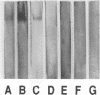
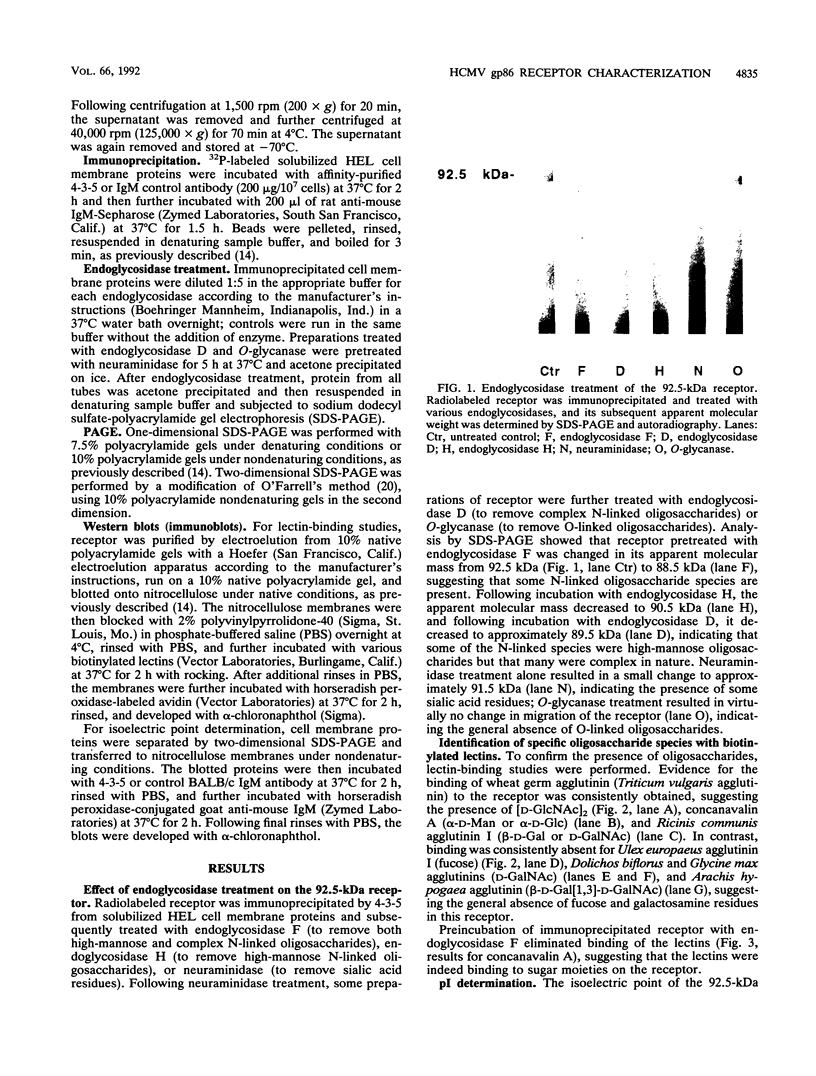
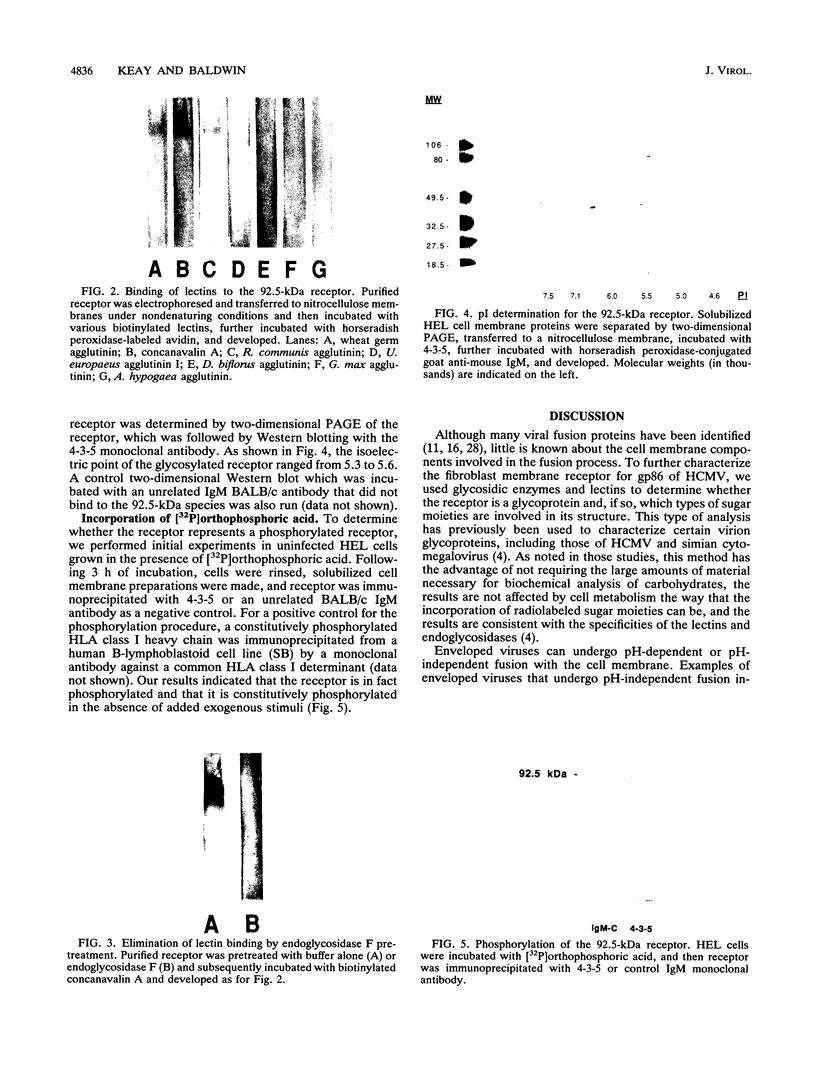
Abstract
A human embryonic lung (HEL) cell receptor for gp86 of human cytomegalovirus that functions in virus-cell fusion was further characterized. Anti-idiotype antibodies that mimic gp86 were used to immunoprecipitate the 92.5-kDa fibroblast membrane receptor for gp86, which was preincubated with various endoglycosidases. The receptor, which has a pI ranging from 5.3 to 5.6, appears to be a glycoprotein with primarily N-linked sugar residues, some of which have high concentrations of mannose and some of which are complex oligosaccharides. Western blots (immunoblots) of electrophoretically transferred receptor incubated with various biotinylated lectins confirmed the presence of sugar moieties, including N-acetylglucosamine, glucose or mannose, and galactose, but not fucose or N-acetylgalactosamine. This gp86 receptor from uninfected HEL cells also incorporated radiolabeled phosphate from orthophosphoric acid, indicating that it is a constitutively phosphorylated receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acres R. B., Conlon P. J., Mochizuki D. Y., Gallis B. Rapid phosphorylation and modulation of the T4 antigen on cloned helper T cells induced by phorbol myristate acetate or antigen. J Biol Chem. 1986 Dec 5;261(34):16210–16214. [PubMed] [Google Scholar]
- Adlish J. D., Lahijani R. S., St Jeor S. C. Identification of a putative cell receptor for human cytomegalovirus. Virology. 1990 Jun;176(2):337–345. doi: 10.1016/0042-6822(90)90003-a. [DOI] [PubMed] [Google Scholar]
- Bar-Zvi D., Branton D. Clathrin-coated vesicles contain two protein kinase activities. Phosphorylation of clathrin beta-light chain by casein kinase II. J Biol Chem. 1986 Jul 25;261(21):9614–9621. [PubMed] [Google Scholar]
- Benko D. M., Gibson W. Primate cytomegalovirus glycoproteins: lectin-binding properties and sensitivities to glycosidases. J Virol. 1986 Sep;59(3):703–713. doi: 10.1128/jvi.59.3.703-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F., Cambiaggi C., Maccari J., Burastero S., Gregory T., Patzer E., Porter J., McDanal C., Matthews T. Antibody raised against soluble CD4-rgp120 complex recognizes the CD4 moiety and blocks membrane fusion without inhibiting CD4-gp120 binding. J Exp Med. 1990 Oct 1;172(4):1143–1150. doi: 10.1084/jem.172.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changelian P. S., Fearon D. T. Tissue-specific phosphorylation of complement receptors CR1 and CR2. J Exp Med. 1986 Jan 1;163(1):101–115. doi: 10.1084/jem.163.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., McKeating J. A., Ward P. J., Sanderson A. R., Griffiths P. D. Beta 2 microglobulin enhances the infectivity of cytomegalovirus and when bound to the virus enables class I HLA molecules to be used as a virus receptor. J Gen Virol. 1987 Mar;68(Pt 3):793–803. doi: 10.1099/0022-1317-68-3-793. [DOI] [PubMed] [Google Scholar]
- Healey D., Dianda L., Moore J. P., McDougal J. S., Moore M. J., Estess P., Buck D., Kwong P. D., Beverley P. C., Sattentau Q. J. Novel anti-CD4 monoclonal antibodies separate human immunodeficiency virus infection and fusion of CD4+ cells from virus binding. J Exp Med. 1990 Oct 1;172(4):1233–1242. doi: 10.1084/jem.172.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K. Sendai virus-erythrocyte membrane interaction: quantitative and kinetic analysis of viral binding, dissociation, and fusion. J Virol. 1986 Apr;58(1):87–95. doi: 10.1128/jvi.58.1.87-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak I. D., Popovic M., Horak E. M., Lucas P. J., Gress R. E., June C. H., Bolen J. B. No T-cell tyrosine protein kinase signalling or calcium mobilization after CD4 association with HIV-1 or HIV-1 gp120. Nature. 1990 Dec 6;348(6301):557–560. doi: 10.1038/348557a0. [DOI] [PubMed] [Google Scholar]
- Keay S., Baldwin B. Anti-idiotype antibodies that mimic gp86 of human cytomegalovirus inhibit viral fusion but not attachment. J Virol. 1991 Sep;65(9):5124–5128. doi: 10.1128/jvi.65.9.5124-5128.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S., Merigan T. C., Rasmussen L. Identification of cell surface receptors for the 86-kilodalton glycoprotein of human cytomegalovirus. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10100–10103. doi: 10.1073/pnas.86.24.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S., Rasmussen L., Merigan T. C. Syngeneic monoclonal anti-idiotype antibodies that bear the internal image of a human cytomegalovirus neutralization epitope. J Immunol. 1988 Feb 1;140(3):944–948. [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. O., Marsh M., Weiss R. A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988 Feb;7(2):513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Structure, function, and intracellular processing of paramyxovirus membrane proteins. Virus Res. 1988 May;10(2-3):113–135. doi: 10.1016/0168-1702(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Nir S., Klappe K., Hoekstra D. Mass action analysis of kinetics and extent of fusion between Sendai virus and phospholipid vesicles. Biochemistry. 1986 Dec 16;25(25):8261–8266. doi: 10.1021/bi00373a020. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Guild B. C., Strominger J. L. Phosphorylation in vivo and in vitro of human histocompatibility antigens (HLA-A and HLA-B) in the carboxy-terminal intracellular domain. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6002–6006. doi: 10.1073/pnas.75.12.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Choppin P. W. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology. 1983 Dec;131(2):518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Sturman L. S., Ricard C. S., Holmes K. V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: activation of cell-fusing activity of virions by trypsin and separation of two different 90K cleavage fragments. J Virol. 1985 Dec;56(3):904–911. doi: 10.1128/jvi.56.3.904-911.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. P., Cooper N. R. The human cytomegalovirus receptor on fibroblasts is a 30-kilodalton membrane protein. J Virol. 1990 Jun;64(6):2484–2490. doi: 10.1128/jvi.64.6.2484-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Abraham N., Caron L., Davidson D. The lymphocyte-specific tyrosine protein kinase p56lck. Semin Immunol. 1991 May;3(3):143–152. [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- White M. F. Structure and function of tyrosine kinase receptors. J Bioenerg Biomembr. 1991 Feb;23(1):63–82. doi: 10.1007/BF00768839. [DOI] [PubMed] [Google Scholar]