Abstract
Tcra gene assembly is characterized by an orderly progression of primary and secondary Vα to Jα recombination events across the Jα array, but the targeting mechanisms responsible for this progression are largely unknown. Previous studies revealed that the TEA promoter plays an important role in targeting primary Tcra rearrangements. We show that TEA and a novel promoter associated with Jα49 target primary recombination to discrete sets of Cα-distal Jα segments and together direct nearly all normal primary recombination events. Further, we show that TEA promoter deletion activates previously suppressed downstream promoters and stimulates primary rearrangement to centrally located Jα segments. Central promoter derepression also occurs following primary rearrangement, thereby providing a mechanism to target secondary recombination events.
The development of T lymphocytes bearing an αβ T cell receptor (TCR) depends on the somatic assembly of variable (V), diversity (D) and joining (J) gene segments by the process of V(D)J recombination1. Tcrb gene rearrangements occur first, in the CD4-CD8- double negative (DN) subset of thymocytes. Production of a functional TCRβ protein then promotes differentiation to the CD4+CD8+ double positive (DP) stage, during which Tcra gene rearrangements occur. Following expression of a cell surface αβ TCR, DP thymocytes are tested by positive selection to identify those with useful TCRs2. Only a small fraction of DP thymocytes are ultimately selected for further maturation.
Tcra rearrangement has several unique features that are thought to increase the likelihood of positive selection2,3. First, Vα to Jα rearrangement occurs on both alleles without allelic exclusion. Second, initial, or primary rearrangements, are targeted to Jα segments at Cα-distal, or 5′ end, of the 70 kb Jα array (polarity is denoted in reference to the sense strand throughout). If primary rearrangement fails to produce a selectable TCR, DP thymocytes may then undergo multiple additional rounds of secondary rearrangement that involve progressively more 5′ Vα gene segments and progressively more 3′ Jα gene segments. Moreover, the 5′ to 3′ progression along the Jα array is nearly synchronous on two Tcra alleles. Tcra rearrangement is ultimately terminated by positive selection, which silences recombinase expression, or by cell death resulting from a lack of positive selection. Thus, secondary Vα to Jα rearrangement provides thymocytes multiple opportunities for positive selection, and is critical for the production of a robust and diverse TCRα repertoire4,5.
The mechanisms that direct primary Tcra rearrangement are only partially understood. The Tcra enhancer (Eα), situated 3′ of Cα, controls the entire Jα array and is required for all Vα to Jα recombination events6. The T early α (TEA) promoter, located at the 5′ end of the Jα array, acts more locally, and controls usage of the most 5' Jα segments (Jα61-Jα53)7. However, TEA-deficient mice have quantitatively normal Jα usage downstream of this region, suggesting the presence of one or more additional cis-acting elements with the potential to target primary rearrangements. We previously analyzed the distribution of Jα usage in the short-lived DP thymocytes of mice lacking transcription factor RORγ(Rorc-/-)5. Jα usage was restricted to the 5′ portion of the Jα array, as would be expected if thymocytes were limited to primary rearrangements. Moreover, the detected rearrangements resolved into two discrete clusters, one just downstream of TEA and one spanning Jα50 to Jα45. The former is presumed to reflect primary rearrangements targeted by the TEA promoter. The latter appears to identify a site of primary targeting that could account for the residual rearrangements in TEA-deficient thymocytes. However, to date, no known regulatory elements have been mapped to this region.
Even less is understood about the targeting of secondary Tcra rearrangements. An orderly 5′ to 3′ progression of rearrangements across the Jα array is implied by allelic synchrony in Jα usage. Two types of models have been proposed, both relying on the ability of promoters to provide local access to the recombinase5,7,8. The developmental windows model envisions a set of embedded germline Jα promoters that are successively activated according to an intrinsic developmental program during DP thymocyte development. An alternative model envisions a Vα promoter-driven chain reaction, in which, following primary rearrangement, successively introduced Vα promoters would target subsequent rounds of secondary rearrangement to progressively more 3′ sets of Jα segments.
To better understand the molecular basis for the ordered propagation of rearrangement events across the Jα array, we searched this portion of the Tcra locus for novel promoter elements. We identified a strong promoter element situated just upstream of Jα49, and tested its functional relevance by deleting it, either alone or in combination with the TEA promoter, using homologous recombination strategies. Our results indicate that the two promoters target discrete sets of 5′ Jα segments for primary rearrangement, and together account for almost all normal primary Jα recombination events. In addition, we found that 5′ promoter deletion leads to the activation of previously suppressed downstream promoters, which can efficiently target rearrangements into the central portion of the Jα array. Central promoter derepression also occurs following primary rearrangement. This provides a previously unappreciated mechanism for the targeting of secondary Vα to Jα recombination events.
RESULTS
Identification of a germline promoter upstream of Jα49
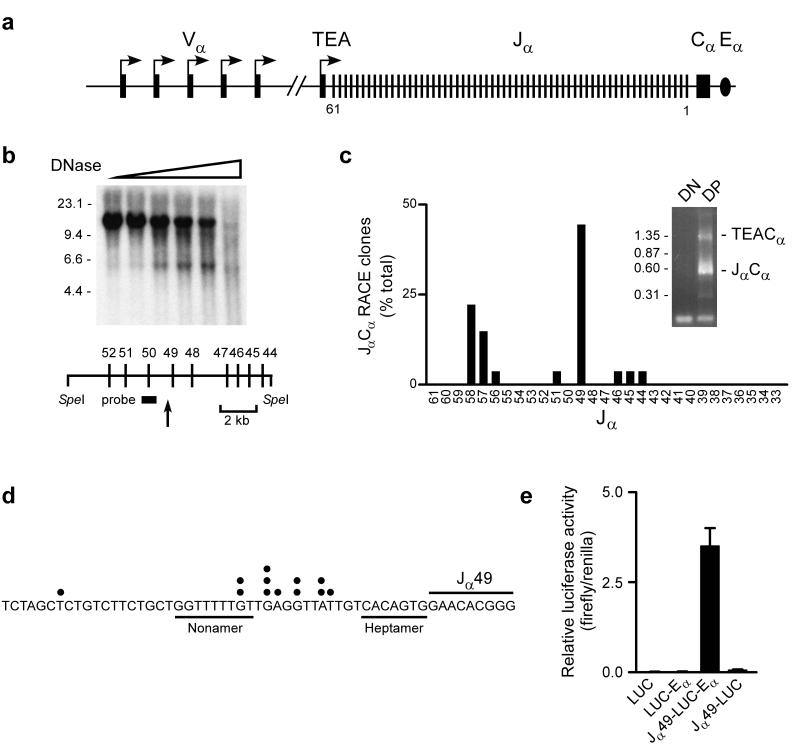
We searched for the presence of promoter elements that could account for the recovery of Jα rearrangements downstream of Jα53 in TEA-deficient mice7. As one approach, DP thymocytes from Rag2-/- mice carrying a functional Tcrb transgene (Rxβ) were analyzed for novel DNase I hypersensitive sites within the Jα array (Fig. 1a,b). These mice provide a pure source of DP thymocytes that retain both a germline Jα array and all associated promoter elements. Based on Southern hybridization using a Jα50 probe, DNase I digestion reduced a 12 kb SpeI fragment to a 5.5 kb species. This mapped a strong hypersensitive site just upstream of the Jα49 gene segment. In the second approach, a Cα primer was used in 5' rapid amplification of cDNA ends (RACE) to amplify full-length germline transcripts that span the Jα array (Fig. 1a,c). Such transcripts are typically spliced from the most 5′ splice donor to Cα. Two major species of 1.3 kb and 0.6 kb were amplified from cDNA of Rxβ DP thymocytes (Fig. 1c, inset). These species were cloned and sequenced. The 1.3 kb products were found to reflect initiation at the TEA promoter at the extreme 5′ end of the Jα array, with the TEA exon spliced to Cα (data not shown). The 0.6 kb products reflected initiation at sites associated with Jα segments (Jα58-Jα44), with splicing from Jα to Cα. The predominant germline Jα transcripts included Jα49 and initiated at several sites clustered within the Jα49 recombination signal sequence (Fig. 1c,d). To test for Jα49 promoter function in vitro, a 900 bp fragment spanning the Jα49 transcription start site was cloned upstream of the luciferase gene in a reporter plasmid that contained or lacked Eα, and was tested for activity following transfection into Jurkat T cells (Fig. 1e). When introduced into LUC-Eα, the promoter fragment stimulated luciferase activity by about 200-fold. However, promoter activity was minimal in reporter substrates lacking Eα. All activity of the 900 bp fragment was contained within a 5′ truncated fragment of 425 bp (data not shown). The position of the Jα49 promoter corresponds to the region of the Jα array in which Jα usage increases to wild-type frequency in TEA-deficient mice7 and to the cluster of primary Jα recombination events observed in the short-lived DP thymocytes of Rorc-/- mice5. In contrast, promoter activity associated with the Jα58, Jα57, and Jα56 gene segments (Fig. 1c) localized within the TEA-dependent zone. Therefore, we focused on the Jα49 promoter as a putative targeting element for primary Vα to Jα recombination.
Figure 1.
A promoter upstream of Jα49. (a) Schematic of the Tcra locus, identifying Vα(≅100 total), Jα and Cα segments and TEA exon (filled rectangles), Vα and TEA promoters (arrows) and Eα (oval). Jα segment numbering (5′ to 3′) is from 61 to 1. (b) Permeabilized Rxβ thymocytes were incubated with increasing amounts of DNase I (wedge) and SpeI digested genomic DNA was analyzed by Southern blot. Size markers (in kb) are indicated to the left of the blot. The arrow locates a DNase I hypersensitive site upstream of Jα49. (c) RNA of Rag2-/- (DN) and Rxβ (DP) thymocytes was subjected to 5′ RACE using a Cα primer. Agarose gel electrophoresis revealed two major species (inset) which were identified by sequencing. Size markers (in kb) are indicated. Frequencies of clones reflecting initiation upstream of or within individual Jα segments is plotted (27 total). (d) Map of 5′ ends of RACE clones (dots) initiating near Jα49. (e) A 900 bp promoter fragment was tested for activity in enhancerless (LUC) or enhancer-containing (LUC-Eα) luciferase plasmids. The mean±SEM of five determinations is presented.
T cell development in promoter-deleted mice
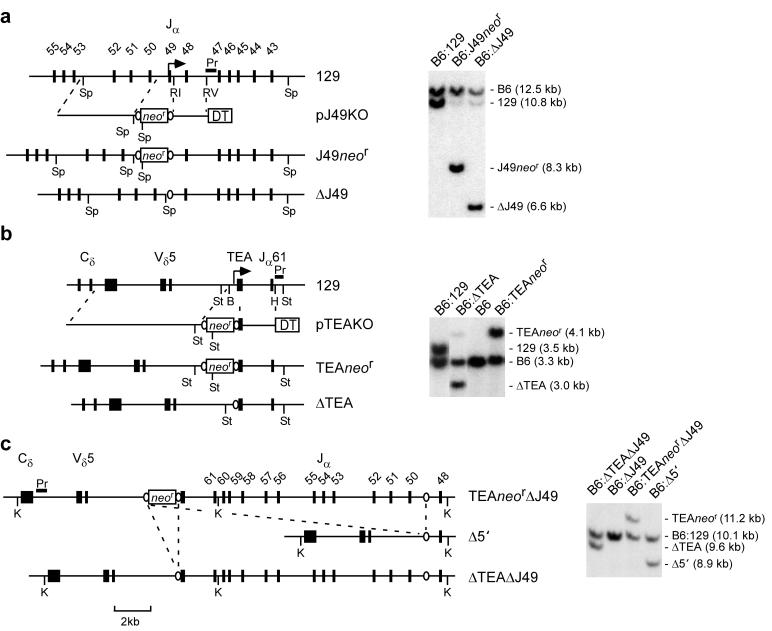
The Jα49 and TEA promoters were individually deleted from embryonic stem (ES) cells derived from 129:C57BL/6 (B6) heterozygous mice using a Cre-loxP homologous recombination strategy (Fig. 2a,b). Chimeric mice carrying a loxP-flanked neor cassette in place of TEA were bred to transgenic mice mice expressing Cre recombinase to delete the cassette, creating a TEA-deficient allele (ΔTEA). The loxP-flanked neor cassette was removed from Jα49 promoter-targeted ES cells by transient expression of Cre recombinase in vitro to create a Jα49-deficient allele (ΔJ49). These cells were then retargeted to delete the TEA promoter as well. The use of heterozygous ES cells insured that the two homologous recombination events would occur on the same (129) allele. Cre-mediated recombination then yielded two different alleles, one with simple deletions of both promoters (ΔTEAΔJ49) and a second with a 16 kb deletion encompassing the entire region between TEA and Jα49 (Δ5′) (Fig. 2c). The behavior of all four targeted alleles was subsequently analyzed in homozygous mice (ΔTEA, ΔJ49, ΔTEAΔJ49, and Δ5′).
Figure 2.
Generation of TEA and Jα49 promoter-deleted mice. Strategies for generation of ΔJ49, ΔTEA, ΔTEAΔJ49 and )5′ mice. V, J, C and TEA exons (filled rectangles) on the wild-type 129 allele are shown, as are neomycin resistance (neor) and diptheria toxin (DT) cassettes (open rectangles) and loxP sites (ovals) of targeting constructs pJ49KO and pTEAKO. Restriction sites are: Sp, SpeI; RI, EcoRI; RV, EcoRV; St, StuI; B, BglII; H, HpaI; K, KpnI. The Southern hybridization probes (Pr) are identified. (a) Genomic DNA isolated from B6:129 ES cells, B6:J49neor ES cells, and B6:ΔJ49 ES cells were digested with SpeI and analyzed by Southern hybridization. (b) B6:TEAneor mice were bred with CMV-Cre transgenic mice (B6 background). As compared to a B6:129 control, Southern hybridization analysis of StuI digested genomic DNA identified progeny carrying a TEAneor allele (B6:TEAneor), two B6 alleles (B6), or a ΔTEA allele (B6:ΔTEA). (c) B6:ΔJ49 ES cells were retargeted to introduce a loxP-flanked neor cassette in place of TEA (B6:TEAneorΔJ49). Cre-mediated recombination in vitro generated B6:Δ5′ and B6:ΔTEAΔJ49 clones, as revealed by Southern hybridization of KpnI digested genomic DNA.
To test for developmental defects in the various strains, CD4, CD8 and TCRβ expression was analyzed in thymocytes and splenocytes of 2-5 week old mice by flow cytometry. No differences were detected in the absolute numbers and percentages of DN, DP, and single positive thymocytes and splenic T lymphocytes as compared to age matched littermate controls (data not shown). Similarly TCRβ expression on thymocytes and splenocytes was indistinguishable from littermate controls. Thus, there were no gross deficiencies in αβ TCR expression or αβ T cell development in promoter-deleted mice.
The Jα49 promoter controls primary rearrangement
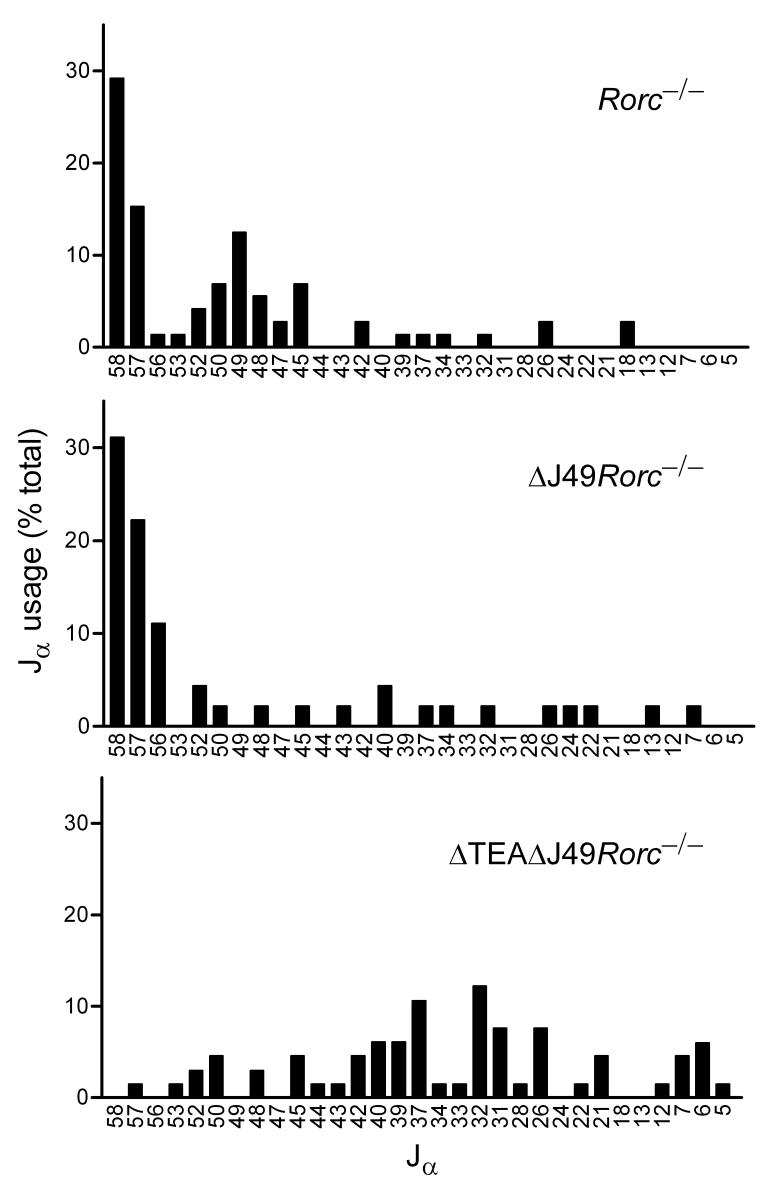
To study the effect of promoter deletions on primary targeting to the Jα locus, ΔJ49 and ΔTEAΔJ49 alleles were bred onto the Rorc-/- background. Vα8 and Cα primers were used to amplify cDNA prepared from Rorc-/-, ΔJ49Rorc-/-, and ΔTEAΔJ49Rorc-/- thymocytes, and polymerase chain reaction (PCR) products were cloned and sequenced to identify the distribution of Jα segments used (Fig. 3). Because the Vα8 primer detects multiple Vα8 family members that are dispersed across the Vα region, Vα8 rearrangements are relatively unbiased with respect to Jα usage5. Consistent with previous results5, two clusters of Jα usage were detected in Rorc-/- thymocytes, one encompassing Jα58 and Jα57, and a second encompassing Jα50 to Jα45. The second cluster was absent in ΔJ49Rorc-/- mice, indicating that the Jα49 promoter is required for primary rearrangements to the region spanning from Jα50 to Jα45. Both clusters were absent in ΔTEAΔJ49Rorc-/- mice, indicating that the TEA promoter is required for primary rearrangements to the most 5′ Jα segments, and that the two promoters together are required for almost all normal primary targeting events into the Jα array. Without these promoters, Jα usage in short-lived DP thymocytes was much more broadly distributed, although nearly 60% of rearrangements involved Jα42 to Jα26.
Figure 3.
Primary Jα usage in TEA and Jα49 promoter-deleted mice. Vα8 to Cα RT-PCR products obtained from thymocytes of 4-5 week old Rorc-/-, ΔJ49Rorc-/- and ΔTEAΔJ49Rorc-/- mice were cloned and sequenced to evaluate Jα usage. The fraction of clones using particular Jα segments is plotted. Numbers of clones analyzed were 72, 45 and 66 for Rorc-/-, ΔJ49Rorc-/- and ΔTEAΔJ49Rorc-/-, respectively.
Altered T cell repertoire in promoter-deleted mice
To analyze the effects of altered primary rearrangement on the Jα repertoire, cDNA was prepared from wild-type, ΔTEA, ΔJ49, ΔTEAΔJ49 and Δ5' thymocytes, and was amplified using Vα8 and Cα primers. PCR products were then fractionated on agarose gels and transferred to nylon filters, and Jα usage was evaluated by hybridization with Jα-specific oligonucleotide probes (Fig. 4). In comparison to wild-type thymocytes, ΔJ49 thymocytes displayed an overrepresentation of Jα segments from Jα58 to Jα50, with normal to slight underrepresentation of Jα usage further downstream. We interpret elevated usage of the most 5′ Jα segments to reflect an increase in primary targeting to the TEA-dependent zone in the absence of the Jα49 promoter. The relatively normal usage of Jα segments across the Jα49 promoter-dependent zone suggests that in the absence of primary targeting by the Jα49 promoter, these Jα segments can still be used efficiently as a consequence of secondary rearrangements that follow primary rearrangements targeted by the TEA promoter.
Figure 4.
Jα repertoires of TEA and Jα49 promoter-deleted mice. Vα8 to Cα RT-PCR products obtained from thymocytes of 4-5 week old ΔJ49, ΔTEAΔJ49, and Δ5′ mice, from thymocytes of 2-5 week old ΔTEA mice, and from thymocytes of age-matched wild-type littermates, were fractionated on agarose gels and immobilized to nylon filters. Jα usage was evaluated by hybridization with 32P-labeled Jα and Cα oligonucleotide probes. Relative Jα usage was calculated as (Jα signal for mutant / Cα signal for mutant) / (Jα signal for wild-type / Cα signal for wild-type). The dashed line indicates wild-type Jα usage (frequency=1). Results represent the mean ± SEM of 4-8 determinations.
ΔTEA mice revealed a profile of Jα usage similar to that reported previously7. By comparison, ΔTEAΔJ49 mice were more substantially impaired, with Jα usage further depressed by an average of 25% between Jα50 and Jα38. Δ5′ mice revealed a nearly identical profile, except that Jα48 usage was suppressed as compared to ΔTEAΔJ49 mice. That Jα49 promoter deletion impairs usage of Jα50 to Jα38 on a ΔTEA but not a TEA+ background must reflect the elimination of secondary rearrangements that follow primary targeting by TEA. Thus, although the TEA and Jα49 promoters target primary rearrangements in nonredundant fashion, they have partially redundant influences on Jα usage as a consequence of secondary rearrangements. Because Jα52 and Jα50 are still used inefficiently on ΔTEAΔJ49 alleles, there may be weak targeting elements in the TEA to Jα49 interval, other than the TEA and Jα49 promoters. These elements must also account for low frequency Jα48 usage on ΔTEAΔJ49 alleles, given that Jα48 usage is minimal on Δ5′ alleles. Downstream of Jα48, there was a gradual increase in Jα usage in both ΔTEAΔJ49 and Δ5′ mice, first to physiologic and then to superphysiologic amounts. This result indicates the presence of additional cis-acting elements that can target primary rearrangements to this region, consistent with the modified profile of primary rearrangements in ΔTEAΔJ49Rorc-/- mice (Fig 3).
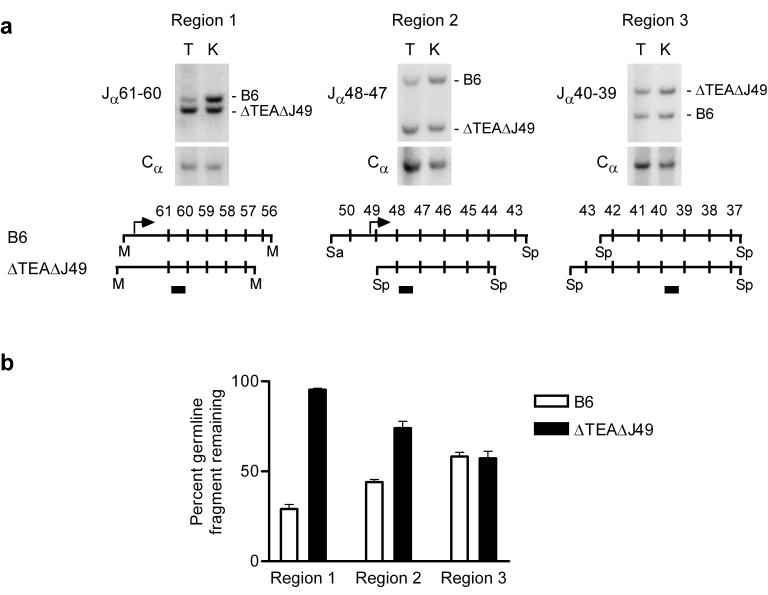
The preceding experiments monitored Jα usage relative to Cα usage in each mouse strain, but did not measure the absolute magnitude of rearrangement across the central portion of the Jα array. For this purpose, we quantitatively analyzed restriction digests of thymocyte genomic DNA by Southern hybridization. We generated heterozygous mice carrying a ΔTEAΔJ49 129 allele and a wild-type B6 allele, and exploited restriction fragment length polymorphisms to simultaneously examine rearrangement of the two alleles in individual DNA samples (Fig 5a). The extent of recombination events involving a set of Jα gene segments was evaluated by measuring loss of the germline (unrearranged) restriction fragment on which they are located. Previous studies of wild-type alleles showed rearrangement to be extensive at the 5′ end of the Jα array, and to diminish gradually from 5′ to 3′7,9,10. Consistent with this, we observed that on the B6 allele, retention of the germline Jα61-Jα56 fragment (Region 1) was only 29%, whereas retention of the germline Jα50-Jα43 fragment (Region 2) was 44% and retention of the germline Jα42-Jα37 fragment was 58% (Fig. 5a,b). Previous analysis of TEA-deficient alleles revealed almost complete retention of the germline signal for Jα61-Jα53, but only 20-25% retention immediately downstream (Jα53-Jα39)7. This indicates that recombination events involving 5′ Jα segments are rare, whereas Jα segments immediately 3′ rearrange at high frequency. Germline signals for the 5′ Jα segments persist despite extensive rearrangement of more 3′ Jα segments because the unrearranged 5′ Jα segments are excised onto extrachromosomal circles that are stably maintained in DP thymocytes. Consistent with repertoire analysis (Fig. 4), rearrangement on the ΔTEAΔJ49 allele was more substantially impaired than on a ΔTEA allele, since the retention of germline signal was 95% for Jα61-Jα56 (Region 1) and 74% for Jα48-Jα44 (Region 2). Nevertheless, rearrangement on the ΔTEAΔJ49 allele was equivalent to B6 for Jα43-Jα37 (Region 3). That recombination efficiency of the mutant allele was similar to wild-type in Region 3 implies that with the TEA and Jα49 promoters missing, downstream elements can efficiently target primary rearrangements to 3′ Jα segments, and thereby promote a physiological amount of Vα to Jα recombination.
Figure 5.
Quantitative Southern blot analysis of Jα rearrangement on a ΔTEAΔJ49 allele. (a) Thymocyte (T) and kidney (K) genomic DNA was isolated from B6:ΔTEAΔJ49 mice and MscI (M), SpeI (Sp), or SacI (Sa) plus SpeI digests were analyzed by Southern blot using Jα61-60, Jα48-47 and Jα40-39 probes (filled rectangles) and a Cα probe. (b) Retention of germline fragment was calculated by first normalizing hybridization signals for Jα to Cα in thymocytes and in kidney. Normalized thymus signals were then expressed as a percentage of the normalized kidney signals. The results represent the mean ± SEM of triplicate determinations.
Jα chromatin structure in promoter-deleted mice
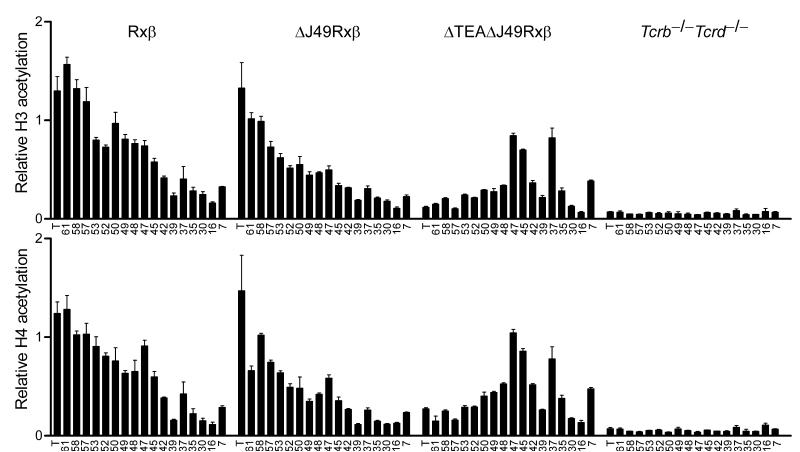
Chromatin that is accessible for V(D)J recombination typically displays increased acetylation of histones H3 and H411. Therefore, to understand the targeting and recovery of rearrangements across the central portion of the Jα array, we analyzed the effects of promoter deletion on the acetylation state of Jα histones. For this purpose, ΔJ49 and ΔTEAΔJ49 alleles were bred onto a Rxβ background, and mononucleosomes were prepared from Rxβ thymocytes, ΔJ49Rxβ thymocytes, ΔTEAΔJ49Rxβ thymocytes, and as a control, from T cell-deficient splenocytes isolated from Tcrb-/-Tcrd-/- mice. Acetylated mononucleosomes were immunoprecipitated using antibodies specific for diacetylated histone H3 and tetraacetylated histone H4, and real-time PCR was used to quantify acetylation at sites along the Jα array (Fig. 6). In Rxβ thymocytes acetylation was found to be high across the most 5′ Jα gene segments and to decrease gradually towards the 3′ portion of the array. In contrast, the entire array Jα array was hypoacetylated in Tcrb-/-Tcrd-/- splenocytes. As compared to the histone acetylation found in Rxβ thymocytes, H3 and H4 acetylation in ΔJ49Rxβ thymocytes was normal at the TEA exon but was partially reduced from Jα61 through Jα45. In contrast, ΔTEAΔJ49Rxβ thymocytes revealed a completely different profile. Relative to ΔJ49Rxβ mice, histone acetylation was substantially reduced from the TEA exon through Jα48, but was substantially increased at multiple sites further 3′. Previous work had shown that TEA deletion reduced Tcra locus acetylation from TEA through Jα5312. The current data imply that the TEA and Jα49 promoters collaborate to maintain a normal amount of histone acetylation through Jα48. More importantly, the detection of normal to elevated acetylation downstream of Jα48 is consistent with the presence of an additional set of cis-acting elements that could target primary rearrangement (Fig. 3) and an increase to a physiologically normal amount of Jα usage (Figs. 4 and 5) across the central portion of the Jα array.
Figure 6.
Jα array histone modifications in TEA and Jα49 promoter-deleted mice. Histone H3 and H4 acetylation was analyzed by chromatin immunoprecipitation from mononucleosomes prepared from thymocytes of Rxβ, ΔJ49Rxβ, and ΔTEAΔJ49Rxβ mice, and as a control, from splenocytes of Tcrb-/-Tcrd-/- mice. Sites analyzed were situated in the TEA exon (T) or at individual Jα segments (numbered). Bound and input fractions were quantified using real time PCR and ratios of bound/input were normalized to that for B2m in each sample. The data represent the mean ± SEM of triplicate PCR reactions.
Activation of downstream promoters
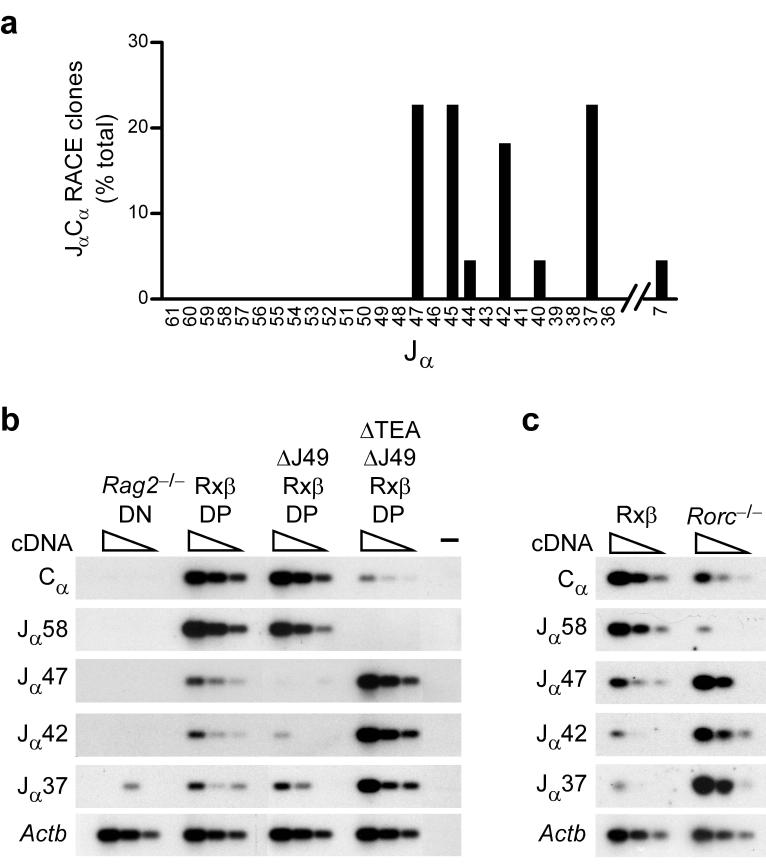
The analysis of germline transcripts in Rxβ DP thymocytes identified a low frequency of germline transcripts initiating at Jα46, Jα45, and Jα44 (Fig. 1c). To assess the activity of centrally located promoter elements following 5′ promoter deletion, germline Cα transcripts in DP thymocytes of ΔTEAΔJ49Rxβ mice were analyzed by 5' RACE (Fig. 7a). Sequence analysis identified germline transcripts initiating upstream of and within Jα47, Jα45, Jα44, Jα42, Jα40, Jα37 and Jα7, as well as just downstream of Jα37. No germline transcripts were identified at Jα58 and Jα57, even though these transcripts were well represented in Rxβ DP thymocytes, and the associated promoters should still have been present. A similar profile of transcripts had previously been described in TEA-deficient DP thymocytes13. However in neither case was it clear whether the transcripts associated with centrally located Jα segments reflected residual transcription that could be more readily detected once 5′ promoters were deleted, or rather, reflected bona fide increases in promoter activity.
Figure 7.
Germline JαCα transcription in TEA and Jα49 promoter-deleted mice. (a) RNA isolated from ΔTEAΔJ49Rxβ thymocytes was subjected to 5′ RACE using a Cα primer. The relative frequencies of RACE clones reflecting initiation upstream of or within individual Jα segments is plotted (22 total). Three of five clones identified as Jα37 initiated 66-105 bp downstream of Jα37 and spliced to Cα using a 5′ splice site 129 bp downstream of Jα37. (b,c) Serial three-fold dilutions of cDNA (wedges) prepared from thymocytes of the indicated genotypes were analyzed by PCR to detect JαCα transcripts, total Cα-containing transcripts, or Actb transcripts. (-) indicates a control PCR lacking cDNA. PCR reactions were analyzed by Southern blot using Cα or Actb probes. The results are representative of two experiments.
To address this issue, we analyzed the abundance of selected germline JαCα transcripts by semiquantitative RT-PCR (Fig. 7b). This analysis allows us to compare how a specific germline transcript varies in abundance among different genotypes. However as presented, it does not provide direct information about the relative abundance of different germline transcripts. The quantity of total germline Cα transcripts was similar in Rxβ and ΔJ49Rxβ DP thymocytes but was reduced by at least 90% in ΔTEAΔJ49Rxβ DP thymocytes. This result is consistent with previous analysis of TEA-deficient mice, which revealed that the majority of germline Cα-containing transcripts depend upon the TEA promoter7. Consistent with the 5' RACE analysis, transcripts initiating from Jα58 were undetectable in ΔTEAΔJ49Rxβ DP thymocytes. Even more striking, transcripts initiating from Jα47, Jα42, and Jα37 were increased about 10-fold in ΔTEAΔJ49Rxβ as compared to Rxβ thymocytes. Because the pattern of transcription on ΔTEAΔJ49 alleles mirrored the pattern previously documented for TEA-deficient alleles7, the inhibition of Jα58 and induction of Jα47, Jα42, and Jα37 transcripts in these thymocytes must reflect TEA promoter deletion per se. This conclusion was strengthened by the finding that Jα49 promoter deletion resulted in downregulation rather than upregulation of Jα47 and Jα42 transcripts. Moreover, because of this downregulation, the magnitude of induction of these transcripts as a consequence of TEA promoter deletion was even greater than the 10-fold increase noted in comparison of ΔTEAΔJ49Rxβ to Rxβ thymocytes. Thus, deletion of the TEA promoter dramatically downregulates transcription initiating at the 5′ end of the Jα array, but reciprocally upregulates transcription initiating within the central portion of the array. This refocusing of promoter activity likely accounts for the retargeting of primary Jα rearrangements in 5′ promoter-deleted mice.
Were central promoter induction to occur when the TEA promoter is deleted by primary rearrangement, it would provide a mechanism for the targeting of secondary rearrangements into the central portion of the Jα array. To test this, we examined germline promoter activity in thymocytes of Rorc-/- mice, since in these mice the majority of DP thymocytes would have lost the TEA promoter due to primary Vα to Jα rearrangement, but would retain the central portion of the Jα array in germline configuration. As compared to Rxβ thymocytes, total Cα-containing transcripts were downregulated in Rorc-/- thymocytes (Fig. 7c), although not to the same extent as in ΔTEAΔJ49Rxβ thymocytes (Fig. 7b). Blunted downregulation likely occurs because during primary rearrangement in Rorc-/- thymocytes the TEA promoter is replaced by a Vα promoter, rather than simply deleted as in ΔTEAΔJ49 thymocytes. Downregulation of Jα58 transcripts is substantial in Rorc-/- thymocytes, owing to deletion of both the TEA and the Jα58 promoters during primary rearrangement. However, despite the general downregulation of total Cα-containing transcripts, Jα47, Jα42, and Jα37 transcripts are much more abundant in Rorc-/- as compared to Rxβ thymocytes (Fig. 7c). Thus, centrally located promoters are induced whether the TEA promoter is eliminated by gene targeting or by primary rearrangement. As such, central promoter induction should provide a physiologically relevant stimulus for the targeting of secondary rearrangements into the central portion of the Jα array.
DISCUSSION
In this study we show that the TEA promoter and a second promoter associated with Jα49 target primary recombination to discrete sets of 5′ Jα segments, and together direct nearly all normal primary recombination events into the Jα array. Numerous studies have implicated germline promoters as local regulators of accessibility14. TEA was shown to be required for recombination and histone acetylation across a 15 kb window at the 5′ end of the Jα array, and was assumed to exert direct effects on accessibility that extend across, but are limited to, this defined region of chromatin7,12. However, due to the presence of redundant downstream elements, the 3′ extent of TEA's influence on accessibility could not have been known with certainty. In fact, our analysis suggests that accessibility control by TEA may be restricted to a much narrower region than was thought previously. Specifically, we found that TEA-dependent primary rearrangements in Rorc-/- mice are limited to Jα58, Jα57 and Jα56. Moreover, since these Jα segments are associated with promoters that are themselves TEA-dependent, accessibility mediated directly by TEA could very well be limited to Jα61 only. Although Jα61 is competent for rearrangement, it was excluded from our analysis since it lacks a functional splice site and could not be surveyed by RT-PCR. We assume that TEA promotes accessibility directly at Jα61, but suggest that it may do so indirectly at Jα58, Jα57 and Jα56 due to its ability to potentiate local promoters at these sites. Thus, the direct influence of TEA on recombinase access could extend over a distance of no greater than 2 kb.
Our study suggests that the Jα49 promoter controls the targeting of primary rearrangements to a window spanning Jα50 to Jα45. However, since Jα47 and Jα45 are associated with promoters whose activity is potentiated by the Jα49 promoter, the Jα49 promoter could conceivably exert a direct influence on accessibility at Jα50, Jα49 and Jα48 only. Thus, the true "windows" for accessibility provided by the TEA and Jα49 promoters may extend no more than 1-2 kb. In terms of range, this would make them more like the Dβ1 promoter, which exerts local control over Dβ1-Jβ1 rearrangement at the Tcrb locus15,16. Because a 1-2 kb window would typically accomodate one to three Jα segments, our findings have important implications for the way in which Jα segments are sequentially activated during DP thymocyte development. The critical remodeling events that target such windows remain to be defined, but likely include both the covalent modification of histone residues and noncovalent changes in nucleosome structure or organization11.
Our data emphasize that primary rearrangements are restricted almost exclusively to the region spanning Jα61 to Jα45, and that use of Jα segments downstream of Jα45 depends on the process of secondary rearrangement. Speculations about the mechanisms driving secondary rearrangements have presumed dominant roles for either downstream germline Jα promoters, or Vα promoters introduced by Vα to Jα rearrangement. In the developmental windows model, downstream Jα promoters would be activated according to an intrinsic developmental program in DP thymocytes. The analysis of allelic Jα usage in TEA promoter heterozygous mice argued against this model, since even T cells using relatively 3′ Jα segments displayed uncoordinated allelic Jα usage, with the TEA-deficient allele substantially more advanced than the TEA+ allele8. These results were more consistent with the predictions of a Vα promoter-dependent model, in which rearrangements on the two alleles would progress independently, as a function of the position of the previously introduced Vα promoter. Nevertheless, direct evidence in support of either model has been lacking. Our data provides indirect support for the role of newly juxtaposed Vα promoters. The argument is perhaps clearest for Jα48, which rearranges normally in ΔJ49 mice but barely at all in Δ5′ mice. Jα48 rearrangement on ΔJ49 alleles is therefore almost completely dependent on upstream promoters, even though these promoters lack the ability to target primary Jα48 rearrangements. It is difficult to explain Jα48 rearrangement on ΔJ49 alleles without the involvement of an appropriately positioned Vα promoter as an upstream targeting element. Furthermore, the enhanced usage of Jα47 to Jα38 on ΔJ49 versus ΔTEAΔJ49 alleles is most easily explained by such a mechanism as well.
Our data provide strong support for a distinct and previously unappreciated mechanism that operates to target secondary rearrangements into the central portion of the Jα array. As a consequence of TEA promoter deletion, we observed both the induction of Jα promoters and the retargeting of primary recombination events within the central portion of the Jα array. Moreover, the same promoters were induced when the TEA promoter was deleted by primary rearrangement in Rorc-/- thymocytes. Since central promoter activation seems almost certain to account for the central targeting of primary rearrangements in 5′ promoter-deleted mice, it follows directly that these promoters should also stimulate secondary rearrangement upon their induction following primary rearrangement in wild-type thymocytes. Although this promoter-deletion and derepression mechanism relies on embedded Jα promoters as in the developmental windows model, they would be activated by prior Vα to Jα rearrangement as opposed to an intrinsic developmental program. Promoter-deletion and derepression could therefore be compatible with the asynchronous rearrangement observed in mice heterozygous for TEA promoter deficiency8.
The promoter-deletion and derepression pathway and the Vα promoter-dependent pathway could synergize in important ways to efficiently propagate rearrangement events across the Jα array. If the true window of accessibility provided by germline Jα and newly juxtaposed Vα promoters is maximally a few Jα segments, a Vα promoter-dependent pathway would require many rounds of rearrangement before 3′ Jα segments could be used. It seems reasonable that there must be a substantial interval between successive rearrangement events on a single allele, because at each step TCRs must be expressed at the cell surface to allow testing by positive selection. Hence, there may not be sufficient time for a Vα promoter-dependent mechanism, by itself, to provide accessibility to 3′ Jα segments. In this light, the jumps along the array afforded by downstream promoter induction may synergize with the stepwise progress provided by introduced Vα promoters to allow more efficient use of central and 3′ Jα segments.
Although our studies reveal an intriguing hierarchy among germline promoters, the mechanisms that enforce this hierarchy remain to be established. The dominant TEA and Jα49 promoters clearly have positive influences on the activities of weaker promoters immediately downstream. One possibility is that the dominant promoters provide a degree of downstream chromatin remodeling that enhances transcription factor occupancy at the downstream sites17,18. Alternatively, the dominant promoters may be uniquely effective in establishing direct contacts with Eα or in some other way establishing a specific physical organizion of the locus that is then taken advantage of by downstream promoters19. The potent suppressive effect of the TEA promoter on centrally located promoters can be explained by either of two mechanisms. One possibility is that suppression reflects promoter competition20,21; TEA could compete more effectively for interaction with Eα and in this way exclude the downstream promoters from interactions that are critical for their activation. Alternatively, suppression could reflect transcriptional interference22-24; abundant TEA-dependent transcription could interfere with transcription factor loading at centrally located promoters. That 5′ promoters can overcome this suppressive effect emphasizes the complexity of hierarchical promoter interactions across the Jα array. Additional studies will be required to fully understand the mechanistic basis for this hierarchy as well as the qualitative and quantitative aspects of promoter function that are critical for its maintentance.
METHODS
Probes for Southern hybridization. The following DNA fragments were generated for use as radiolabeled probes in Southern hybridization: 3′Cδ, an 867 bp HindIII-PstI fragment spanning nucleotodes 11817 to 12683 of Genbank file M64239; Jα60-61, a 446 bp HpaI-StuI fragment spanning nucleotides 20991 to 21436 of M64239; Jα53, a 1011 bp BglII-EcoRI fragment spanning nucleotides 27714 to 28724 of M64239; Jα50, a 682 bp fragment generated by PCR using primers 5′-CCTGCAGTGTGTCAGAGGTTC-3′ and 5′-CCACGGCACTATTTAAGGACC-3′; Jα48-47, a 677 bp EcoRV-NcoI fragment spanning 36623 to 37298 of M64239; and Jα40-39, a 1018 bp fragment generated by PCR using primers 5′-CTCTCAACCCACATGCTTCC-3′ and 5′-CTTCTCCACCTTGCAGTTTG-3′.
DNase I hypersensitivity. Unfractionated thymoctyes (5×107) were permeabilized with lysolecithin and treated with DNase I as described25,26, with minor modifications. DNase I (Worthington Biomedical) was used at 0, 2.5, 5, 10, 12, 14, and 16 U/ml. SpeI digested genomic DNA was fractionated on a 0.7% agarose gel and immobilized onto a nylon membrane. Southern hybridization was performed using the Jα50 probe.
5' RACE analysis. 5′ RACE was performed using a GeneRacer™ Kit (Invitrogen) according to the manufacturer's instructions, making use of a Cα-specific 3′ primer (5′-TGGGAGTCAGGCTCTGTCAGTCTT-3′) and a touchdown PCR strategy. PCR products were purified through agarose gels, were cloned using a TOPO TA Cloning Kit for Sequencing (Invitrogen) according to the manufacturer's instructions, and were sequenced using a Model 3730 DNA Analyzer (Applied Biosystems).
Luciferase assay. Test fragments were inserted into the pXPG firefly luciferase reporter plasmid (a gift from P. Cockerill, University of Leeds, UK)27. Human Eα was amplified by PCR using primers 5′-GTCTCCGAATTCCCTCCAGGTGTTTGG-3′ and 5′-CAAGCGCTGAATTCGGTGAGATCAAGG-3 (which include introduced EcoRI sites). Following EcoRI digestion, the fragment was cloned into the unique EcoRI site downstream of the luciferase gene in pXPG (also referred to as LUC) to generate pXPG-Eα (also referred to as LUC-Eα) A promoter fragment was generated by PCR using primers 5′-CTGATGTCCCGGGTGTCTGATATGTGACCC-3′ (with an introduced SmaI site) and 5′-GACAGTCAAGCTTGTTCCTTTCCC-3′ (with an introduced HindIII site). The 900 bp SmaI-HindIII fragment was cloned into the unique SmaI and HindIII sites upstream of the luciferase gene in either pXPG or pXPG-Eα. Jurkat T cells (4×106) were co-transfected with 5 μg of CsCl-purified pXPG derivatives and 10 ng of pRL-TK (renilla luciferase plasmid) (Promega) using Superfect® (Qiagen) as recommended by the manufacturer. Cells were cultured at 37°C with 5% CO2 for 24h, after which cells were lysed and luciferase activity was measured using a Dual Luciferase Kit (Promega).
Generation of TEA and Jα49 promoter knockout mice. Homology arms were generated by PCR using pfu polymerase (Stratagene) and were sequenced to confirm PCR fidelity. The long and short arms of pTEAKO extend from nucleotides 12960 to 18300 and 18973 to 20990 of Genbank file M64239. The long arm was introduced between the NotI and XmaI sites upstream of the loxP-flanked phosphoglycerate kinase (PGK) promoter-neomycin resistance (neor) cassette of plasmid PGKneolox2DTA.2 (http://www.fhcrc.org/labs/soriano/vectors/PGKneolox2DTA.2.html)(gift of Y.W. He, Duke University Durham NC), whereas the short arm was introduced between the NheI and EcoRV sites downstream of the cassette. Homologous recombination deletes 678 bp from the TEA promoter. The long and short arms of pJ49KO extend from nucleotides 28711 to 33461 and 34422 to 36622 of Genbank file M64239. The long arm was introduced between the SacII and XmaI sites upstream of PGK-neor, whereas the short arm was introduced between the HindIII and EcoRV sites downstream of PGK-neor. Homologous recombination deletes 961 bp encompassing the Jα49 promoter and gene segment. Targeting constructs were linearized with XhoI and electroporated into the EF1 129SvEv/C57B6 ES cell line (gift of F. Alt, Harvard Medical School, Boston MA). ES clones correctly targeted at the TEA promoter were initially screened by PCR and were subsequently confirmed by Southern blot using the 3′Cδ probe in BamHI-KpnI digests and the Jα61-60 probe in StuI digests. Clones correctly targeted at the Jα promoter were similarly screened by PCR and confirmed by Southern blot using the Jα53 probe in XbaI digests and the Jα48-47 probe in SpeI digests. StuI and SpeI polymorphisms between 129 and B6 insured targeting to the 129 allele. Chimeric mice carrying the TEA deletion were bred to CMV-Cre transgenic mice (B6 background) for germline transmission and to delete neor. Neor was removed from Jα49 targeted ES cells by transient expression of Cre recombinase in vitro. Targeted ES cells lacking neor were used directly to produce chimeric mice and were bred for germline transmission, or were retargeted for TEA deletion. The doubly targeted ES cells were screened by PCR and confirmed by Southern hybridization using the 3′Cδ probe in KpnI digests. Doubly targeted ES cells were then treated to delete neor in vitro or were used directly to produce chimeric mice that were bred to delete neor in vivo. ΔTEAΔJ49 and Δ5′ alleles were generated through both strategies. Gene targeted mice were subsequently bred with Rorc-/-28, Rag2-/-xTCRβ transgene29 and 129 mice. Breeding schemes insured that littermate controls always segregated wild-type 129 alleles. All mice were used in accordance with protocols approved by the Duke University Animal Care and Use Committee.
Flow cytometric analysis. Suspensions of thymocytes and splenocytes (2×106) were incubated for 5 min at 4°C with anti-mouse CD16 to minimize non-specific staining. They were then stained for 20 min at 4° with cocktails of monoclonal antibodies and were diluted with 1 ml of cold RPMI containing 10% fetal bovine serum. Pellets were resuspended in 300 μl of PBS containing 1% formaldehyde. Flow cytometric analysis was performed using a FACStar Plus (BD-PharMingen). Antibodies used were fluorescein isothiocyanate (FITC) conjugated anti-mouse CD4 (GK1.5), phycoerythrin (PE) conjugated anti-mouse CD8α (53-6.7), and Cy-Chrome conjugated anti-mouse TCRβ chain (H57-597). FITC conjugated rat IgG2a, PE conjugated rat IgG2, and Cy-Chrome conjugated hamster IgG2 were used as negative controls. All antibodies were purchased from BD Pharmingen.
RT-PCR analysis of Jα usage. RNA was isolated from total thymocytes and splenocytes using TRIzol (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized using M-MLV reverse transcriptase (Invitrogen) and oligo dT primers. PCR conditions for amplifying Vα8 (5′-CAGACAGAAGGCCTGGTCAC-3′) to Cα (5′-TGGCGTTGGTCTCTTTGAAG-3′) products were 94°C for 2 min, 33 to 35 cycles of 92°C for 30 s, 55°C for 30 s and 72°C for 30 s, and a 4 min extension at 72°C. PCR products were fractionated on 1.0% agarose gels, transferred to nylon membranes and hybridized with 32P-labeled Jα specific oligonucleotides. Primers, hybridization and washing conditions are provided in Supplementary Table 1 online. Quantification was performed using a PhosphorImager. Alternatively, PCR products were gel purified and cloned using a TOPO TA Cloning Kit for Sequencing (Invitrogen). Sequence analysis was performed using Cα primer 5′-CGGCACATTGATTTGGGAGTC-3′.
Chromatin immunoprecipitation. Chromatin immunoprecipitation was performed from mononucleosomal DNA as described30, using rabbit antisera to diacetylated histone H3 (AcH3) and tetraacetylated histone H4 (AcH4) (Upstate Biotechnology), and control rabbit IgG (Sigma-Aldrich). Bound and input DNA was quantified by real-time PCR using a Roche LightCycler and a FastStart DNA Master Syber Green I Kit (Roche Diagnostics). Primers and annealing temperatures are provided in Supplementary Table 2 online. The PCR program consisted of denaturation for 4 min at 95°C followed 50 cycles of 20 s at 95°C, 20 s at annealing temperature, and 20 s at 72°C. Acetylation values were expressed as (bound/total for experimental)/(bound/total for mouse B2m).
RT-PCR analysis of germline transcription. Thymocyte RNA was converted to cDNA using oligo dT primers and SuperScript (Invitrogen) according to the manufacturer's instructions. PCR conditions were 94°C for 5 min, 22 to 32 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 45 sec, followed by a 10 min extension at 72°C. Actb primers were 5′-GTCAGAAGGACTCCTATGTG-3′ and 5′-TCGTAGATGGGCACAGTGTG-3′. Amplification of germline Cα transcripts was performed using a 3′ Cα primer 5′-GAGTCGGCTCTGTCAGTCTT-3′ in conjunction with 5′ primers for Cα (5′-AGAACCTGCTGTGTACCAGTTA-3′), Jα58 (5′-ACTGGGTCTAAGCTGTCATTTG-3′), Jα47 (5′-CTTTGGCTTGGGAACCATTTTG-3′), Jα42 (5′-GAGGAAGCAATGCAAAGCTAAC-3′), and Jα37 (5′-GACTGGGGACAACTTTACAAGT-3′) (Fig. 7b). Upstream Jα58 (5′-TGCAAAGCCCTTCAGTGCAGT-3′), Jα47 (5′-GTCACAGGAGTTTGAGGCTGT-3′), Jα42 (5′-CCCAAATGACTGTGAATTCTGG-3′), and Jα37 (5′-AAAGTGCAGCATTGGGGTGTAA-3′) primers were used for analysis on a recombinase positive background so that transcripts of germline Jα segments could be distinguished from those of rearranged Jα segments (Fig. 7c). Following agarose gel electrophoresis and transfer to nylon membranes, PCR products were detected by hybridization with a 32P-labeled Actb PCR fragment generated using the 5′ primer noted above and 5′-AGGTAGTCTGTCAGGTCCCG-3′ as a 3′ primer, or with 32P-labeled Cα oligonucleotide 5′-CGGCACATTGATTTGGGAGTC-3′.
Supplementary Material
ACKNOWLEDGEMENTS
We thank E. Oltz (Vanderbilt University, Nashville TN), B. Sleckman (Washington University, St. Louis MO), Y. Zhuang and I. Abarrategui for critical review of the manuscript. This work was supported by NIH grant GM41052 (to MSK).
REFERENCES
- 1.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109:S45–S55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 2.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 3.Krangel MS, Carabana J, Abarrategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor α/δ locus. Immunol. Rev. 2004;200:224–232. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 4.Yannoutsos N, et al. The role of recombination activating gene (RAG) reinduction in thymocyte development in vivo. J. Exp. Med. 2001;194:471–480. doi: 10.1084/jem.194.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo J, et al. Regulation of the TCRα repertoire by the survival window of CD4+CD8+ thymocytes. Nat. Immunol. 2002;3:469–476. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 6.Sleckman BP, Bardon CG, Ferrini R, Davidson L, Alt FW. Function of the TCRα enhancer in αβ and γδ T cells. Immunity. 1997;7:505–515. doi: 10.1016/s1074-7613(00)80372-6. [DOI] [PubMed] [Google Scholar]
- 7.Villey I, Caillol D, Selz F, Ferrier P, de Villartay J-P. Defect in rearrangement of the most 5′ TCR-Jα following targeted deletion of T early α (TEA): implications for TCR α locus accessibility. Immunity. 1996;5:331–342. doi: 10.1016/s1074-7613(00)80259-9. [DOI] [PubMed] [Google Scholar]
- 8.Mauvieux L, Villey I, de Villartay J-P. T early alpha (TEA) regulates initial TCRVAJA rearrangements and leads to TCRJA coincidence. Eur. J. Immunol. 2001;31:2080–2086. doi: 10.1002/1521-4141(200107)31:7<2080::aid-immu2080>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Petrie HT, Livak F, Burtrum D, Mazel S. T cell receptor gene recombination patterns and mechanisms: cell death, rescue and T cell production. J. Exp. Med. 1995;182:121–127. doi: 10.1084/jem.182.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livak F, Petrie HT, Crispe IN, Schatz DG. In frame TCR δ gene rearrangements play a critical role in the αβ/γδ T cell lineage decision. Immunity. 1995;2:617–627. doi: 10.1016/1074-7613(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 11.Krangel MS. Gene segment selection in V(D)J recombination: accessibility and beyond. Nature Immunol. 2003;4:624–630. doi: 10.1038/ni0703-624. [DOI] [PubMed] [Google Scholar]
- 12.Mauvieux L, Villey I, de Villartay J-P. TEA regulates local TCR-Jα accessibility through histone acetylation. Eur. J. Immunol. 2003;33:2216–2222. doi: 10.1002/eji.200323867. [DOI] [PubMed] [Google Scholar]
- 13.Villey I, Quartier P, Selz F, de Villartay J-P. Germ-line transcription and methylation status of the TCR-Jα locus in its accessible configuration. Eur. J. Immunol. 1997;27:1619–1625. doi: 10.1002/eji.1830270705. [DOI] [PubMed] [Google Scholar]
- 14.Krangel MS, Schlissel MS. Allelic exclusion, isotypic exclusion, and the developmental regulation of V(D)J recombination, in Molecular Biology of B Cells. In: Alt FW, Honjo T, Neuberger MS, editors. Elsevier Science Ltd.; London: 2004. pp. 127–140. [Google Scholar]
- 15.Whitehurst CE, Chattopadhyay S, Chen J. Control of V(D)J recombinational accessibility of the Dβ1 gene segment at the TCRβ locus by a germline promoter. Immunity. 1999;10:313–322. doi: 10.1016/s1074-7613(00)80031-x. [DOI] [PubMed] [Google Scholar]
- 16.Sikes ML, Meade A, Tripathi R, Krangel MS, Oltz EM. Regulation of V(D)J recombination: A dominant role for promoter positioning in gene segment accessibility. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12309–12314. doi: 10.1073/pnas.182166699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SA, Kingston RE. Disruption of downstream chromatin directed by a transcriptional activator. Genes Dev. 1997;11:3116–3121. doi: 10.1101/gad.11.23.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travers A. Chromatin modification by DNA tracking. Proc. Natl. Acad. Sci. USA. 1999;96:13634–13637. doi: 10.1073/pnas.96.24.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolhuis B, Palstra R-J, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active β-globin locus. Mol. Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 20.Foley KP, Engel JD. Individual stage selector element mutations lead to reciprocal changes in β- vs. ε-globin gene transcription: genetic confirmation of promoter competition during globin gene switching. Genes Dev. 1992;6:730–744. doi: 10.1101/gad.6.5.730. [DOI] [PubMed] [Google Scholar]
- 21.Ohtsuki S, Levine M, Cai HN. Different core promoters possess distinct regulatory activities in the Drosophila embryo. Genes Dev. 1998;12:547–556. doi: 10.1101/gad.12.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullen BR, Lomedico PT, Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984;307:241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- 23.Proudfoot NJ. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986;322:562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- 24.Greger IH, Demarchi F, Giacca M, Proudfoot NJ. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic Acids Res. 1998;26:1294–1301. doi: 10.1093/nar/26.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyes J, Felsenfeld G. Tissue-specific factors additively increase the probability of the all-or-none formation of a hypersensitive site. EMBO J. 1996;15:2496–2507. [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez-Munain C, Sleckman BP, Krangel MS. A developmental switch from TCRδ enhancer to TCRα enhancer function during thymocyte maturation. Immunity. 1999;10:723–733. doi: 10.1016/s1074-7613(00)80071-0. [DOI] [PubMed] [Google Scholar]
- 27.Bert AG, Burrows J, Osborne CS, Cockerill PN. Generation of an improved luciferase reporter gene plasmid that employs a novel mechanism for high-copy replication. Plasmid. 2000;44:173–182. doi: 10.1006/plas.2000.1474. [DOI] [PubMed] [Google Scholar]
- 28.Sun Z, et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 29.Shinkai Y, et al. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993;259:822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 30.McMurry MT, Krangel MS. A role for histone acetylation in the developmental regulation of V(D)J recombination. Science. 2000;287:495–498. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.