Abstract
Purpose
To evaluate the 1-year results of using triamcinolone acetonide (TA) in pars plana vitrectomy (PPV).
Design
Multicenter prospective controlled clinical trial.
Methods
SETTING and STUDY POPULATION: the study population comprised 774 eyes from patients treated at eight Japanese hospitals, among which 391 eyes underwent TA-assisted PPV and 383 control eyes underwent conventional PPV. The patients were assigned to the two groups using a single-blind quasi-randomization approach within the participating clinical centers. INTERVENTION: intra-operative use of TA to aid visualization of the vitreous. MAIN OUTCOME MEASURES: changes of visual acuity, post-operative complications (including additional surgery), and adverse events occurring within 1 year of the operation were compared between the TA-PPV group and the conventional PPV group.
Results
The visual acuity improved over time, and no significant differences were found between the two groups (log-rank versus TA, P = 0.98 for improvement, P = 0.26 for deterioration). The logistic regression model also showed that the intra-operative use of TA was not a significant factor for the improvement of visual acuity [P = 0.91, odds ratio (OR) = 1.10, 95% confidence interval (95%CI) = 0.860–1.183)] after adjustments for age, gender, and diagnosis. Intra-operative TA was not a significant factor for the need for additional surgery (log-rank test P = 0.45, logistic regression test P = 0.35, OR = 1.23, 95%CI = 0.797–1.911]. No serious adverse events related to surgery were observed.
Conclusions
This 1-year follow-up study of a controlled clinical trial showed that TA-assisted PPV had neither a positive nor a negative effect on visual acuity, the incidence of additional surgeries, or adverse events compared with conventional PPV.
Keywords: Proliferative vitreoretinopathy, Steroid, Adjuvant, Diabetic retinopathy, Retinal detachment
Introduction
Recently, vitrectomy has been used to treat various vitreoretinal diseases. It still remains a demanding procedure requiring a skilled and experienced surgeon, despite advances in surgical instrumentation and techniques. One major difficulty with this procedure is the transparency of the vitreous. Intra-operative triamcinolone acetonide (TA) has been used to address this problem, as it can be injected into the vitreous to visualize the posterior hyaloid or the internal limiting membrane (ILM) [1, 2]. Several previous reports have demonstrated the usefulness of TA as an adjunct to pars plana vitrectomy (PPV) [3–13]. Although their findings supported the use of TA-assisted PPV, these studies involved relatively small numbers of eyes and were not randomized. In addition, more recent reports on the use of intravitreous TA in patients not undergoing vitrectomy raised concerns about the risks of elevated intra-ocular pressure (IOP) [14–19].
We therefore undertook a multicenter prospective controlled study of TA-assisted pars plana vitrectomy (PPV). Considering the enduring nature of the effect of PPV on ocular tissue, it is important to investigate the long-term results in order to evaluate TA-assisted PPV. Moreover, the crucial issue of the effects on patients’ vision must be explored before TA-assisted PPV can be recommended. The current report presents the 1-year results of the use of TA in PPV, and evaluates the procedure in light of the aforementioned issues.
Methods
Study design, randomization and sample size
The prospective controlled clinical trial took place at eight hospitals in Japan as previously described (see Acknowledgements below) [13].
The patients were assigned to the two groups using a single-blind quasi-randomization approach within the participating clinical centers. Our preliminary study showed that the most important factor influencing the surgical outcome was not the nature of the disease for which PPV was used, but rather the hospital in which the surgery was performed (data not shown). To minimize this bias, equal numbers of patients from each hospital were assigned to each of the study groups. Thus, the patients were assigned by a single-blind quasi-randomization within the participating clinical centers. Briefly, after the patients passed the inclusion/exclusion criteria and gave consent for the study, they were numbered serially at each hospital, and alternate numbers were assigned to the TA-assisted and conventional PPV groups, respectively, as previously described [13]. Data analysis was performed by an intent-to-treat analysis method. To conceal the allocations at the participating hospitals, the surgeons were not informed of the group to which a patient had been assigned until immediately before surgery.
This is the second report of controlled clinical trial of intraocular TA in PPV. Primary outcome was whether the use of TA might reduce the incidence of intraoperative complications. The sample size was determined based upon our preliminary study, and the method has been described elsewhere [13]. Briefly, the incidence of intraoperative retinal breaks was found to be approximately 15% in the participating hospitals. The sample size calculation was based on our pilot study data, and was aimed to detect a difference in short-term postoperative complications as reported previously [13]
Eligibility criteria and surgery
Institutional review board (IRB)/ethics committee approval was obtained from all of the participating clinical establishments, such that all patients undergoing PPV at these hospitals were eligible to enroll in the study. Each patient was fully informed about the nature of the treatment, and provided written consent. The following groups were excluded: (1) patients with diagnoses that included macular holes; patients with uveitis, (2) patients with phakic lenses, (3) patients who were known to be steroid responders, to have glaucoma, or to have apparent infectious endophthalmitis, and (4) patients who were undergoing a second PPV. For ethical reasons, if at the time of surgery a surgeon felt that TA was necessary to achieve a successful PPV outcome, it was included in the procedure; for the purposes of the intent-to-treat analysis, these cases were included within the conventional PPV group.
The details of the surgical protocols varied between the hospitals, but the basic procedure was similar. PPV was performed according to a previously reported methodology, either with or without the use of a TA suspension prepared as described elsewhere [2, 13]. The recording of the operations, control of surgical quality, use of antibiotics, and termination of the study have been described elsewhere [13].
During the follow-up period, additional surgeries were performed at each hospital as and when they were needed. For example, filtering surgeries were performed when the intra-ocular pressure (IOP) remained at an uncontrollably high level (25 mmHg or greater) even with full medication. Full medication included the use of all latanoprost, beta-blocker, and dorzolamide hydrochloride eyedrops and acetazolamide medicine.
Data collection and documentation were performed as previously described [13]. For the purposes of this study, the adverse events related to surgery were defined as follows: retinal breaks, macular pucker, retinal fibrous membrane formation, retinal detachment, vitreous hemorrhage (VH), rubeosis iridis, optic-disc damage, corneal diseases, and after cataract. The serious adverse events related to surgery were defined as follows: infectious endophthalmitis, retinal degeneration, unexplained optic-disc damage, and unexplained deterioration of vision.
Masking and outcome assessment
The study records were collected by the principal investigator (TS) up until November 2006. After checking the records for harmful events, the data were sent to the controllers (YN) at Kyushu University. The remaining investigators were not permitted access to any information on the outcomes before completion of the analysis. Visual acuity was measured by ophthalmic technicians using the Snellen high-contrast acuity test. IOP was also measured by the ophthalmic technicians, and the findings were reviewed by the physicians. IOP was measured with pneumotonometer. The ophthalmic technicians were not informed of the purpose of the study or the assignment schedule. Anti-glaucoma eye drops and/or acetazolamide were used when an IOP of 22 mmHg or higher persisted for 3 days.
Statistical analysis
The visual acuity was converted to the logarithm of the minimum angle of resolution (LogMAR), and the baseline value was compared with those at 1 week, 1 month, 3 months, 6 months, and 1 year. The eyes were categorized into the following three groups: (1) the improved group, in which patients showed a 0.3 logMAR or greater improvement of visual acuity at the final examination compared with baseline, (2) the deteriorated group, in which patients showed a 0.3 logMAR or worse deterioration of visual acuity at the final examination compared with baseline, and (3) the unchanged group, in which patients did not meet the criteria of either the improved or the deteriorated group.
A Kaplan-Meier survival analysis was used to estimate the probability of improved visual acuity with time after surgery. The improvement or deterioration was analyzed by the log rank test. The earliest time point after surgery at which the events in each category were reported was designated as the time point of each event.
We also estimated the multiple-adjusted odds ratio (OR) and the 95% confidence interval (95%CI) both for TA use and for the other variables, using a logistic regression model. The parameters that were adjusted for included age, gender, and disease diagnosis, which was categorized into the following five groups: proliferative diabetic retinopathy (PDR), diabetic macular edema (DME), rhegmatogenous retinal detachment (RRD), retinal vein occlusion (RVO), and others. A diagnosis of PDR was used as a reference. Even when a single eye received multiple additional surgeries, it was counted as one eye. All statistical analyses were performed using SAS® (Proprietary Software Release 8.2; SAS Institute Inc., Cary, NC, USA). The differences in diagnosis or surgical intervention between the two groups were compared using a two-tailed Chi-squared test with the Yates’ correction. P values < 0.05 were considered statistically significant.
Results
Number of eyes
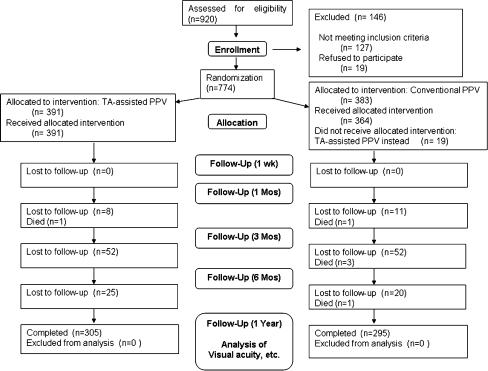
In total, 920 patients were initially registered for PPV during this period. Among these, 146 eyes were not included in the study because they did not meet the eligibility criteria or because the patient declined to participate. In total, 774 eyes were randomized, with 391 eyes (from 216 male and 175 female patients) assigned to the TA-assisted PPV group and 383 eyes (201 male and 182 female patients) assigned to the conventional PPV group. The mean ages of the patients were 63.5 ± 12.1 years in the TA-assisted PPV group and 63.0 ± 11.3 years in the conventional PPV group. In total, 19 eyes that were originally assigned to the conventional PPV group received unplanned TA based on surgical judgment. The 1-year post-operative follow-up was completed for a total of 305 eyes in the TA-assisted PPV group (78.0%) and 295 eyes in the conventional PPV group (77.0%). The overall follow-up rate was 77.5% (Fig. 1).
Fig. 1.
Schematic diagram of the study. TA: triamcinolone acetonide, PPV: pars plana vitrectomy
Diagnoses
The diagnoses for each group are shown in Table 1. In both groups, PDR accounted for the largest number of cases, followed by RRD. In both groups, the pattern at the 1-year follow-up was similar to that at the baseline, and the different diagnoses had significantly different distributions within the two groups: the TA-assisted PPV group had a relatively larger number of patients with DME (P = 0.018 at baseline, P = 0.006 at 1-year follow-up) and a smaller number of patients with epiretinal membrane (ERM) formation (P = 0.031 at baseline, P = 0.008 at 1-year follow-up).
Table 1.
Diseases of each group at baseline and 1 year after surgery
| TA-assisted PPV | Conventional PPV | |||
|---|---|---|---|---|
| Baseline | 1 year | baseline | 1 year | |
| N (%) | N (%) | N (%) | N (%) | |
| AMD | 7 (1.8%) | 3 (1.0%) | 3 (0.8%) | 3 (1.0%) |
| BRVO | 50 (12.8%) | 41 (13.4%) | 37 (9.7%) | 29 (9.8%) |
| CRVO | 11 (2.8%) | 9 (3.0%) | 7 (1.8%) | 6 (2.0%) |
| DME | 67 (17.1%) | 60 (19.7%) | 42 (11.0%) | 34 (11.5%) |
| ERM | 24 (6.1%) | 19 (6.2%) | 41 (10.7%) | 37 (12.5%) |
| Lens luxation | 16 (4.1%) | 11 (3.6%) | 16 (4.2%) | 12 (4.1%) |
| Macroaneurysm | 10 (2.6%) | 8 (2.6%) | 9 (2.3%) | 4 (1.4%) |
| PDR | 113 (28.9%) | 85 (27.9%) | 131 (34.2%) | 101 (34.2%) |
| RRD | 80 (20.5%) | 59 (19.3%) | 77 (20.1%) | 54 (18.3%) |
| VH | 6 (1.5%) | 6 (2.0%) | 14 (3.7%) | 11 (3.7%) |
| Others | 7 (1.8%) | 4 (1.3%) | 6 (1.6%) | 4 (1.4%) |
| total | 383 (100%) | 305 (100%) | 391 (100%) | 295 (100%) |
TA; triamcinolone acetonide: PPV; pars plana vitrectomy: AMD; age-related macular degeneration: BRVO;branch retinal vein occlusion: CRVO: central retinal vein occlusion: DME; diabetic macular edema: ERM; epiretinal membrane: PDR; proliferative diabetic retinopathy: RRD; rhegmatogenous retinal detachment: VH; vitreous hemorrhage.
There were no significant differences between the two groups in the usage of surgical methods, such as cataract surgery (phacoemulsification P = 0.724 and pars plana lensectomy P = 0.272), ILM peeling (P = 0.075) and endolaser-photocoagulation (P = 0.413), or in the use of post-operative tamponade (sulfur hexafluoride gas P = 0.810, octa-fluorepropane gas P = 0.756, room air P > 0.999, and silicone oil P = 0.831) [13].
Visual acuity
The visual acuity in both groups improved over time. In the TA-assisted PPV group, 322 eyes were categorized as improved, 21 were categorized as unchanged, and 48 were categorized as deteriorated. In the conventional PPV group, 312 eyes were categorized as improved, 45 eyes were categorized as unchanged, and 26 eyes were categorized as deteriorated. The log-rank test revealed no significant difference between the two groups regarding the improvement (P = 0.98) or deterioration (P = 0.26) of visual acuity. The logistic regression model also showed that the intra-operative use of TA was not a significant factor for the improvement of visual acuity (P = 0.91) after adjusting for age, gender, and diagnosis (Table 2). Neither gender (P = 0.54) nor age (P = 0.31) was a significant factor for the improvement of visual acuity. However, PDR diagnosis was a significant factor for the improvement of visual acuity compared with DME (OR = 0.63, 95%CI = 0.478–0.817, P = 0.0006; Table 2).
Table 2.
Factors affecting improvement of visual acuity
| Variable (no. of improved eyes/total eyes) | Odds ratio (95%CI) | P-value |
|---|---|---|
| TA-assisted PPV (322/391) vs conventional PPV (312/383) | 1.10 (0.860–1.183) | 0.91 |
| Age | 1.00 (0.991–1.005) | 0.54 |
| Gender (male vs female) | 0.92 (0.785–1.080) | 0.31 |
| Diseases | ||
| RD vs PDR | 1.04 (0.834–1.340) | 0.71 |
| DME vs PDR | 0.63 (0.478–0.817) | 0.0006* |
| RVO vs PDR | 1.21 (0.938–1.561) | 0.14 |
| ERM vs PDR | 0.78 (0.568–1.061) | 0.11 |
| Others vs PDR | 1.21 (0.924–1.579) | 0.17 |
CI; confidence interval: TA; triamcinolone acetonide: PPV; pars plana vitrectomy: RVO; branch retinal vein occlusion + central retinal vein occlusion: DME; diabetic macular edema: ERM; epiretinal membrane: PDR; proliferative diabetic retinopathy: RRD; rhegmatogenous retinal detachment. * statistically significant
Post-operative complications
Subgroup analysis
In terms of post-operative complications, there was not any statistically significant difference. Fibrous ERM was observed in five eyes (1.4%) in the conventional PPV group, but in only one eye (0.3%) in the TA-assisted PPV group. A subgroup analysis showed that this difference was not significant (P = 0.10). Among the other complications, an IOP increase was found more frequently in the TA-assisted PPV group (23 eyes, 5.9%) than in the conventional PPV group (13 eyes, 3.4%), although this difference was not statistically significant (P = 0.10). There were no significant differences in the other complications between the two groups (Table 3).
Table 3.
Post-operative complications in each group
| TA-assisted PPV (total 391 eyes): n (%) | Conventional PPV (total 383 eyes): n (%) | P-value* | |
|---|---|---|---|
| After cataract | 18 (4.6%) | 17 (4.4%) | 0.91 |
| Macular pucker | 23 (5.9%) | 22 (5.7%) | 0.93 |
| IOP increase | 23 (5.9%) | 13 (3.4%) | 0.10 |
| Retinal detachment | 9 (2.3%) | 9 (2.3%) | 0.96 |
| Vitreous hemorrhage | 22 (5.6%) | 29 (7.8%) | 0.28 |
| Fibrous membrane | 1 (0.3%) | 5 (1.4%) | 0.10 |
| Iris synechiae | 6 (1.5%) | 4 (1.0%) | 0.55 |
| Others | 8 (2.0%) | 12 (3.1%) | 0.34 |
TA; triamcinolone acetonide: PPV; pars plana vitrectomy: PPL; pas plana lensectomy: IOP; intraocular pressure: * Chi-square test with the Yates’ correction.
Additional surgery
Although the filtering surgery was more performed in TA-assisted PPV and the removal of vitreous hemorrhage was more performed in conventional PPV, there was not any statistically significant difference between the two groups with respect to causes of additional surgery after PPV. In the TA-assisted PPV group, 22 surgeries were performed (excluding filtering surgery). The most common reasons for additional surgery were removal of silicone oil (11 eyes), followed by retinal detachment (eight eyes), VH (six eyes), and others (seven eyes). In the conventional PPV group, 26 surgeries were performed (excluding filtering surgery). The most common reasons were VH (12 eyes), followed by retinal detachment (nine eyes), removal of silicone oil (seven eyes), and others (six eyes). Although there was a tendency for VH to be less common in TA-assisted PPV eyes, no significant difference was found in comparison to conventional PPV (P = 0.13; Table 4). Filtering surgery was more frequently performed in the TA-assisted PPV group (15 cases, 3.8%) than the conventional PPV group (seven cases, 1.8%), although this difference was not significant (P = 0.10). Ten eyes received multiple additional surgeries in TA-assisted PPV group and seven eyes in conventional PPV group.
Table 4.
Causes of additional surgery after PPV
| TA-assisted PPV (total 391 eyes): n (%) | Conventional PPV (total 383 eyes) : n (%) | P-value* | |
|---|---|---|---|
| Silicone removal | 11 (2.8%) | 7 (1.8%) | 0.36 |
| Retinal detachment | 8 (2.0%) | 9 (2.3%) | 0.77 |
| Vitreous hemorrhage | 6 (1.5%) | 12 (3.1%) | 0.14 |
| Filtering surgery | 15 (3.8%) | 7 (1.8%) | 0.10 |
| Others | 7 (1.8%) | 6 (1.6%) | 0.81 |
| Total | 37 (9.5%)✝ | 32 (8.6%)✝✝ | 0.68 |
TA; triamcinolone acetonide: PPV; pars plana vitrectomy: * Chi-square test with the Yates’ correction: ✝ Ten eyes received multiple additional surgeries: ✝✝ Seven eyes received multiple additional surgeries.
The survival curve produced by the log-rank test showed that the incidence of additional surgery was not significantly different between the two groups (P = 0.45). The logistic regression test also showed that the use of TA was not a significant factor for additional surgery after adjustments for gender, age, and diagnosis. However, a diagnosis of PDR was a significant factor for additional surgery in comparison to DME (P = 0.005), RVO (P = 0.006), and ERM (P = 0.02; Table 5). None of the variables were related to the requirement for filtering surgery (Table 6).
Table 5.
Factors affecting additional surgery
| Variable (no. of additional surgery/total) | Odds ratio (95%CI) | P-value |
|---|---|---|
| TA-assisted PPV (37/391) vs conventional PPV (32/383) | 1.23 (0.797–1.911) | 0.35 |
| Age | 0.97 (0.950–0.984) | 0.0001* |
| Gender (male vs female) | 1.15 (0.733–1.797) | 0.55 |
| Diseases | ||
| RD vs PDR | 0.60 (0.345–1.029) | 0.06 |
| DME vs PDR | 0.29 (0.122–0.681) | 0.005* |
| RVO vs PDR | 0.23 (0.084–0.657) | 0.006* |
| ERM vs PDR | 0.087 (0.012–0.631) | 0.02* |
| Others vs PDR | 0.64 (0.299–1.374) | 0.25 |
CI; confidence interval: RVO; branch retinal vein occlusion + central retinal vein occlusion: DME; diabetic macular edema: ERM; epiretinal membrane: PDR; proliferative diabetic retinopathy: RRD; rhegmatogenous retinal detachment. * statistically significant
Table 6.
Factors affecting filtering surgery
| Variable (no. of filtering surgery/total) | Odds ratio (95%CI) | P-value |
|---|---|---|
| TA-assisted PPV (12/391) vs conventional PPV (5/383) | 1.74 (0.708–4.265) | 0.23 |
| Age | 0.98 (0.941–1.018) | 0.28 |
| Gender (male vs female) | 1.81 (0.696–4.728) | 0.22 |
| Diseases | ||
| RRD vs PDR | – | 0.99 |
| DME vs PDR | – | 0.99 |
| RVO vs PDR | – | 0.99 |
| ERM vs PDR | – | 0.99 |
| Others vs PDR | 0.83 (0.266–2.573) | 0.74 |
CI; confidence interval: TA; triamcinlone acetonide: PPV; pars plana vitrectomy: RVO; branch retinal vein occlusion + central retinal vein occlusion: DME; diabetic macular edema: ERM; epiretinal membrane: PDR; proliferative diabetic retinopathy: RRD; rhegmatogenous retinal detachment
Serious adverse events
None of the serious adverse events related to surgery defined in this study (such as retinal degeneration, endophthalmitis, an unexplained decrease in visual acuity, or optic-disc atrophy) were observed in either group during the 1-year follow-up.
Discussion
The first report on this study showed that the intra-operative use of TA significantly decreased the incidence of intra-operative complications associated with PPV, and no adverse events related to surgery were found over a 3-month observation period [13]. A decreased incidence of intra-operative complications is beneficial; however, the critical issue is whether the surgery improves the patients’ vision. We therefore followed up the visual acuity, which is a crucial factor influencing vision, for 1 year after surgery. Our results revealed no significant difference in visual acuity between the TA-assisted PPV group and the conventional PPV group after 1 year. As described in the earlier report, which analyzed data from the same eyes, intra-operative retinal breaks and retinal detachment were significantly less frequent in the TA-assisted PPV group than in the conventional PPV group. Nonetheless, the decreased incidence of intra-operative complications did not significantly affect the visual acuity after 1 year. This finding can be explained by the following facts. First, surgical skill is the most important factor for surgical success. As all of the operations in this study were performed by qualified and experienced surgeons, the intra-operative complications were managed so as not to cause serious post-operative complications. Thus, the influence of intra-operative complications on visual acuity might have been minimal. Second, a patient’s vision after vitrectomy might be more strongly affected by a disease diagnosis than by intra-operative complications. Indeed, eyes with PDR were significantly more likely to show improved visual acuity compared with baseline than eyes with DME in the current study. As our present case series included various diseases, the impact of intra-operative complications on post-operative visual acuity might have been masked by another strong factor, such as disease diagnosis, even after adjustments. Third, the eyes in the current study were primarily chosen for the detection of intra-operative retinal breaks, based on the hypothesis that the intra-operative use of TA might decrease their incidence from 15% to 8% [13]. Thus, the sample size might not have been large enough to detect the superiority or inferiority of TA-assisted PPV. For example, in order to detect the difference of post-operative improvement of vision in TA-assisted PPV and conventional PPV based upon the present results, approximately 29,315 cases in each group are necessary with α power of 0.05 and 1-β of 0.8. However, considering the relative stability of the visual acuity observed here, we are confident that the intra-operative use of TA does not have a positive or negative effect on postoperative visual acuity for 1 year.
Additional surgeries carry associated risks and can be costly; they are thus important when justifying the intra-operative use of TA in PPV. The incidence of additional surgery after vitrectomy was found to be equivalent using both methods. Considering the IOP increase after surgery, the opportunity to use anti-glaucoma eye drops was high in the TA-assisted PPV group [13], and the number of filtering surgeries performed during 1 year after TA-assisted PPV was also high; however, the incidence of additional surgeries was not statistically different between the groups. Several previous reports showed that intravitreous TA injection required filtering surgery, and its frequency was less than 1% of all treated eyes [18, 19]. In contrast to intravitreous TA injection, most of the TA was removed from the eye at the end of the surgery. Thus, it was plausible that the necessity of filtering surgery in TA-assisted PPV was not significant. The other causes of additional surgery occurred at similar frequencies, and there were no significant differences between the groups. A diagnosis of PDR was a significant factor for the requirement for additional surgery, as it was for the improvement of visual acuity. Furthermore, in our earlier report on the 3-month follow-up study, a diagnosis of PDR was found to be a significant risk factor for the occurrence of intra-operative complications. PDR eyes thus appear to have several poor prognostic factors, consistent with the present results.
With regard to the post-operative complications, pre-retinal fibrosis appeared to be less common in the TA-assisted PPV group than in the conventional PPV group, although this was not statistically significant. This advantage of TA-assisted PPV has been described in previous reports [3, 5–9]. The intra-operative use of TA visualizes the posterior hyaloid clearly, which facilitates its removal [6, 7]; as the residual posterior hyaloid can act as a scaffold for pre-retinal fibrous-membrane formation, its removal could reduce the incidence of post-operative pre-retinal fibrosis [5–7]. In the current study group, the removal of the residual posterior hyaloid was carried out according to the surgeons’ preference—some surgeons removed it completely, while others did not. This might have obscured any potential advantage of the intra-operative use of TA in inhibiting post-operative pre-retinal fibrosis.
There were clear limitations to our analysis. The number of cases in each study group was primarily chosen to allow the detection of intra-operative complications; thus, the significance of the intra-operative use of TA might only become clear when studying larger numbers of patients. In addition, the sample size was too small to detect the risk of endophthalmitis. The incidence of endophthalmitis related to PPV was 0.046% in the conventional PPV group and 0.053% in the TA-assisted PPV group [20, 21]. An analysis of a greater number of cases will thus be necessary to evaluate the risk of endophthalmitis related to TA-assisted PPV. However, the present sample size was large enough to detect several factors that affected post-operative visual acuity or the need for additional surgery. Another limitation was that the post-operative visual acuity was strongly influenced by the diagnosis of disease. Eyes with simple VH clearly had a greater chance of surgery improving vision above the baseline level than did eyes with complicated PDR. In order to evaluate the influence of the intra-operative use of TA on visual acuity more precisely, we are currently performing a study on eyes with the same disease diagnosis. Furthermore, the subgroup of DME or ERM in each group was not completely matched. The interpretation of the results should be done carefully. Additionally, caution must be applied when making generalizations on the basis of our current results, and their application to clinical practice must be undertaken with care. Other factors, such as invasiveness and cost, are also important in seeking justification for this treatment. The success of surgery depends upon various factors, and surgical skill is critical. When considering the likelihood of retinal breaks and their recovery without damaging vision, comparisons of the hazard ratios of TA-assisted PPV and conventional PPV would be valid only if made by individuals who qualify as PPV specialists.
In summary, the intra-operative use of TA in PPV did not affect visual acuity over 1 year. As negative or adverse events were not observed in this case series, a more detailed study is warranted to establish the value of TA in PPV.
Acknowledgements
This study was supported, in part, by a Grant-in-Aid from the Ministry of Education, Science and Culture, Japan.
The authors indicate no financial conflict of interest.
Institutional review board (IRB)/ethics committee approval was obtained from all of the following participating clinical establishments, The prospective controlled clinical trial took place at the following eight hospitals in Japan; Kagoshima University Hospital, Miyata Eye Hospital, Amatasu Hospital, Imamura General Hospital, Kimura Eye and Internal Medicine Hospital, Shinjo Eye Hospital, Kamiiida Daiichi General Hospital, and the University Hospital of Occupational and Environmental Health, Kitakyushu, Japan. All patients undergoing PPV at these hospitals were eligible to enroll in the study. Each patient was fully informed about the nature of the treatment, and provided written consent.
References
- 1.Peyman GA, Cheema R, Conway MD et al (2000) Triamcinolone acetonide as an aid to visualization of the vitreous and the posterior hyaloid during pars plana vitrectomy. Retina 20:554–555 [DOI] [PubMed]
- 2.Sakamoto T, Miyazaki M, Hisatomi T et al (2002) Triamcinolone-assisted pars plana vitrectomy improves the surgical procedures and decreases the postoperative blood-ocular barrier breakdown. Graefes Arch Clin Exp Ophthalmol 240:423–429 [DOI] [PubMed]
- 3.Sonoda KH, Sakamoto T, Enaida H et al (2004) Residual vitreous cortex after surgical posterior vitreous separation visualized by intravitreous triamcinolone acetonide. Ophthalmology 111:226–230 [DOI] [PubMed]
- 4.Enaida H, Hata Y, Ueno A et al (2004) Visualization of the Cloquet canal during triamcinolone-assisted vitrectomy. Arch Ophthalmol 122:1564–1565 [DOI] [PubMed]
- 5.Enaida H, Hata Y, Ueno A et al (2003) Possible benefits of triamcinolone-assisted pars plana vitrectomy for retinal diseases. Retina 23:764–770 [DOI] [PubMed]
- 6.Ueno A, Enaida H, Hata Y et al (2007) Long-term clinical outcomes and therapeutic benefits of triamcinolone-assisted pars plana vitrectomy for proliferative vitreoretinopathy: A case study. Eur J Ophthalmol 17:392–398 [DOI] [PubMed]
- 7.Sonoda KH, Enaida H, Ueno A et al (2003) Pars plana vitrectomy assisted by triamcinolone acetonide for refractory uveitis: a case series study. Br J Ophthalmol 87:1010–1014 [DOI] [PMC free article] [PubMed]
- 8.Furino C, Micelli Ferrari T et al (2003) Triamcinolone-assisted pars plana vitrectomy for proliferative vitreoretinopathy. Retina 23:771–776 [DOI] [PubMed]
- 9.Ikuno Y, Sayanagi K, Ohji M et al (2004) Vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Am J Ophthalmol 137:719–724 [DOI] [PubMed]
- 10.Kimura H, Kuroda S, Nagata M (2004) Triamcinolone acetonide-assisted peeling of the internal limiting membrane. Am J Ophthalmol 137:172–173 [DOI] [PubMed]
- 11.Shah GK, Rosenblatt BJ, Smith M (2004) Internal limiting membrane peeling using triamcinolone acetonide: histopathologic confirmation. Am J Ophthalmol 138:656–657 [DOI] [PubMed]
- 12.Tognetto D, Zenoni S, Sanguinetti G et al (2005) Staining of the internal limiting membrane with intravitreal triamcinolone acetonide. Retina 25:462–467 [DOI] [PubMed]
- 13.Yamakiri K, Sakamoto T, Noda Y et al (2007) Reduced incidence of intraoperative complications in a multicenter controlled clinical trial of triamcinolone in vitrectomy. Ophthalmology 114:289–296 [DOI] [PubMed]
- 14.Jonas JB, Hayler JK, Sofker A, Panda-Jonas S (2001) Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol 131:468–471 [DOI] [PubMed]
- 15.Moshfeghi DM, Kaiser PK, Scott IU et al (2003) Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol 136:791–796 [DOI] [PubMed]
- 16.Smithen LM, Ober MD, Maranan L, Spaide RF (2004) Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol 138:740–743 [DOI] [PubMed]
- 17.Singh IP, Ahmad SI, Yeh D et al (2004) Early rapid rise in intraocular pressure after intravitreal triamcinolone acetonide injection. Am J Ophthalmol 138:286–287 [DOI] [PubMed]
- 18.Rhee DJ, Peck RE, Belmont J et al (2006) Intraocular pressure alterations following intravitreal triamcinolone acetonide. Br J Ophthalmol 90:999–1003 [DOI] [PMC free article] [PubMed]
- 19.Jonas JB, Degenring RF, Kreissig I et al (2005) Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology 112:593–598 [DOI] [PubMed]
- 20.Aaberg TM Jr, Flynn HW Jr, Schiffman J, Newton J (1998) Nosocomial acute-onset postoperative endophthalmitis survey. A 10-year review of incidence and outcomes. Ophthalmology 105:1004–1010 [DOI] [PubMed]
- 21.Sakamoto T, Enaida H, Kubota T et al (2004) Incidence of acute endophthalmitis after triamcinolone-assisted pars plana vitrectomy. Am J Ophthalmol 138:137–138 [DOI] [PubMed]