Abstract
Objective
To establish whether a diet based on the usage of low-protein products for renal patients (LPP) is associated with higher energy expenditure (EE) than a free low-protein diet (NO-LPP) by calculating 24 h EE by indirect calorimetry using an electronic armband monitor.
Design
Randomized, cross-over, single-blind, pilot clinical trial performed comparing two different low-protein dietary regimens.
Subjects
Forty-two days with LPP and 42 days with NO-LPP regimen in six patients with Parkinson's disease with levodopa.
Methods
Monitoring patient response to two different nutritional schemes through indirect calorimetry (armband), BMI, Patient Global Improvement Scale.
Results
Mean total EE was 1731 ± 265 kcal/day with NO-LPP vs. 1903 ± 265 kcal/day with LPP (p = 0.02).
Conclusions
The usage of LPP increases EE and improves motor function in PD patients to a greater extent than NO-LPP dietary regimen. Calorie intake should be increased to prevent malnutrition in the long-term.
Sponsorship
Fondazione Grigioni per il Morbo di Parkinson.
Keywords: Parkinson's disease, diet, energy expenditure, levodopa
Introduction
Parkinson's disease (PD) is a common movement disorder (worldwide prevalence: 3–4:1000), which develops in the second half of life and is characterized by bradykinesia, rigidity, resting tremor and postural instability (Zhang and Roman 1993; Quinn 1995). The disorder is the result of a neurodegenerative process that leads to the death of dopaminergic neurons in the substantia nigra located in the midbrain. The degenerative process is progressive and inevitably leads to major disability and morbidity associated with high healthcare expenditure (Schapira 1999). Its etiology has not been elucidated; it is believed that the neuronal degeneration is due to a number of environmental factors in genetically susceptible subjects (Sherer et al. 2001). Current therapy is symptomatic and consists in the replacement of dopamine, the neurotransmitter that the degenerated dopaminergic neurons no longer produce, by administering either a precursor of dopamine (levodopa) and/or other compounds that stimulate dopaminergic receptors (dopamine agonists); levodopa is the most effective replacement therapy and sooner or later it is added to the therapeutic regimen of all PD patients (Thanvi and Lo 2004). Also surgical symptomatic therapy exists, namely deep brain stimulation that consists in the stimulation of the damaged neuronal circuits via implanted electrodes; its use is confined to advanced cases that no longer respond to pharmacological therapy (Ahlskog 2001).
Most patients suffering from PD on treatment with levodopa experience frequent postprandial motor blocks, i.e. periods of loss of efficacy of pharmacological treatment, associated with a reduction in quality of life (Thanvi and Lo 2004).
The phenomenon has been ascribed also to the intake of amino acids during a protein-rich meal, which compete with levodopa, a neutral amino acid, for the same carriers during absorption from the gut and passage through the blood–brain barrier. Studies have shown that a low-protein meal at midday improves motor fluctuations and increases ON time (Juncos et al. 1987; Riley and Lang 1988; Carter et al. 1989; Duarte et al. 1993; Simon et al. 2004). Indeed, a low-protein diet is recommended by the guidelines for the management of PD (Olanow et al. 2001; Italian Neurological Society 2003).
In a previous 4-month study (Barichella et al. 2006) a diet with a controlled protein content (0.8 g/kg body weight) was compared with a low-protein diet based on the usage of low-protein food marketed for renal patients. The results showed that consumption of these foods reduced daily time in OFF and enabled a reduction in pharmacological therapy in some cases. A reduction in body weight during the first two months of consumption of the special food was observed. A possible explanation was that the improvement in motor function may have been associated with an increase in energy consumption that was not compensated by adequate calory intake. This hypothesis, however, was not clearly supported by evidence.
The objective of this study was to establish whether a low-protein diet based on the usage of low-protein food for renal patients (LPP) is associated with higher energy expenditure (EE) than a free low-protein diet (NO-LPP) by calculating 24 h EE by indirect calorimetry using an electronic armband monitor (Jakicic et al. 2004).
Methods
This was a randomized, cross-over, single-blind clinical trial performed comparing two different low-protein dietary regimens. It was performed in the month of February 2006.
Six out of the 18 patients (30%) who took part in the previous study with low-protein food for renal patients were included (Barichella et al. 2006). The flow chart of the study is shown in Table I.
Table I.
Study flow-chart.
| Group 1 patients 1-3-5 | LPP | Crossover | NO-LPP |
|---|---|---|---|
| Group 2 patients 2-4-6 | NO-LPP | LPP | |
| Evaluations | Day 0: baseline | Day 7 | Day 14: end of study |
| Body weight | X | X | |
| Height | X | ||
| Body mass index | X | X | |
| Nutritional status | X | X | |
| Mini Mental State examination | X | ||
| Dietary instructions | X | X | |
| PGI | X | X | |
| Diary dispensing | X | X | |
| Diary collection | X | X | |
| Armband | X | X | X |
They were PD patients diagnosed according to Brain Bank Criteria (Hughes et al. 1992) attending the ICP Parkinson Institute, on treatment with levodopa, who were experiencing post-prandial motor blocks of at least 30 min during the 5 h after the midday meal.
The patient population included three women and three men, median age 66 (50;76) years, mean body weight 64.3 ± 11.1 kg, body mass index (BMI) 24.1 ± 2.6 kg/m2, median duration of disease 21 (11;27) years, mean levodopa dosage 579 ± 293 mg/day; all patients were also receiving a dopamine agonist. No patients had dementia. Further details are provided in Table II.
Table II.
Patient population details.
| Patient | Sex | Age | Years of disease | kcal/day: 1st week | kcal/day: 2nd week | Weight (kg) | Height (cm) | BMI (kg/m2) | Levodopa dose (mg/day) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 76 | 21 | 1523 with LPP | 1550 NO LPP | 59 | 151 | 25.9 | 450 |
| 2 | M | 69 | 11 | 1795 NO LPP | 1800 with LPP | 83 | 172 | 28.1 | 1000 |
| 3 | M | 58 | 27 | 1800 with LPP | 1800 NO LPP | 67 | 164 | 24.9 | 500 |
| 4 | F | 64 | 23 | 1660 NO LPP | 1650 with LPP | 54 | 159 | 21.4 | 250 |
| 5 | M | 50 | 11 | 2250 with LPP | 2250 NO LPP | 69 | 177 | 22.1 | 400 |
| 6 | F | 68 | 13 | 1800 NO LPP | 1800 with LPP | 54 | 156 | 22.2 | 875 |
All patients were examined by a physician specialized in nutrition and were interviewed by a dietician at baseline, after a Mini Mental State examination had been performed to exclude dementia. They were also interviewed by a dietician so that she could prepare a dietary regimen tailored to the tastes of the patient in terms of source of protein for the evening meal and sauce for the pasta at midday. Patients were weighed and their height was measured so that their BMI could be calculated. Calory requirements were calculated on the basis of basal metabolism estimated using the formula of Harris and Benedict (1919) and adding 20–30% according to reported physical activity.
Patients were randomized, using a randomization code prepared by the Nutrition service of the Parkinson Institute, to one of two low-protein dietary regimens:
-
—
a low-protein dietary regimen (0.8–1 g/kg ideal body weight) achieved using low-protein food marketed for renal patients (LPP). These products (pasta, bread and milk to be used for breakfast and for lunch) were given to the patient by a physician specialized in nutrition. Their composition in
-
—
a low-protein dietary regimen (0.8–1 g/kg ideal body weight) achieved by diminishing the consumption of protein-rich food and not resorting to the usage of any special kind of food (NO-LPP).
Both dietary regimens provided on average the intake of 31.2 kcal/kg ideal body weight (range 30.0–34.0 kcal/kg), with calories spread out throughout the day; they were both in compliance with the guidelines for healthy nutrition in the Italian population (Guidelines for healthy nutrition 2003).
Patients were given detailed instructions so that direct comparisons between low-protein food and common food could be made (see example of 1800 kcal diet in Table III showing the difference in terms of protein content). Each diet was followed for 7 days before assessments. The content of LPP and common foods used for patient dietary regimens in terms of amino acids competing with levodopa for absorption is provided in Tables IVA and IVB.
Table III.
A comparison between dietary regimen of 1800 kcal with and without LPP products.
| Diet 1800 kcal with LPP | Diet 1800 kcal NO LPP | |||
|---|---|---|---|---|
| Proteins 10.1% | Proteins 13.9% | |||
| Lipids 28.7% | Lipids 26.7% | |||
| Carbohydrates 61.2% | Carbohydrates 59.4% | |||
| Breakfast | ||||
| Tea or coffee | S.Q. | Tea or coffee | S.Q. | |
| Jam | g 25 | Jam | g 25 | |
| Biscuits LPP | g 50 | Biscuits | g 50 | |
| Lunch | ||||
| Pasta LPP | g 80 | Pasta | g 80 | |
| Vegetables | S.Q. | Vegetables | S.Q. | |
| Oil | g 15 | Oil | g 15 | |
| Fruits | g 150 | Fruits | g 150 | |
| Snack | ||||
| Biscuits LPP | g 50 | Biscuits | g 50 | |
| Dinner | ||||
| Bread | g 50 | Bread | g 50 | |
| Vegetables | S.Q. | Vegetables | S.Q. | |
| Oil | g 15 | Oil | g 15 | |
| Fruits | g 150 | Fruits | g 150 | |
| Monday | Meat g 150 | Monday | Meat g 150 | |
| Tuesday | Cheese g 100 | Tuesday | Cheese g 100 | |
| Wednesday | Fish g 250 | Wednesday | Fish g 250 | |
| Thursday | Two eggs | Thursday | Two eggs | |
| Friday | Fish g 250 | Friday | Fish g 250 | |
| Saturday | Legumes g 100 | Saturday | Legumes g 100 | |
| Sunday | HAM g 100 | Sunday | HAM g 100 | |
| Diet 1800 kcal with LPP | Diet 1800 kcal with NO LPP | |||
| Proteins (g) | Percentage of protein (percentage total kcal) | Protein (g) | Percentage of protein (percentage of total kcal) | |
| Breakfast | 0.50 | 0.16 | 3.55 | 0.80 |
| Lunch | 4.75 | 1.00 | 16.23 | 3.60 |
| Snack | 0.50 | 0.16 | 2.51 | 0.61 |
| Dinner | 39.72 | 8.82 | 39.72 | 8.82 |
| Total value | 45.49 | 10.10 | 62.01 | 13.83 |
Table IVA.
A comparison in protein content between LPP and common foods.
| LPP products | Proteins (g/100 g) | kcal/100 g | Common foods | Proteins (g/100 g) | kcal/100 g |
|---|---|---|---|---|---|
| Breakfast | |||||
| Semi-sweet biscuits | < 1 | 449 | Semi-sweet biscuits | 6.6 | 418 |
| Plain biscuits | < 1.35 | 488.5 | Plain biscuits | 7.4 | 493 |
| Wafers | < 1.2 | 539.5 | Wafers | 7.1 | 454 |
| Lunch | |||||
| Breadsticks (grissini) | < 1.4 | 419 | Breadsticks (grissini) | 12.3 | 433 |
| Toasted bread | < 1.6 | 399 | Rice | 7 | 362 |
| Melba toast | < 1 | 421 | Melba toast | 11.3 | 410 |
| Pasta | < 0.7 | 354 | Pasta | 10.8 | 356 |
Table IVB.
Comparison between LPP and common foods in terms of content in aminoacids competing with levodopa for absorption.
| LPP products | Phe (mg/100 g) | Tyr (mg/100 g) | Trp (mg/100 g) | Leu (mg/100 g) | Iso (mg/100 g) | Val (mg/100 g) | Common foods* | Phe (mg/100 g) | Tyr (mg/100 g) | Trp (mg/100 g) | Leu (mg/100 g) | Iso (mg/100 g) | Val (mg/100 g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breakfast | Breakfast | ||||||||||||
| Semi-sweet biscuits | < 60 | < 60 | – | – | – | – | Semi-sweet biscuits | 644 | 462 | 133 | 1141 | 625 | 710 |
| Plain biscuits | < 80 | < 55 | – | – | – | – | Plain biscuits | – | – | – | – | – | – |
| Wafers | < 45 | < 35 | – | – | – | – | Wafers | – | – | – | – | – | – |
| Lunch | Lunch | ||||||||||||
| Breadsticks (grissini) | < 40 | < 20 | 7.7 | 36.1 | 18.7 | 26.8 | Breadsticks (grissini) | – | – | – | – | – | – |
| Toasted bread | < 50 | < 50 | 8.4 | 39 | 20.2 | 28.9 | Rice | 360 | 228 | 84 | 590 | 306 | 438 |
| Melba toast | < 60 | < 40 | 8.6 | 41.7 | 21.9 | 30.7 | Melba toast | 595 | 297 | ||||
| Pasta | < 30 | < 30 | 3.7 | 60.3 | 19.6 | 26.3 | Pasta | 542 | 310 | 105 | 834 | 455 | 544 |
From: “Tables of food composition” Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione.
All assessments were made by staff blind to the dietary regimen of the patient, who was instructed not to mention it to the examiners.
Patients were given study diaries and were instructed to write down the following information everyday: hours of sleep; waking hours in ON, i.e. times when medication was working and motor symptoms are controlled (with and without dyskinesias) and hours in OFF, i.e. times when the medication was not working and symptoms reappeared; time of antiparkinsonian drug intake; time of meals; any deviations from the dietary regimen.
An armband (Bodymedia Sensewear Pro2) was positioned on the right triceps of the patients for the whole 14 day period (24 h per day) of the study, so that it could measure EE continuously. The SenseWear Pro Armband™ (Body Media, Pittsburgh, PA) is a newly developed commercially available device to assess EE. It has already been extensively used for research purposes and its use has been validated not only for usage in sports medicine (Fruin and Rankin 2004) and in particular environments, such as under water, but also during normal daily activity (Mignault et al 2005; Levine and Foster 2005). The device is worn on the right upper arm over the triceps muscle and monitors various physiological and movement parameters. Data from a variety of parameters including heat flux, accelerometry, galvanic skin response, skin temperature, near-body temperature and demographic characteristics (gender, age, height and body weight) are used to estimate EE utilizing proprietary equations developed by the manufacturer. Due to its lightness and wear ability, the armband monitor is particularly suitable for continuous patient monitoring for several days. The software data analysis was carried out at the end of each of the 7 days of the dietary regimen period.
At the end of each dietary regimen the patient global improvement (PGI) questionnaire was given to the patients, who completed it by themselves. The PGI served as an independent, yet patient-based assessment of a treatment effect.
The primary endpoint was EE. The secondary endpoints were: 24 hOFF time, 24 h ON time with and without dyskinesias.
The statistical analysis compared data related to days on balanced diet with data related to days on LPP diet using ANOVA.
Diary cards were coded with the number of the patient and the allocated sequence (AB or BA). The person who analyzed the data was blind to sequence.
Results
All six patients completed the study as per protocol and provided 84 valid diaries, 42 with LPP and 42 with NO-LPP regimen.
Diary results
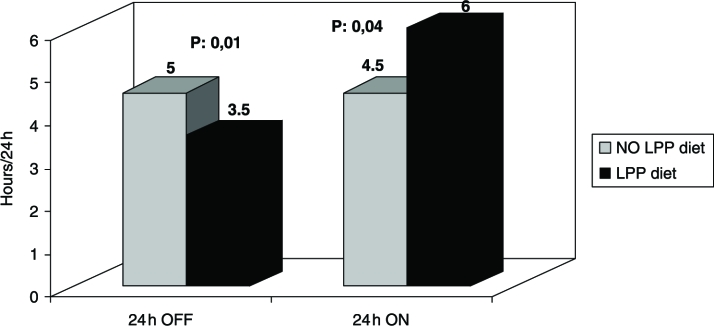
Twenty-four hours OFF time was significantly shorter after LPP diet than after NO-LPP diet (3.5 h vs. 5 h, p = 0.01); 24 h dyskinetic ON time was significantly longer after LPP diet (6 h vs. 4.5 h, p = 0.04) (Figure 1).
Figure 1.
Diary results: 24 h OFF time is significantly shorter after LPP diet than after NO-LPP diet (P = 0.01); 24 h dyskinetic ON time is significantly longer after LPP diet (P = 0.04).
Armband results
The armband was worn by the patients for 98% of the time in both the evaluation periods associated with the two nutritional schemes.
The daily hours of sleep were similar in the two groups (7.68 ± 1.94 with NO-LPP vs. 8.02 ± 2.2 h with LPP). These results are consistent with the sleep hours estimated from the patient diary analysis.
An increase in total EE of about 10% was noticed for the LPP dietary regimen compared to the NO-LPP diet: mean total EE was 1731 ± 265 kcal/day with NO-LPP diet vs. 1903 ± 265 kcal/day with LPP (p = 0.02). Also the time spent in physical activity was longer with LPP than with NO-LPP diet (1.75 ± 1.33 vs. 1.38 ± 1.32 h; p = 0.05).
PGI results
According to PGI questionnaires, all patients expressed a benefit with LPP regimen (very much better n = 2, much better n = 4) while no benefit or worsening were expressed with the NO-LPP diet.
Discussion
This study shows that the LPP dietary regimen is associated with a significant increase in EE in fluctuating PD patients, as measured by the armband. This finding is consistent with the additional evidence of improvement in motor function in such patients, expressed as a significant reduction in 24 h OFF time, according to both armband and patient diary data.
The only difference between the two low-protein dietary regimens (one using low-protein food for renal patients and the other being a free low-protein diet) was the midday meal protein content (with lower protein content for the low-protein product food nutritional scheme); the protein intake in the evening meal was the same. It appears that the increase in EE and greater improvement in motor function with LPP was due to better absorption of levodopa at midday, less hindered by lower protein intake. Calorie intake and hours of sleep were similar in the two groups and should not have influenced results. Furthermore, these results suggest that the evening meal does not play an important role in determining motor performance.
An additional finding was the increase in ON periods with dyskinesias, according to the patient diary data. The output data of the armband did not enable us to address the issue of whether the higher EE comes from an increase in physiological physical activity or in dyskinesias. Accurate tuning of the algorithm elaborating the accelerometer signals should be implemented to be able to distinguish between the two in subsequent studies. In any case, the improvement in PGI suggests that the increase in dyskinesias did not counteract the benefit of improved motor function. Indeed, dyskinesias do not always cause disability and actually show that levodopa is being absorbed and is effective. In addition, it is well known that fluctuating patients (motor fluctuations were an inclusion criterion) prefer dyskinesias to OFF episodes, which were another inclusion criterion, as patients had to have postprandial OFF episodes (Palmer et al. 2000).
The improvements achieved in OFF and ON time are consistent with those recorded in the previous study (Barichella et al. 2006), in which a diet with a controlled protein content (0.8 g/kg body weight) was compared with a low-protein diet based on the usage of low-protein food marketed for renal patients.
A key issue in this study is the subjectivity of the patient diary data and the novelty of the armband used for the measurement of the primary endpoint, EE. The use of patient diariesis a generally accepted method by regulatory authorities for the assessment of medicinal products (EMEA, CPMP/EWP/563/1995). Sensewear Pro2 has already been extensively used for research purposes and its use has been validated not only for usage in sports medicine and in particular environments, such as under water, but also during normal daily activity (Mignault et al. 2005; Levine and Foster 2005). Consequently, its measurement may be considered to be reliable. At present no alternative methods and devices for medium-term daily EE monitoring are available.
It would have been useful to measure the blood levels of levodopa and of the amino acids that compete with the drug, as this would enable us to understand where the competition occurs (at the blood–intestine and/or blood–brain barrier). However, the primary objective of our study was different, namely to establish whether the body weight loss that occurs during LPP consumption is due to greater EE or not; our secondary objectives were to establish whether the ON periods associated with dyskinesias increase and whether the evening meal has an influence or not. We have already planned another study focusing on amino acid/levodopa competition, in which blood levels will be measured.
Thus, the findings of this study suggest that the consumption of LPP for renal patients is a simple way to improve the therapeutic efficacy of levodopa, which does not appear to have any important drawbacks, as dyskinesias are not a major problem and malnutrition can easily be prevented by increasing calory intake. Its rationale is based on the recommendation to reduce protein intake at midday in order to prevent their interference with levodopa absorption associated with postprandial OFF episodes, which is included in international guidelines for the management of PD (Olanow 2001; Italian Neurological Society 2003). Indeed, PD patients should consume these products, which have been on the market for more thana decade, only at midday and not throughout the day as renal patients (Kopple, 2001), so the overall risk of malnutrition in PD patients is lower than in renal patients.
In conclusion, the consumption of renal LPP is associated with an improvement in motor function and an increase in EE in PD patients to a greater extent than NO-LPP dietary regimen alone. The increase in EE should be taken into account for the overall management of PD patients: calorie intake should be increased to prevent malnutrition in the long-term.
Acknowledgments
We wish to thank the Jennifer Hartwig, MD, for assistance in drafting the manuscript; the Fondazione Grigioni per il Morbo di Parkinson for financial support; and Aproten Heinz-Italia for supplying the low-protein products free of charge.
References
- Ahlskog JE. Parkinson's disease: Medical and surgical treatment. Neurol Clin. 2001;19:579–605. doi: 10.1016/s0733-8619(05)70036-0. [DOI] [PubMed] [Google Scholar]
- Barichella M, Marczewska A, De Notaris R, Vairo A, Baldo C, Mauri A, Savardi C, Pezzoli G. Special low-protein foods ameliorate postprandial off in patients with advanced Parkinson's disease. Mov Disord. 2006;21(10):1682–1687. doi: 10.1002/mds.21003. [DOI] [PubMed] [Google Scholar]
- Carter JH, Nutt JG, Woodward WR, Hatcher LF, Tratman TL. Amount and distribution of dietary protein affects clinical response to levodopa in Parkinson's disease. Neurology. 1989;39(4):552–556. doi: 10.1212/wnl.39.4.552. [DOI] [PubMed] [Google Scholar]
- Duarte J, Moreno C, Coria F, Perez A, Claveria LE. Efficacy of the proteic redistribution diet (PRD) in the antiparkinsonian effect of L-dopa. Neurologia. 1993;8(8):248–251. [PubMed] [Google Scholar]
- Fruin ML, Rankin JW. Validity of a multi-sensor armband in estimating rest and exercise energy expendiure. Med Sci Sports Exerc. 2004;36(6):1063–1069. doi: 10.1249/01.mss.0000128144.91337.38. [DOI] [PubMed] [Google Scholar]
- Harris J, Benedict F. Abiometric study of basal metabolism in man. Washington DC: Carnegie Institute of Washington; 1919. [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinicopathologic study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italian Neurological Society. Italian society of clinical neurophysiology. Treatment of Parkinson's disease. Neurol Sci. 2003;24(Suppl 3):S165–S213. doi: 10.1007/s100720300068. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Marcus M, Gallagher KI. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004 May;36(5):897–904. doi: 10.1249/01.mss.0000126805.32659.43. 2004. [DOI] [PubMed] [Google Scholar]
- Juncos JL, Fabbrini G, Mouradian MM, Serrati C, Chase TN. Dietary influences on the antiparkinsonian response to levodopa. Arch Neurol. 1987;44(10):1003–1005. doi: 10.1001/archneur.1987.00520220009006. [DOI] [PubMed] [Google Scholar]
- Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001;37(1 Suppl 2):S66–S70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- Levine JA, Foster LM. Interindividual variation in posture allocation: Possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- Mignault D, Onge MS, Karelis AD. Evaluation of the Portable HealthWear Armband. Diabetes Care. 2005;28:225–227. doi: 10.2337/diacare.28.1.225-a. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture and Forest Policy, National Institute of Research on Food and Nutrition. Guidelines for healthy nutrition. 2003 www.inran.it.
- Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson's disease (2001): Treatment guidelines. Neurology. 2001;56(11 Suppl 5):S1–S88. doi: 10.1212/wnl.56.suppl_5.s1. [DOI] [PubMed] [Google Scholar]
- Palmer CS, Schmier JK, Snyder E, Scott B. Patient preferences and utilities for off-time outcomes in the treatment of Parkinson's disease. Qual Life Res. 2000;9:819–827. doi: 10.1023/a:1008903126315. [DOI] [PubMed] [Google Scholar]
- Quinn N. Parkinsonism—recognition and differential diagnosis. BMJ. 1995;310:447–452. doi: 10.1136/bmj.310.6977.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D, Lang AE. Practical application of a low-protein diet for Parkinson's disease. Neurology. 1988;38(7):1026–1031. doi: 10.1212/wnl.38.7.1026. [DOI] [PubMed] [Google Scholar]
- Schapira AHV. Parkinson's disease. BMJ. 1999;318:311–314. doi: 10.1136/bmj.318.7179.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Greenamyre JT. The pathogenesis of Parkinson's disease. Curr Opin Investig Drugs. 2001;2:657–662. [PubMed] [Google Scholar]
- Simon N, Gantcheva R, Bruguerolle B, Viallet F. The effects of a normal protein diet on levodopa plasma kinetics in advanced Parkinson's disease. Parkinsonism Relat Disord. 2004;10(3):137–142. doi: 10.1016/j.parkreldis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Thanvi BR, Lo TCN. Long termmotor complications of levodopa: Clinical features, mechanisms, and management strategies. Postgrad med J. 2004;80:452–458. doi: 10.1136/pgmj.2003.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZX, Roman GC. Worldwide occurrence of Parkinson's disease: A review. Neuroepidemiology. 1993;12:195–208. doi: 10.1159/000110318. [DOI] [PubMed] [Google Scholar]