pg/mL.
Statistical analysis
The Kolmogorov–Smirnov test was employed to determine whether the data was normally distributed. Comparisons among groups were performed using the Mann-Whitney Test. A p-value <0.05 was considered statistically significant. The statistical package used was SPSS v.14.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinical and obstetrical characteristics of women in each group are displayed in Table I. The median serum concentration of CXCL10/IP-10 was significantly higher in pregnant patients with acute pyelonephritis than in normal pregnant women (acute pyelonephritis: median 318.5 pg/mL, range 78.8–2459.2 vs. normal pregnancy: median 116.1 pg/mL, range 40.7–1314.3; p<0.001) (Figure 1).
Table I.
Clinical and obstetrical characteristics of the study groups
| Normal pregnancy (N=89) | Pyelonephritis (N=41) | p | |
|---|---|---|---|
| Maternal age (years) | 23 (17 – 34) | 22 (17–41) | NS |
| Nulliparity | 21.3 (19/89) | 19.5 (8/41) | NS |
| Smoking | 20.5 (17/83) | 21.4 (6/28) | NS |
| Gestational age at blood draw (weeks) | 31.1 (19.4 – 38.3) | 31.2 (17 – 41.9) | NS |
| Gestational age at delivery (weeks) | 39.6 (37 – 42) | 39.1 (28.7 – 42.7) | NS |
| Birth weight (g) | 3342 (2550 – 4050) | 3140 (1080 – 4090) | 0.02 |
Values are expressed as percentage (number) or median (range). NS: not significant.
Figure 1.

Maternal serum concentrations of CXCL10/IP-10 in patients with normal pregnancies and those with pyelonephritis. The median serum concentration of CXCL10/IP-10 in pregnant patients with pyelonephritis was significantly higher than normal pregnant women (median: 318.5 pg/mL, range: 78.8–2459.2 vs. median: 116.1 pg/mL, range: 40.7–1314.3, respectively; p<0.001).
The most common microorganism isolated from urine cultures of patients with pyelonephritis was Escherichia. coli (30 cases). Other microorganisms less frequently isolated included mixed flora (2), Klebsiella pneumoniae (2), E. coli and Streptococcus viridans (1), Enterobacter aerogenes (1), Pseudomonas aeruginosa (1), Gram negative bacilli (1), Proteus mirabilis (1), Streptococcus agalactiae (1), and Citrobacter koseri (1).
Among the 36 women with acute pyelonephritis in which blood cultures were performed, the results were positive in 16 cases (44.4%). The following microorganisms were isolated: E. Coli (11), Coaugulase negative Staphylococcus (2), Gram positive cocci (1), Klebsiella pneumoniae (1), and Enterobacter aerogenes (1).
Among patients with acute pyelonephritis, no significant difference in the median serum concentration of CXCL10/IP-10 was observed between those who had positive blood cultures and those who did not (positive blood cultures: median 362.6 pg/mL, range 100.2–2459.2 vs. negative blood cultures: median 298.9 pg/mL, range 108.5–1148.7, respectively; p=0.3) (Figure 2).
Figure 2.
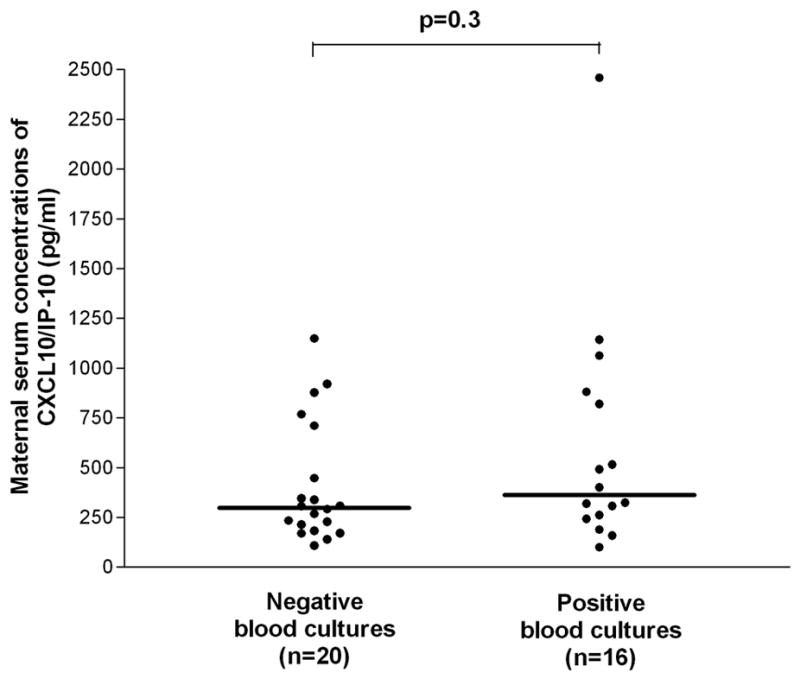
Maternal serum concentrations of CXCL10/IP-10 in patients with pyelonephritis. No significant differences in maternal median serum concentrations of CXCL10/IP-10 were observed between patients with positive and negative blood cultures (median: 362.6 pg/mL, range: 100.2–2459.2 vs. median: 298.9 pg/mL, range: 108.5–1148.7, respectively; p=0.3).
Patients with acute pyelonephritis delivered neonates with a lower birth weight than those in the control group (pyelonephritis: median 3140.0 g, range 1080–4090 g vs. normal pregnancy: median 3342.5 g, range 2550–4050 g; p=0.02). However, the mean gestational age at delivery was not different between the two groups.
Discussion
Principal findings of this study
The median serum concentration of CXCL10/IP-10 in pregnant women with acute pyelonephritis was significantly higher than that of normal pregnant women;
among patients with pyelonephritis, no significant difference in the median serum concentration of CXCL10/IP-10 was observed between those who had positive blood cultures and those who did not. This is the first report documenting an increase in the maternal serum concentration of CXCL10/IP-10 in women with an acute infection during pregnancy.
The chemokine family
The superfamily of chemokines includes secreted proteins with a molecular weight of 8–10 kD that selectively target and activate specific cell populations and attract them to inflamed tissues in which microbial invasion has occurred [19,20,22,61,62].
Until recently, there was much discussion about the specificity of subsets of chemokines for a particular leukocyte population (e.g. neutrophils, lymphocytes, etc.), and whether or not a particular set of chemokines was involved in the disease processes with a characteristic type of white blood cell infiltration. Recent findings indicate that chemokines and their receptors are expressed by a wide variety of cells of non-hematopoietic origin, and that the functions of chemokines extend far beyond white blood cell attraction. A fundamental observation, which has added complexity to the field, is the ‘promiscuity’ of this family of molecules and their receptors. Indeed, chemokines can bind several different receptors, while chemokine receptors can bind more than one chemokine [19].
Traditionally, two main subfamilies of chemokines have been described on the basis of the number and arrangement of the conserved cystine residues, the CC and the CXC chemokines [19,20,22,61,62]. The CC chemokines preferentially attract monocytes, basophils, eosinophils and lymphocytes, but have no effect on neutrophils [63]. The CXC chemokines are subdivided according to the presence or absence of the glutamic acid-leucine-arginine (ELR) sequence. ELR-containing CXC chemokines (ELR+) are chemotactic for neutrophils and promoters of angiogenesis. In contrast, non-ELR containing CXC chemokines (ELR-) are chemotactic mainly for lymphocytes, and characterized by anti-angiogenic activity [21,23–26,64,65].
The chemokine CXCL10/IP-10
IP-10 (CXCL10) is an ELR- CXC chemokine [15] first described as the product of a gene induced in response to recombinant IFN-γ in several cell populations [28].
The biological properties of IP-10 are mediated through the interaction with a trans-membrane G protein-coupled receptor, CXCR3 [66,67], shared by two other IFN-γ inducible CXC chemokines, CXCL9 (MIG) and CXCX11 (ITAC), whose distinct biological activities are related to different signal transduction pathways [68–71].
CXCL10/IP-10 gene and protein expression is modulated by pro-inflammatory stimuli. Indeed, IFNγ is an inducer of the gene and protein expression of this chemokine by mononuclear cells [28], neutrophils [36,37], eosinophils [37,72], keratinocytes[28,31,32,34], fibroblasts[28], endothelial cells[28–30], pancreatic βcells [38,39], and animal astrocytes/microglia [33,35]. TNFα [37,40–46], Interleukin (IL-1β) [37,38], as well as viral [33,48,49] and microbial products [37,47,50–52], can also stimulate the production of IP-10. Interestingly, in vitro infection of epithelium of the urinary tract with E. coli results in an increase (as measured in the conditioned media with ELISA) in both CXCL10/IP-10 secretion and epithelial cells CXCL10/IP-10 mRNA expression [73]. Activation of the NF-kB pathway [48,49,53–56] after ligation of pattern recognition receptors (TLR4 [50] and TLR3 [56]) can also upregulate gene and protein expression of IP-10. Thus, IP-10 is considered an ‘NF-kB responsive gene’[74].
IP-10 in inflammation
The pro-inflammatory activities of CXCL10/IP-10 include chemotaxis and endothelial adhesion of activated T cells [75], as well as chemotaxis [76] and enhancement of natural killer (NK) cells mediated cytolysis[76]. However, this chemokine is a poor neutrophil activator [75,77], and its effects on monocytes [66,75], and B cells [78,79] is a controversial topic.
IP-10 in presence of infections
Infection is associated with an increase in serum and/or plasma concentrations of CXCL10/IP-10. Evidence in support of this statement includes: (1) median serum concentration of CXCL10/IP-10, determined on the first postnatal day, are significantly higher in neonates with perinatal infections than in controls (p<0.001) [80]; (2) median serum concentrations of CXCL10/IP-10 are significantly higher in neonates with nosocomial infections as compared to controls [80]; (3) neonatal plasma IP-10 =1250 pg/mL has been reported to identify all preterm infants with sepsis and necrotizing enterocolitis[81]; (4) it has been proposed that the severity of neonatal infection is associated with the magnitude of upregulation of circulating CXCL10/IP-10. Indeed, CXCL10/IP-10 plasma concentrations in infants who died of sepsis were all markedly elevated (range: 23,817–34,415 pg/ml); [81]; and (5) in adults, plasma CXCL10/IP-10 concentration has been used to monitor the disease activity and the response to therapy of Mycobacterium tuberculosis infection [82].
Interestingly, serum concentrations of CXCL10/IP-10 have been reported to be significantly higher in those with severe ARDS than in control patients (patients with bacterial pneumonia). Chemokine and cytokine disregulation has been implicated in the pathogenesis of ARDS [81,83,84], and CXCL10/IP-10 has been proposed as a useful marker of lung injury in patients with severe acute respiratory syndrome [85].
IP-10 in urinary tract infections
Previous studies reported higher serum [86] and urine [86,87] CXCL10/IP-10 concentrations (determined by ELISA) in the presence of both culture-proven Gram-negative urosepsis [86] and febrile urinary tract infections than in control patients [73,87]. Furthermore, serum CXCL10/IP-10 concentrations did not change significantly during the follow-up (72 h after beginning of treatment). However, a significant decrease in the urinary concentrations was observed [86]. In serum and in urine, CXCL10/IP-10 concentrations were not different in patients with positive or negative blood cultures [86,87]. Of interest is that in experimental endotoxemia in humans, CXCL10/IP-10 concentrations were elevated in both serum and urine [86]. The finding of increased concentrations in urine, raises questions about how the changes in the peripheral circulation are reflected in urine. It is possible that this reflects renal excretion of increased available CXCL10/IP-10 in the peripheral circulation, local production or both.
IP-10: Maternal serum concentrations increase in pregnancies complicated with pyelonephritis
This is the first report on the topic of serum CXCL10/IP-10 concentrations in pregnant women with pyelonephritis. Our finding that pyelonephritis in pregnancy is associated with a significant increase in the serum concentration of this chemokine is consistent with what has been previously reported in non-pregnant patients [86]. The mechanisms responsible for the increase in CXCL10/IP-10 during normal pregnancy [88], and in acute infection during gestation are unknown. In addition, the physiologic function of CXCL10/IP-10 during pregnancy as well as the role of CXCL10/IP-10 in acute infection during gestation remains to be determined. Recently, it has been demonstrated that CXCL10/IP-10 has antimicrobial properties [89]. Thus, it is tempting to postulate that this chemokine participates in host defense not only by orchestrating leukocyte chemotaxis, but also by inducing other biological activities which may have a protective effect during infection. In this study, no significant differences were observed in the median maternal serum concentrations of CXCL10/IP-10 between patients with and without positive blood cultures. Otto et al. [87] reported similar results when quantifying urinary CXCL10 in response to febrile urinary tract infections. Indeed, only some chemokines such as CXCL1, CXCL3, CXCL5, CXCL8, and CCL2, but not CXCL10/IP-10, were significantly higher in the presence of bacteremia. One interpretation is that the elevation in the serum concentration of CXCL10/IP-10 in the context of pyelonephritis results from systemic inflammation and it is not affected by the presence of microorganisms in the circulation. However, one possible explanation for this negative result is a limited statistical power.
IP-10: a local first line defense mechanism in the urinary tract mucosae
Migration of immunocompetent cells to sites of mucosal infection requires expression of molecules by the epithelial cells of the affected tissues [90]. Chemokines, particularly those belonging to the CXC family, have been shown to play an important role in this process [20,90–94]. Indeed, infections of the mucosae are associated with a local increase in CXCL10/IP-10. Evidence in support of this statement includes: 1) exposure of uro-epithelial cells to E. coli is followed by a significant increase in CXCL10/IP-10 mRNA expression (quantification with an RNA protection assay) and protein secretion (ELISA )[73]; 2) intravesical instillation of Mycobacterium bovis bacillus Calmette-Guerin (BCG) is followed by detection of urinary CXCL10/IP-10 in patients undergoing immunotherapy for bladder cancer (by ELISA) [95,96]; 3) conditioned media collected from culture of human uroepithelial cell lines exposed to BCG or BCG-derived cytokines such as IFNγ, IL-1β, and TNFα contains a significantly higher amount of CXCL10/IP-10 (ELISA) than control media[95]; and 4) intrapulmonary administration of pneumoniae in mice is followed by upregulation of CXCL10/IP-10 mRNA expression in the lung. Moreover, neutralization of CXCL10/IP-10 activity with an antibody results in reduced bacterial clearance and decreased survival, while, in contrast, over-expression of CXCL10/IP-10 (via adenovirus) results in improved bacterial clearance [97].
Antimicrobial activity of IP-10
Recent studies have reported structural similarities between chemokines and defensins (a family of antimicrobial peptides serving as the first line of defense in epithelia before the activation of adaptive immunity) [98–105]. The following evidence suggests an overlap between the biological functions of defensins and CXCL10/IP-10: (1) chemokines share many properties with defensins such as structure, size, charge, disulfide bonding, and IFN-inducibility [89]; (2) the CXC motif, present in CXCL10/IP-10, is contained and conserved in α-defensins-1 and -2; these defensins, as well as a structurally related β-defensin, have been shown to be chemotactic for leukocytes at subnanomolar concentrations [106–109]; (3) CXCL10/IP-10 has antimicrobial properties similar to those of defensins: in a radial diffusion assay, CXCL10/IP-10 has been demonstrated to have antimicrobial activity against E. coli and Listeria monocytogenes [89]. Moreover, this antimicrobial activity, similarly to the activity of defensins and many other antimicrobial peptides[110], is salt dependent (inhibited by NaCl) [89]; and (4) calculations of the amount of CXCL10/IP-10 produced by infiltrating inflammatory cells indicate that the tissue concentrations of this chemokines may reach the threshold necessary for microbicidal activity locally and directly inactivate microbes before attracting other host defense cells to the area [89].
IP-10, pyelonephritis and lung injury
Compared to non-pregnant subjects with pyelonephritis, mothers with pyeloneprhtis are at an increased risk for ARDS [7,12–14,111–115]. This has been attributed to an enhanced baseline activation of the innate immunoresponse during pregnancy [116,117], although other explanations may be possible [118]. Indeed, an excessive inflammatory response (in particular neutrophil activation) has been implicated in the pathogenesis of ARDS [118]. Nevertheless, the precise mechanism for the association between pyelonephritis and ARDS remains to be determined. Neumann et al. [119] caused lung injury in female mice by inducing continuous leakage of bacteria into the peritoneal cavity. The investigators demonstrated that this procedure causes, in as early as three hours, massive recruitment of granulocytes to the lung and a significant increase in lung CXCL10/IP-10 mRNA.
The authors concluded that CXCL10/IP-10 may contribute to the accumulation of mononuclear phagocytes in the lung [119]. Thus, CXCL10/IP-10 may be involved in the pathogenesis of ARDS in pregnant women with sepsis and/or pyelonephritis. This hypothesis is strengthened by the observation that, in patients with SARS, the serum concentrations of CXCL10/IP-10 increase before the development of chest involvement, reaching a peak concentration before abnormalities are detected in the chest X-ray [85].
Conclusion
Maternal serum concentrations of CXCL10/IP-10 are higher in the context of acute bacterial infection (pyelonephritis) than in normal pregnancy. The role of this chemokine in host defense during infection requires further study.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Pitukkijronnakorn S, Chittacharoen A, Herabutya Y. Maternal and perinatal outcomes in pregnancy with acute pyelonephritis. Int J Gynaecol Obstet. 2005;89:286–287. doi: 10.1016/j.ijgo.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Wing DA. Pyelonephritis in pregnancy: treatment options for optimal outcomes. Drugs. 2001;61:2087–2096. doi: 10.2165/00003495-200161140-00006. [DOI] [PubMed] [Google Scholar]
- 3.Hill JB, Sheffield JS, McIntire DD, Wendel GD., Jr Acute pyelonephritis in pregnancy. Obstet Gynecol. 2005;105:18–23. doi: 10.1097/01.AOG.0000149154.96285.a0. [DOI] [PubMed] [Google Scholar]
- 4.Cox SM, Shelburne P, Mason R, Guss S, Cunningham FG. Mechanisms of hemolysis and anemia associated with acute antepartum pyelonephritis. Am J Obstet Gynecol. 1991;164:587–590. doi: 10.1016/s0002-9378(11)80027-x. [DOI] [PubMed] [Google Scholar]
- 5.Cavenee MR, Cox SM, Mason R, Cunningham FG. Erythropoietin in pregnancies complicated by pyelonephritis. Obstet Gynecol. 1994;84:252–254. [PubMed] [Google Scholar]
- 6.Gilstrap LC, Leveno KJ, Cunningham FG, Whalley PJ, Roark ML. Renal infection and pregnancy outcome. Am J Obstet Gynecol. 1981;141:709–716. doi: 10.1016/s0002-9378(15)33316-0. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham FG, Lucas MJ. Urinary tract infections complicating pregnancy. Baillieres Clin Obstet Gynaecol. 1994;8:353–373. doi: 10.1016/s0950-3552(05)80325-6. [DOI] [PubMed] [Google Scholar]
- 8.Cavanagh D, Rao PS. Septic shock (endotoxic shock) Clin Obstet Gynecol. 1973;16:25–39. doi: 10.1097/00003081-197306000-00004. [DOI] [PubMed] [Google Scholar]
- 9.DILWORTH EE, WARD JV. Bacteremic shock in pyelonephritis and criminal abortion. Obstet Gynecol. 1961;17:160–167. [PubMed] [Google Scholar]
- 10.Cavanagh D, Knuppel RA, Shepherd JH, Anderson R, Rao PS. Septic shock and the obstetrician/gynecologist. South Med J. 1982;75:809–813. doi: 10.1097/00007611-198207000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Mabie WC, Barton JR, Sibai B. Septic shock in pregnancy. Obstet Gynecol. 1997;90:553–561. doi: 10.1016/s0029-7844(97)00352-9. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham FG, Leveno KJ, Hankins GD, Whalley PJ. Respiratory insufficiency associated with pyelonephritis during pregnancy. Obstet Gynecol. 1984;63:121–125. [PubMed] [Google Scholar]
- 13.Catanzarite VA, Willms D. Adult respiratory distress syndrome in pregnancy: report of three cases and review of the literature. Obstet Gynecol Surv. 1997;52:381–392. doi: 10.1097/00006254-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham FG, Lucas MJ, Hankins GD. Pulmonary injury complicating antepartum pyelonephritis. Am J Obstet Gynecol. 1987;156:797–807. doi: 10.1016/0002-9378(87)90335-8. [DOI] [PubMed] [Google Scholar]
- 15.Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8:207–219. doi: 10.1016/s1359-6101(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 16.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van DJ, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 17.Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995;4:155–160. doi: 10.1097/00024382-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van DJ, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol. 1995;57:752–762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 19.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 20.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 21.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 22.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 23.Bernardini G, Ribatti D, Spinetti G, Morbidelli L, Ziche M, Santoni A, Capogrossi MC, Napolitano M. Analysis of the role of chemokines in angiogenesis. J Immunol Methods. 2003;273:83–101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 24.Rosenkilde MM, Schwartz TW. The chemokine system -- a major regulator of angiogenesis in health and disease. APMIS. 2004;112:481–495. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- 25.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 28.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 29.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luster AD, Ravetch JV. Genomic characterization of a gamma-interferon-inducible gene (IP-10) and identification of an interferon-inducible hypersensitive site. Mol Cell Biol. 1987;7:3723–3731. doi: 10.1128/mcb.7.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan G, Luster AD, Hancock G, Cohn ZA. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J Exp Med. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottlieb AB, Luster AD, Posnett DN, Carter DM. Detection of a gamma interferon-induced protein IP-10 in psoriatic plaques. J Exp Med. 1988;168:941–948. doi: 10.1084/jem.168.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanguri P, Farber JM. IFN and virus-inducible expression of an immediate early gene, crg-2/IP-10, and a delayed gene, I-A alpha in astrocytes and microglia. J Immunol. 1994;152:1411–1418. [PubMed] [Google Scholar]
- 34.Sarris AH, Esgleyes-Ribot T, Crow M, Broxmeyer HE, Karasavvas N, Pugh W, Grossman D, Deisseroth A, Duvic M. Cytokine loops involving interferon-gamma and IP-10, a cytokine chemotactic for CD4+ lymphocytes: an explanation for the epidermotropism of cutaneous T-cell lymphoma? Blood. 1995;86:651–658. [PubMed] [Google Scholar]
- 35.Vanguri P. Interferon-gamma-inducible genes in primary glial cells of the central nervous system: comparisons of astrocytes with microglia and Lewis with brown Norway rats. J Neuroimmunol. 1995;56:35–43. doi: 10.1016/0165-5728(94)00131-7. [DOI] [PubMed] [Google Scholar]
- 36.Cassatella MA, Gasperini S, Calzetti F, Bertagnin A, Luster AD, McDonald PP. Regulated production of the interferon-gamma-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur J Immunol. 1997;27:111–115. doi: 10.1002/eji.1830270117. [DOI] [PubMed] [Google Scholar]
- 37.Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella MA. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162:4928–4937. [PubMed] [Google Scholar]
- 38.Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Hollander GA, Piali L. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med. 2002;8:1414–1420. doi: 10.1038/nm1202-792. [DOI] [PubMed] [Google Scholar]
- 39.Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia. 2003;46:255–266. doi: 10.1007/s00125-002-1017-0. [DOI] [PubMed] [Google Scholar]
- 40.Narumi S, Yoneyama H, Inadera H, Nishioji K, Itoh Y, Okanoue T, Matsushima K. TNF-alpha is a potent inducer for IFN-inducible protein-10 in hepatocytes and unaffected by GM-CSF in vivo, in contrast to IL-1beta and IFN-gamma. Cytokine. 2000;12:1007–1016. doi: 10.1006/cyto.1999.0672. [DOI] [PubMed] [Google Scholar]
- 41.Kraft M, Riedel S, Maaser C, Kucharzik T, Steinbuechel A, Domschke W, Luegering N. IFN-gamma synergizes with TNF-alpha but not with viable H. pylori in up-regulating CXC chemokine secretion in gastric epithelial cells. Clin Exp Immunol. 2001;126:474–481. doi: 10.1046/j.1365-2249.2001.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Algood HM, Lin PL, Yankura D, Jones A, Chan J, Flynn JL. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J Immunol. 2004;172:6846–6857. doi: 10.4049/jimmunol.172.11.6846. [DOI] [PubMed] [Google Scholar]
- 43.Villagomez MT, Bae SJ, Ogawa I, Takenaka M, Katayama I. Tumour necrosis factor-alpha but not interferon-gamma is the main inducer of inducible protein-10 in skin fibroblasts from patients with atopic dermatitis. Br J Dermatol. 2004;150:910–916. doi: 10.1111/j.1365-2133.2004.05937.x. [DOI] [PubMed] [Google Scholar]
- 44.Hardaker EL, Bacon AM, Carlson K, Roshak AK, Foley JJ, Schmidt DB, Buckley PT, Comegys M, Panettieri RA, Jr, Sarau HM, et al. Regulation of TNF-alpha- and IFN-gamma-induced CXCL10 expression: participation of the airway smooth muscle in the pulmonary inflammatory response in chronic obstructive pulmonary disease. FASEB J. 2004;18:191–193. doi: 10.1096/fj.03-0170fje. [DOI] [PubMed] [Google Scholar]
- 45.Sheng WS, Hu S, Ni HT, Rowen TN, Lokensgard JR, Peterson PK. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J Leukoc Biol. 2005;78:1233–1241. doi: 10.1189/jlb.0405221. [DOI] [PubMed] [Google Scholar]
- 46.Berthier-Vergnes O, Bermond F, Flacher V, Massacrier C, Schmitt D, Peguet-Navarro J. TNF-alpha enhances phenotypic and functional maturation of human epidermal Langerhans cells and induces IL-12 p40 and IP-10/CXCL-10 production. FEBS Lett. 2005;579:3660–3668. doi: 10.1016/j.febslet.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 47.Shin HS, Drysdale BE, Shin ML, Noble PW, Fisher SN, Paznekas WA. Definition of a lipopolysaccharide-responsive element in the 5′-flanking regions of MuRantes and crg-2. Mol Cell Biol. 1994;14:2914–2925. doi: 10.1128/mcb.14.5.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nazar AS, Cheng G, Shin HS, Brothers PN, Dhib-Jalbut S, Shin ML, Vanguri P. Induction of IP-10 chemokine promoter by measles virus: comparison with interferon-gamma shows the use of the same response element but with differential DNA-protein binding profiles. J Neuroimmunol. 1997;77:116–127. doi: 10.1016/s0165-5728(97)00070-2. [DOI] [PubMed] [Google Scholar]
- 49.Cheng G, Nazar AS, Shin HS, Vanguri P, Shin ML. IP-10 gene transcription by virus in astrocytes requires cooperation of ISRE with adjacent kappaB site but not IRF-1 or viral transcription. J Interferon Cytokine Res. 1998;18:987–997. doi: 10.1089/jir.1998.18.987. [DOI] [PubMed] [Google Scholar]
- 50.Gasper NA, Petty CC, Schrum LW, Marriott I, Bost KL. Bacterium-induced CXCL10 secretion by osteoblasts can be mediated in part through toll-like receptor 4. Infect Immun. 2002;70:4075–4082. doi: 10.1128/IAI.70.8.4075-4082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen Q, Zhang R, Bhat NR. MAP kinase regulation of IP10/CXCL10 chemokine gene expression in microglial cells. Brain Res. 2006;1086:9–16. doi: 10.1016/j.brainres.2006.02.116. [DOI] [PubMed] [Google Scholar]
- 52.Durand SH, Flacher V, Romeas A, Carrouel F, Colomb E, Vincent C, Magloire H, Couble ML, Bleicher F, Staquet MJ, et al. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J Immunol. 2006;176:2880–2887. doi: 10.4049/jimmunol.176.5.2880. [DOI] [PubMed] [Google Scholar]
- 53.Ohmori Y, Hamilton TA. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem. 1993;268:6677–6688. [PubMed] [Google Scholar]
- 54.Ohmori Y, Hamilton TA. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J Immunol. 1995;154:5235–5244. [PubMed] [Google Scholar]
- 55.Osawa Y, Iho S, Takauji R, Takatsuka H, Yamamoto S, Takahashi T, Horiguchi S, Urasaki Y, Matsuki T, Fujieda S. Collaborative action of NF-kappaB and p38 MAPK is involved in CpG DNA-induced IFN-alpha and chemokine production in human plasmacytoid dendritic cells. J Immunol. 2006;177:4841–4852. doi: 10.4049/jimmunol.177.7.4841. [DOI] [PubMed] [Google Scholar]
- 56.Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–256. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- 57.Gangur V, Simons FE, Hayglass KT. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-gamma over IL-4 responses. FASEB J. 1998;12:705–713. doi: 10.1096/fasebj.12.9.705. [DOI] [PubMed] [Google Scholar]
- 58.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, Toyoda M, Seki T, Morohashi M, Matsushima K, et al. Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J Leukoc Biol. 2000;68:568–574. [PubMed] [Google Scholar]
- 60.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 61.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 62.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 63.Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 64.Keane MP, Arenberg DA, Lynch JP, III, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159:1437–1443. [PubMed] [Google Scholar]
- 65.Strieter RM, Belperio JA, Keane MP. CXC chemokines in angiogenesis related to pulmonary fibrosis. Chest. 2002;122:298S–301S. doi: 10.1378/chest.122.6_suppl.298s. [DOI] [PubMed] [Google Scholar]
- 66.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu L, Callahan MK, Huang D, Ransohoff RM. Chemokine receptor CXCR3: an unexpected enigma. Curr Top Dev Biol. 2005;68:149–181. doi: 10.1016/S0070-2153(05)68006-4. [DOI] [PubMed] [Google Scholar]
- 68.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cox MA, Jenh CH, Gonsiorek W, Fine J, Narula SK, Zavodny PJ, Hipkin RW. Human interferon-inducible 10-kDa protein and human interferon-inducible T cell alpha chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol Pharmacol. 2001;59:707–715. doi: 10.1124/mol.59.4.707. [DOI] [PubMed] [Google Scholar]
- 70.Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD. CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell alpha chemoattractant (CXCL11) J Immunol. 2001;167:7084–7093. doi: 10.4049/jimmunol.167.12.7084. [DOI] [PubMed] [Google Scholar]
- 71.Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–30227. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- 72.Dajotoy T, Andersson P, Bjartell A, Lofdahl CG, Tapper H, Egesten A. Human eosinophils produce the T cell-attracting chemokines MIG and IP-10 upon stimulation with IFN-gamma. J Leukoc Biol. 2004;76:685–691. doi: 10.1189/jlb.0803379. [DOI] [PubMed] [Google Scholar]
- 73.Godaly G, Otto G, Burdick MD, Strieter RM, Svanborg C. Fimbrial lectins influence the chemokine repertoire in the urinary tract mucosa. Kidney Int. 2007 doi: 10.1038/sj.ki.5002076. [DOI] [PubMed] [Google Scholar]
- 74.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 75.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 77.Dewald B, Moser B, Barella L, Schumacher C, Baggiolini M, Clark-Lewis I. IP-10, a gamma-interferon-inducible protein related to interleukin-8, lacks neutrophil activating properties. Immunol Lett. 1992;32:81–84. doi: 10.1016/0165-2478(92)90203-z. [DOI] [PubMed] [Google Scholar]
- 78.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, Hiepe F, Radbruch A, Arce S, Manz RA. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105:3965–3971. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 80.Fotopoulos S, Mouchtouri A, Xanthou G, Lipsou N, Petrakou E, Xanthou M. Inflammatory chemokine expression in the peripheral blood of neonates with perinatal asphyxia and perinatal or nosocomial infections. Acta Paediatr. 2005;94:800–806. doi: 10.1111/j.1651-2227.2005.tb01988.x. [DOI] [PubMed] [Google Scholar]
- 81.Ng PC, Li K, Chui KM, Leung TF, Wong RP, Chu WC, Wong E, Fok TF. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr Res. 2007;61:93–98. doi: 10.1203/01.pdr.0000250207.95723.96. [DOI] [PubMed] [Google Scholar]
- 82.Azzurri A, Sow OY, Amedei A, Bah B, Diallo S, Peri G, Benagiano M, D’Elios MM, Mantovani A, Del PG. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:1–8. doi: 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2005;288:L3–15. doi: 10.1152/ajplung.00405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med. 1994;42:640–651. [PubMed] [Google Scholar]
- 85.Chien JY, Hsueh PR, Cheng WC, Yu CJ, Yang PC. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11:715–722. doi: 10.1111/j.1440-1843.2006.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olszyna DP, Prins JM, Dekkers PE, De JE, Speelman P, Van Deventer SJ, Van Der PT. Sequential measurements of chemokines in urosepsis and experimental endotoxemia. J Clin Immunol. 1999;19:399–405. doi: 10.1023/a:1020554817047. [DOI] [PubMed] [Google Scholar]
- 87.Otto G, Burdick M, Strieter R, Godaly G. Chemokine response to febrile urinary tract infection. Kidney Int. 2005;68:62–70. doi: 10.1111/j.1523-1755.2005.00381.x. [DOI] [PubMed] [Google Scholar]
- 88.Gotsch F, Romero R, Friel L, Kusanovic JP, Espinoza J, Erez O, Than NG, Mittal P, Edwin S, Yoon BH, et al. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia? J. Matern Fetal Neonatal Med. 2007;20:777–792. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 90.Godaly G, Bergsten G, Hang L, Fischer H, Frendeus B, Lundstedt AC, Samuelsson M, Samuelsson P, Svanborg C. Neutrophil recruitment, chemokine receptors, and resistance to mucosal infection. J Leukoc Biol. 2001;69:899–906. [PubMed] [Google Scholar]
- 91.Hang L, Haraoka M, Agace WW, Leffler H, Burdick M, Strieter R, Svanborg C. Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J Immunol. 1999;162:3037–3044. [PubMed] [Google Scholar]
- 92.Olszyna DP, Florquin S, Sewnath M, Branger J, Speelman P, Van Deventer SJ, Strieter RM, Van Der PT. CXC chemokine receptor 2 contributes to host defense in murine urinary tract infection. J Infect Dis. 2001;184:301–307. doi: 10.1086/322030. [DOI] [PubMed] [Google Scholar]
- 93.Frendeus B, Godaly G, Hang L, Karpman D, Lundstedt AC, Svanborg C. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J Exp Med. 2000;192:881–890. doi: 10.1084/jem.192.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Godaly G, Hang L, Frendeus B, Svanborg C. Transepithelial neutrophil migration is CXCR1 dependent in vitro and is defective in IL-8 receptor knockout mice. J Immunol. 2000;165:5287–5294. doi: 10.4049/jimmunol.165.9.5287. [DOI] [PubMed] [Google Scholar]
- 95.Luo Y, Chen X, O’donnell MA. Mycobacterium bovis bacillus Calmette-Guerin (BCG) induces human CC- and CXC-chemokines in vitro and in vivo. Clin Exp Immunol. 2007;147:370–378. doi: 10.1111/j.1365-2249.2006.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Poppas DP, Pavlovich CP, Folkman J, Voest EE, Chen X, Luster AD, O’donnell MA. Intravesical bacille Calmette-Guerin induces the antiangiogenic chemokine interferon-inducible protein 10. Urology. 1998;52:268–275. [PubMed] [Google Scholar]
- 97.Zeng X, Moore TA, Newstead MW, Deng JC, Kunkel SL, Luster AD, Standiford TJ. Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect Immun. 2005;73:8226–8236. doi: 10.1128/IAI.73.12.8226-8236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshio H, Lagercrantz H, Gudmundsson GH, Agerberth B. First line of defense in early human life. Semin Perinatol. 2004;28:304–311. doi: 10.1053/j.semperi.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 99.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 100.Svinarich DM, Gomez R, Romero R. Detection of human defensins in the placenta. Am J Reprod Immunol. 1997;38:252–255. doi: 10.1111/j.1600-0897.1997.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 101.Svinarich DM, Wolf NA, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynecol. 1997;176:470–475. doi: 10.1016/s0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- 102.Cole AM, Ganz T. Human antimicrobial peptides: analysis and application. Biotechniques. 2000;29:822–1. doi: 10.2144/00294rv01. [DOI] [PubMed] [Google Scholar]
- 103.Papagianni M. Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol Adv. 2003;21:465–499. doi: 10.1016/s0734-9750(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 104.Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- 105.Chen H, Xu Z, Peng L, Fang X, Yin X, Xu N, Cen P. Recent advances in the research and development of human defensins. Peptides. 2006;27:931–940. doi: 10.1016/j.peptides.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 106.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- 108.Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 109.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 110.Ganz T. Defensins and host defense. Science. 1999;286:420–421. doi: 10.1126/science.286.5439.420. [DOI] [PubMed] [Google Scholar]
- 111.Elkington KW, Greb LC. Adult respiratory distress syndrome as a complication of acute pyelonephritis during pregnancy: case report and discussion. Obstet Gynecol. 1986;67:18S–20S. doi: 10.1097/00006250-198603001-00006. [DOI] [PubMed] [Google Scholar]
- 112.Soisson AP, Eldridge E, Kopelman JN, Duff P. Acute pyelonephritis complicated by respiratory insufficiency. A case report. J Reprod Med. 1986;31:525–527. [PubMed] [Google Scholar]
- 113.Towers CV, Kaminskas CM, Garite TJ, Nageotte MP, Dorchester W. Pulmonary injury associated with antepartum pyelonephritis: can patients at risk be identified? Am. J Obstet Gynecol. 1991;164:974–978. doi: 10.1016/0002-9378(91)90568-c. [DOI] [PubMed] [Google Scholar]
- 114.Yazigi R, Lerner S, Tejani N. Association of acute pyelonephritis with pulmonary complications in pregnancy. A report of two cases. J Reprod Med. 1990;35:562–564. [PubMed] [Google Scholar]
- 115.Pruett K, Faro S. Pyelonephritis associated with respiratory distress. Obstet Gynecol. 1987;69:444–446. [PubMed] [Google Scholar]
- 116.Soto E, Richani K, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Goncalves L, et al. Increased concentration of the complement split product C5a in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med. 2005;17:247–252. doi: 10.1080/14767050500072805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–1123. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 118.Windsor AC, Mullen PG, Fowler AA, Sugerman HJ. Role of the neutrophil in adult respiratory distress syndrome. Br J Surg. 1993;80:10–17. doi: 10.1002/bjs.1800800106. [DOI] [PubMed] [Google Scholar]
- 119.Neumann B, Zantl N, Veihelmann A, Emmanuilidis K, Pfeffer K, Heidecke CD, Holzmann B. Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int Immunol. 1999;11:217–227. doi: 10.1093/intimm/11.2.217. [DOI] [PubMed] [Google Scholar]


