Abstract
The formation of stable cell–cell contacts is required for the generation of barrier-forming sheets of epithelial and endothelial cells. During various physiological processes like tissue development, wound healing or tumorigenesis, cellular junctions are reorganized to allow the release or the incorporation of individual cells. Cell–cell contact formation is regulated by multiprotein complexes which are localized at specific structures along the lateral cell junctions like the tight junctions and adherens junctions and which are targeted to these site through their association with cell adhesion molecules. Recent evidence indicates that several major protein complexes exist which have distinct functions during junction formation. However, this evidence also indicates that their composition is dynamic and subject to changes depending on the state of junction maturation. Thus, cell–cell contact formation and integrity is regulated by a complex network of protein complexes. Imbalancing this network by oncogenic proteins or pathogens results in barrier breakdown and eventually in cancer. Here, I will review the molecular organization of the major multiprotein complexes at junctions of epithelial cells and discuss their function in cell–cell contact formation and maintenance.
Keywords: Adherens junction, Tight junction, Cell polarity, Cell–cell adhesion, Protein complexes, JAM, PAR proteins
Introduction
In multicellular organisms, cell–cell adhesion is involved in most developmental processes. It is necessary for example for the assembly of coherent sheets of barrier-forming epithelial and endothelial cells which line the inner and outer surfaces of the organism like those of the intestine, the skin or the blood vessels. However, also in adult tissues cell–cell contacts are far from being static structures which maintain the barriers by simply holding cells together. During the turnover of growing tissues such as the intestine or the skin, they are constantly remodeled to allow the extrusion of “old” cells and the incorporation of “young” cells derived from stem cells without a concomitant loss of the barrier function (Fuchs et al. 2004). Similarly, during leukocyte extravasation in secondary lymphoid organs or at sites of an ongoing immune response, the homotypic interactions between endothelial cells must be altered to allow the passage of the leukocytes without affecting the barrier properties of the endothelium (Ley et al. 2007). Finally, during wound healing and tissue repair, cells undergo a coordinated movement, proliferate and establish new cell–cell contacts once they encounter cells from the opposing site of the wound (Perez-Moreno and Fuchs 2006). These different demands in different cell types and different physiological situations require a sophisticated regulatory network which enables a partial dismantling and re-establishment of cell–cell contacts while simultaneously preventing the loss of an epithelial phenotype which in adult tissues frequently correlates with tumor progression and metastasis (Thiery 2002).
Not surprisingly, the organization of cell–cell contacts of epithelial has attracted a great deal of attention. Due to the easy accessibility of cultured epithelial cells many discoveries have been made in epithelial cells. Epithelial cell–cell contacts contain three major adhesive structures which can be identified at the ultrastructural level, the tight junctions (TJs), the adherens junctions (AJs), and the desmosomes. In polarized epithelial cells of certain tissues like the intestine, TJs and AJs are asymmetrically distributed at the apical region of the lateral cell contact forming the apical junctional complex (AJC) which encircles the apex of the cells and demarkates the border between the apical and the basolateral membrane domains (Nelson 2003). Common to all three types of structures is the presence of several adhesion molecules that link the neighbouring cells through homophilic and heterophilic adhesive interactions, and the presence of cytoplasmic scaffolding proteins that organize signaling complexes and which provide a mechanical link to the actin cytoskeleton (or intermediate filaments in the case of desmosomes). The scaffolding proteins might also link different protein complexes—at least temporarily—and thus organize supramolecular protein complexes. It should be noted that these protein complexes are dynamic, and that their composition is subject to regulation depending on junctional maturation and integrity. During the last few years, a rapid progress has been made in identifying new proteins at cell–cell contacts and in particular in deciphering the molecular composition of the TJs. Among the most exciting findings was probably the discovery of protein complexes at TJs which are highly conserved through evolution and which regulate cellular polarization in different organisms and various cell types. Here, I will review the major multiprotein complexes present at cell–cell contacts of vertebrate epithelial cells and highlight the most recent advances in the understanding of their role in the organization and functions of epithelial cell–cell contacts in vertebrates.
Adherens junctions
The function of adherens junctions
A main function of AJs is to connect cells to regulate tissue formation and morphogenesis during development as well as the maintenance of solid tissues in the adult organism (Gumbiner 1996). The major cell adhesion molecules at AJs, the classical cadherins, connect adjacent cells through homophilic interactions and are linked to the cytoskeleton through proteins associated with their cytoplasmic tail, the catenins. This link generates a transcellular network of actin filaments running through the entire sheet of cells with the cadherin–catenin complexes serving as connectors of the actin filaments bundles at the intercellular space. During morphogenetic events, for example during neural tube formation, mechanical forces can thus be applied to the whole cellular sheet. In adult tissues, cadherin-mediated cell adhesion is absolutely required for cell–cell adhesion to be maintained as a loss of cadherin adhesion by Ca2+-depletion results in a loss of cell–cell interaction and rounding up of the cells despite a number of other adhesion molecules (which are not Ca2+-dependent) at cell–cell contacts (Takeichi 1977).
Cadherins and catenins
Since the cadherin–catenin complex has been the subject of a number of reviews, I will only summarize the central interactions and refer the reader to recent comprehensive reviews (Gumbiner 2005; Halbleib and Nelson 2006; Perez-Moreno and Fuchs 2006; Perez-Moreno et al. 2003; Pokutta and Weis 2007). As mentioned above, the cytoplasmic tail of classical cadherins like E-cadherin forms a multiprotein complex with β-catenin, γ-catenin and p120 catenin (p120ctn), members of the armadillo repeat domain-containing family of proteins. In this complex, β-catenin and p120ctn are directly associated with the membrane-distal and membrane-proximal region, respectively (Fig. 1). Both regions are highly conserved among classical cadherins underscoring the importance of these interactions. Under conditions where β-catenin is limiting, its binding site in E-cadherin can be occupied by the β-catenin-related plakoglobin/γ-catenin which otherwise associates preferentially with desmosomal cadherins (Zhurinsky et al. 2000). The interaction with β-catenin is required for the transport of E-cadherin from the ER to the basolateral cell surface (Chen et al. 1999). It is also required for the adhesive function of E-cadherin as post-translational modifications of β-catenin which alter its affinity towards E-cadherin alter the strength of E-cadherin adhesive activity (Perez-Moreno et al. 2003; Pokutta and Weis 2007). The adhesive activity is thus dependent on its association with β-catenin. Conversely, this association is also necessary to stabilize β-catenin which is otherwise rapidly degraded by the ubiquitin-proteasome pathway (Nelson and Nusse 2004).
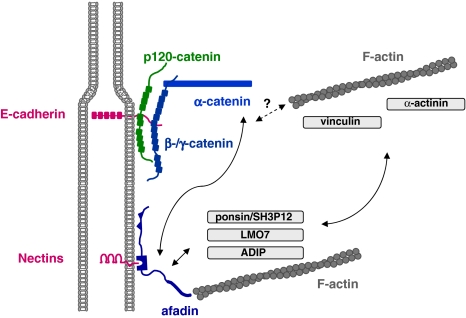
Fig. 1.
Major protein complexes at adherens junctions. Two major protein complexes exist at AJs of epithelial cells. The cadherin–catenin complex consist of the Ca2+-dependent adhesion molecule E-cadherin and the armadillo repeat proteins p120ctn and β/γ-catenin which directly bind to the cytoplasmic domain of E-cadherin. α-catenin directly associates with β-catenin but not simultaneously with F-actin. The nectin–afadin complex consists of the Ca2+-independent adhesion molecule nectin and the PDZ protein afadin. Afadin contains a F-actin-binding domain and thus can link the nectin–afadin system to the actin cytoskeleton. The two adhesion complexes can be linked through several molecular interactions. Afadin can directly interact with α-catenin. It also interacts with ponsin/SH3P12 which can interact with the F-actin-binding protein vinculin, and it interacts with LMO7 and ADIP which both can interact with the F-actin binding protein α-actinin. The nature of the link between the cadherin–catenin complex and the actin cytoskeleton is still unclear. Double arrows indicate direct interactions, the question mark symbolizes the missing link
The association of E-cadherin with p120ctn is subject to a similar reciprocal regulation of cell–cell contact localization: The cadherin molecule is necessary to localize p120ctn at the cell contact, and p120ctn is required for the stable localization of the cadherin molecule at AJs (Reynolds and Roczniak-Ferguson 2004). In contrast to β-catenin, p120ctn stabilizes cadherin which is constitutively endocytosed (Bryant and Stow 2004) by regulating its turnover rate at the surface (Davis et al. 2003). Besides its additional role in regulating transcription by interacting with transcription factors (van Roy and McCrea 2005), p120ctn has a also a function in regulating the activity of Rho small GTPases. The formation of early cell–cell contacts correlates with the activation of Cdc42 and Rac1 and the inhibition of RhoA (Noren et al. 2001), and these changes in activities of the small GTPases are at least in part regulated by p120ctn which interacts with the Rac activator Vav2 (Noren et al. 2000) and the RhoA inhibitor p190RhoGAP (Wildenberg et al. 2006). The activation of Cdc42/Rac1 and the downregulation of RhoA activity serves to facilitate new cell adhesion by increasing the cell surface interacting with membranes of neighbouring cells through lamellipodia and filopodia formation and simultaneously inhibiting cell migration by inhibiting stress fiber formation, respectively (Perez-Moreno and Fuchs 2006).
The role of α-catenin is less clear. For a long time considered to bridge the AJs to the actin cytoskeleton through direct interactions with both β-catenin and F-actin, this role has been challenged by the observations that its binding to F-actin (occurs only as homodimer) and its heterodimeric association with β-catenin are mutually exclusive (Drees et al. 2005; Yamada et al. 2005). Therefore, the physical bridge between the cadherin–catenin complex and the actin cytoskeleton remains to be identified. It is possible that α-catenin present in the cadherin-associated heterodimeric β-catenin–α-catenin complex is able to bind to other proteins which associate with F-actin. The putative linker is required to fulfill two critera: to bind directly to α-catenin and simultaneously–either indirectly or directly—to F-actin. From several proteins which fulfill these requirements, vinculin and α-actinin turned out not to mediate actin binding to the cadherin–catenin complex excluding them from the list of possible candidates (Yamada et al. 2005). Other candidate proteins including AF-6/afadin (Pokutta et al. 2002), ZO-1 (Itoh et al. 1997), formin (Kobielak et al. 2004), spectrin (Pradhan et al. 2001) or the LIM protein ajuba (Marie et al. 2003) remain to be tested.
Nectins and afadin
The second major adhesive protein complex at AJs consists of members of the nectin family of adhesion molecules and a scaffolding protein that is directly associated with the cytoplasmic domain of nectins named AF-6/afadin (Takai and Nakanishi 2003). Nectins are immunoglobulin-like proteins and comprise a family consisting of four members (nectin-1 to -4), which are localized at AJs of epithelial cells (Reymond et al. 2001; Sakisaka and Takai 2004). Unlike classical cadherins which undergo only homophilic interactions in trans, nectins undergo both trans-homophilic and trans-heterophilic interactions. The major heterophilic binding partners are other members of the nectin family as well as members of nectin-related adhesion molecules Nectin-like (Necl)-1 to -5 (Sakisaka et al. 2007). Nectins are “true” adhesion molecules as they support cell aggregation when ectopically expressed in cells (Aoki et al. 1997; Lopez et al. 1998; Satoh-Horikawa et al. 2000; Takahashi et al. 1999). They also seem to influence the E-cadherin-mediated adhesion (Martinez-Rico et al. 2005; Sato et al. 2006) suggesting that they are contributing to the overall strength of cell–cell adhesion. Like E-cadherin, nectin-2 appears very early at cell–cell contacts during junction formation and is present at so-called primordial, spot-like AJs (pAJs) or puncta (Asakura et al. 1999), which are formed at the tips of protrusions of two contacting cells (Perez-Moreno and Fuchs 2006). An important function of nectins which is similar to that of cadherins is their ability to activate Cdc42 and Rac1 small GTPases. Trans-interaction of nectins results in the recruitment and activation of c-Src, followed by the activation of the two guanine-nucleotide exchange factors “FGD-1-related Cdc42 GEF” (FRG) and Vav2, which are specific for Cdc42 and Rac1, respectively (Fukuhara et al. 2004; Kawakatsu et al. 2002, 2005). As pointed out above, the activation of these small GTPases is probably required to facilitate junction formation suggesting that nectins cooperate with cadherins in the regulation of the actin cytoskeleton at sites of cell adhesion. However, in addition they might also help to regulate the formation of tight junctions and their physical separation from AJs during junctional maturation (see below).
All nectins directly associate with afadin (Reymond et al. 2001; Takahashi et al. 1999). L-Afadin, the longer version of two afadin isoforms with a F-actin-binding domain, is a scaffolding protein which interacts with both nectins and F-actin through independent domains suggesting that it directly links nectin-based adhesion sites to the actin cytoskeleton (Mandai et al. 1997) (Fig. 1). However, through additional protein interactions, it might also establish an indirect link to the actin cytoskeleton as well as to the cadherin–catenin complex by its interaction with “classical” AJ-associated proteins. Afadin directly associates with α-catenin (Pokutta et al. 2002; Tachibana et al. 2000), with vinculin through its association with ponsin/SH3P12 (Mandai et al. 1999), and with α-actinin through its association with “afadin Dil domain-interacting protein” (ADIP) and “Lim domain only 7” (LMO7) (Asada et al. 2003; Ooshio et al. 2004) (Fig. 1). It should be mentioned that some of these protein interactions might occur specifically in certain tissues, and that the molecular mechanisms underlying these interactions are not revealed in detail. Nevertheless, it is likely that through these multiple interactions the two major protein complexes at AJs are physically linked and that they influence each other in their localization and activity (Sakisaka et al. 2007).
Tight junctions
The function of TJs
In polarized epithelial cells, the TJs forms a belt-like structure at the apical region of the cellular junction and represent a boundary between the apical and the basolateral membrane domains which differ in the composition of lipids and proteins (Tsukita et al. 2001). At the TJ area, two structures can be distinguished by ultrathin electron microscopy: sites where the intercellular space is basically obliterated and where the outer leaflets of the adjacent membranes appear to be in direct contact, and regions where the membranes of the two adjacent cells are separated by intercellular space. By freeze-fracture electron microscopy, the TJ area appears as a branched network of strands where the strands reflect the sites of direct membrane contacts (Tsukita et al. 2001). Two major functions are attributed to TJs: First, the regulation of the paracellular permeability of the epithelial sheet for ions and small solutes, which is an organ-specific function and which varies for different epithelia depending on the specific requirements of the organ (Furuse and Tsukitas 2006; Van Itallie and Anderson 2006); second, the formation of a physical barrier to prevent intramembrane diffusion of lipids and proteins, a rather cell-autonomous function which is necessary for a cell to maintain an asymmetric distribution of membrane components and to develop membrane polarity (Tsukita et al. 2001). In the recent years it has become clear that the molecular basis of the TJ strands are claudins (Fig. 2), a family of integral membrane proteins at TJs which are constituents of the TJ strands and which induce the formation of TJ strands upon ectopic expression in fibroblasts (Furuse et al. 1998). Claudins do not just create TJ strands, but within the strands form a selective permeability barrier by forming size- and charge-selective aqueous pores (Tsukita and Furuse 2000; Van Itallie and Anderson 2006). Surprisingly, the TJ strands do not seem to be the basis for the second major function of TJs, the diffusion barrier for intramembrane particles (Umeda et al. 2006). It has also become clear that the TJs harbor peripheral membrane proteins which regulate cell polarity and membrane asymmetry suggesting that these proteins are involved in the role of TJs in regulating apico-basal polarity.
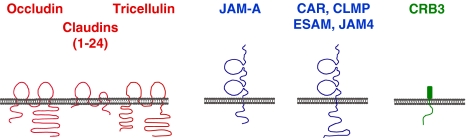
Fig. 2.
Integral membrane proteins at tight junctions of epithelial and endothelial can be grouped into three classes based on their overall organizations. The first class is characterized by two extracellular loops, four transmembrane regions, and two cytoplasmic tails (occludin, claudins, tricellulin). The second class consists of Ig-SF members which all contain two Ig-like domains. The third class (contains only one member, CRB3) is characterized by a short extracellular domain (36 AA), a single transmembrane domain and a short cytoplasmic tail. In contrast to the other integral membrane proteins, the function of the extracellular domain of CRB3 is not clear
Integral membrane proteins at TJs
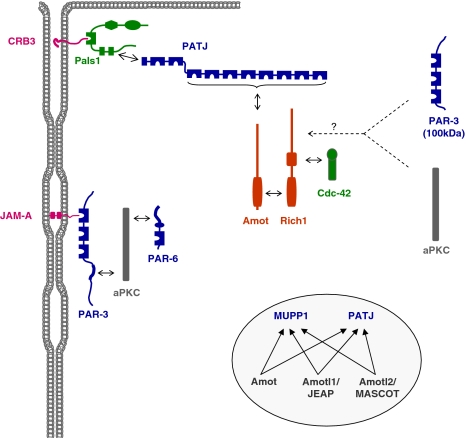
Three different classes of integral membrane proteins have been identified at TJs (Fig. 2). One class comprises occludin, claudins and tricellulin, which all contain four transmembrane domains, two extracellular loops, and the N-terminal and C-terminal ends are localized in the cytoplasm. Twenty-four claudins have been identified in humans. The major role of claudins is to form paired strands through homophilic and heterophilic cis and trans interactions. The large number of claudins, their ability to undergo heterophilic interactions and their ion selectivity allows for the formation of TJ strands with specific permeability properties depending on the needs of a specific tissue (Furuse and Tsukita 2006). Occludin is incorporated into TJ strands but its ectopic expression does not induce strand formation (Furuse et al. 1998) suggesting that it has rather an accessory role in TJ strand formation (Yu et al. 2005). Tricellulin differs from claudins and occludin in its specific enrichment at tricellular contact sites (Ikenouchi et al. 2005). The second class of integral membrane proteins comprises members of the CTX subfamily of the immunoglobulin superfamily (Fig. 2) which is characterized by one V-type and one C2-type Ig-like domain (Williams and Barclay 1988). Based on sequence homology, the length of their cytoplasmic tails and the type of the C-terminal PDZ domain motifs, the Ig-SF proteins at TJs can be further subdivided into a group consisting of JAM-A, JAM-B and JAM-C, and in a second group consisting of CAR, CLMP, ESAM, and JAM4 (Ebnet et al. 2004). Except for JAM-A, there is only little information on the role of these proteins in the development or the function of tight junctions. All but CLMP interact with TJ-associated scaffolding proteins like ZO-1 (CAR, JAM-A, -B, -C (Cohen et al. 2001; Ebnet et al. 2000, 2003), PAR-3 (JAM-A, -B, -C (Ebnet et al. 2001, 2003) or MAGI-1 (ESAM, JAM4; (Hirabayashi et al. 2003; Wegmann et al. 2004) (Fig. 3), and in some cases, their ectopic expression increases the transepithelial electrical resistance (TER) or decreases the paracellular permeability suggesting a regulatory role in TJ formation (Cohen et al. 2001; Hirabayashi et al. 2003; Mandicourt et al. 2007; Raschperger et al. 2003). For JAM-A, there is good evidence that it regulates TJ formation through its direct association with the scaffolding protein PAR-3 (Ebnet et al. 2001; Itoh et al. 2001). JAM-A localizes very early at sites of cell adhesion during cell–cell contact formation (Ebnet et al. 2001; Suzuki et al. 2002), and this localization probably serves to recruit PAR-3 to these site to initiate the polarization of the lateral membrane resulting in TJ formation (see below for details). Both RNA interference-mediated downregulation of JAM-A and ectopic expression of a JAM-A dominant-negative mutant that mislocalizes PAR-3 result in decreased TER, increased paracellular permeability and a defect in the development of membrane asymmetry (Mandell et al. 2005; Rehder et al. 2006) pointing to a general defect in the formation of functional TJs. The third class of integral membrane proteins comprises only one protein, Crumbs3 (CRB3), a homologue of the Drosophila Crumbs protein with a very short extracellular domain of only 36 AA and a short cytoplasmic domain of 41 AA (Makarova et al. 2003; Medina et al. 2002). CRB3 directly associates with two peripheral membrane proteins, Pals1 (Roh et al. 2002b) and PAR-6 (Lemmers et al. 2004), which are components of the two major cell polarity protein complexes localized at TJs, i.e. the Pals1–PATJ complex and the PAR-3–aPKC–PAR-6 complex (Fig. 3, see also below). Its overexpression delays TJ formation (Lemmers et al. 2004; Roh et al. 2003), and its ectopic expression in a cell line that expresses only little endogenous CRB3 results in the development of functional TJs (Fogg et al. 2005). These effects can most likely be attributed to its association with cell polarity proteins and the regulation of their subcellular localization (see below).
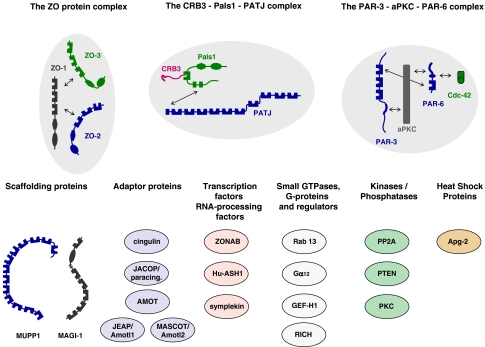
Fig. 3.
Major protein complexes and functional classes of molecules at tight junctions. The TJs contain three major multi-protein complexes consisting largely of scaffolding proteins, the ZO protein complex, the CRB3–Pals1–PATJ complex and the PAR-3–aPKC–PAR-6 complex. Besides these three protein complexes which seem to be constitutively associated at TJs, a number of proteins with different functions has been identified at TJs. These include additional scaffolding proteins like MUPP1 and MAGI-1, adaptor proteins, transcription regulators and RNA processing factors, regulatory proteins like small GTPases and G-proteins, kinases and phosphatases, and heat shock proteins. Double arrows indicate direct interactions. Not all direct interactions that have been identified are depicted
Multiprotein complexes at TJs
Since the discovery of ZO-1 as the first protein at TJs and its molecular cloning (Itoh et al. 1993; Stevenson et al. 1986), the number of proteins that are localized at TJs has steadily increased. These proteins comprise scaffolding and adapter proteins, regulatory proteins like small GTPases, G-proteins, kinases and phosphatases, as well as transcription factors or factors regulating RNA processing (Fig. 3). The large number and the functional diversity of these proteins suggest that TJs are a focus of incoming and outgoing signals and that their composition is dynamic. In accordance with this, many proteins identified at TJs are found at other compartments of the cell as well including the nucleus or the cytoskeleton and are actively shuttling between these compartments and the TJs (Matter and Balda 2007). The organization of these networks is regulated by proteins containing multiple protein–protein interaction domains such as PDZ domains, GuK domains, SH2 or SH3 domains (Pawson and Nash 2003). At the TJs three major protein complexes exist which involve one or several scaffolding proteins, the ZO protein complex, the Pals1–PATJ complex, and the PAR-3–aPKC–PAR-6 complex (Fig. 3).
The ZO protein complex
ZO-1 is a classical scaffolding protein of the MAGUK family with three PDZ domains, one SH3 domain and one GuK domain (Funke et al. 2005). It can directly associate with several integral membrane proteins at TJs including occludin, claudins, JAMs and CAR through independent domains (Fig. 4), and it probably serves to cluster these proteins at the TJs. It also interacts with other cytoplasmic proteins including its homologues ZO-2 and ZO-3, and in addition with the actin cytoskeleton (Fanning et al. 1998; Fanning et al. 2002; Wittchen et al. 1999). The exact composition of the ZO complex is not completely understood, yet. ZO-1 forms independent complexes with ZO-2 and ZO-3 (Wittchen et al. 1999), and both ZO-2 and ZO-3 can also interact with F-actin and share with ZO-1 some of the integral membrane proteins at TJs like occludin and claudins (Itoh et al. 1999; Wittchen et al. 1999). Alltogether, the ZO complex provides the major link to the actin cytoskeleton at the TJs (Fig. 4). However, regarding the role of the individual ZO proteins, some redundancy might exist. In agreement with this, the absence of ZO-1 results in a slight delay in TJ formation but does not impair the formation of functional TJs in two epithelial cell lines (McNeil et al. 2006; Umeda et al. 2004). Only when all three ZO proteins are absent the formation of TJs is blocked as indicated by the absence of TJ strands, the lack of other TJ proteins like occludin, claudin-3 and JAM-A, and the complete loss of the barrier function (Umeda et al. 2006). Together, these findings indicate a critical role for the ZO proteins for the development of TJ strands, probably by forming the physical scaffold for the strand-forming proteins like claudins and occludin. As will be discussed below, ZO proteins also form a platform for signaling proteins to regulate epithelial proliferation and morphogenesis.
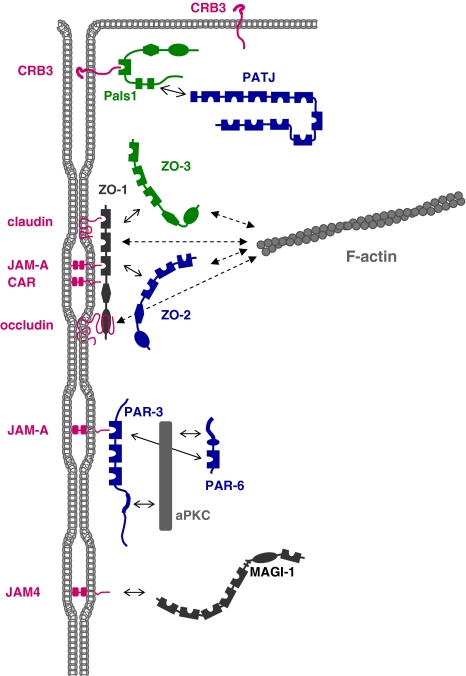
Fig. 4.
Organization of the tight junctional plaque. The major protein complexes at TJs interact with specific transmembrane proteins. CRB3 recruits the Pals1–PATJ complex to TJs. CRB3 interacts with the PDZ domain of Pals1, Pals1 interacts with PATJ through a heterodimeric L27 domain interaction. CRB3 is also localized at the apical membrane domain of epithelial cells (Makarova et al. 2003). The CRB3–Pals1–PATJ complex regulates TJ formation but the mechanism is largely unknown. The ZO complex is associated with the membrane through multiple interactions of ZO-1 with various integral membrane proteins including occludin, claudins, JAM-A and CAR. ZO-2 and ZO-3 can interact with both ZO-1 and also with claudins. The ZO protein complex probably serves to link TJs to the cytoskeleton as all three ZO proteins directly interact with F-actin. The PAR-3–aPKC–PAR-6 complex is associated with the membrane through the interaction of PAR-3 with JAM-A. PAR-3 interacts with aPKC through its aPKC-interacting domain, PAR-6 interacts with aPKC through a PB1–PB1 domain interaction. A direct interaction between the PDZ domain of PAR-6 and PDZ domain 1 of PAR-3 has also been described. The PAR complex regulates the formation of TJs and apico-basal polarity. The JAM-A-related Ig-SF member JAM4 interacts directly with MAGI-1; the role of this protein complex is not clear. It should be noted that this drawing is incomplete as it does not depict interactions among the various protein complexes which have been described (e.g. PAR-6 can also associate with CRB3 and Pals1, ZO-3 can associate with PATJ). Also, the multiple PDZ domain protein MUPP1 (not depicted in this Figure, see Fig. 3 for a schematic representation) associates with claudins, JAM-A and CAR, as well as with angiomotin family members (Coyne et al. 2004; Hamazaki et al. 2002; Sugihara-Mizuno et al. 2007). Double arrows with solid lines indicate direct protein–protein interactions, double arrows with broken lines indicate interactions with F-actin
The CRB3–Pals1–PATJ complex
The CRB3–Pals1–PATJ complex has originally been described in Drosophila as a protein complex (the Crumbs–Stardust–Discs lost complex; this complex is now called Crumbs–Stardust (Sdt)–dPATJ complex (Pielage et al. 2003)) involved in the regulation of apico-basal polarization (Tepass et al. 2001). In Drosophila epithelial cells, this complex localizes to the apical region of the lateral membrane domain, called subapical region (SAR) or marginal zone (Knust and Bossinger 2002), that is positionally analogous to the TJs in vertebrate epithelial cells. The localization of the CRB3–Pals1–PATJ complex at the TJ is mediated through a direct and PDZ domain-dependent interaction of Pals1 with the C-terminal PDZ domain motif in CRB3, and a direct interaction between Pals1 and PATJ involving the L27N domain of Pals1 and the L27 domain present at the NH2-terminal region of PATJ (Fig. 3) (Roh et al. 2002b). RNA interference-mediated knockdown of Pals1 leads to defects in the formation of TJs as well as in the development of lumen-containing epithelial cysts (an assay system for apico-basal polarity development (O’Brien et al. 2002)), and is accompagnied by a loss of PATJ protein expression (Straight et al. 2004). Conversely, knockdown of PATJ impairs the barrier function of TJs and results in a loss of Pals1 at TJs, an internalization of CRB3, and a redistribution of other TJ components like occludin and ZO-3 (Michel et al. 2005; Shin et al. 2005). These observations strongly suggest that the CRB3–Pals1–PATJ complex is important for the development of functional TJs in vertebrate epithelial cells.
The PAR-3–aPKC–PAR-6 complex
In evolutionary terms, the PAR-3–aPKC–PAR-6 complex is the most ancient among the three major protein complexes at TJs. As opposed to the ZO protein complex and the CRB3–Pals1–PATJ complex, all three proteins of this complex exist in C.elegans where they cooperate to regulate the development of membrane asymmetry in the zygote (Kemphues 2000). The acronym Par stands for partitioning-defective and reflects the lack of partition of cytoplasmic P granules in C.elegans mutant embryos in response to sperm entry (Kemphues et al. 1988). The initial screen identified six par genes, and their molecular characterization revealed that they encoded proteins of different structures and functions which include scaffolding/adapter proteins with several protein–protein interaction domains (PAR-3, PAR-6), serine/threonine kinases (PAR-1, PAR-4), a protein containing a RING finger domain typical for E3 ubiquitin ligases (PAR-2) and a member of the 14-3-3 family of signaling proteins (PAR-5) (reviewed in (Goldstein and Macara 2007; Suzuki and Ohno 2006)). With the exception of PAR-2, all PAR proteins exist in Drosophila and vertebrates. Two PAR proteins, PAR-3 and PAR-6, form a functional unit with aPKC, the PAR-3–aPKC–PAR-6 complex (Ohno 2001). In this complex, PAR-3 and PAR-6 undergo direct interactions with aPKC (Fig. 3). The interaction of PAR-6 with aPKC is mediated by a heterotypic PB1–PB1 domain interaction, the interaction of PAR-3 with aPKC by the CR3 domain of PAR-3 and the kinase domain of aPKC (Ohno 2001). The interactions of the two scaffolding proteins PAR-3 and PAR-6 with aPKC are assumed to regulate the localization and the activity of aPKC, respectively. Among all integral membrane proteins tested, PAR-3 binds specifically to JAM-A, -B, and -C (Ebnet et al. 2001, 2003), and the interaction with JAM-A might serve to anchor the PAR–aPKC complex to TJs. The interaction with PAR-6 is assumed to regulate the activity of aPKC (Lin et al. 2000). In the absence of small GTPases like Cdc42 or Rac1 aPKC is inactive. The binding of active Cdc42 or Rac1 to the CRIB domain of PAR-6 activates aPKC, probably by inducing a conformational change of PAR-6 which allows aPKC to become active (Yamanaka et al. 2001).
What is the function of the PAR–aPKC complex in TJ physiology? A large body of evidence indicates a critical role of the PAR complex in TJ formation rather than in TJ maintenance (Chen and Macara 2005, 2006; Gao et al. 2002; Hirose et al. 2002; Joberty et al. 2000; Mizuno et al. 2003; Nagai-Tamai et al. 2002; Ooshio et al. 2007; Suzuki et al. 2001, 2002; Yamanaka et al. 2001). Many of these studies applied dominant-negative mutants of either PAR-3 or PAR-6 or aPKC. The negative effects on TJ formation were only observed when these mutants were expressed during the process of cell–cell contact formation but not when expressed in fully polarized epithelial cells where TJ formation had already been completed (Gao et al. 2002; Nagai-Tamai et al. 2002; Suzuki et al. 2001, 2002; Yamanaka et al. 2001). This strongly suggests that the PAR–aPKC complex develops its polarizing activity at an early stage of cell–cell contact formation and that it is critical for the formation of TJs rather than for their maintenance.
Regulation of membrane asymmetry and TJ formation by the PAR-3–aPKC–PAR-6 complex
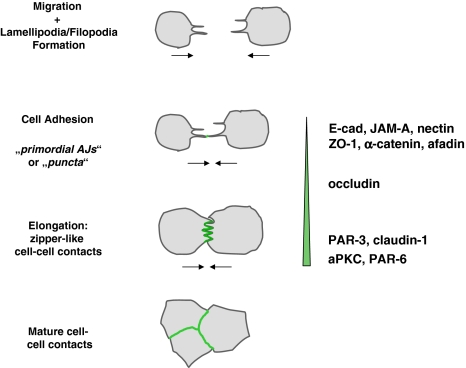
The formation of cell–cell contacts and the development of intercellular junctions with distinct structures like AJs and TJs is a step-wise process (Fig. 5). In the absence of cell–cell contacts, cells form thin protrusions filled by axial actin filaments which upon encountering protrusions of other cells form multiple transient contacts which are subsequently stabilized (McNeill et al. 1993). The first sites of cell–cell contact formation are called “primordial, spot-like AJs” (pAJs) or “puncta” (Adams et al. 1996; Yonemura et al. 1995). The formation of multiple pAJs between the protrusions of adjacent cells results in a zipper-like appearance of the early cell contact sites (McNeill et al. 1993) (Fig. 5). During maturation of cell–cell contacts, the pAJs gradually fuse to form a linear contact region, the cells start to polarize and eventually develop cell junctions with AJs and TJs.
Fig. 5.
A step-wise recruitment of proteins to cell–cell contacts. The earliest sites of stable physical interaction during cell–cell contact formation are primordial, spot-like AJs (pAJs) or puncta at the tips of cellular protrusions. During junctional maturation, the cellular protrusions of adjacent cells interdigitate, and multiple puncta are formed along the sides of protrusions. These puncta gradually fuse to form linear arrangements of cell–cell contacts sites thus generating a zipper-like appearance. During further maturation, cell–cell contacts are formed along the entire lateral cell surface, and the zipper-like cell–cell structure disappears. Cell contact-associated proteins are recruited in a step-wise manner. The pAJs/puncta are positive for integral membrane proteins (E-cadherin, JAM-A, nectin-2), but also peripheral membrane proteins (ZO-1, α-catenin, afadin) and contain proteins associated with AJs as well as TJs in polarized cells. During the formation of zipper-like cell contacts, occludin is recruited, probably through its interaction with ZO-1. Thereafter, PAR-3 is recruited by JAM-A and/or nectin-2, and claudins are recruited, probably through interaction with ZO-1. Indirect evidence suggests that aPKC and PAR-6 appear slightly later than PAR-3. The vertical bar reflects the increase in the contacting membrane area during cell–cell contact formation
The pAJs are positive for typical AJ proteins like E-cadherin, α-catenin, β-catenin, nectin-2, AF-6/afadin and ponsin but also for typical TJ proteins like ZO-1 and JAM-A (Adams et al. 1996; Asakura et al. 1999; Ebnet et al. 2001; Suzuki et al. 2002; Yonemura et al. 1995). During maturation, occludin is recruited to these sites, and during further maturation, claudin-1, PAR-3 and aPKC appear (Suzuki et al. 2002) (Fig. 5). Although direct comparison has not been performed yet, it is likely that aPKC together with PAR-6 appear slightly later than PAR-3 (Suzuki et al. 2002). The formation of cadherin-based pAJs marks the early sites of cell–cell adhesion and probably serves as a “landmark” or “positional cues” for membrane growth and for the recruitment of other integral and peripheral membrane proteins (Yeaman et al. 1999). After the localization of the first set of proteins at pAJs other proteins can be recruited through direct physical interactions with those already present. For example, α-catenin-associated ZO-1 could serve to recruit occludin and claudins, afadin could serve to recruit JAM-A and nectin-2 (or vice versa), and JAM-A or nectin-2 could recruit PAR-3 which serves as scaffold to assemble the PAR-3–aPKC–PAR-6 complex.
Once the PAR-3–aPKC–PAR-6 complex has been recruited to nascent cell–cell contacts aPKC has to be activated as suggested by the observation that ectopic expression of a kinase-dead, dominant-negative mutant of aPKC prevents the maturation of pAJs into belt-like AJs and TJs (Suzuki et al. 2001, 2002). The activation occurs most likely by the Rho GTPases Cdc42 and Rac1 which bind to the Crib domain of PAR-6 thereby inducing a conformational change which leads to the activation of PAR-6-associated aPKC (Garrard et al. 2003; Ohno 2001; Peterson et al. 2004; Yamanaka et al. 2001). Both E-cadherin and nectin-2 could be responsible for the activation of Cdc42 and Rac1. Whereas E-cadherin seems to activate Rac1 but not Cdc42 (Betson et al. 2002; Kovacs et al. 2002; Nakagawa et al. 2001; Noren et al. 2001; Yamada and Nelson 2007), nectin-2 induces the activation of both Cdc42 and Rac1 after ectopic expression in cultured epithelial cells (Fukuhara et al. 2003, 2004; Fukuyama et al. 2005; Kawakatsu et al. 2002, 2005). The association of the Rac1 GEF Tiam1 with PAR-3 (Chen and Macara 2005; Mertens et al. 2005) could regulate a locally restricted activation of Rac1 specifically at those sites where cell–cell adhesion has occured and where the activity of aPKC is required to promote the maturation of cell–cell contacts and the development of TJs from pAJs.
The exact mechanism how the maturation of cell–cell contacts is regulated by aPKC is not clear. One could imagine that aPKC phosphorylates components of TJs and thereby regulates their specific localization or their specific functions at the TJs. Phosphorylation of occludin, claudin-1 and ZO-1 by aPKCζ has been found in vitro (Nunbhakdi-Craig et al. 2002). However, a physiological relevance of these phosphorylations has not been demonstrated, yet. Alternatively, aPKC could regulate TJ formation indirectly by regulating the development of membrane asymmetry along the lateral cell–cell contacts. The following example might serve to illustrate an example for this activity. In polarized epithelial cells, aPKC and PAR-1, another Ser/Thr kinase, are separately localized along the apico-basal axis: aPKC localizes to TJs whereas PAR-1 localizes to the basolateral membrane domain (Bohm et al. 1997). PAR-1 is a substrate for aPKC, and aPKC-mediated phosphorylation of PAR-1 leads to its dissociation from the membrane into the cytoplasm (Hurov et al. 2004; Suzuki et al. 2004). As a result, PAR-1 is absent from aPKC-containing membrane domains. A reciprocal mechanism has been described in Drosphila epithelial cells (Benton and St Johnston 2003). Drosphila PAR-1 phosphorylates PAR-3/Bazooka thereby inhibiting its dimerization and blocking its ability to assemble a functional PAR-3–aPKC–PAR-6 complex. As a result, the PAR-3–aPKC–PAR-6 complex is absent from PAR-1-containing membrane domains. Through these reciprocal inhibitory interactions two distinct membrane domains are generated characterized by the mutual exclusion of aPKC and PAR-1. Thus, by regulating the formation of a specific membrane domain from which certain proteins are actively excluded, aPKC could indirectly promote the formation of TJs. It is not clear, yet, if the abilities of the PAR-3–aPKC–PAR-6 complex to regulate TJ formation and to regulate membrane asymmetry are mechanistically linked.
Regulation of TJ maintenance by the Rich1–Amot complex
Recently, a new protein complex has been identified which has been suggested to regulate the maintenance rather than the formation of TJs. The functional core of this complex is a set of two proteins which regulate the activity of Cdc42, the Rich1 and angiomotin (Amot) proteins (Wells et al. 2006) (Fig. 6). Rich1 is a Rho GTPase activating protein (RhoGAP) for Cdc42 and Rac1 with a Cdc42-selective activity in epithelial cells; it contains a BAR domain and a RhoGAP domain (Richnau and Aspenstrom 2001; Richnau et al. 2004; Wells et al. 2006). Amot is a scaffolding/adapator protein with a coiled-coil domain region and a C-terminal PDZ domain-binding motif (Bratt et al. 2002). The Rich1–Amot complex is targeted to TJs through a PDZ domain-dependent interaction of Amot with PATJ (Fig. 6). Both overexpression of Amot and partial downregulation of Rich1 by RNA interference affect the barrier function of TJs. More importantly, the loss of the barrier function in response to Ca2+-removal is accelerated after partial Rich1 downregulation (Wells et al. 2006) suggesting that the Rich1–Amot complex is important for the maintenance of functional TJs. This function of the Rich1–Amot complex probably resides in regulating the cycling of Cdc42 and maintaining the pool of active Cdc42 at TJs at a low level.
Fig. 6.
The Rich1–Amot complex at TJs. Rich1 is a RhoGAP with specificity for Cdc42 in epithelial cells. Rich1 interacts with Amot through a reciprocal BAR domain-dependent interaction, and Amot binding regulates Rich1 activity. Rich1 associates with Cdc42 through its GAP domain. Amot directly interacts with one (or several) of the PDZ domains 3–10 of PATJ that in turn associates with CRB3–Pals1. Surprisingly, Rich1 is directly or indirectly associated with PAR-3 and aPKC, and this PAR complex is distinct form the PAR-3–aPKC–PAR-6 complex because it does not contain PAR-6 and because aPKC cannot be associated with the 100 kDa isoform of PAR-3 which lacks the aPKC-binding domain. All three Amot-like proteins (Amot, Amotl1, Amotl2) form Rich1-independent protein complexes with MUPP1 and PATJ. Double arrows with solid lines indicate direct protein–protein interactions, arrows with broken lines indicate the presence of the two proteins in the same complex but the nature of the interaction has not been characterized in detail, yet
Besides Amot, PATJ and Pals1, the Rich1 immunoprecipitates contain PAR-3 and aPKC (Wells et al. 2006). Surprisingly, the Rich1 immunoprecipitates do not contain PAR-6, and they contain the 100 kDa isoform of PAR-3 which lacks the aPKC-interacting domain and thus cannot directly associate with aPKC (Lin et al. 2000). This suggests that PAR-3 and aPKC can undergo interactions with the Rich1–Amot complex which are independent of the PAR-3–aPKC–PAR-6 interaction with the Pals1–PATJ complex (Hurd et al. 2003) (Fig. 6). The functional relevance of this interaction is not clear. Two Amot-like (Amotl) proteins—Amotl1/JEAP and Amotl2/MASCOT—which have been described earlier as TJ components (Nishimura et al. 2002; Patrie 2005) are present in Amot but not Rich1-containing protein complexes. All three Amot proteins directly interact with the scaffolding protein MUPP1 and its paralogue PATJ (Sugihara-Mizuno et al. 2007) (Fig. 6). These findings suggest that additional Amot protein-containing complexes exist with functions different from regulating Cdc42 activity.
Signaling from TJs
In addition to the relatively stable protein complexes described so far (Fig. 4), many protein complexes at TJs assemble only transiently. In addition, some proteins are not exclusively associated with TJ but shuttle between the TJ and other compartments in the cell. The identification of such proteins has revealed that TJ proteins are engaged in receiving signals but also in delivering signals to the cell interiour and thereby regulate epithelial proliferation and differentiation (Matter and Balda 2003). The mechanism by which TJ proteins influence for example gene expression is most likely indirect through binding and sequestration of regulatory molecules at the TJs as exemplified by ZO-1.
ZO-1 associates with ZONAB/DbpA, a transcription factor which promotes proliferation of epithelial cells, in part by interacting with the cell division kinase CDK4 and also by regulating the expression of genes involved in proliferation like cyclin D1 and PCNA (Balda et al. 2003; Balda and Matter 2000; Sourisseau et al. 2006). In proliferating cells, which have little ZO-1, ZONAB/DbpA expression is high and ZONAB/DbpA protein is localized in the nucleus. When cells reach confluence and develop intercellular junctions, ZO-1 is accumulating at cell–cell contacts and recruits ZONAB/DbpA to the junctions thus sequestering it away from the nucleus (Balda and Matter 2000). Interestingly, during cellular stress ZONAB/DbpA associated with ZO-1 can be re-activated. The heat shock protein Apg-2 that is distributed in the cytoplasm under normal conditions is recruited to cell–cell contacts in response to heat shock where it binds directly to ZO-1 using the same binding interface like ZONAB/DbpA, i.e. the SH3 domain of ZO-1 (Tsapara et al. 2006). This leads to a loss of ZONAB/DbpA from cell junctions and in activation of the transcriptional activity of ZONAB/DbpA (Tsapara et al. 2006). Thus, ZO-1 influences gene expression and cell cycle progression in a cell density-dependent manner, and this function can be regulated during cellular stress.
ZO-2 is another scaffolding proteins at TJs which is involved in signaling. In contrast to ZO-1, however, ZO-2 seems to actively shuttle between TJs and the nucleus. ZO-2 contains functional nuclear localization and nuclear export signals (Gonzalez-Mariscal et al. 2006; Jaramillo et al. 2004) and interacts with various proteins that have nuclear functions including the transcription factors AP-1 and C/EBP (Betanzos et al. 2004), the DNA-binding protein SAF-B (Traweger et al. 2003), and the p120ctn family member ARVCF (Kausalya et al. 2004). ZO-2 probably inhibits the activity of the transcription factors AP-1 and C/EBP by regulating their export from the nucleus which is consistent with the predominant nuclear localization of ZO-2 in sparse cells and the localization of ZO-2 as well as AP-1 and C/EBP at TJs in confluent cells (Betanzos et al. 2004). In the case of ARVCF, ZO-2 regulates its nuclear import (Kausalya et al. 2004) where it might regulate transcription similar to other p120ctn family members (Hatzfeld 2005). In summary, the identification of protein complexes formed by typical TJ proteins and typical nuclear proteins involved in the regulation of transcription indicates that the TJs participate in the regulation of proliferation and differentiation.
A protein complex at the lateral membrane which regulates TJ formation: the Scribble–Discs Large–Lethal Giant Larvae complex
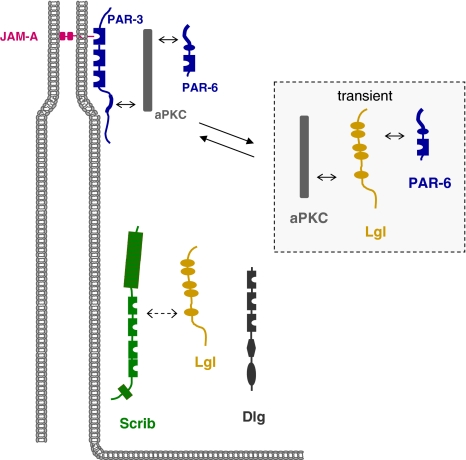
The Scribble complex comprises the proteins Scribble (Scrib), Discs Large (Dlg), and Lethal Giant Larvae (Lgl) (Fig. 7) which have originally been identified in Drosophila as tumor suppressor proteins (Bilder 2004). Mutations in the scrib, dlg or lgl genes result in overgrowth of certain tissues ultimately leading to a “giant larvae” phenotype and also in a disruption of apico-basal polarity (Bilder 2004). All three proteins are membrane-associated in Drosophila epithelial cells. Scrib and Dlg localize to the Septate Junctions (SJ), a structure that is a functional homologue of vertebrate TJs but which is localized basally of the AJs; Lgl localizes along the lateral membrane domain and is excluded from the SAR (Tepass et al. 2001). Importantly, the Scrib complex genetically interacts with the PAR-3–aPKC–PAR-6 and the Crumbs–Sdt–PATJ complexes to regulate apico-basal polarity (Bilder et al. 2003; Tanentzapf and Tepass, 2003). All three proteins are conserved and exist in vertebrates. In mammals, one homologue of Drosophila Scrib, four homologues of Dlg (Dlg1–Dlg4) and two homologues of Lgl (Lgl1, Lgl2) exist, and the proteins localize to the basolateral membrane domain of epithelial cells (Dow and Humbert 2007; Humbert et al. 2003). As opposed to the PAR-3–aPKC–PAR-6 and the CRB3–Pals1–PATJ complexes, Scrib, Dlg and Lgl do not seem to form a ternary complex through direct interactions. Recent evidence indicates that Scrib exists in a complex with Lgl2 (Kallay et al. 2006), but it is not clear if this interaction is direct or indirect as described in Drosophila (Mathew et al. 2002). The role of the Srib protein complex in regulating apico-basal polarity and cell–cell contact formation seems to be conserved in vertebrates. Scrib knockdown results in a delayed TJ formation (Qin et al. 2005), and knockdown of Dlg1 disturbs TJ formation after Ca2+-switch-induced new cell–cell contact formation (Stucke et al. 2007). Furthermore, Lgl has been described to regulate cell–cell contact and apico-basal polarity formation by forming a complex with PAR-6 and aPKC (Plant et al. 2003; Yamanaka et al. 2003) (Fig. 7). The interaction of Lgl with PAR-6 and aPKC precludes the binding of PAR-3 to PAR-6 and aPKC. Also, the Lgl–aPKC–PAR-6 complex counteracts the activity of the PAR-3–aPKC–PAR-6 complex by suppressing not only its formation but also its activation by Cdc42 (Yamanaka et al. 2006). According to the current model (Fig. 7), Lgl forms a complex with aPKC and PAR-6 early during cell–cell contact formation and blocks the formation of the PAR-3–aPKC–PAR-6 complex at this stage. Upon phosphorylation by aPKC, Lgl dissociates from the complex thus allowing for the formation and activation of the PAR-3–aPKC–PAR-6 complex and the development of apical TJs harboring the PAR–aPKC complex and a basolateral domain harboring Lgl (Yamanaka et al. 2003, 2006).
Fig. 7.
The Scribble (Scrb), Discs large (Dlg) and Lethal giant larvae (Lgl) proteins localize to the basolateral membrane domain in polarized epithelial cells. Scrb and Lgl exist in a complex but it is not clear if the interaction is direct. Inset: During the process of cell–cell contact formation, Lgl forms a transient complex with aPKC and PAR-6 from which PAR-3 is excluded. After aPKC-induced dissociation of Lgl from the complex, PAR-3 associates with PAR-6 and aPKC which promotes TJ formation and the development of apical and basolateral membrane domains. Double arrows with solid lines indicate direct protein–protein interactions, double arrows with broken lines indicate the presence of the two proteins in the same complex but the nature of the interaction has not been characterized in detail, yet
Protein complexes at cell junctions and cancer
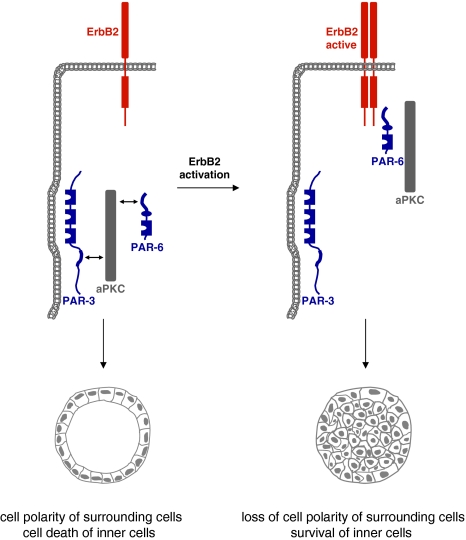
Given the important role of protein complexes localized at cell junctions of epithelial cells in regulating cell–cell contact formation, cell polarity and cell proliferation, it is not surprising that altering the compositions of these protein complexes will result in changes in cell–cell adhesion, a loss in cell–cell contact integrity and eventually in uncontrolled proliferation and cancer. A loss of cell polarity is frequently associated with cancer (Bissell and Radisky 2001; Wodarz and Nathke 2007). As pointed out above, many tumor suppressor genes identified in Drosophila including dlg, scrib and lgl turned out to encode proteins which regulate epithelial cell polarity (Bilder 2004), and loss of their expression correlates with more invasive and aggressive cancers in mammalian cells (Dow and Humbert 2007). Recent evidence identified the PAR-3–aPKC–PAR-6 complex at TJs as a target for the oncogenic receptor tyrosine kinase ErbB2. When cultured on reconstituted basement membrane, MCF-10A mammary epithelial cells form three-dimensional spheroids that resemble glandular structures (acini) with a single-layered, polarized epithelium surrounding a luminal space (Debnath and Brugge 2005). ErbB2 signaling leads to multiacinar structures as a result of hyperproliferation and to filling of the luminal space in individual acini due to a block of apoptosis of the inner cells. The latter of these two effects turned out to be regulated by the PAR-3–aPKC–PAR-6 complex (Aranda et al. 2006). Although the molecular mechanism has not been revealed in detail, the findings indicate that in response to ErbB2 activation ErbB2 physically interacts with the PAR-3–aPKC–PAR-6 complex which leads to a removal of PAR-3 and the formation of a ErbB2–PAR-6–aPKC complex (Fig. 8). The newly formed ErbB2–PAR-6–aPKC complex disrupts apico-basal polarity and blocks apoptosis of inner acinar cells through aPKC activity (Aranda et al. 2006). A role of PAR-6–aPKC in regulating apoptosis of inner cells has been observed in MDCK cysts as well (Kim et al. 2007). Thus, the ErbB2 oncogene exploits the PAR–aPKC system to regulate survival of the ErbB2-transformed cells.
Fig. 8.
The ErbB2 oncogene targets the PAR complex. In normal cells, PAR-3, aPKC and PAR-6 form a stable complex at TJs of epithelial cells (left panel). This complex is required for the fomation of TJs and the development of apico-basal polarity. ErbB2 activation triggers the association of the ErbB2 homodimer with PAR-6 and aPKC thereby disrupting the PAR-3–aPKC–PAR-6 complex. As a consequence, the development of apico-basal polarity is inhibited, and inner cells do not undergo apoptosis (right panel)
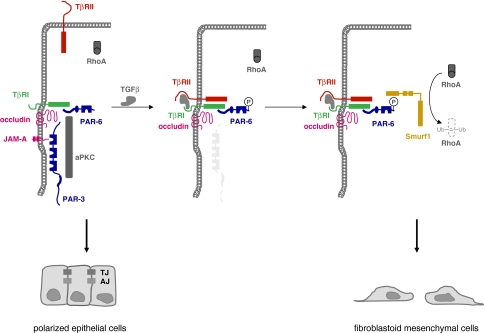
Besides increased proliferation and reduced apoptosis, epithelial-mesenchymal transition (EMT) is another hallmark of tumor progression (Thiery 2002). During EMT, polarized epithelial cells adopt a mesenchymal or fibroblastoid, highly motile phenotype, and this is required during phases of embryonic development when epithelial cells leave a primitive epithelium to migrate to a distinct site in the embryo in order to induce new organ formation. Not surprisingly, typical characteristics of EMT are transcriptional repression of E-cadherin expression, profound changes in the cytoskeleton concomitant with a loss of apico-basal polarity (Thiery 2002). When this developmentally regulated programme is re-activated in the adult organism, it easily contributes to tumor progression by facilitating invasion and metastasis. Among the various physiological inducers of EMT, TGFβ signaling turned to be critically involved in EMT through cooperation with receptor tyrosine kinases (RTKs) and oncogenic Ras (Huber et al. 2005). Recent evidence indicates that TGFβ signaling affects not only the integrity of AJs through its known effect on E-cadherin expression but also the integrity of TJs by targeting the PAR-3–aPKC–PAR-6 complex. TGFβ signaling is mediated by two TGFβ receptors, the Ser/Thr kinases TGFβ-receptor I (TβRI) and TβRII. In polarized NMuMG cells, TGFβ induces EMT by recruiting TβRII to TJs which results in TJ dissolution. TβRI localizes to TJ through a direct interaction with occludin (Barrios-Rodiles et al. 2005). Interestingly, TβRI interacts also directly with PAR-6 (Ozdamar et al. 2005) (Fig. 9). A TGFβ signal triggers heterodimeric complex formation between the two TGFβ receptors bringing TβRII in close vicinity of the PAR complex. PAR-6 is then phosphorylated by TβRII, and this leads to the recruitement of the ubiquitin ligase Smurf1 which in turn mediates ubiquitination of RhoA. RhoA, however, is critical for junctional integrity (Sahai and Marshall 2002), and its localized degradation might thus lead to TJ dissolution. TGFβ does not only induce RhoA degradation via PAR-6 and Smurf1 but also induces the downregulation of PAR-3 (Wang et al. 2007) (Fig. 9). Although the fate of aPKC during this process has not been clarified, these findings suggest that the PAR-3–aPKC–PAR-6 complex is a major target of TGFβ signaling at TJs during TGFβ-induced EMT.
Fig. 9.
TGFβ signaling targets the PAR complex at TJs. Left panel: Under normal conditions, TGFβ receptor I (TβRI) localizes to TJ through direct interactions with occludin and PAR-6. The cells maintain a polarized morrphology. Middle panel: TGFβ induces heterodimer formation of the two TGFβ receptors TβRI and TβR2 leading to activation of TβRII folllowed by TβRII-mediated phosphorylation of PAR-6 at Ser345. Right panel: Ser345-phosphorylated PAR-6 recruits Smurf1 leading to ubiquitination and degradation of the local pool of RhoA. As a consequence, the integrity of TJs is disturbed, the polarized morphology can not be maintained and the development of a fibroblastoid morphology is facilitated. In addition to PAR-6 phosphorylation, TGFβ signaling also induces downregulation of PAR-3. By targeting the PAR-3–aPKC–PAR-6 complex at TJs, TGFβ impairs the ability of cells to maintain a polarized morphology
Protein complexes as targets for pathogens
Many pathogens need to overcome epithelial and endothelial barriers to invade the host and establish infection. For this purpose, various strategies have evolved to disrupt the barrier and allow the pathogen the passage into tissues. These strategies include the release of proteolytic enzymes that cleave adhesion molecules like occludin, E-cadherin or desmoglein (Hanakawa et al. 2004; Pentecost et al. 2006; Wu et al. 1998, 2000), or the release of toxins that act via cell surface receptors to induce intracellular changes (e.g. of the actin cytoskeleton) which eventually lead to alterations of the barrier (Hopkins et al. 2003; Nusrat et al. 2001). More “advanced” strategies involve the delivery of pathogen-derived proteins via secretion systems into the host cell cytoplasm where these proteins directly associate with host cell proteins to influence their function. One example is the Helicobacter pylori (H.pylori) effector protein “Cytotoxin-associated gene A antigen” (CagA). H.pylori induces morphological changes of epithelial cells, alterations of the composition of the apical junctional complex as well as a breakdown of the epithelial barrier function (Amieva et al. 2003; Bagnoli et al. 2005), and H.pylori infections can result in mucosal damage (ulceration), inflammation (gastritis) and cancer (gastric carcinoma) (Peek and Blaser 2002). The CagA protein is involved in many of these processes through multiple interactions with a number of host proteins. After the translocation into the host cell, CagA is phosphorylated by src-family kinases and recruits the phosphotyrosine phosphatase SHP-2 (Higashi et al. 2002). In addition, CagA associates with several proteins involved in the regulation of TJs and in the formation of apico-basal polarity. First, it recruits and thereby mislocalizes the TJ proteins ZO-1 and JAM-A to the site of bacterial attachment (Amieva et al. 2003). Second, it directly interacts with the serine/threonine kinase PAR-1 (Saadat et al. 2007). Under normal conditions, PAR-1 cooperates with the PAR-3–aPKC–PAR-6 complex through reciprocal phosphorylations to regulate the formation of distinct membrane domains (Hurov et al. 2004; Suzuki et al. 2004) (see also above). The binding of CagA to PAR-1 blocks the kinase activity of PAR-1 thus preventing the phosphorylation of PAR-3; at the same time, it prevents the phosphorylation of itself by aPKC. As a result, the integrity of cell–cell contacts is disturbed and cells are extruded from the monolayer (Saadat et al. 2007). Thus, through its multiple interactions with signaling molecules, scaffolding proteins and cell polarity proteins, CagA disregulates critical cellular functions to enter the sub-epithelial tissues which also leads to inflammation and eventually to cancer (Hatakeyama 2004).
Conclusions and perspectives
The last decade has witnessed a steady increase in the number of new proteins localized at cell–cell contacts of epithelial cells. The identification of claudins at TJs has strongly increased the understanding of the molecular basis of TJ function. The identification of cell polarity protein complexes like the PAR-3–aPKC–PAR-6 complex and the CRB3–Pals1–PATJ complex at TJs has added new aspects on the mechanisms underlying the development of TJs. The identification of the nectin–afadin system provided evidence for a second major adhesive system besides the cadherin–catenin system at AJs. It also became evident that TJs and AJs are signaling centers which are actively engaged in regulating proliferation and differentiation through feed-back mechanisms with the cytoskeleton and the nucleus.
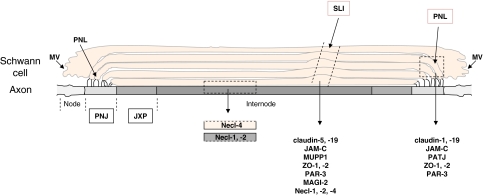
Meanwhile, many of the proteins that regulate formation and maintenance of cell–cell contacts in epithelial cells have been found in other cellular systems as well suggesting a general function in cell–cell contact regulation. Claudins, nectins, and JAMs are used by cells of the male reproductive system to mediate homotypic Sertoli–Sertoli cell as well as heterotypic Sertoli cell–spermatid interactions (Gliki et al. 2004; Gow et al. 1999; Takai and Nakanishi 2003), and similar functions are performed by these molecules as those proposed in epithelial cells, i.e. formation of TJ strands, regulation of the actin cytoskeleton and regulation of cellular polarization, respectively (Gliki et al. 2004; Gow et al. 1999; Ozaki-Kuroda et al. 2002). In the peripheral nervous system (PNS), a large number of proteins typically associated with TJs or AJs was found to mediate autotypic Schwann cell interactions within the myelin sheath as well as heterotypic Schwann cell–axon interactions. For example, different claudins, JAM-C, Necl-1, Necl-2, Necl-4, and various scaffolding proteins like MUPP1, PATJ, ZO-1, ZO-2, PAR-3 and MAGI-2 are localized at areas of non-compact myelin including Schmidt-Lanterman-incisures, paranodal loops, and mesaxons, and some proteins preferentially localize to some but not other areas (Maurel et al. 2007; Poliak et al. 2002; Spiegel et al. 2007) (Fig. 10). The absence of claudin-19 or JAM-C in mice results in defective nerve conduction indicating that both are critically important for the proper functioning of the PNS (Miyamoto et al. 2005; Scheiermann et al. 2007). Necl-1 and Necl-4 mediate heterotypic interactions between axons and Schwann cells during myelination (Maurel et al. 2007; Spiegel et al. 2007). And PAR-3 has recently been identified at the interface between pre-myelinating Schwann cells and axons along the internodal region (Chan et al. 2006). PAR-3 is expressed by the Schwann cells, not by the axon, and recruits the p75 neurotrophin receptor to the glial–axon junction to regulate myelination of the axon.
Fig. 10.
Proteins at autotypic and heterotypic cell–cell contacts in the peripheral nervous system (PNS). A multitude of proteins localized at TJs of polarized epithelial cells localizes to autotypic glial–glial cell contacts and heteroytpic glial cell–axon contacts. Abbreviations: JXP juxtaparanodal region, MV microvilli, PNL paranodal loops, PNJ paranodal junction, SLI Schmidt-Lanterman incisure
It is not clear if these proteins form the same complexes like in epithelial cells, and it is likely that differences exist in the composition of protein complexes to regulate the specific requirements in the context of the given cell or tissue. As one example, JAM-C deficiency in mice leads to a mislocalization of PAR-6, aPKC and PATJ bot not PAR-3 in spermatids (Gliki et al. 2004) suggesting that PAR-3 is not part of the JAM-C-associated polarity complex in spermatids despite its ability to interact with PAR-6, aPKC and also directly with JAM-C (Ebnet et al. 2003; Suzuki and Ohno 2006). As another example, in endothelial cells two PAR protein complexes have been identified, a “conventional” PAR-3–aPKC–PAR-6 complex and a second PAR complex in which PAR-3 and PAR-6 are independently associated with VE-cadherin and which lacks aPKC (Iden et al. 2006). Nevertheless, the use of a set of conserved proteins by morphologically diverse cell types to regulate cell–cell contact formation highlights both the importance of the proteins for cellular function as well as their versatility that allows the regulation of similar aspects in different cell types.
Many open questions remain. For example, what is the molecular nature of the intramembrane diffusion barrier (fence function) of the TJs? The absence of TJ strands in cells lacking all three ZO proteins results in a complete loss of the barrier function of the epithelial sheet but, unexpectedly, the fence function which has been attributed to the presence of TJ strands is retained (Umeda et al. 2006). Membrane diffusion barriers exist also in other cells even in the absence of a physical cell–cell contact, for example at the axonal hillock of neurons to separate somatodendritic and axonal membrane domains (Winckler et al. 1999). It has been suggested that in these cells the accumulation of integral membrane proteins that are anchored to the submembranous cytoskeleton function as rows of pickets which prevent the free diffusion of even small molecules (Nakada et al. 2003). A second unresolved issue is if the activity of the PAR-3–aPKC–PAR-6 complex to regulate membrane asymmetry is mechanistically linked to its role in TJ formation. Finally, the functional interrelationship of the three major polarity complexes at TJs (depicted in Figs 3, 4) has not been resolved, yet. Members of individual complexes interact with each other. For example, CRB3 can also interact with PAR-6 (Lemmers et al. 2004), PAR-6 can also interact with Pals1 (Hurd et al. 2003), and ZO-3 can interact with PATJ (Roh et al. 2002a). Genetic evidence in Drosophila suggests a functional hierarchy among the protein complexes (Johnson and Wodarz 2003) but it is unclear if a similar hierarchy exists in vertebrate epithelial cells and which aspects of cell–cell contact formation are regulated by these interactions. Most likely, many of these interactions are dynamically regulated and occur in a temporally and spatially restricted manner. The large number of scaffolding and signaling molecules identified at cell–cell contacts and the multitude of physical interactions described so far among these proteins indicates that cell–cell contact formation, the development of TJs from pAJs and the aquisition of membrane polarity is a highly dynamic process the complexity of which is still far from being completely understood.
Acknowledgments
I would like to thank Volker Gerke for discussions and continuous support. I would also like to thank Karl Matter for helpful discussions and Atsushi Suzuki for critically reading the manuscript and for ongoing collaborations. I apologize to authors of work in the field that could not be cited due to space limitations. Work from my group is supported by grants from the German Research Foundation (DFG) and from the Medical Faculty of the University Münster.
References
- Adams CL, Nelson WJ, Smith SJ (1996) Quantitative analysis of cadherin-catenin-actin reorganization during development of cell–cell adhesion. J Cell Biol 135:1899–1911 [DOI] [PMC free article] [PubMed]
- Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S (2003) Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430–1434 [DOI] [PMC free article] [PubMed]
- Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A (1997) Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res 235:374–384 [DOI] [PubMed]
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, Pawson T, Muthuswamy SK (2006) Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol 8:1235–1245 [DOI] [PubMed]
- Asada M, Irie K, Morimoto K, Yamada A, Ikeda W, Takeuchi M, Takai Y (2003) ADIP, a novel Afadin- and alpha-actinin-binding protein localized at cell–cell adherens junctions. J Biol Chem 278:4103–4111 [DOI] [PubMed]
- Asakura T, Nakanishi H, Sakisaka T, Takahashi K, Mandai K, Nishimura M, Sasaki T, Takai Y (1999) Similar and differential behaviour between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells 4:573–581 [DOI] [PubMed]
- Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR (2005) Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci USA 102:16339–16344 [DOI] [PMC free article] [PubMed]
- Balda MS, Garrett MD, Matter K (2003) The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol 160:423–432 [DOI] [PMC free article] [PubMed]
- Balda MS, Matter K (2000) The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. Embo J 19:2024–2033 [DOI] [PMC free article] [PubMed]
- Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW, Luga V, Przulj N, Robinson M, Suzuki H, Hayashizaki Y, Jurisica I, Wrana JL (2005) High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307:1621–1625 [DOI] [PubMed]
- Benton R, St Johnston D (2003) Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115:691–704 [DOI] [PubMed]
- Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L (2004) The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res 292:51–66 [DOI] [PubMed]
- Betson M, Lozano E, Zhang J, Braga VM (2002) Rac activation upon cell–cell contact formation is dependent on signaling from the epidermal growth factor receptor. J Biol Chem 277:36962–36969 [DOI] [PubMed]
- Bilder D (2004) Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev 18:1909–1925 [DOI] [PubMed]
- Bilder D, Schober M, Perrimon N (2003) Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol 5:53–58 [DOI] [PubMed]
- Bissell MJ, Radisky D (2001) Putting tumours in context. Nat Rev Cancer 1:46–54 [DOI] [PMC free article] [PubMed]
- Bohm H, Brinkmann V, Drab M, Henske A, Kurzchalia TV (1997) Mammalian homologues of C. elegans PAR-1 are asymmetrically localized in epithelial cells and may influence their polarity. Curr Biol 7:603–606 [DOI] [PubMed]
- Bratt A, Wilson WJ, Troyanovsky B, Aase K, Kessler R, Van Meir EG, Holmgren L (2002) Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene 298:69–77 [DOI] [PubMed]
- Bryant DM, Stow JL (2004) The ins and outs of E-cadherin trafficking. Trends Cell Biol 14:427–434 [DOI] [PubMed]
- Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M (2006) The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science 314:832–836 [DOI] [PubMed]
- Chen X, Macara IG (2005) Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 7:262–269 [DOI] [PubMed]
- Chen X, Macara IG (2006) Par-3 mediates the inhibition of LIM kinase 2 to regulate cofilin phosphorylation and tight junction assembly. J Cell Biol 172:671–678 [DOI] [PMC free article] [PubMed]
- Chen YT, Stewart DB, Nelson WJ (1999) Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol 144:687–699 [DOI] [PMC free article] [PubMed]
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM (2001) The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA 98:15191–15196 [DOI] [PMC free article] [PubMed]
- Coyne CB, Voelker T, Pichla SL, Bergelson JM (2004) The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J Biol Chem 279:48079–48084 [DOI] [PubMed]
- Davis MA, Ireton RC, Reynolds AB (2003) A core function for p120-catenin in cadherin turnover. J Cell Biol 163:525–534 [DOI] [PMC free article] [PubMed]
- Debnath J, Brugge JS (2005) Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer 5:675–688 [DOI] [PubMed]
- Dow LE, Humbert PO (2007) Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol 262:253–302 [DOI] [PubMed]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI (2005) Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123:903–915 [DOI] [PMC free article] [PubMed]
- Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, Meyer Zu Brickwedde MK, Suzuki A, Imhof BA, Vestweber D (2003) The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: A possible role for JAMs in endothelial cell polarity. J Cell Sci 116:3879–3891 [DOI] [PubMed]
- Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D (2000) Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem 275:27979–27988 [DOI] [PubMed]
- Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D (2001) The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). Embo J 20:3738–3748 [DOI] [PMC free article] [PubMed]
- Ebnet K, Suzuki A, Ohno S, Vestweber D (2004) Junctional adhesion molecules (JAMs): More molecules with dual functions? J Cell Sci 117:19–29 [DOI] [PubMed]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273:29745–29753 [DOI] [PubMed]
- Fanning AS, Ma TY, Anderson JM (2002) Isolation and functional characterization of the actin-binding region in the tight junction protein ZO-1. Faseb J 16:1835–1837 [DOI] [PubMed]
- Fogg VC, Liu CJ, Margolis B (2005) Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J Cell Sci 118:2859–2869 [DOI] [PubMed]
- Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116:769–778 [DOI] [PubMed]
- Fukuhara A, Shimizu K, Kawakatsu T, Fukuhara T, Takai Y (2003) Involvement of nectin-activated Cdc42 small G protein in organization of adherens and tight junctions in Madin-Darby canine kidney cells. J Biol Chem 278:51885–51893 [DOI] [PubMed]
- Fukuhara T, Shimizu K, Kawakatsu T, Fukuyama T, Minami Y, Honda T, Hoshino T, Yamada T, Ogita H, Okada M, Takai Y (2004) Activation of Cdc42 by trans interactions of the cell adhesion molecules nectins through c-Src and Cdc42-GEF FRG. J Cell Biol 166:393–405 [DOI] [PMC free article] [PubMed]
- Fukuyama T, Ogita H, Kawakatsu T, Fukuhara T, Yamada T, Sato T, Shimizu K, Nakamura T, Matsuda M, Takai Y (2005) Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. J Biol Chem 280:815–825 [DOI] [PubMed]
- Funke L, Dakoji S, Bredt DS (2005) Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem 74:219–245 [DOI] [PubMed]
- Furuse M, Sasaki H, Fujimoto K, Tsukita S (1998) A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143:391–401 [DOI] [PMC free article] [PubMed]
- Furuse M, Tsukita S (2006) Claudins in occluding junctions of humans and flies. Trends Cell Biol 16:181–188 [DOI] [PubMed]
- Gao L, Joberty G, Macara IG (2002) Assembly of epithelial tight junctions is negatively regulated by Par6. Curr Biol 12:221–225 [DOI] [PubMed]
- Garrard SM, Capaldo CT, Gao L, Rosen MK, Macara IG, Tomchick DR (2003) Structure of Cdc42 in a complex with the GTPase-binding domain of the cell polarity protein, Par6. Embo J 22:1125–1133 [DOI] [PMC free article] [PubMed]
- Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH (2004) Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature 431:320–324 [DOI] [PubMed]
- Goldstein B, Macara IG (2007) The par proteins: fundamental players in animal cell polarization. Dev Cell 13:609–622 [DOI] [PMC free article] [PubMed]
- Gonzalez-Mariscal L, Ponce A, Alarcon L, Jaramillo BE (2006) The tight junction protein ZO-2 has several functional nuclear export signals. Exp Cell Res 312:3323–3335 [DOI] [PubMed]
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA (1999) CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 99:649–659 [DOI] [PubMed]
- Gumbiner BM (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345–357 [DOI] [PubMed]
- Gumbiner BM (2005) Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6:622–634 [DOI] [PubMed]
- Halbleib JM, Nelson WJ (2006) Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20:3199–3214 [DOI] [PubMed]
- Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S (2002) Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem 277:455–461 [DOI] [PubMed]
- Hanakawa Y, Schechter NM, Lin C, Nishifuji K, Amagai M, Stanley JR (2004) Enzymatic and molecular characteristics of the efficiency and specificity of exfoliative toxin cleavage of desmoglein 1. J Biol Chem 279:5268–5277 [DOI] [PubMed]
- Hatakeyama M (2004) Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer 4:688–694 [DOI] [PubMed]
- Hatzfeld M (2005) The p120 family of cell adhesion molecules. Eur J Cell Biol 84:205–214 [DOI] [PubMed]
- Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M (2002) SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683–686 [DOI] [PubMed]
- Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y (2003) JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol 23:4267–4282 [DOI] [PMC free article] [PubMed]
- Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, Suzuki Y, Yamanaka T, Suzuki A, Mizuno K, Ohno S (2002) Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci 115:2485–2495 [DOI] [PubMed]
- Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A (2003) Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci 116:725–742 [DOI] [PubMed]
- Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558 [DOI] [PubMed]
- Humbert P, Russell S, Richardson H (2003) Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays 25:542–553 [DOI] [PubMed]
- Hurd TW, Gao L, Roh MH, Macara IG, Margolis B (2003) Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol 5:137–142 [DOI] [PubMed]
- Hurov JB, Watkins JL, Piwnica-Worms H (2004) Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol 14:736–741 [DOI] [PubMed]
- Iden S, Rehder D, August B, Suzuki A, Wolburg-Buchholz K, Wolburg H, Ohno S, Behrens J, Vestweber D, Ebnet K (2006) A distinct PAR complex associates physically with VE-cadherin in vertebrate endothelial cells. EMBO Rep 7:1239–1246 [DOI] [PMC free article] [PubMed]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S (2005) Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171:939–945 [DOI] [PMC free article] [PubMed]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147:1351–1363 [DOI] [PMC free article] [PubMed]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S (1997) Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol 138:181–192 [DOI] [PMC free article] [PubMed]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S (1993) The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol 121:491–502 [DOI] [PMC free article] [PubMed]
- Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S (2001) Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol 154:491–498 [DOI] [PMC free article] [PubMed]
- Jaramillo BE, Ponce A, Moreno J, Betanzos A, Huerta M, Lopez-Bayghen E, Gonzalez-Mariscal L (2004) Characterization of the tight junction protein ZO-2 localized at the nucleus of epithelial cells. Exp Cell Res 297:247–258 [DOI] [PubMed]
- Joberty G, Petersen C, Gao L, Macara IG (2000) The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2:531–539 [DOI] [PubMed]
- Johnson K, Wodarz A (2003) A genetic hierarchy controlling cell polarity. Nat Cell Biol 5:12–14 [DOI] [PubMed]
- Kallay LM, McNickle A, Brennwald PJ, Hubbard AL, Braiterman LT (2006) Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem 99:647–664 [DOI] [PubMed]
- Kausalya PJ, Phua DC, Hunziker W (2004) Association of ARVCF with zonula occludens (ZO)-1 and ZO-2: binding to PDZ-domain proteins and cell–cell adhesion regulate plasma membrane and nuclear localization of ARVCF. Mol Biol Cell 15:5503–5515 [DOI] [PMC free article] [PubMed]
- Kawakatsu T, Ogita H, Fukuhara T, Fukuyama T, Minami Y, Shimizu K, Takai Y (2005) Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J Biol Chem 280:4940–4947 [DOI] [PubMed]
- Kawakatsu T, Shimizu K, Honda T, Fukuhara T, Hoshino T, Takai Y (2002) Trans-interactions of nectins induce formation of Filopodia and Lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J Biol Chem 277:50749–50755 [DOI] [PubMed]
- Kemphues K (2000) PARsing embryonic polarity. Cell 101:345–348 [DOI] [PubMed]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS (1988) Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52:311–320 [DOI] [PubMed]
- Kim M, Datta A, Brakeman P, Yu W, Mostov KE (2007) Polarity proteins PAR6 and aPKC regulate cell death through GSK-3beta in 3D epithelial morphogenesis. J Cell Sci 120:2309–2317 [DOI] [PubMed]
- Knust E, Bossinger O (2002) Composition and formation of intercellular junctions in epithelial cells. Science 298:1955–1959 [DOI] [PubMed]
- Kobielak A, Pasolli HA, Fuchs E (2004) Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 6:21–30 [DOI] [PMC free article] [PubMed]
- Kovacs EM, Ali RG, McCormack AJ, Yap AS (2002) E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem 277:6708–6718 [DOI] [PubMed]
- Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, Le Bivic A (2004) CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell 15:1324–1333 [DOI] [PMC free article] [PubMed]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7:678–689 [DOI] [PubMed]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T (2000) A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2:540–547 [DOI] [PubMed]
- Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P (1998) The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood 92:4602–4611 [PubMed]
- Makarova O, Roh MH, Liu CJ, Laurinec S, Margolis B (2003) Mammalian Crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1). Gene 302:21–29 [DOI] [PubMed]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y (1997) Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol 139:517–528 (published erratum appears in J Cell Biol 1997 Nov 17;139(4):1060) [DOI] [PMC free article] [PubMed]
- Mandai K, Nakanishi H, Satoh A, Takahashi K, Satoh K, Nishioka H, Mizoguchi A, Takai Y (1999) Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell–cell and cell-matrix adherens junctions. J Cell Biol 144:1001–1017 [DOI] [PMC free article] [PubMed]
- Mandell KJ, Babbin BA, Nusrat A, Parkos CA (2005) Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem 280:11665–11674 [DOI] [PubMed]
- Mandicourt G, Iden S, Ebnet K, Aurrand-Lions M, Imhof BA (2007) JAM-C regulates tight junctions and integrin-mediated cell adhesion and migration. J Biol Chem 282:1830–1837 [DOI] [PubMed]
- Marie H, Pratt SJ, Betson M, Epple H, Kittler JT, Meek L, Moss SJ, Troyanovsky S, Attwell D, Longmore GD, Braga VM (2003) The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with alpha-catenin. J Biol Chem 278:1220–1228 [DOI] [PubMed]
- Martinez-Rico C, Pincet F, Perez E, Thiery JP, Shimizu K, Takai Y, Dufour S (2005) Separation force measurements reveal different types of modulation of E-cadherin-based adhesion by nectin-1 and -3. J Biol Chem 280:4753–4760 [DOI] [PubMed]
- Mathew D, Gramates LS, Packard M, Thomas U, Bilder D, Perrimon N, Gorczyca M, Budnik V (2002) Recruitment of scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr Biol 12:531–539 [DOI] [PMC free article] [PubMed]
- Matter K, Balda MS (2003) Signalling to and from tight junctions. Nat Rev Mol Cell Biol 4:225–236 [DOI] [PubMed]
- Matter K, Balda MS (2007) Epithelial tight junctions, gene expression and nucleo-junctional interplay. J Cell Sci 120:1505–1511 [DOI] [PubMed]
- Maurel P, Einheber S, Galinska J, Thaker P, Lam I, Rubin MB, Scherer SS, Murakami Y, Gutmann DH, Salzer JL (2007) Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol 178:861–874 [DOI] [PMC free article] [PubMed]
- McNeil E, Capaldo CT, Macara IG (2006) Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 17:1922–1932 [DOI] [PMC free article] [PubMed]
- McNeill H, Ryan TA, Smith SJ, Nelson WJ (1993) Spatial and temporal dissection of immediate and early events following cadherin-mediated epithelial cell adhesion. J Cell Biol 120:1217–1226 [DOI] [PMC free article] [PubMed]
- Medina E, Lemmers C, Lane-Guermonprez L, Le Bivic A (2002) Role of the Crumbs complex in the regulation of junction formation in Drosophila and mammalian epithelial cells. Biol Cell 94:305–313 [DOI] [PubMed]
- Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG (2005) The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol 170:1029–1037 [DOI] [PMC free article] [PubMed]
- Michel D, Arsanto JP, Massey-Harroche D, Beclin C, Wijnholds J, Le Bivic A (2005) PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J Cell Sci 118:4049–4057 [DOI] [PubMed]
- Miyamoto T, Morita K, Takemoto D, Takeuchi K, Kitano Y, Miyakawa T, Nakayama K, Okamura Y, Sasaki H, Miyachi Y, Furuse M, Tsukita S (2005) Tight junctions in Schwann cells of peripheral myelinated axons: a lesson from claudin-19-deficient mice. J Cell Biol 169:527–538 [DOI] [PMC free article] [PubMed]
- Mizuno K, Suzuki A, Hirose T, Kitamura K, Kutsuzawa Y, Futaki M, Amano Y, Ohno S (2003) Self-association of PAR-3 mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctionsr. J Biol Chem 278:31240–31250 [DOI] [PubMed]
- Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S (2002) Regulated protein–protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells 7:1161–1171 [DOI] [PubMed]
- Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, Kasai RS, Yamaguchi K, Fujiwara T, Kusumi A (2003) Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol 5:626–632 [DOI] [PubMed]
- Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K (2001) Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell–cell adhesion sites. J Cell Sci 114:1829–1838 [DOI] [PubMed]
- Nelson WJ (2003) Adaptation of core mechanisms to generate cell polarity. Nature 422:766–774 [DOI] [PMC free article] [PubMed]
- Nelson WJ, Nusse R (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483–1487 [DOI] [PMC free article] [PubMed]
- Nishimura M, Kakizaki M, Ono Y, Morimoto K, Takeuchi M, Inoue Y, Imai T, Takai Y (2002) JEAP, a novel component of tight junctions in exocrine cells. J Biol Chem 277:5583–5587 [DOI] [PubMed]
- Noren NK, Liu BP, Burridge K, Kreft B (2000) p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 150:567–580 [DOI] [PMC free article] [PubMed]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K (2001) Cadherin engagement regulates Rho family GTPases. J Biol Chem 276:33305–33308 [DOI] [PubMed]
- Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL 3rd, Sontag E (2002) Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol 158:967–978 [DOI] [PMC free article] [PubMed]
- Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA (2001) Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun 69:1329–1336 [DOI] [PMC free article] [PubMed]
- O’Brien LE, Zegers MM, Mostov KE (2002) Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3:531–537 [DOI] [PubMed]
- Ohno S (2001) Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol 13:641–648 [DOI] [PubMed]
- Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, Okamoto R, Nakata S, Miki A, Irie K, Takai Y (2007) Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J Cell Sci 120:2352–2365 [DOI] [PubMed]
- Ooshio T, Irie K, Morimoto K, Fukuhara A, Imai T, Takai Y (2004) Involvement of LMO7 in the association of two cell–cell adhesion molecules, nectin and E-cadherin, through Afadin and alpha-actinin in epithelial cells. J Biol Chem 279:31365–31373 [DOI] [PubMed]
- Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, Nishimune Y, Takai Y (2002) Nectin couples cell–cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol 12:1145–1150 [DOI] [PubMed]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL (2005) Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307:1603–1609 [DOI] [PubMed]
- Patrie KM (2005) Identification and characterization of a novel tight junction-associated family of proteins that interacts with a WW domain of MAGI-1. Biochim Biophys Acta 1745:131–144 [DOI] [PubMed]
- Pawson T, Nash P (2003) Assembly of cell regulatory systems through protein interaction domains. Science 300:445–452 [DOI] [PubMed]
- Peek RM Jr, Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2:28–37 [DOI] [PubMed]
- Pentecost M, Otto G, Theriot JA, Amieva MR (2006) Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoS Pathog 2:29–40 [DOI] [PMC free article] [PubMed]
- Perez-Moreno M, Fuchs E (2006) Catenins: keeping cells from getting their signals crossed. Dev Cell 11:601–612 [DOI] [PMC free article] [PubMed]
- Perez-Moreno M, Jamora C, Fuchs E (2003) Sticky business: orchestrating cellular signals at adherens junctions. Cell 112:535–548 [DOI] [PubMed]
- Peterson FC, Penkert RR, Volkman BF, Prehoda KE (2004) Cdc42 regulates the Par-6 PDZ domain through an allosteric CRIB-PDZ transition. Mol Cell 13:665–676 [DOI] [PubMed]
- Pielage J, Stork T, Bunse I, Klambt C (2003) The Drosophila cell survival gene discs lost encodes a cytoplasmic Codanin-1-like protein, not a homolog of tight junction PDZ protein Patj. Dev Cell 5:841–851 [DOI] [PubMed]
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T (2003) A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol 5:301–308 [DOI] [PubMed]
- Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI (2002) Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem 277:18868–18874 [DOI] [PMC free article] [PubMed]
- Pokutta S, Weis WI (2007) Structure and mechanism of cadherins and catenins in cell–cell contacts. Annu Rev Cell Dev Biol 23:237–261 [DOI] [PubMed]
- Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E (2002) Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J Cell Biol 159:361–372 [DOI] [PMC free article] [PubMed]
- Pradhan D, Lombardo CR, Roe S, Rimm DL, Morrow JS (2001) alpha-Catenin binds directly to spectrin and facilitates spectrin-membrane assembly in vivo. J Biol Chem 276:4175–4181 [DOI] [PubMed]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG (2005) The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol 171:1061–1071 [DOI] [PMC free article] [PubMed]
- Raschperger E, Engstrom U, Pettersson RF, Fuxe J (2003) CLMP, a novel member of the CTX family and a new component of epithelial tight junctions. J Biol Chem 279:796–804 [DOI] [PubMed]
- Rehder D, Iden S, Nasdala I, Wegener J, Brickwedde MK, Vestweber D, Ebnet K (2006) Junctional adhesion molecule-A participates in the formation of apico-basal polarity through different domains. Exp Cell Res 312:3389–3403 [DOI] [PubMed]
- Reymond N, Fabre S, Lecocq E, Adelaide J, Dubreuil P, Lopez M (2001) Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem 276:43205–43215 [DOI] [PubMed]
- Reynolds AB, Roczniak-Ferguson A (2004) Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene 23:7947–7956 [DOI] [PubMed]
- Richnau N, Aspenstrom P (2001) Rich, a rho GTPase-activating protein domain-containing protein involved in signaling by Cdc42 and Rac1. J Biol Chem 276:35060–35070 [DOI] [PubMed]
- Richnau N, Fransson A, Farsad K, Aspenstrom P (2004) RICH-1 has a BIN/Amphiphysin/Rvsp domain responsible for binding to membrane lipids and tubulation of liposomes. Biochem Biophys Res Commun 320:1034–1042 [DOI] [PubMed]
- Roh MH, Fan S, Liu CJ, Margolis B (2003) The Crumbs3-Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J Cell Sci 116:2895–2906 [DOI] [PubMed]
- Roh MH, Liu CJ, Laurinec S, Margolis B (2002a) The carboxyl terminus of zona occludens-3 binds and recruits a Mammalian homologue of discs lost to tight junctions. J Biol Chem 277:27501–27509 [DOI] [PubMed]
- Roh MH, Makarova O, Liu CJ, Shin K, Lee S, Laurinec S, Goyal M, Wiggins R, Margolis B (2002b) The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J Cell Biol 157:161–172 [DOI] [PMC free article] [PubMed]
- Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A, Ohno S, Hatakeyama M (2007) Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447:330–333 [DOI] [PubMed]
- Sahai E, Marshall CJ (2002) ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol 4:408–415 [DOI] [PubMed]
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y (2007) The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol 19:593–602 [DOI] [PubMed]
- Sakisaka T, Takai Y (2004) Biology and pathology of nectins and nectin-like molecules. Curr Opin Cell Biol 16:513–521 [DOI] [PubMed]
- Sato T, Fujita N, Yamada A, Ooshio T, Okamoto R, Irie K, Takai Y (2006) Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J Biol Chem 281:5288–5299 [DOI] [PubMed]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y (2000) Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell–cell adhesion activities. J Biol Chem 275:10291–10299 [DOI] [PubMed]
- Scheiermann C, Meda P, Aurrand-Lions M, Madani R, Yiangou Y, Coffey P, Salt TE, Ducrest-Gay D, Caille D, Howell O, Reynolds R, Lobrinus A, Adams RH, Yu AS, Anand P, Imhof BA, Nourshargh S (2007) Expression and function of junctional adhesion molecule-C in myelinated peripheral nerves. Science 318:1472–1475 [DOI] [PMC free article] [PubMed]
- Shin K, Straight S, Margolis B (2005) PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J Cell Biol 168:705–711 [DOI] [PMC free article] [PubMed]
- Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, Balda MS (2006) Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol 26:2387–2398 [DOI] [PMC free article] [PubMed]
- Spiegel I, Adamsky K, Eshed Y, Milo R, Sabanay H, Sarig-Nadir O, Horresh I, Scherer SS, Rasband MN, Peles E (2007) A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat Neurosci 10:861–869 [DOI] [PMC free article] [PubMed]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA (1986) Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103:755–766 [DOI] [PMC free article] [PubMed]
- Straight SW, Shin K, Fogg VC, Fan S, Liu CJ, Roh M, Margolis B (2004) Loss of PALS1 expression leads to tight junction and polarity defects. Mol Biol Cell 15:1981–1990 [DOI] [PMC free article] [PubMed]
- Stucke VM, Timmerman E, Vandekerckhove J, Gevaert K, Hall A (2007) The MAGUK protein MPP7 binds to the polarity protein hDlg1 and facilitates epithelial tight junction formation. Mol Biol Cell 18:1744–1755 [DOI] [PMC free article] [PubMed]
- Sugihara-Mizuno Y, Adachi M, Kobayashi Y, Hamazaki Y, Nishimura M, Imai T, Furuse M, Tsukita S (2007) Molecular characterization of angiomotin/JEAP family proteins: interaction with MUPP1/Patj and their endogenous properties. Genes Cells 12:473–486 [DOI] [PubMed]
- Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, Miwa Y, Ohno S (2004) aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol 14:1425–1435 [DOI] [PubMed]
- Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S (2002) aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci 115:3565–3573 [DOI] [PubMed]
- Suzuki A, Ohno S (2006) The PAR-aPKC system: lessons in polarity. J Cell Sci 119:979–987 [DOI] [PubMed]
- Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S (2001) Atypical protein kinase C is involved in the evolutionary conserved PAR protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol 152:1183–1196 [DOI] [PMC free article] [PubMed]
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y (2000) Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol 150:1161–1176 [DOI] [PMC free article] [PubMed]
- Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y (1999) Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol 145:539–549 [DOI] [PMC free article] [PubMed]
- Takai Y, Nakanishi H (2003) Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci 116:17–27 [DOI] [PubMed]
- Takeichi M (1977) Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol 75:464–474 [DOI] [PMC free article] [PubMed]
- Tanentzapf G, Tepass U (2003) Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol 5:46–52 [DOI] [PubMed]
- Tepass U, Tanentzapf G, Ward R, Fehon R (2001) Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet 35:747–784 [DOI] [PubMed]
- Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454 [DOI] [PubMed]
- Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H (2003) The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J Biol Chem 278:2692–2700 [DOI] [PubMed]
- Tsapara A, Matter K, Balda MS (2006) The heat-shock protein Apg-2 binds to the tight junction protein ZO-1 and regulates transcriptional activity of ZONAB. Mol Biol Cell 17:1322–1330 [DOI] [PMC free article] [PubMed]
- Tsukita S, Furuse M (2000) Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol 149:13–16 [DOI] [PMC free article] [PubMed]
- Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293 [DOI] [PubMed]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M (2006) ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126:741–754 [DOI] [PubMed]
- Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S (2004) Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem 279:44785–44794 [DOI] [PubMed]
- Van Itallie CM, Anderson JM (2006) Claudins and Epithelial Paracellular Transport. Annu Rev Physiol 68:403–429 [DOI] [PubMed]
- van Roy FM, McCrea PD (2005) A role for Kaiso-p120ctn complexes in cancer? Nat Rev Cancer 5:956–964 [DOI] [PubMed]
- Wang X, Nie J, Zhou Q, Liu W, Zhu F, Chen W, Mao H, Luo N, Dong X, Yu X (2008) Downregulation of Par-3 expression and disruption of Par complex integrity by TGF-beta during the process of epithelial to mesenchymal transition in rat proximal epithelial cells. Biochim Biophys Acta 1782:51–59 [DOI] [PubMed]
- Wegmann F, Ebnet K, Du Pasquier L, Vestweber D, Butz S (2004) Endothelial adhesion molecule ESAM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp Cell Res 300:121–133 [DOI] [PubMed]
- Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, Colwill K, Starostine A, Metalnikov P, Pawson T (2006) A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 125:535–548 [DOI] [PubMed]
- Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB (2006) p120-catenin and p190RhoGAP regulate cell–cell adhesion by coordinating antagonism between Rac and Rho. Cell 127:1027–1039 [DOI] [PubMed]
- Williams AF, Barclay AN (1988) The immunoglobulin superfamily-domains for cell surface recognition. Annu Rev Immunol 6:381–405 [DOI] [PubMed]
- Winckler B, Forscher P, Mellman I (1999) A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397:698–701 [DOI] [PubMed]
- Wittchen ES, Haskins J, Stevenson BR (1999) Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem 274:35179–35185 [DOI] [PubMed]
- Wodarz A, Nathke I (2007) Cell polarity in development and cancer. Nat Cell Biol 9:1016–1024 [DOI] [PubMed]
- Wu S, Lim KC, Huang J, Saidi RF, Sears CL (1998) Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci U S A 95:14979–14984 [DOI] [PMC free article] [PubMed]
- Wu Z, Nybom P, Magnusson KE (2000) Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cellular microbiology 2:11–17 [DOI] [PubMed]
- Yamada S, Nelson WJ (2007) Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol 178:517–527 [DOI] [PMC free article] [PubMed]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ (2005) Deconstructing the cadherin-catenin-actin complex. Cell 123:889–901 [DOI] [PMC free article] [PubMed]
- Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S (2006) Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci 119:2107–2118 [DOI] [PubMed]
- Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S (2003) Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol 13:734–743 [DOI] [PubMed]
- Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, Ohno S (2001) Par-6 regulates aPKC activity in a novel way and mediates cell–cell contact-induced formation of epithelial junctional complex. Genes Cells 6:721–731 [DOI] [PubMed]
- Yeaman C, Grindstaff KK, Nelson WJ (1999) New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev 79:73–98 [DOI] [PubMed]
- Yonemura S, Itoh M, Nagafuchi A, Tsukita S (1995) Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci 108:127–142 [DOI] [PubMed]
- Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE (2005) Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 288:C1231–1241 [DOI] [PubMed]
- Zhurinsky J, Shtutman M, Ben-Ze’ev A (2000) Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci 113(Pt 18):3127–3139 [DOI] [PubMed]