Abstract
α-Synuclein has been implicated in the pathogenesis of Parkinson’s disease (PD). Previous studies have shown that α-synuclein is involved in the regulation of dopamine (DA) metabolism, possibly by down-regulating the expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in DA biosynthesis. In this study, we constructed α-synuclein stably silenced MN9D/α-SYN− cells by vector mediated RNA interference and examined its effects on DA metabolism. We found that there were no significant differences in TH protein and mRNA levels between MN9D, MN9D/α-SYN− and MN9D/CON cells, suggesting that silencing α-synuclein expression does not affect TH gene expression. However, significant increases in phosphorylated TH, cytosolic 3, 4-dihydroxyphenylalanine (l-DOPA) and DA levels were observed in MN9D/α-SYN− cells. Our data show that TH activity and DA biosynthesis were enhanced by down-regulation of α-synuclein, suggesting that α-synuclein may act as a negative regulator of cytosolic DA. With respect to PD pathology, a loss of functional α-synuclein may result in increased DA levels in neurons that may lead to cell injury or even death.
Keywords: α-Synuclein, RNA interference, Dopamine, Parkinson’s disease
Introduction
α-Synuclein is a 140-amino acid protein, which has been implicated in the pathogenesis of Parkinson’s disease (PD) [20, 30]. Previous studies have shown that α-synuclein mutations (A53T, A30P, E46K) are associated with some autosomal-dominant PD [14, 15, 27] and that aggregated α-synuclein is the major component of Lewy bodies and Lewy neurites, pathological hallmarks of PD [2, 21, 34]. Although the normal function of α-synuclein and its role in the pathogenesis of PD remain unclear, several hypotheses have been proposed based on its physical properties or interacting partners [17–19, 25, 28, 31, 36]. One theory suggested that α-synuclein might be linked to PD via the regulation of dopamine (DA) homeostasis [1, 18, 25, 31, 41]. Several studies have shown that α-synuclein may be involved in regulating the biosynthesis, vesicular storage and release, as well as reuptake of DA [3, 9, 26, 32, 33, 38, 40, 42]. However, some of these findings remain controversial. It has also been reported that α-synuclein may down-regulate the gene expression of tyrosine hydroxylase (TH), the rate-limiting enzyme involved in DA biosynthesis [3, 42]. Others report that α-synuclein regulates DA biosynthesis by reducing TH activity [26]. Another study showed that the tyrosine-mediated enhancement of phosphorylated TH in nigrostriatal DA neurons was inhibited in α-synuclein knockout mice, suggesting that α-synuclein may either enhance TH phosphorylation or hinder TH inactivation during accelerated neuronal activity [9]. Although it has been established that α-synuclein plays an important role in enhancing TH activity, conflicting reports indicate that further studies are required to examine the role of α-synuclein in regulating DA metabolism.
RNA interference (RNAi) is a new strategy for silencing gene expression that uses short double-stranded (ds) RNA to mediate the degradation of sequence specific mRNA [10–12]. It has been reported that introducing small interfering RNAs (siRNAs) or short hairpin RNA (shRNA) into mammalian cells could specifically silence the expression of target genes [7, 23]. The high specificity, efficiency and convenience of this technique means that it is a powerful tool for studying gene function.
In the present study, we silenced the expression of α-synuclein using vector mediated RNAi in dopaminergic MN9D cells and examined the effect of down-regulation of α-synuclein on TH and DA biosynthesis.
Materials and Methods
Construction of shRNA Expression Plasmids
Short hairpin RNA were designed to target specific regions of mouse α-synuclein mRNA (GenBank accession no. AF179273). We identified two 19-nucleotide stretches within the coding region of the α-synuclein gene that were 40–50% GC rich, located within one exon and unique within the mouse genome. A nine-nucleotide loop structure was designed to form the hairpin. We designed three sets of oligonucleotides as instructed by the BLOCK-iT™ Inducible H1 RNAi Entry Vector Kit (Invitrogen, Carlsbad, CA, USA): SH1/α-SYN: 5′-CACCGAGCAAGTGACAAATTTGTTCAAGAGACAACATTTGTCACTTGCTC-3′ (forward), 5′-AAAAGAGCAAGTGACAAATGTTGTCTCTTGAACAACATTTGTCACTTG CTC-3′ (reverse); SH2/α-SYN: 5′-CACCAGGCTACCAAGACTATGAGTTCAAGAG ACTCATAGTCTTGGTAGCC-3′ (forward), 5′-AAAAGGCTACCAAGACTATGA GTCTCTTGAACTCATAGTCTTGGTAGCCT-3′ (reverse); and SH/CON: 5′-CACCGGATCGCCAGAACAAGTATTTCAAGAGAATACTTGTTCTGGCGATCC-3′ (forward), 5′-AAAAGGATCGCCAGAACAAGTATTCTCTTGAAATACTTGTTCTGG CGATCC-3′ (reverse). Single-stranded (ss) DNA oligos (BioSia, Co., Ltd, Shanghai, China) were synthesized. Equal amounts of each pair of ss oligos were annealed to generate ds oligos. The ds oligos were inserted into the pENTRTM/H1/TO vector (Invitrogen) using T4 DNA ligase and transformed into One Shot® TOP10 chemically competent Escherichiacoli (Invitrogen). The transformants were verified by PCR and sequencing. The positive plasmids were named pSH1/α-SYN, pSH2/α-SYN and pSH/CON, respectively.
Cell Culture
The MN9D dopaminergic cell line was generated by fusion of rostral mesencephalic neurons from embryonic C57BL/6J (embryonic day 14) mice with N18TG2 neuroblastoma cells [8] (gift from Dr Bastian Hengerer, Novartis AG). Cells were cultured in DMEM/F12 media supplemented with 10% neonatal calf serum (GIBCO BRL, Grand Island, NY, USA; pH 7.2) and incubated at 37°C in an atmosphere of 5% CO2. MN9D cells endogenously express α-synuclein and TH, and produce measurable levels of DA.
Transfection and Selection
MN9D cells were transfected with pSH1/α-SYN, pSH2/α-SYN, pSH/CON or pcDNA3.1 containing human wild-type (WT) α-synuclein cDNA (constructed in our laboratory). For stable transfection of pSH1/α-SYN, pSH2/α-SYN and pSH/CON, cells were seeded in 6-well tissue culture plates pre-coated with poly-l-lysine (Sigma, St Louis, MO, USA) at 80–85% confluency. Transfections were performed using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s instructions. Four to six hours after transfection, the media was replaced with fresh growth media. The following day, cells were trypsinized and replated into a larger sized tissue culture format. Stably transfected MN9D cells were selected using 400 μg/ml Zeocin™ (Invitrogen) for 12 days. The Zeocin™ resistant clones were then selected and expanded. Cells were maintained with media containing 50 μg/ml Zeocin™. Seventy-two hours post-transfection, cells transfected with pcDNA3.1 containing human WT α-synuclein cDNA were collected and named MN9D/α-SYN+.
RNA Isolation, RT-PCR and Real-time RT-PCR
Total RNA was extracted from pSH1/α-SYN, pSH2/α-SYN and pSH/CON stably transfected MN9D cells and MN9D/α-SYN+ cells using Trizol reagent. First-strand cDNA was synthesized from 5 μg of total RNA, using the SuperScript™ First-strand synthesis System for RT-PCR (Invitrogen). The reaction mixture for PCR consisted of 1 μl of cDNA template, 17 μl of sterile ddH2O and 10 pmol (1 μl) of each specific primer. The following primers were used: α-synuclein: 5′-ATAAGAATGCGGCCGCATGGATGTATTCATGAAAG-3′ (forward) and 5′-CCGCTCGAGGCTTCAGGTTCGTAGTCTTGA-3′ (reverse). As an internal control, β-actin cDNA was co-amplified using the following primer sequences: 5′-CCCATCTACGAGGGCTACGC-3′ (forward) and 5′-AGGAAGGAGGGCTGGAACA-3′ (reverse). Each PCR was started by predenaturation at 96°C for 3 min. Each PCR cycle consisted of 94°C for 30 s, 50°C for 55 s and 72°C for 55 s, followed by a final 10 min extension at 72°C. PCR amplification was carried out for 35 cycles for α-synuclein and 22 cycles for β-actin using a Perkin–Elmer DNA thermal cycler 480 (Applied Biosystems, Foster City, CA, USA).
The reaction mixture (20 μl) for real-time PCR consisted of 10 μl SYBR GREEN PCR Master Mix (Applied Biosystems), 0.5 μl of cDNA template, 0.4 μl of each specific primer (10 μM) and 9.7 μl of sterile ddH2O. The following primers were used: α-synuclein: 5′-TGACGGGTGTGACAGCAGTAG-3′ (forward) and 5′-CAGTGGCTGCTGCAATGC-3′ (reverse); TH: 5′-CAGCCCTACCAAGATCAAAC-3′ (forward) and 5′-TACGGGTC AAACTTCACAGA-3′ (reverse); and β-actin: 5′-ACCACCATGTACCCAGGCATT-3′ (forward) and 5′-CCACACAGAGTACTTGCGCTCA-3′ (reverse). Quantitative PCR was performed in duplicate or triplicate using a 7300 real-time PCR thermal cycler (Applied Biosystems). Each reaction was started by 50°C for 2 min, 95°C for 10 min and 96°C for 3 min, followed by 40 cycles of 94°C for 15 s, 59°C for 20 s and 72°C for 30 s.
Immunofluorescence Staining
Cells were washed with 0.01 M PBS, fixed in 4% paraformaldehyde (30 min), permealized with 0.5% Triton-X (20 min) and blocked in 5% normal goat serum (30 min). Samples were incubated with a specific monoclonal antibody against α-synuclein (2E3; 1:1000) (gift from Dr Shun Yu, Xuanwu Hospital, Capital Medical University, Beijing, China) overnight at 4°C. Cells were washed with 0.01 M PBS and incubated with goat anti-mouse IgG conjugated to cyanine 3 (Cy3TM; 1:400, Sigma) at 37°C for 30 min. After a final wash, cells were examined by fluorescence microscopy.
Cell Viability Assay
Cell viability was assessed using a MTT Kit (Promega, WI, USA). Briefly, the transfected MN9D cells were plated into 96-well plates (1.0 × 104 cells per well) and cultured for 24 h prior to the assay. Sixteen wells were measured for each stably transfected MN9D cell line.
Western Blot Analysis
Lysates of MN9D cells were prepared as previously described [39]. Protein concentrations were determined using BCA relative to BSA protein standards according to the manufacturer (Pierce, Rockford, IL, USA). Whole cell protein extracts were resolved by SDS-PAGE and electrophoretically transferred to nitrocellulose membrane. Prestained protein standards were used to determine the relative molecular mass of proteins. The membranes were blocked in 5% nonfat dry milk in Tris-buffered saline, then incubated with the TH specific monoclonal antibody (1:5000, Sigma) or α-synuclein specific monoclonal antibody (2E3; 1:1000) at room temperature for 3 h, followed by a 1-h incubation with a peroxidase-conjugated secondary antibody (1:5000, ZhongShan, Beijing, China) at room temperature. The blots were washed and immunodetection was carried out by chemiluminescence using SuperSignal (Pierce). Blots were then stripped and re-probed with an antibody against β-actin (1:500, Zhongshan). Blots were scanned using Kodak Image Station (440 CF; Kodak, Rochester, MN, USA) and the optical densities of the bands relative to β-actin within each lane were obtained.
HPLC for Assaying DA and 3, 4-Dihydroxyphenylalanine (l-DOPA) Levels
MN9D cells were collected and re-suspended in HPLC buffer consisting of 0.1 N perchloric acid, 0.3 mM EDTA and 0.1% l-lysine. Cells were freeze-thawed three times, then centrifugated at 15,000 g for 15 min at 4°C to remove particulates. The supernatants were collected and stored at −80°C until analysis. Briefly, the supernatant samples (20–50 μl) were injected onto a Phase II Column (ODS, 3.2 × 100 mm cartridge, Φ3.2 μm, Bioanalytical Systems, Inc, USA). The mobile phase consisted of 0.05 M CH3COONa, 0.05 M citric acid, 1 mM sodium octyl sulfate, 5 mM TEA, 0.075 mM Na2EDTA and 10% methanol (v/v), pH 2.7. The mobile phase was pumped through the system at 0.4 ml/min using a PM-80 pump (Bioanalytical Systems, Inc). Compounds were detected and quantified with an LC-4C detector (Bioanalytical Systems, Inc). The limit of detection for DA was 2 nmol/20 μl. Peaks were identified by retention times set to known standards.
Tyrosine hydroxylase activity was assessed by measuring the accumulation of l-DOPA within cells treated with the aromatic l-amino acid decarboxylase (AADC) inhibitor n-hydroxybenzylhydrazine dihydrochloride (NSD-1015) and measured by HPLC as described above. Briefly, MN9D cells were cultured in 12-well tissue culture plates, washed twice in artificial CSF (ACSF containing 147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2 and 1.0 mM MgCl2) and equilibrated for 20 min at 37°C before addition of 200 μM NSD-1015 in ACSF for 30 min. ACSF samples were collected and assayed by HPLC as described above.
Statistical Analysis
ANOVA followed by Bonferroni post hoc multiple comparisons were used to examine the significance between differences among the experimental groups. A value of p < 0.05 was considered to be statistically significant. All data are expressed as mean ± SE.
Results
Construction of the shRNA Expression Plasmids
After synthesis, each pair of the ss oligos was annealed to generate ds oligos. The annealing efficiency was analyzed by 4% agarose gel electrophoresis. Each ds oligo annealing reactant showed a clearly detectable molecular weight band around 50 bp, as expected for the length of the designed ds oligos. The 50 bp bands were brighter than the corresponding lower molecular weight band, which represented the unannealed ss oligos in the annealing reactant. These data indicate that each pair of ss oligos annealed effectively to form ds oligos. The ds oligos were ligated with linearized pENTR™/H1/TO vectors and the positive clones were expanded and verified by PCR. Bands near 350 bp were found in all PCR reactants, indicating that the ds oligos were ligated into the pENTR/H1/TO vectors. Further analysis by sequencing confirmed that the orientation and sequence of the inserted ds oligos were correct.
α-Synuclein Expression in MN9D Transfected Cells
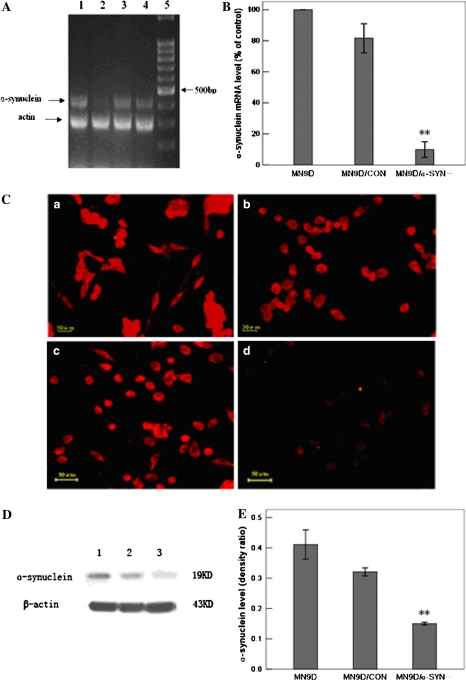
The gene expression of α-synuclein in the transfected MN9D cells was assayed by RT-PCR, real-time RT-PCR, immunofluorescence and Western blot. RT-PCR analysis revealed that α-synuclein mRNA levels were decreased in pSH2/α-SYN-transfected MN9D cells compared with MN9D and pSH/CON-transfected MN9D cells (Fig. 1a). However, α-synuclein mRNA levels in pSH1/α-SYN-transfected cells were not significantly different to levels observed in control MN9D and pSH/CON-transfected MN9D cells (Fig. 1a). These data suggest that α-synuclein expression may be inhibited by transfection with pSH2/α-SYN. In addition, real-time RT-PCR revealed that α-synuclein mRNA levels in pSH2/α-SYN-transfected cells (9.8 ± 2.4%) were significantly lower than in MN9D and MN9D/CON-transfected (81.6 ± 4.9%) cells (Fig. 1b). The immunofluorescence data also indicated that α-synuclein gene expression was down-regulated in pSH2/α-SYN-transfected cells (Fig. 1c). Weak immunofluorescence staining was observed in pSH2/α-SYN-transfected cells compared to strong α-synuclein staining in control MN9D, pSH/CON-transfected and pSH1/α-SYN-transfected MN9D cells. Furthermore, western blot analysis revealed that α-synuclein protein levels were significantly lower in pSH2/α-SYN-transfected cells (0.150 ± 0.004) than in MN9D cells (0.401 ± 0.038) (Fig. 1d, e). Thus, α-synuclein expression was inhibited after transfection of MN9D cells with pSH2/α-SYN. These data suggest that SH2/α-SYN was an effective siRNA sequence and that α-synuclein expression was stably silenced in pSH2/α-SYN-transfected MN9D cells. The pSH2/α-SYN and pSH/CON stably transfected cells were then named MN9D/α-SYN- and MN9D/CON, respectively.
Fig. 1.
Inhibition of α-synuclein expression in pSH2/α-SYN-transfected cells. (a) α-Synuclein mRNA levels were measured by RT-PCR. Lane 1 pSH1/α-SYN-transfected MN9D cells; Lane 2 pSH2/α-SYN-transfected MN9D cells; Lane 3 pSH/CON-transfected MN9D cells; Lane 4 MN9D cells; Lane 5 100 bp DNA ladder. (b) Statistical analysis of α-synuclein gene expression by real-time RT-PCR. α-Synuclein mRNA levels were decreased by approximately 90% in pSH2/α-SYN-transfected cells compared with normal MN9D cells [n = 5, F = 216.167, df(total) = 14, **p < 0.001 compared with MN9D cells, **p < 0.001 compared with MN9D/CON cells]. α-Synuclein mRNA levels were not significantly different in pSH1/α-SYN-transfected cells compared with MN9D and MN9D/CON cells. (c) Immunofluorescence staining of normal MN9D cells (a) and MN9D cells transfected with pSH1/α-SYN (b), pSH/CON (c) and pSH2/α-SYN (d) using an antibody against α-synuclein. Strong α-synuclein staining was observed in MN9D, pSH1/α-SYN-transfected and pSH/CON-transfected MN9D cells, whereas weak staining was observed in pSH2/α-SYN-transfected MN9D cells. Bar = 50 μm. (d) Western blot analysis of α-synuclein protein levels. Lane 1 MN9D cells; Lane 2 MN9D/CON cells; Lane 3 MN9D/α-SYN− cells. (e) Statistical analysis of α-synuclein protein levels in MN9D, MN9D/CON and MN9D/α-SYN− cells using the linear density ratio of α-synuclein/β-actin. α-Synuclein protein levels were significantly decreased in MN9D/α-SYN− cells (0.150 ± 0.004) compared with MN9D (0.401 ± 0.038) and MN9D/CON (0.321 ± 0.011) cells [n = 5, F = 162.034, df(total) = 14, **p < 0.001 compared with MN9D cells, **p < 0.001 compared with MN9D/CON cells]
Effects of Silencing α-Synuclein Expression on Cell Viability
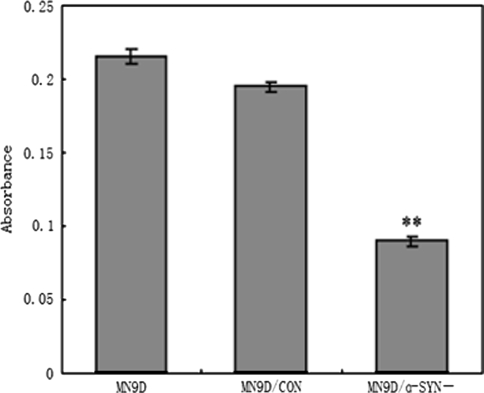
No significant differences in cell morphology were observed between the three groups of MN9D cells (data not shown). As shown in Fig. 2, the cell viability of MN9D/α-SYN− cells was significantly decreased compared with control MN9D and MN9D/CON cells [n = 5, F = 421.457, df(total) = 14, **p < 0.001 compared with MN9D cells, **p < 0.001 compared with MN9D/CON cells]. Cell viability was decreased by 58.3 and 50% in MN9D/α-SYN− cells (0.091 ± 0.006) compared with MN9D (0.217 ± 0.012) and MN9D/CON (0.189 ± 0.005) cells, respectively (Fig. 2). These data suggest that α-synuclein may be important for the viability of MN9D cells.
Fig. 2.
Effects of silencing α-synuclein expression on cell viability. Cell viability was measured using an MTT assay. Cell viability was decreased in MN9D/α-SYN− cells compared with MN9D and MN9D/CON cells [n = 5, F = 421.457, df(total) = 14, **p < 0.001 compared with MN9D cells, **p < 0.001 compared with MN9D/CON cells]
TH Expression and Activity in MN9D Transfected Cells
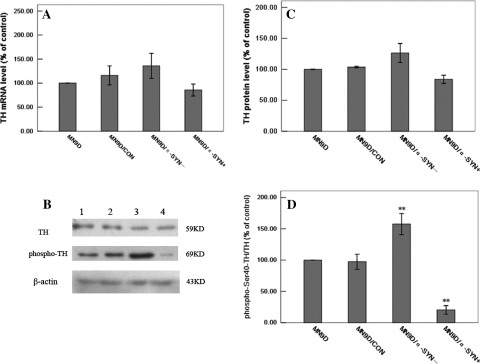
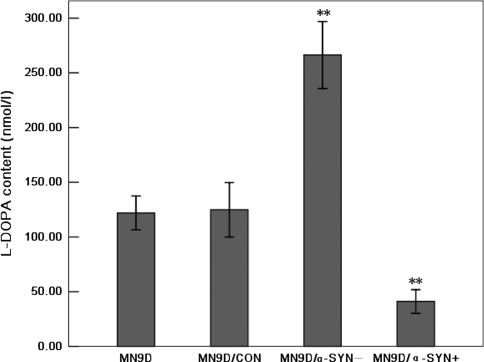
Previous studies have reported that TH expression may be down-regulated by over-expression of α-synuclein in MES23.5 and M17 cells [3, 42]. Here, we examined whether a similar effect occurred after the silencing of α-synuclein expression in MN9D/α-SYN− cells. TH mRNA, protein and phosphorylation levels were measured by real-time RT-PCR and Western blot analysis, respectively. Real-time RT-PCR showed that there were no significant differences in TH mRNA levels between MN9D, MN9D/CON (116.00 ± 10.23%), MN9D/α-SYN− (135.89 ± 14.58%) and MN9D/α-SYN+ (85.67 ± 5.55%) cells (Fig. 3a). These observations show that silencing α-synuclein expression did not alter TH gene expression, suggesting that α-synuclein does not regulate expression of TH. Similar TH protein levels were observed in MN9D, MN9D/CON (103.55 ± 0.84%), MN9D/α-SYN− (126.40 ± 10.81%) and MN9D/α-SYN+ (83.89 ± 4.06%) cells by Western blot analysis (Fig. 3b, c). However, TH phosphorylation levels were significantly increased in MN9D/α-SYN− (157.63 ± 8.36%) cells compared with MN9D and MN9D/CON (97.45 ± 5.06%) cells, and significantly decreased in MN9D/α-SYN+ cells (20.36 ± 2.86%) (Fig. 3b, d). To directly assess TH activity, we used NSD-1015 to inhibit AADC, thereby blocking the conversion of l-DOPA to DA. We found that l-DOPA levels were increased in MN9D/α-SYN− cells (266.33 ± 11.05 nmol/l) compared with MN9D (121.92 ± 5.61 nmol/l) and MN9D/CON (124.89 ± 8.94 nmol/l) cells (Fig. 4). In contrast, a significant decrease in l-DOPA levels was observed in MN9D/α-SYN+ cells (41.14 ± 3.89 nmol/l). Since accumulation of l-DOPA could only occur if TH actively converted tyrosine to l-DOPA, these data suggest that TH activity was increased in MN9D/α-SYN− cells and diminished in cells over-expressing α-synuclein. These data support the hypothesis that silencing α-synuclein expression does not affect TH gene expression but can enhance TH activity by increasing TH Ser40 phosphorylation.
Fig. 3.
Effects of silencing α-synuclein expression on TH and phospho-Ser40-TH levels. (a) Statistical analysis of TH gene expression by real-time RT-PCR. Differences in TH mRNA levels between the transfected MN9D cells were not statistically significant (n = 5). (b) Western blot analysis of TH, phospho-Ser40-TH and β-actin protein levels. Lane 1 normal MN9D; Lane 2 MN9D/CON; Lane 3 MN9D/α-SYN−; Lane 4 MN9D/α-SYN+ cells. (c) Statistical analysis of TH protein levels in MN9D, MN9D/CON, MN9D/α-SYN− and MN9D/α-SYN+ cells using the linear density percent of TH/β-actin. No significant differences in TH protein levels were observed between the transfected MN9D cells (n = 5). (d) Statistical analysis of phospho-Ser40-TH protein levels in MN9D, MN9D/CON, MN9D/α-SYN− and MN9D/α-SYN+ cells using the linear density percent of phospho-Ser40-TH/TH. Phospho-Ser40-TH protein levels were significantly increased in MN9D/α-SYN− cells and significantly decreased in MN9D/α-SYN+ cells compared with MN9D and MN9D/CON cells [n = 5, F = 84.505, df(total) = 19, **p < 0.001 MN9D/α-SYN− cells compared with MN9D cells, **p < 0.001 MN9D/α-SYN− cells compared with MN9D/CON cells; **p < 0.001 MN9D/α-SYN+ cells compared with MN9D cells, **p < 0.001 MN9D/α-SYN+ cells compared with MN9D/CON cells]
Fig. 4.
Effects of silencing α-synuclein expression on l-DOPA levels. Cell lysates were prepared from transfected MN9D cells incubated in the presence of 200 μM NSD-1015 for 30 min. l-DOPA levels were measured by HPLC. l-DOPA levels were significantly increased in MN9D/α-SYN− cells and significantly decreased in MN9D/α-SYN+ cells compared with MN9D and MN9D/CON cells [n = 6, F = 140.944, df(total) = 23, **p < 0.001 MN9D/α-SYN− cells compared with MN9D cells, **p < 0.001 MN9D/α-SYN− cells compared with MN9D/CON cells; **p < 0.001 MN9D/α-SYN+ cells compared with MN9D cells, **p < 0.001 MN9D/α-SYN+ cells compared with MN9D/CON cells]
Effects of Silencing α-Synuclein Expression on DA Levels
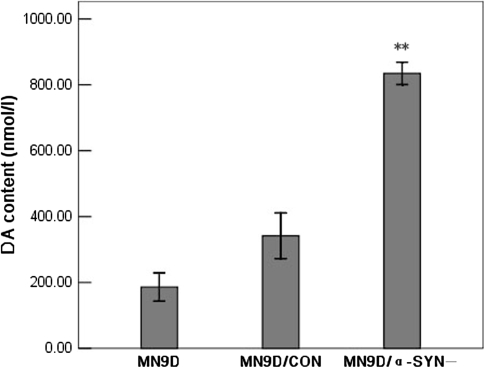
To assess differences in cytosolic DA levels between the transfected MN9D cells, we prepared cell extracts and measured DA content by HPLC with electrochemical detection. As shown in Fig. 5, DA levels were significantly increased in MN9D/α-SYN− cells compared with MN9D and MN9D/CON cells. The DA content was approximately 4.8-fold higher in MN9D/α-SYN− cells (834.72 ± 24.72 nmol/l) than in normal MN9D cells (186.04 ± 31.38 nmol/l) and 2.4-fold higher than in MN9D/CON cells (341.87 ± 46.25 nmol/l). No significant differences in DA content were observed between MN9D and MN9D/CON cells (Fig. 5). These results indicate that silencing α-synuclein expression leads to an increase in DA levels in MN9D cells.
Fig. 5.
Effects of silencing α-synuclein expression on DA levels. Changes in DA levels were detected by HPLC. DA levels were significantly increased in MN9D/α-SYN− cells compared with MN9D and MN9D/CON cells [n = 6, F = 161.774, df(total) = 17, **p < 0.001 compared with MN9D cells, **p < 0.001 compared with MN9D/CON cells]. Differences in DA levels between the MN9D and MN9D/CON cells were not statistically significant
Discussion
In the present study, we silenced the expression of α-synuclein in MN9D dopaminergic cells by vector mediated RNAi using the BLOCK-iT™ inducible H1 RNAi entry vector. This vector was selected for several reasons. Firstly, the vector contains a kanamycin resistance gene for selection in E.coli and a ZeocinTM resistance marker to allow generation of stable cell lines that express the shRNA of interest. Secondly, the cloning site of the vector contains a 4-nucleotide overhang on the 5′ end of each DNA strand, allowing directional cloning of the shRNA of interest. Thirdly, the H1 promoter of the vector is recognized by RNA polymerase III resulting in high-level, constitutive expression of shRNA in most mammalian cell types. We identified an effective targeting sequence for RNAi, which localized in the C-terminal coding sequence of the mouse α-synuclein gene, and used this to generate MN9D/α-SYN− cells in which the expression of α-synuclein was stably silenced. Due to the endogenous expression of α-synuclein and the dopaminergic characteristics of MN9D cells, MN9D/α-SYN− is a good cellular model for studying the normal function of α-synuclein and examining its role in PD pathogenesis.
We found that the cell viability of MN9D/α-SYN− cells was significantly decreased compared with MN9D and MN9D/CON cells, suggesting that loss of α-synuclein may induce cell injury directly or indirectly. Based on subsequent observations, we propose that a marked increase in cytosolic DA levels may account for decreased cell viability. DA can readily oxidize to generate hydrogen peroxide, superoxide and reactive DA quinones [16, 35], which are cytotoxic due to their inhibitory effects on the mitochondrial respiratory system [5, 13]. In addition, superfluous DA itself may be cytotoxic via inhibition of mitochondrial complex I activity [4]. Thus, it is also possible that increased cytosolic DA levels induced mitochondrial dysfunction resulting in decreased viability in MN9D/α-SYN− cells.
α-Synuclein may be involved in the regulation of TH, the rate-limiting enzyme of DA biosynthesis. Several studies have shown that α-synuclein regulates TH gene expression. In α-synuclein over-expressing MES23.5 dopaminergic cells, TH mRNA and protein levels were significantly reduced [42]. A similar effect was observed in M17 dopaminergic cells transfected with WT α-synuclein [3]. However, in the current study, no significant differences in TH mRNA and protein levels were observed between the four groups of MN9D cells. Interestingly, a previous study using MN9D cells reported that α-synuclein over-expression did not diminish endogenous TH levels [26], which is consistent with our results. We propose that the difference in TH levels between the present α-synuclein silenced system and the previously described α-synuclein over-expression system is mainly due to the different cell types and not due to the different ways in which the cells were manipulated.
Although TH protein levels remained unchanged in MN9D/α-SYN− cells, phosphorylation of TH at its Ser40 site was significantly enhanced. Furthermore, l-DOPA levels were significantly increased after NSD-1015 treatments. These results suggest that TH activity was enhanced in MN9D/α-SYN− cells, which is consistent with previous reports [24, 26]. In addition, we also found that cytosolic DA levels were significantly increased in MN9D/α-SYN− cells. Our data show that TH activity was enhanced in α-synuclein-silenced MN9D/α-SYN− cells, and that this was associated with an increase in DA biosynthesis followed by increased cytosolic DA levels. The mechanisms by which α-synuclein may regulate TH activity have not been elucidated. It is known that only the phosphorylated form of TH is active, and that the phosphorylation and dephosphorylation of TH are important in regulating DA biosynthesis [29, 37]. Furthermore, phosphorylation of TH at Ser40 results in the most prominent activation of TH [37]. Thus, it has been suggested that α-synuclein regulates TH activity by regulating the phosphorylation of TH [24]. Protein phosphatase 2A (PP2A), an important enzyme required for dephosphorylation of TH, can be activated by α-synuclein, resulting in a significant increase in TH dephosphorylation and reduction in TH activity [6]. In addition, other factors may also be involved in the regulation of TH phosphorylation by α-synuclein. For example, 14-3-3 enhances TH activity by binding directly to phosphorylated TH. α-Synuclein may directly interact with 14-3-3 [22], thereby accelerating the dissociation of 14-3-3 from phosphorylated TH. α-Synuclein may also affect TH activity by directly interacting with TH [26].
In conclusion, our data demonstrate that in PD pathology, silencing of α-synuclein expression results in enhanced TH activity and increased DA levels in neurons. We propose that in cells that unable to remove excess DA from the cytosol, DA itself and its oxidized products including reactive oxygen species and reactive DA quinones may lead to cell injury or even death. Further understanding of the normal function of α-synuclein in maintaining DA homeostasis may help to identify novel preventative or therapeutic strategies for PD.
Acknowledgments
This work was supported by grants from The National Basic Research Program of China (2006CB500706), National Natural Science Foundation of China (30670655, 30430280, 30700199, 7082011), National Ministry of Education (20060025004), and Natural Science Foundation and Municipal Education Commission of Beijing (KM200610025002).
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Abeliovich A, Schmitz Y, Farinas I et al (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252 [DOI] [PubMed]
- 2.Baba M, Nakajo S, Tu PH et al (1998) Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152:879–884 [PMC free article] [PubMed]
- 3.Baptista MJ, O’Farrell C, Daya S et al (2003) Co-ordinate transcriptional regulation of dopamine synthesis genes by alpha-synuclein in human neuroblastoma cell lines. J Neurochem 85:957–968 [DOI] [PubMed]
- 4.Ben-Shachar D, Zuk R, Gazawi H et al (2004) Dopamine toxicity involves mitochondrial complex I inhibition: implications to dopamine-related neuropsychiatric disorders. Biochem Pharmacol 67:1965–1974 [DOI] [PubMed]
- 5.Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem 73:1127–1137 [DOI] [PubMed]
- 6.Berresheim U, Kuhn DM (1994) Dephosphorylation of tyrosine hydroxylase by brain protein phosphatases: a predominant role for type 2A. Brain Res 637:273–276 [DOI] [PubMed]
- 7.Brummelkamp TR, Bernards R, Agami RA (2002) System for stable expression of short interfering RNAs in mammalian cells. Science 296:550–553 [DOI] [PubMed]
- 8.Choi HK, Won LA, Kontur PJ et al (1991) Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res 552:67–76 [DOI] [PubMed]
- 9.Drolet RE, Behrouz B, Lookingland KJ et al (2006) Substrate-mediated enhancement of phosphorylated tyrosine hydroxylase in nigrostriatal dopamine neurons: evidence for a role of alpha-synuclein. J Neurochem 96:950–959 [DOI] [PubMed]
- 10.Dykxhoorn DM, Novina CD, Sharp PA (2003) Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol 4:457–467 [DOI] [PubMed]
- 11.Elbashir SM, Lendeckel W, Tuschl T et al (2001) RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 15:188–200 [DOI] [PMC free article] [PubMed]
- 12.Fire A, Xu S, Montgomery MK et al (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811 [DOI] [PubMed]
- 13.Gluck M, Ehrhart J, Jayatilleke E et al (2002) Inhibition of brain mitochondrial respiration by dopamine: involvement of H2O2 and hydroxyl radicals but not glutathione-protein-mixed disulfides. J Neurochem 82:66–74 [DOI] [PubMed]
- 14.Greenbaum EA, Graves CL, Mishizen-Eberz AJ et al (2005) The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem 280:7800–7807 [DOI] [PubMed]
- 15.Kruger R, Kuhn W, Muller T et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18:106–108 [DOI] [PubMed]
- 16.LaVoie MJ, Hastings TG (1999) Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci 19:1484–1491 [DOI] [PMC free article] [PubMed]
- 17.Lotharius J, Brundin P (2002) Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum Mol Genet 11:2395–2407 [DOI] [PubMed]
- 18.Lotharius J, Brundin P (2002) Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3:932–942 [DOI] [PubMed]
- 19.Lykkebo S, Jensen PH (2002) Alpha-synuclein and presynaptic function: implications for Parkinson’s disease. Neuromolecular Med 2:115–129 [DOI] [PubMed]
- 20.Martin FL, Williamson SJ, Paleologou KE et al (2004) Alpha-synuclein and the pathogenesis of Parkinson’s disease. Protein Pept Lett 11:229–237 [DOI] [PubMed]
- 21.Mezey E, Dehejia AM, Tresser N et al (1998) Alpha synuclein is present in Lewy bodies in sporadic Parkinson’s disease. Mol Psychiatry 3:493–499 [DOI] [PubMed]
- 22.Ostrerova N, Petrucelli L, Farrer M et al (1999) Alpha-synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci 19:5782–5791 [DOI] [PMC free article] [PubMed]
- 23.Paddison PJ, Caudy AA, Bernstein E et al (2002) Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 16:948–958 [DOI] [PMC free article] [PubMed]
- 24.Peng X, Tehranian R, Perez RG et al (2005) Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci 118:3523–3530 [DOI] [PubMed]
- 25.Perez RG, Hastings TG (2004) Could a loss of alpha-synuclein function put dopaminergic neurons at risk? J Neurochem 89:1318–1324 [DOI] [PubMed]
- 26.Perez RG, Waymire JC, Lin E et al (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22:3090–3099 [DOI] [PMC free article] [PubMed]
- 27.Polymeropoulos MH, Lavedan C, Leroy E et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047 [DOI] [PubMed]
- 28.Rajagopalan S, Andersen JK (2001) Alpha-synuclein aggregation: is it the toxic gain of function responsible for neurodegeneration in Parkinson’s disease? Mech Ageing Dev 122:1499–1510 [DOI] [PubMed]
- 29.Ramsey AJ, Hillas PJ, Fitzpatrick PF (1996) Characterization of the active site iron in tyrosine hydroxylase: redox states of the iron. J Biol Chem 271:24395–24400 [DOI] [PubMed]
- 30.Recchia A, Debetto P, Negro A et al (2004) Alpha-synuclein and Parkinson’s disease. FASEB J 18:617–626 [DOI] [PubMed]
- 31.Rochet JC, Outeiro TF, Conway KA et al (2004) Interactions among alpha-synuclein, dopamine, and biomembranes: some clues for understanding neurodegeneration in Parkinson’s disease. J Mol Neurosci 23:23–34 [DOI] [PubMed]
- 32.Sidhu A, Wersinger C, Vernier P (2004) Alpha-synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson’s disease. FEBS Lett 565:1–5 [DOI] [PubMed]
- 33.Sidhu A, Wersinger C, Vernier P (2004) Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J 18:637–647 [DOI] [PubMed]
- 34.Spillantini MG, Schmidt ML, Lee VM et al (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840 [DOI] [PubMed]
- 35.Stokes AH, Hastings TG, Vrana KE et al (1999) Cytotoxic and genotoxic potential of dopamine. J Neurosci Res 55:659–665 [DOI] [PubMed]
- 36.Tabner BJ, Turnbull S, El-Agnaf OM et al (2002) Formation of hydrogen peroxide and hydroxyl radicals from A(beta) and alpha-synuclein as a possible mechanism of cell death in Alzheimer’s disease and Parkinson’s disease. Free Radic Biol Med 32:1076–1083 [DOI] [PubMed]
- 37.Vrana KE (1996) Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem 67:443–462 [DOI] [PubMed]
- 38.Wersinger C, Sidhu A (2003) Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci Lett 340:189–192 [DOI] [PubMed]
- 39.Wersinger C, Sidhu A (2003) Differential cytotoxicity of dopamine and H2O2 in a human neuroblastoma divided cell line transfected with alpha-synuclein and its familial Parkinson’s disease-linked mutants. Neurosci Lett 342:124–128 [DOI] [PubMed]
- 40.Yavich L, Tanila H, Vepsalainen S et al (2004) Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci 24:11165–11170 [DOI] [PMC free article] [PubMed]
- 41.Yu S, Ueda K, Chan P (2005) Alpha-synuclein and dopamine metabolism. Mol Neurobiol 31:243–254 [DOI] [PubMed]
- 42.Yu S, Zuo X, Li Y et al (2004) Inhibition of tyrosine hydroxylase expression in alpha-synuclein-transfected dopaminergic neuronal cells. Neurosci Lett 367:34–39 [DOI] [PubMed]