Abstract
Five ocean vessels were investigated for the characterization and quantification of gaseous compounds emitted during ocean transportation of wood pellets in closed cargo hatches from Canada to Sweden. The study was initiated after a fatal accident with several injured during discharge in Sweden. The objective with the investigation was to better understand the off-gassing and issues related to workers' exposure. Air sampling was done during transport and immediately before discharge in the undisturbed headspace air above the wood pellets and in the staircase adjacent to each hatch. The samples were analyzed with Fourier transform infrared spectroscopy and direct reading instruments. The following compounds and ranges were detected in samples from the five ships: carbon monoxide (CO) 1460–14650 ppm, carbon dioxide (CO2) 2960–21570 ppm, methane 79.9–956 ppm, butane equivalents 63–842 ppm, ethylene 2–21.2 ppm, propylene 5.3–36 ppm, ethane 0–25 ppm and aldehydes 2.3–35 ppm. The oxygen levels were between 0.8 and 16.9%. The concentrations in the staircases were almost as high as in the cargo hatches, indicating a fairly free passage of air between the two spaces. A potentially dangerous atmosphere was reached within a week from loading. The conclusions are that ocean transportation of wood pellets in confined spaces may produce an oxygen deficient atmosphere and lethal levels of CO which may leak into adjacent access spaces. The dangerous combination of extremely high levels of CO and reduced oxygen produces a fast-acting toxic combination. Measurement of CO in combination with oxygen is essential prior to entry in spaces having air communication with cargo hatches of wood pellets. Forced ventilation of staircases prior to entry is necessary. Redesign, locking and labeling of access doors and the establishment of rigorous entry procedures and training of onboard crew as well as personnel boarding ocean vessels are also important.
Keywords: confined space, emissions, exposure measurements, oxygen deficiency, seamen
INTRODUCTION
The use of wood pellets as an energy source for domestic, municipal and industrial use has increased dramatically during the last few years. A direct consequence of this is that ocean transportation of the commodity in large bulk has become commonplace, and in 2007, ∼40% of the total global wood pellet production of 9 million tons underwent shorter or longer ocean transportation (Melin, 2007). The majority of wood pellets are produced by milling wood chips, bark, planer shavings or sawdust into a fine powder, which after drying is compressed into pellets of 6- or 8-mm diameter. The dense wood pellet has moisture content of 10% or less. The pellets described in this paper were made from sawdust and planer shavings from Lodgepole pine (Pinus Contorta).
The risks associated with the transportation of seemingly harmless cargo such as wood and wood products, including wood pellets, are neither well-known nor properly understood. This study was initiated after a fatal accident onboard a vessel in the Port of Helsingborg, Sweden, in November 2006, which occurred while the vessel was discharging wood pellets from British Columbia, Canada. One seaman was killed, a stevedore was seriously injured and several rescue workers were slightly injured after entering an unventilated stairway next to a cargo hold. The dead seaman was removed after ∼15 min. The injured stevedore lost consciousness and was pulseless when removed from the contaminated atmosphere after ∼10 min. He was treated with hyperbaric oxygen but his condition deteriorated with the onset of neurological symptoms that later proved to be caused by a cerebral vascular lesion which eventually left him bound to a wheel chair. It was first believed that the workers were affected by lack of oxygen, but on arrival at the hospital, it was concluded that they had also been exposed to carbon monoxide (CO).
The vessels used for these 7- to 9-week ocean voyages are classified as box-shaped dry-cargo vessels and used for transport of pulp, lumber, wood pellets etc. in the Pacific as well as in the Atlantic Oceans. Wood pellets have been transported between British Columbia and European ports since 1997. In 2002, one stevedore died and several other workers were injured in the Port of Rotterdam under similar circumstances, but to our knowledge, that accident has not been reported in the scientific literature, nor has there been any systematic quantification of gas emissions during other shipments of wood pellets.
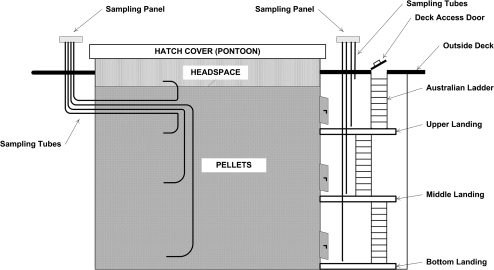
A typical vessel of this type has up to 10 cargo holds, each of which can hold ∼3000 tons of wood pellets. Adjacent to the each hold is an access stairway (Australian ladder type) leading from the outside deck down to the bottom of the vessel, see Fig. 1. This ladder is entered through a deck-access door. The stairway has three landings, each equipped with a door in to the cargo hold. These doors are closed and sometimes taped during the voyage. Wood pellets must at all times be protected from water and the hatch covers (pontoon type) and the stairway access doors on deck are closed during the voyage. The doors are labeled on the outside and sometimes on the inside with warnings of a possible oxygen-deficient atmosphere, but there is no reference to the potential presence of hazardous gases.
Fig. 1.
Schematics of sampling points in the cargo hold and stairway on Vessel #4.
At the time of the fatal accident in the Port of Helsingborg, no air measurements had been taken prior to entering the stairway, contrary to the usual routine of making an oxygen check. The deck-access door had been left open for ∼8 h on the previous day while another cargo hold was discharged. However, due to the risk of rain, all doors were closed after the shift ended at 22:00 and opened again at 05:45 the next morning, the day of the accident. The accident occurred around 08:00.
It has previously been reported that CO and other one-carbon compounds, such as methanol, formic acid and formaldehyde, are emitted from wood pellets during storage in warehouses (Svedberg et al., 2004). The authors also reported emissions of aldehydes, predominantly hexanal and pentanal, which they suggested were formed by a radical-induced oxidative degradation of natural lipids, particularly the polyunsaturated linoleic acid. The biochemical mechanism by which the one-carbon compounds were formed was not clear but again the oxidation of fatty acids and other components in the wood seemed likely. The oxidative processes occurred below room temperature but were accelerated by elevated temperature.
The immediate objective of the present study was to investigate and describe the composition and concentrations of gaseous compounds in the stairways and cargo holds of vessels carrying wood pellets, in order to better understand the off-gassing and issues related to the exposure of the workers.
METHODS
Five different vessels carrying wood pellets from British Columbia were investigated after arrival in the Port of Helsingborg, Sweden. Before the hatch covers were removed, air samples were collected through a small side door leading to the headspace air above the pellet bed. Air samples were collected 2 days after the accident from an undisturbed cargo hold from the vessel on which the fatal accident took place (Vessel #1). On Vessel #3 and #5, samples were also collected in the undisturbed air of the respective stairways by lowering sampling tubes through a slightly opened access door. The samples were collected by pumping the air into Tedlar sample bags which were analyzed immediately on the dockside in a mobile laboratory installed in a minivan or within the next few days in one of our laboratories in Sweden. Vessel #4 was sampled by pumping the air through long nylon tubing directly to the analytical cell in the mobile laboratory. All samples were analyzed using Fourier transform infrared spectroscopy (FTIR) instruments (Bomem MB-series, Bomem, Quebec, Canada), equipped with gas cells (White type) and nitrogen-cooled mercury–cadmium–telluride detectors. The FTIR technique detects gaseous compounds in the 2.5- to 11.5-μm infrared wavelength region. The concentrations of CO, carbon dioxide (CO2) and methane (CH4) in the spectra were analyzed by fitting a set of calibration spectra based on the Hitran2000 spectral database (Griffith, 1996; Rothman et al., 2003). Alkanes (CnH2n+2), ethylene, propylene, methanol and propanal concentrations were retrieved using the PNNL database (Sharpe et al., 2004). The uncertainty in the concentration retrievals is made up of the absolute uncertainty in the absorption cross sections in the databases, and the uncertainty in the fitting procedure itself. For CO, CO2, CH4, ethylene and alkanes (C3 and higher), the overall uncertainty was 5%, for propylene 6% and 8% for methanol. Ethane and propanal concentrations were associated with a 15% uncertainty in the present study. Alkanes were retrieved in the 3.2- to 3.7-μm wavelength region, where the absorption is due to the C–H bond. The specificity among the different alkanes are thus somewhat limited, but the total alkane mass can be retrieved well, fitting a set of different alkanes. Propane and pentane were used in the fit, whereas the total alkane mass is presented as butane equivalents.
Upon discharge of the wood pellets, oxygen was measured using a direct-reading, hand-held instrument, RKI-GX-2001 (Riken Keiki Co. Ltd, Tokyo, Japan), with a galvanic cell sensor, calibrated with ambient air and 12% oxygen span gas.
In an attempt to describe the dynamics of the off-gassing during the voyage, a system of sampling points was installed in one cargo hold and the respective stairway prior to the loading of Vessel #4, schematically outlined in Fig. 1. Sampling could thus be carried out from two sample panels without opening the deck-access door and hatch cover. Measurement of CO and oxygen were taken three times a day by the vessel's crew during the ocean transport, using a gas meter, model ATX620 (Industrial Scientific, Oakdale, PA, USA), equipped with an electrochemical sensor and calibrated with zero-gas (20.9% O2 and 79.1% N2) and 100 ppm CO.
Oxygen depletion in a cargo hold is believed to be caused by several mechanisms. Corrosion consumes oxygen. Microbiological activity both consumes oxygen and produces CO2. Oxygen may also dissolve in water. However, our belief is that the primary mechanisms for oxygen depletion in wood pellets are triggered by oxidative reactions with wood constituents.
Linoleic acid (CAS 60-33-3) is the predominant unsaturated fatty acid in wood and presumably also in wood pellets. It is difficult to buy the pure fatty acids present in wood in larger quantities, but they are readily available in mixture in commercial linseed oil. The major constituents of the linseed oil sold in Sweden are the unsaturated fatty acids linolenic acid (CAS 463-40-1) ∼56%, oleic acid (CAS 112-80-1) ∼18% and the above mentioned linoleic acid ∼15.4%. To explore the hypothesis that oxidative degradation of fatty acids occurs and to identify gaseous emissions, a simple experiment was staged where the headspace air of common raw linseed oil was investigated. A sealed 1-l plastic bottle containing linseed oil was acquired from a paint shop. The bottle showed clear signs of deflation, indicating oxygen depletion in the headspace air. With an airtight syringe, a sample of headspace air was collected and analyzed, without dilution, in a 50-ml FTIR gas cell with a 1-m optical path (Axiom Analytical Inc., Irvine, CA, USA). The oxygen level in the bottle was measured with the hand-held instrument, RKI-GX-2001.
Hydrogen sulfide, (H2S), is formed during anaerobic bacterial decomposition of the sulfurous material in organic matter. The FTIR method used in this project was not sensitive enough to measure hydrogen sulfide in levels <100 ppm. Screening measurements with H2S monitors prior to this study indicated no H2S in a cargo of wood pellets and it was therefore omitted from further evaluation unless its presence was indicated in the FTIR spectra.
RESULTS
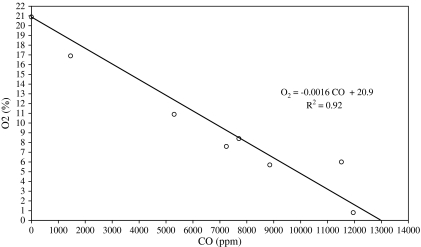
The results from the measurements aboard the vessels and from the headspace of the linseed oil are summarized in Table 1. Particularly notable are the high CO and CO2 concentrations and the low oxygen levels. No indication of the presence of H2S was seen. When the oxygen level from each vessel was plotted as function of the CO level, a high degree of correlation was obtained (r2) = 0.92, see Fig. 2. Good correlation was also found between CO and butane equivalents (r2 = 0.93) and CO2 and propylene (r2 = 0.98).
Table 1.
Summary of all measurements from each vessel and headspace of linseed oil
| Vessel #1 Hold |
Vessel #2 Hold |
Vessel #3 Hold |
Vessel #3 Stair |
Vessel #4 Staira |
Vessel #5 Hold 2 |
Vessel #5 Stair 2 |
Vessel #5 Hold 4 |
Vessel # 5 Stair 4 |
Vessel #5 Hold 6 |
Vessel #5 Stair 6 |
Linseed Oil Headspace |
|
| November 2006 | January 2007 | February 2007 | February 2007 | March 2007 | October 2007 | October 2007 | October 2007 | October 2007 | October 2007 | October 2007 | ||
| CO | 5850 | 6980 | 14 650 | 10 960 | 11 510 | 11 950 | 7710 | 7240 | 1460 | 8860 | 5300 | 1090 |
| CO2 | 9340 | 3240 | 7070 | 5450 | 5160 | 21 570 | 12 360 | 17 430 | 2960 | 18 820 | 8690 | 570 |
| Methane | 614 | 216 | 632 | 468 | 204 | 956 | 589 | 388 | 79.9 | 454 | 246 | 40 |
| Ethylene | 12.2 | 5.3 | 9.7 | 7.4 | 3.3 | 21.2 | 12.3 | 11.2 | 2.0 | 11.5 | 5.9 | 2.0 |
| Propylene | 12.8 | 7.6 | 12.3 | 9.3 | 8.0 | 36 | 21.4 | 28.3 | 5.3 | 32.6 | 15.9 | <0.5 |
| Methanol | 2.2 | 0.2 | 0.5 | 0.4 | 0.7 | 4.5 | 1.4 | 16.9 | 2.1 | 16.6 | 3.2 | 1.1 |
| Propanal (aldehydes) | 14 | 6.9 | 4.8 | 6.0 | 2.7 | 19 | 8.8 | 29 | 2.3 | 35 | 7.8 | 120 |
| Ethane | 18 | 32 | <5.0 | <5.0 | 15 | 23 | 13 | 25 | 9.2 | 32 | 15 | 380 |
| Butane equivalentsb | 237 | 275 | 842 | 626 | 591 | 512 | 321 | 265 | 63 | 393 | 219 | 83 |
| Oxygen % | 6% | |||||||||||
| 5%c | 0.8% | 8.4% | 7.6% | 16.9% | 5.7% | 10.9% | 7.0% |
All components except oxygen were measured with FTIR spectroscopy. All values in ppm except oxygen which is in %.
The results from Vessel #4 are means from measurements at four different levels of the stairway.
Sum concentration of alkanes (CnH2n+2) with three or more carbons expressed as ppmv butane equivalents.
Parallel reading from head space in cargo hold.
Fig. 2.
The correlation between oxygen and CO concentrations in hatches and stairways. The point representing 20.9% oxygen and 0% CO is added manually.
The aldehyde concentrations aboard the vessels were much lower than in previous findings in pellet warehouses. The qualitative analysis of headspace air above linseed oil revealed the presence of all the gas species found in the cargo holds and the reduction of the oxygen level was similar. The relative concentrations of aldehydes and ethane were higher.
The concentrations in the stairways were considerably lower than the levels found in the adjacent cargo holds, (Vessel #3 and #5). The stairway on Vessel #4 was sampled on four different levels, showing <1% stratification throughout the air column of the stairway.
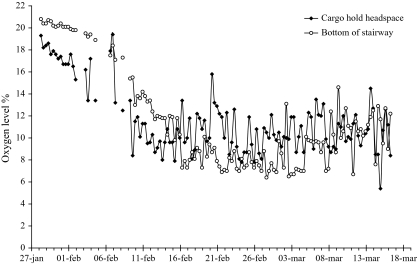
The results from the measurements aboard the vessels of oxygen at the bottom of the stairway and in the headspace of the cargo hold on Vessel #4 are described in Fig. 3. A steady state with ∼10% oxygen level was reached after ∼3 weeks at sea. The CO level in the headspace of the hold reached 900 ppm after 7 days. The onboard CO instrumentation was found to be unreliable over 1000 ppm and further evaluation of this parameter was not possible until the FTIR analysis was available at the time the cargo was discharged in the Port of Helsingborg.
Fig. 3.
The oxygen level in cargo hold headspace and bottom of stairway measured during ocean voyage (Vessel #4).
While Vessel #4 was discharging, the decay of CO at the bottom level of the stairway was monitored at 2.5-min intervals over a period of 2 h. The sample air was pumped to the mobile FTIR unit located alongside the vessel. Initially, the stairway deck-access door was closed and almost no decay could be seen. The door was then opened to simulate the usual self-ventilation procedure. Over the next 45 min, an exponential decay (r2 = 1.00) was recorded corresponding to a ventilation rate of 0.23 air exchanges per hour. In order for the CO concentration at the bottom level of the stairway to reach an acceptable 8-h occupational exposure limit of 35 ppm according to Swedish standards, 26 h of ventilation would have been required.
DISCUSSION
The results from this study strongly suggest that immediate action needs to be taken in the maritime industry to prevent fatal accidents aboard cargo vessels. The extremely high CO concentrations and low oxygen levels found in the stairways are the most important findings from the point of view of workers’ health. The combined toxic effect of CO and low oxygen content is the most likely explanation for the rapid course of events leading to the tragic fatality and severe injury aboard Vessel # 1. The recorded CO2 concentrations were occasionally above accepted short-term occupational exposure limits but are not considered life threatening by itself, although synergistic health effects with CO and O2 may exist. The other chemicals identified were below or well below accepted exposure limits. The low aldehydes’ levels compared to previous findings in warehouses can probably be explained by the fact that this particular emission decreases with time more rapidly than the others, and the several reloads of the pellets between production and final discharge.
Wood pellets undergo drying and heat treatment during processing, which may alter the chemical composition of the fatty acids in the wood. However, the results from the headspace analysis of linseed oil support the hypothesis that oxidative degradation of fatty acids occurs, and may explain at least part of the formation of gaseous compounds identified in headspace above wood pellets. The formation of CO explains only a fraction of the oxygen depletion. The majority of the oxygen is believed to be chemically bound in the oxidative degradation process of fatty acids and other organic materials present in wood. The recording of 0.8% oxygen in hold 2 on Vessel #5 shows that wood pellets may trap almost all available oxygen. One possible explanation for the fact that such low levels were not seen in every hold is that this particular hold may have been well sealed, with limited air communication to ambient air.
It is believed that the mechanisms behind oxygen depletion and the formation of gaseous compounds are quite complex and a deeper analysis is beyond the scope of this study. The high correlation found between several of the gaseous compounds may provide additional leads to an understanding of these mechanisms. In spite of its limitations, the information obtained from the experimental study with linseed oil provides important information, which may be useful in designing future studies of these processes.
CO is an odorless and colorless gas. CO binds to hemoglobin (Hb) forming a COHb complex that inhibits the ability of hemoglobin to bind oxygen. The level of CO saturation in the blood is measured as % COHb. Normal levels are 0.3–0.8% COHb, smokers have 3–8% COHb and most people who die from CO poisoning have >60% COHb (NAS/COT, 2005) The injured stevedore in the accident on Vessel #1 had 43.8% COHb when admitted to the hospital. For the working population, a maximum of 3.5% COHb at the end of shift and 20 ppm in end-exhaled air is suggested (ACGIH, 2007).
The occupational exposure limits for CO by inhalation in Sweden is 35 ppm for 8 h and 100 ppm for 15 min (Arbetarskyddsstyrelsen, 2005). The current ACGIH's time weighted average is 25 ppm (ACGIH, 2007). A 10-min exposure at 1700 ppm or 30-min exposure at 600 ppm has been proposed as the airborne concentration of CO, above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death (NAS/COT, 2005). This proposal assumes a normal oxygen level. With reference to Fig. 2, it is seen that a predicted CO level of 600 ppm is reached when the oxygen level is 19.9%, a condition reached after only 3 to 4 days at sea on Vessel #4.
Scientific information is readily available regarding the toxic effects of CO exposure and oxygen deficiency when studied separately. However, the information about any combined effects is very sparse and appears to be limited to remarks about possible effects at higher altitudes (WHO, 1999). CO binds competitively to hemoglobin ∼200 times more strongly than oxygen. It is likely that reduced oxygen partial pressure increases the available sites for CO binding and a toxic effect is caused more quickly and at lower CO concentrations. Physical activity will increase the uptake of CO. The US National Institute for Occupational Safety and Health defines an oxygen-deficient atmosphere as one with an oxygen partial pressure of <132 torr, equivalent to ∼17.2% oxygen at sea level. Above this level, no physiological effects are expected as a result of oxygen deficiency. A minimum of 19.5% oxygen level is recommended for most work situations and provides a margin of safety (ACGIH, 2007). This recommendation is only applicable if there are no other toxic gases present, such as CO.
Since no wood pellets were present and no corrosion was discovered in the stairways at discharge, which could have presented an alternative mechanism for oxygen depletion, it was obvious that gas moved quite freely between the cargo holds and the related stairways. The concentrations in the stairways were lower than in the cargo holds but still remained at very high levels. The results from one stairway showed that there was no stratification of concentrations; however, defective seals around doors may in other cases increase leakage and generate low levels at the top and toxic levels at the bottom of a stairway, creating a potential danger on entry. The difference in gas concentrations observed between the cargo holds on Vessel #5 is most probably an indication of such variation in gas leakage to ambient air, rather than any differences in emission rates.
The vessels’ instruction manuals specified 2 h of self-ventilation prior to entry to the stairways. The slow decay of CO, which we recorded, shows that this is not sufficient. The vessels that arrived after the accident, introduced various methods to ventilate their stairways. From observation, it was clear that some crews were unaccustomed to the procedures and had difficulties due to the use of inappropriate, heavy and bulky equipment. The working space was frequently cramped, particularly when a ventilation hose was temporarily inserted and connected to a fan unit placed near the deck-access door.
Before entering the stairway, oxygen and CO were measured by the crew, by lowering a meter to the upper landing of the stairway. The lower landings could not be ventilated until it was deemed safe to enter the upper landing, whereupon a crew member was sent down with the ventilation equipment to proceed to the next level. In an unfortunate situation, pockets of toxic gases could be released into a previously cleared workspace. Understanding of the time required to clear the toxic gases was the subject of significant uncertainty among the crew as was the understanding that if a ventilated stairway is closed before the cargo of wood pellets is discharged it must be ventilated and monitored again before anyone enters it.
The combined observations indicate the need for the installations of well-designed, automatic, mechanical ventilation systems for the stairways. Such systems would significantly increase the safety of the workers and prevent loss of expensive time in port. The most appropriate type of ventilation for stairways is probably a balanced-type ventilation with mechanical supply and exhaust air, thus, limiting the risks of pressure differences in relation to the cargo holds. The second best solution is forcing air to the bottom level of the stairway thereby displacing contaminated air to the top level and further to a free outside air space. Drawing air from the bottom of the stairways should be avoided since there is a risk that contaminated air will be drawn from the cargo holds. On some vessels, the stairway deck-access doors are located in semi-enclosed rooms. The release of stairway air into such a space might create a dangerous atmosphere.
A ventilation system should not be operated during the voyage in order to avoid the risk of oxygenation of the nearby cargo hold through leakage. Increased oxidation of the pellets may lead to an elevated temperature and uncontrolled thermal conditions in the cargo, particularly when passing through warmer tropical waters. The low oxygen content is in itself a safeguard against uncontrolled oxidation. A fire at sea would be a disastrous scenario and very difficult to control, similar to fires in silos.
Despite improved ventilation, it is important that both oxygen and CO are measured before entry into confined spaces in contact with wood pellets. Ideally, instrumentation should be calibrated for the oxygen and CO levels that might occur. If the calibration is made at a very different concentration than the level to be measured, it may lead to a potentially gross under- or over-estimation. However, instrumentation designed for use with high concentrations may be less accurate at low concentrations where clearance of workspaces is required. It may be difficult to find a single instrument that can accurately measure the whole range from 0 to 15 000 ppm CO. Given the circumstances, it is of primary importance that the measuring instruments are accurate at ∼ 19.5% oxygen and at ∼35 ppm CO. The performance of the instruments, such as recovery time and accuracy, after a shock exposure to extreme concentrations of CO should be specified by the manufacturers. To avoid shock exposure of the instruments, it is possible to use color indicator tubes designed for high CO levels, before checking with electrochemical and galvanic cell detectors.
A temporary and mobile workforce is characteristic of the shipping industry. New crew members must be effectively informed about the hazards associated with wood materials. The provision of a Shipper Cargo Information Sheet, which identifies the inherent hazards associated with the handling of wood pellets, is an important key to safe-guarding workers. It also appears that emergency crews attending accidents of this nature need to be better prepared in order to avoid exposure when entering the scene of an accident. The redesign of access doors would allow easier entry and exit procedures and provide better conditions during emergency rescue operations. It is very difficult to maneuver an unconscious person via vertical ladders and through narrow doors, particularly if the rescuer is wearing cylinders with breathing gas. Protective masks and filters are not effective against CO and oxygen depletion, only self-contained breathing apparatuses will work.
An immediate and cost-effective safety precaution is to padlock and label all access doors to stairways and establish and enforce the strict entry procedures which are already stipulated in existing regulations. Leaving entry doors open for ventilation is an invitation for people to enter a toxic atmosphere. Door openings could be fitted with protective mesh, letting air out but preventing entry during ventilation. Warning signs placed on top of doors will be hidden when the door is open unless there is also a sign on the inside of such doors. Repeated safety training or a certification system for the personnel in question is recommended.
CONCLUSIONS
Ocean transportation of wood pellets in confined spaces may rapidly produce lethal levels of CO and an oxygen-deficient atmosphere that may leak into adjacent access spaces. This is the first report to recognize this danger. The oxygen depletion and CO formation are probably caused by oxidative degradation of natural lipids and other organic materials naturally present in wood pellets. The oxygen depletion may have a value in that it reduces the fire hazard associated with this cargo.
Measurement of both CO and oxygen levels is essential prior to entry into spaces with air communication with a cargo of wood pellets. Measurement of oxygen alone is not safe. Self-ventilation of stairways is not sufficient. Forced ventilation is necessary in order to achieve safe entry conditions within a reasonable time. The provision of proper gas monitoring instruments and training and education of crews are of vital importance as well as the proper design, labeling and locking of access doors leading to confined spaces. The conclusions drawn here also apply generally to other confined spaces in contact with wood pellets.
References
- ACGIH. TLVs and BEIs. Threshold limit values for chemical substances and physical agents. Biological exposure limits. Cincinnati: ACGIH; 2007. [Google Scholar]
- Arbetarskyddsstyrelsen. AFS 2005:17 Hygieniska gränsvärden och åtgärder mot luftföroreningar. Solna: Arbetarskyddsstyrelsen; 2005. [Google Scholar]
- Griffith D. Synthetic calibration and quantitative analysis of gas-phase FT-IR spectra. App Spectrosc. 1996;50:59–70. [Google Scholar]
- Melin S. Canbio/IEA Conference. Toronto, Canada: 2007. Ocean transportation of pellets. [Google Scholar]
- NAS/COT. 2005. Interim acute exposure guideline levels (AEGLs) carbon monoxide: National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances. http://www.epa.gov/oppt/aegl/pubs/tsd306.pdf. February 2008. [Google Scholar]
- Rothman LS, Barbe A, Benner Chris D, et al. The HITRAN molecular spectroscopic database: edition of 2000 including updates through 2001. J Quant Spectrosc Radiat Transf. 2003;82:5–44. [Google Scholar]
- Sharpe SW, Johnson TJ, Sams RL, et al. Gas-phase databases for quantitative infrared spectroscopy. App Spectrosc. 2004;58:1452–61. doi: 10.1366/0003702042641281. [DOI] [PubMed] [Google Scholar]
- Svedberg U, Högberg H-E, Högberg J, et al. Emission of hexanal and carbon monoxide from storage of wood pellets, a potential occupational and domestic health hazard. Ann Occup Hyg. 2004;48:339–49. doi: 10.1093/annhyg/meh015. [DOI] [PubMed] [Google Scholar]
- WHO. Environmental Health Criteria 213 Carbon Monoxide. Geneva: WHO; 1999. [Google Scholar]