Abstract
Androgen receptors are expressed in many different neuronal populations in the central nervous system where they often act as transcription factors in the cell nucleus. However, recent studies have detected androgen receptor immunoreactivity in neuronal and glial processes of the adult rat neocortex, hippocampal formation, and amygdala as well as in the telencephalon of Eastern Fence and green anole lizards. This review discusses previously published findings on extranuclear androgen receptors, as well as new experimental results that begin to establish a possible functional role for androgen receptors in axons within cortical regions. Electron microscopic studies have revealed that androgen receptor immunoreactive processes in the rat brain correspond to axons, dendrites and glial processes. New results show that lesions of the dorsal CA1 region by local administration of ibotenic acid reduce the density of androgen receptor immunoreactive axons in the cerebral cortex and the amygdala, suggesting that these axons may originate in the hippocampus. Androgen receptor immunoreactivity in axons is also decreased by the intracerebroventricular administration of colchicine, suggesting that androgen receptor protein is transported from the perikaryon to the axons by fast axonal transport. Androgen receptors in axons located in the cerebral cortex and amygdala and originating in the hippocampus may play an important role in the rapid behavioral effects of androgens.
Keywords: androgen receptor, androgen, cerebral cortex, hippocampus, axons, extranuclear steroid receptors, rapid actions of steroids
Introduction
The most commonly studied role of steroid receptors has unquestionably been that of their participation as nuclear transcription factors, modulating gene transcription. However, in the 1970s and 1980s, parallel evidence began to emerge that steroids could act rapidly on cellular functions, independent of gene transcription ( Kelly, et al., 1977; Dufy, et al., 1979; Nabekura, et al., 1986). Among the first reports to show the rapid, nongenomic actions of steroid hormone receptors was an electrophysiological study published by Kelly (Kelly et al., 1977) that demonstrated the rapid effects of estradiol on neuronal firing in the rat hypothalamus. Others went on to demonstrate that estradiol potentiates excitatory post-synaptic potentials in hippocampal neurons (Foy and Teyler, 1983; Wong and Moss, 1991; Wong and Moss, 1992), potentiates glutamate responses in the cerebellum (Smith, et al., 1987; Smith, et al., 1989), suppresses μ-opioid and GABAB receptor based hyperpolarization of arcuate neurons (Kelly, et al., 1992), and potentiates K+ stimulated dopamine release in rat nucleus accumbens (Thompson and Moss, 1994), all without affecting gene transcription.
Over the last 30 years, biochemical, molecular, and additional physiological studies have added to our understanding of the rapid, nongenomic actions of estrogen, progesterone, glucocorticoids, mineralcorticoids, thyroid hormone, vitamin D, and androgens (for review, see Schmidt, et al., 2000). This evidence has broadened the view of what the steroid receptor family of proteins is capable of, although the mechanisms remain to be fully characterized. Newer behavioral evidence from several labs (for example, see Bass and Remage-Healey, this issue) has provided a systems-level analysis of what the rapid actions of steroids mean in the context of the nervous system and whole organism. In contrast, relatively few studies have been able to establish a neuroanatomical basis for these rapid actions. With the immunohistochemical observations on extranuclear localization of androgen receptor (AR) reviewed below and other information about the rapid actions of androgens in cells and whole organisms, we may begin to obtain a clear understanding of the role of extranuclear AR in the nervous system.
Evidence of androgen receptors in axons and other non-nuclear locations
Recently, we identified AR immunoreactivity in axons and dendrites in the adult male rat cerebral cortex and amygdala (DonCarlos, et al., 2003; DonCarlos, et al., 2006) (Figure 1–2). No AR-immunoreactive (AR-ir) neurites were present in the hypothalamus, preoptic area or bed nucleus of the stria terminalis, brain regions with abundant nuclear AR. At the light microscopic level, we identified these neurites as axons. The axons were small in diameter and punctate in appearance. At the ultrastructural level, extranuclear AR-immunoreactivity was observed within the cytoplasm of axons, not embedded in the axonal membrane. In addition, the ultrastructural studies revealed extranuclear AR in dendrites and astrocytes. Gonadectomy of male rats resulted in a nearly complete loss of the extranuclear AR. AR-ir axons were observed in neonatal male and female rats beginning on postnatal day 6, with abundant axonal AR by postnatal day 10 (unpublished observations). The regional specificity of the label, corroboration at the ultrastructural level, hormonal regulation and developmental progression, as well as extensive immunohistochemical controls and multiple antibodies, led us to postulate that the immunoreactivity represented true ARs, potentially with a functional role in axons.
Fig. 1. Morphology of AR-ir axons in the adult male rat brain.
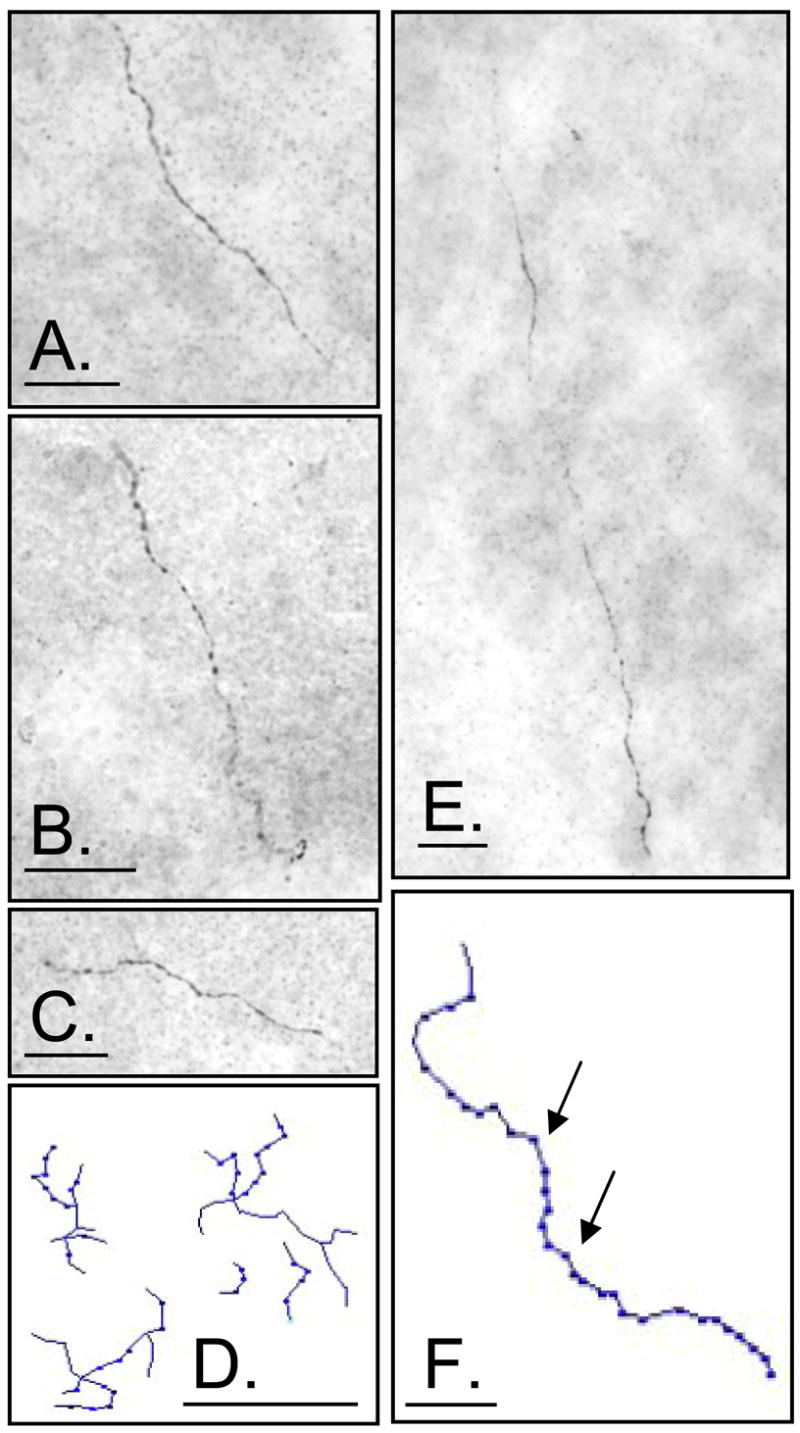
A–C, E: Digital images of AR-ir axons in the A. piriform cortex, B. amygdala, C. hippocampus E. entorhinal cortex, D. Tracing of AR-ir terminal field in the entorhinal cortex; E. Tracing of AR-ir axons in the piriform cortex with arrows indicating punctate stain. Scale bars represent 25 μm.
Fig. 2. Distribution of AR-ir axons in the rat forebrain and brainstem.
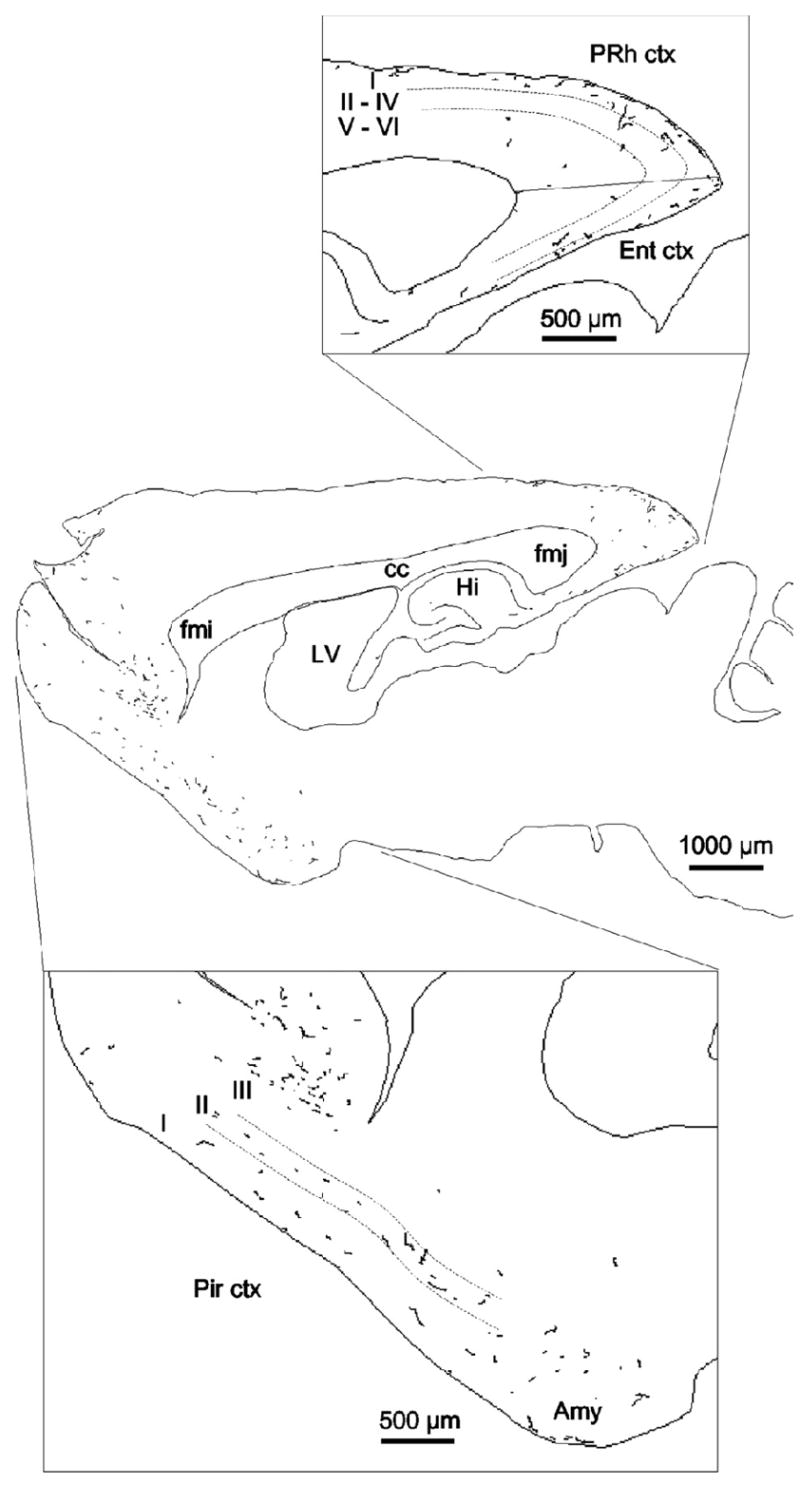
This map was produced using NeuroLucida software (MicroBrightField Inc.) and illustrates a single 40-μm parasagittal section, 2.9 mm lateral to bregma. The entire section was scanned at x40, and all AR immunoreactive axons observed were traced. An adjacent Nissl-stained section was used to delineate cortical layers. Amy, Amygdala; cc, corpus callosum; ctx, cortex; Ent, entorhinal cortex; fmi, forceps minor of the corpus callosum; fmj, forceps major of the corpus callosum. Hi, hippocampus; LV, lateral ventricle; Pir, piriform cortex; PRh, perirhinal cortex. Reproduced from (DonCarlos, et al, 2003), Copyright 2003, The Endocrine Society.
At about the time we first observed these AR-ir axons in rats, two other reports were published that identified AR in neurites, one from Moga’s lab in 2000 (Moga, et al., 2000) on AR-immunoreactivity in the eastern fence lizard, and another from Wade and coauthors (Rosen, et al., 2002) on green anole lizards. These studies mapped AR-immunoreactivity as part of investigations into the more potent role of androgens, compared with estrogenic metabolites of androgens, in activating male reproductive behaviors in these species (Crews, et al., 1978; Tokarz, 1986). In the eastern fence lizard, AR-ir neurites were widely distributed in telencephalic regions, and most abundant in the area triangularis, medial septal nucleus, dorsal and medial cortex. Some AR-ir neurites were located in the periventricular and lateral hypothalamus, but as in the rat, in regions with abundant nuclear AR (including the arcuate nucleus, premammillary nucleus, medial preoptic area, ventral posterior amygdala, and ventromedial hypothalamic nucleus), AR-ir neurites were few or absent. The neurites were punctuated by varicosities, and the images reveal a more dense distribution of AR-ir neurites in the cortex than what we observed in the rat. In the green anole lizard, the distribution of AR-ir fibers was characterized as “remarkably similar” to that reported in the eastern fence lizard (Rosen et al., 2002).
Milner and colleagues subsequently published two ultrastructural studies demonstrating extranuclear AR-immunoreactivity within axons and dendrites in the hippocampal formation (Tabori, et al., 2005) and axons in a rostral medullary region that regulates blood pressure (Milner, et al., 2007). Extranuclear AR immunoreactivity was also observed to be abundant in glia in both studies, and in agreement with our findings ( Lorenz, 2005; DonCarlos et al., 2006).
Other evidence for extranuclear localization of other steroid receptors in the nervous system, most notably, for estrogen receptor (ERα), has been more fully developed (Blaustein, et al., 1992; Milner, et al., 2001; Wagner, et al., 1998), and a few examples are mentioned here. Cytoplasmic ERα is unlike AR in that it is abundant in neurons in the hypothalamus ( Silverman, et al., 1991;Blaustein et al., 1992), whereas ERα and ERβ are similar to AR in that all are abundant in the adult hippocampus (Milner et al., 2001; Milner et al., 2005). The extranuclear ERα is located in vesicles in axon terminals (Blaustein et al., 1992; Hart, et al., 2007) where estrogen promotes a redistribution of the ERα immunoreactive vesicles in the terminal toward synaptic membranes (Hart et al., 2007). Extranuclear ERα and ERβ are also present in dendrites of cultured hippocampal neurons and estradiol, in addition to increasing glutamatergic synapses along these dendrites, also increases the density of extranuclear estrogen receptors (Jelks et al., 2007). There are subtle differences in the extranuclear distribution of ERα and AR at the cellular level (Milner et al., 2001; Tabori et al., 2005); for example, although both are present in axon terminals, dendritic spines and astrocytes in the hippocampus, the cytoplasmic AR was found exclusively in asymmetric synapses, whereas cytoplasmic ERα was observed in axon terminals of both symmetric and asymmetric synapses. Therefore, although the cellular functions of extranuclear ERα and AR may be similar in some respects, differences in cellular and regional concentrations of these two yextranuclear receptors suggest different functions, perhaps through activating different signaling pathways, binding to different intracellular proteins (in vesicles vs lipid rafts, for example) or differentially impacting mitochondrial gene expression.
Despite the increasing number of observations of extranuclear steroid receptors in the nervous system, linking these anatomical results to specific cellular, physiological or behavioral functions has been less forthcoming. We have taken a functional neuroanatomical approach to begin to provide clues as to what role AR in axons may play in mediating the effects of androgens in cortical function in mammals, and the next sections of this review detail the results of some of these previously unpublished experiments.
AR-ir axons may arise from regions with abundant neuronal, nuclear AR
Our more recent studies have corroborated our initial observation that AR-ir axons in the rat are predominately localized in cortical regions and the amygdala, avoiding the preoptic area and hypothalamus. It has not been possible to follow AR-ir axons from the temporal region of the cortex to a cell body of origin, and therefore, other methods had to be used to determine what brain regions may give rise to the AR-ir axons. Clues to the possible origin of AR-ir axons were found in the morphology and distribution of these axons. Punctate, small caliber fibers were distributed throughout the cortex, hippocampus, and amygdala, specifically in the caudal temporal region (DonCarlos et al., 2003). Axons with a similar morphology and distribution arise from several regions, including the CA1 region of the hippocampus (Buzsaki, 1996; Witter, et al., 2000; von Bohlen und Halbach and Albrecht, 2002), the contralateral amygdala (von Bohlen und Halbach and Albrecht, 2002), the contralateral temporal cortical regions (Witter et al., 2000), the substantia innominata (Audet, et al., 1988; Kritzer, 2003), the locus coeruleus (Fallon, et al., 1978; Saper, 1984), the dorsal raphe (Fallon et al., 1978; Vertes, 1991; Vertes, et al., 1999;Kritzer, 2003), and the ventral tegmental area (Descarries, et al., 1987; Goldsmith and Joyce, 1994; Kritzer, 2003). These areas also contain nuclear AR in neurons, and have been implicated in androgen regulated behavioral or physiological functions.
Anterograde tracing studies, using Fluororuby combined with immunofluorescence to detect AR in the anterogradely labeled axons, provided some hints as to the possible location of the cell bodies of origin of the axons; however, in extensive trials, it proved quite difficult to document the colocalization because of bleaching of the two fluorophores. These technical difficulties prompted a switch in methods to the ibotenic acid lesion approach. Ibotenic acid is an excitotoxin that destroys cell bodies, leaving axons of passage intact (Johnston, et al., 1968; Markowska, et al., 1985). Therefore, the regions giving rise to axons with similar appearance and trajectory to those observed in initial mapping studies were lesioned to determine if damage to each of these regions would have the expected effect of eliminating AR-ir axons from the cerebral cortex and amygdala. All animal procedures described below adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and had been previously approved by the Institutional Animal Care and Use Committee of the Loyola University Medical Center.
Ibotenic acid was injected unilaterally into the brain of adult male Sprague-Dawley rats. The injections were made into the CA1 region of the hippocampus, dorsal raphe, amygdala, or the piriform/entorhinal cortex (n=5 animals for each region); another group underwent surgical procedures without receiving injections (n=5 animals) because preliminary studies determined that surgery, with or without vehicle injections, had no effect on the density of AR-ir axons. The regions selected were based on preliminary results from the anterograde tracing trials. Standard stereotaxic procedures were used and the coordinates for each lesion were determined using the Paxinos and Watson rat brain atlas (1986). Animals were anesthetized with sodium pentobarbital (50mg/ml, 42mg/kg; ip) and then received an ibotenic acid injection (0.5μl; 10mg/ml in 25mM PBS, via a 0.5 μl Hamilton syringe) over 15 minutes.
One week later, the animals were reanesthetized and perfused with 4% paraformaldehyde in 25mM PBS. Brains were removed, and processed for AR localization with the PG-21 antiserum (3micrograms/ml, gift from Dr. Gail Prins, University of Illinois, Chicago, IL; (Prins, et al., 1991)) according to previously published procedures (DonCarlos et al., 2003), using standard controls. Additional sections were stained with toluidine blue to confirm the location of lesions at the light microscopic level by observation of a reduction in Nissl substance and the presence of cellular debris.
AR-ir axon density was analyzed in coronal sections through the perirhinal cortex, piriform cortex, entorhinal cortex, and amygdala using a Leica DMR microscope and StereoInvestigator imaging software (MicroBrightField; Burlington, VT), which we used to randomly define counting frames to be sampled. Between 15 and 30 counting frames, depending on the size of the designated brain region, were randomly chosen within that region. Each counting frame was 200μm × 200μm and the total sample size was approximately 40% of each designated brain region, which had been previously determined to accurately represent the total number of AR-ir axons (+/− less than 10%). The observer focused through the section, following individual axons and counting each only once, as it came into focus. The total number of AR-ir axons was then estimated for each brain region based on the sample and this number was used to calculate axon density. Statistical analysis was performed using a one-way ANOVA, with a Dunnet’s post-hoc test for each brain region to determine whether significant differences existed between the AR-ir axon density in the selected regions from control vs lesioned brains. A Tukey’s post-hoc test was used to determine differences between the ipsilateral and contralateral sides of the brain. The p value was set at <0.05.
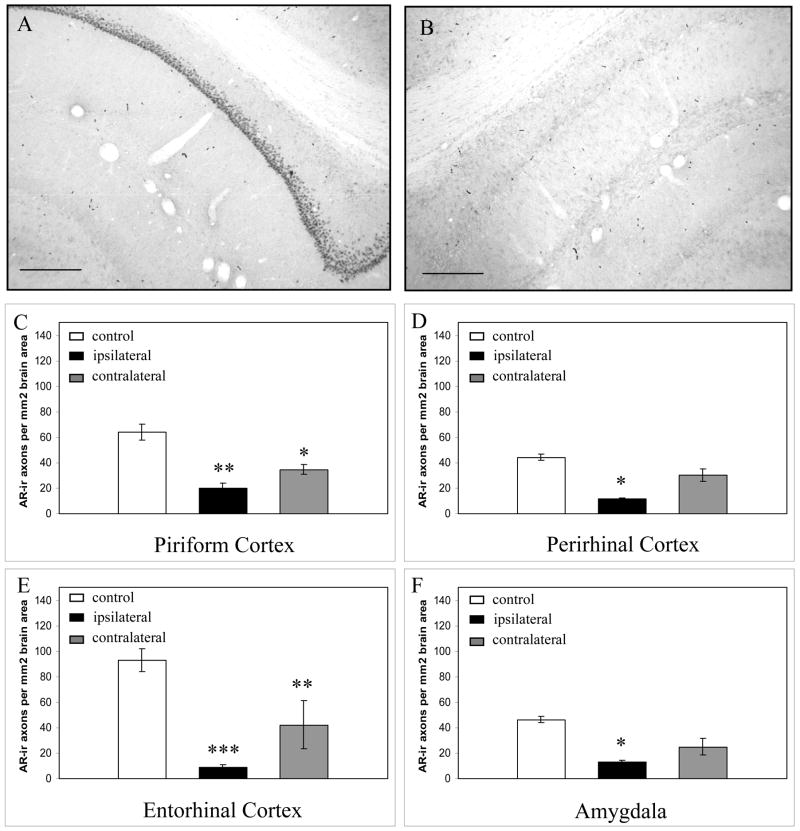
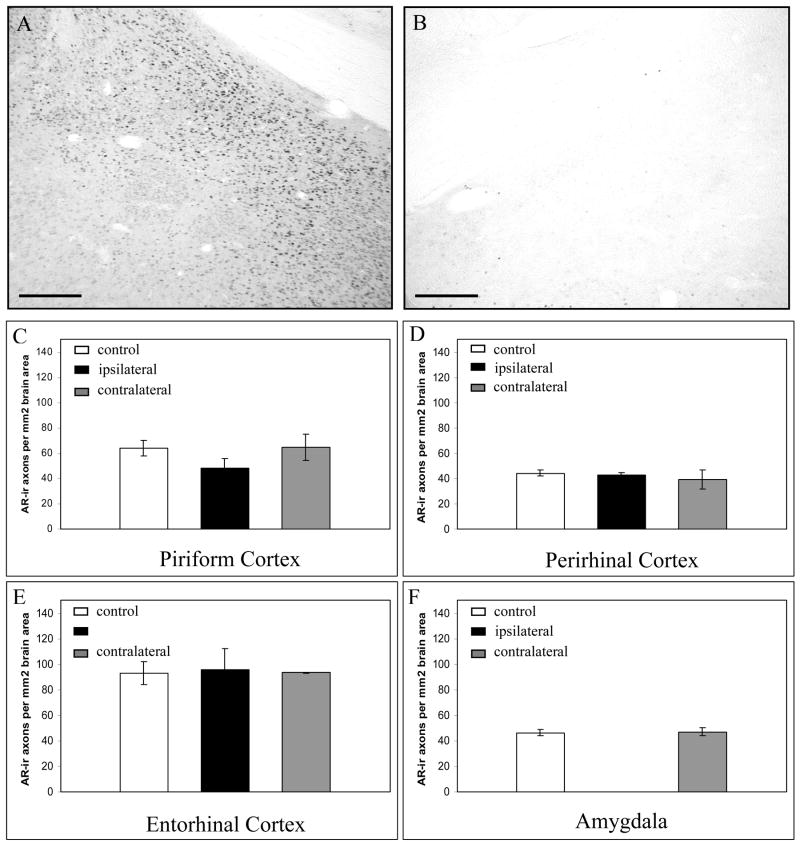
Lesions of the dorsal CA1 region of the hippocampus reduced AR-ir axon density both ipsilaterally and contralaterally (Figure 3). Ipsilaterally, AR-ir density was significantly reduced in the perirhinal cortex, piriform cortex, entorhinal cortex and amygdala. Contralateral to the CA1 lesions, the density of AR-ir axons was significantly reduced in the piriform cortex and entorhinal cortex alone. In contrast, none of the other lesions produced significant changes in the density of AR-ir axons (Figure 4), demonstrating not only that these other regions do not give rise to AR-ir axons, but also that the lesions do not produce a generalized, non-specific effect due to stress or immune system involvement. Therefore, AR-ir axons in the cortex and amygdala may originate in the hippocampus, but do not appear to arise from the dorsal raphe, amygdala, or piriform/entorhinal cortex.
Fig 3. Ibotenic acid lesions of the hippocampal CA1 region significantly reduced the density of AR-ir axons.
A and B. Localization of the ibotenic acid lesions A., demonstrated using AR-immunostained material. Nuclear AR-immunostaining in the CA1 region contralateral to a lesion, seen in (B). B. Nuclear AR-immunostaining was reduced in the CA1, and subiculum region following ibotenic acid lesion into this male rat brain. C–F. Effect of CA1 lesion on AR-ir axon density in temporal brain regions. Control = control brain region with no lesion. Ipsilateral = brain region on the same side as the lesion. Contralateral = brain region on the opposite side of the lesion. Scale bars represent 250μm. * p<0.05, **p<0.01, ***p<0.001 from control. Error bars represent standard error of the mean.
Fig 4. Ibotenic acid lesions of amygdala had no effect on the density of AR-ir axons in any of the regions examined.
A and B. Localization of the ibotenic acid lesions, demonstrated using AR-immunostained material. A. Nuclear AR-immunostaining in the medial amygdala contralateral to a lesion, seen in (B). B. Nuclear AR-immunostaining was reduced in the male rat amygdala following ibotenic acid lesion. C–F. Effect of amygdala lesion on AR-ir axon density in temporal brain regions. Control = control brain region with no lesion. Ipsilateral = brain region on the same side as the lesion. Contralateral = brain region on the opposite side of the lesion. Scale bars represent 250μm. Error bars represent standard error of the mean.
The loss of AR-ir axons in the ipsilateral cortex following CA1 lesions correlated with the projection pattern of the dorsal and intermediate CA1 neurons into temporal brain areas (Knowles, 1992; Witter et al., 2000; von Bohlen und Halbach and Albrecht, 2002; Cenquizca and Swanson, 2007). The dorsal third of the CA1 has direct projections to the entorhinal cortex, and to the retrosplenial and perirhinal cortex, especially to Layer 1, with less extensive projections to Layers 2–4. The dorsal CA1 also projects to the olfactory bulb, and amygdala. The reduction in AR-ir axons contralateral to the lesions may have resulted from destruction of the cells giving rise to the sparse commissural projections from the dorsal CA1 that target the contralateral piriform and entorhinal cortex in the contralateral hemisphere (Knowles, 1992; Cenquizca and Swanson, 2007). The intermediate CA1 fields provide strong inputs to the subiculum as well as terminating extensively in Layers 4 and 5 of the entorhinal region; the loss of AR-ir axons in this area suggests that the lesions partially destroyed the cell bodies in intermediate CA1 that give rise to AR-ir axons heading toward the entorhinal cortex. In the present experiments, primarily the dorsal hippocampus was lesioned, although strong nuclear AR as well as AR in axons are observed in the ventral CA1. However, the ventral CA1 projects strongly to septal nuclei and hypothalamic regions (Cenquizca and Swanson, 2006) that are devoid of AR-ir axons, and therefore, we consider that the ventral CA1 is not likely to be a major source of AR-ir output pathways.
Alternative explanations for our results exist. For one, the reduction in AR-ir axon density could be due to indirect effects of the CA1 lesions on an afferent or efferent cell group that gives rise to the AR-ir axons. Outputs of the CA1 region include the temporal cortex, lateral septum, olfactory nuclei, nucleus accumbens, amygdala, and hypothalamus (Fallon et al., 1978; Ottersen, 1982; Deacon, et al., 1983; Saper, 1984; Canteras, et al., 1992; Knowles, 1992; Kowianski, et al., 1999; Johnson, et al., 2000; Witter et al., 2000; von Bohlen und Halbach and Albrecht, 2002; Cenquizca and Swanson, 2007). Of these output regions, perhaps the most likely to send projections on to all of the other areas that have dense AR-ir axons and receive CA1 projections is the amygdala. However, if indirect effects of CA1 lesions altered maintenance and transport of AR from cell bodies of the amygdala, then direct lesions of the amygdala should have decreased the density of AR-ir axons even more profoundly, but this was not the case; lesions of the amygdala did not reduce the density of AR-ir axons (Figure 4). Therefore, it is possible, but unlikely, than an indirect effect of the CA1 hippocampal lesions was a factor in the lesion-induced reduction of AR-ir axon density. For the reductions in AR-ir density contralateral to the injection, toxin injected into one side but near the midline, could have partially affected neurons in the opposite CA1 region, but this also seems unlikely, since there was no apparent loss of neurons in the CA1 region contralateral to the lesion (with the exception of a slight loss in one animal with an ibotenic acid lesion quite close to the midline).
The cell bodies of origin of AR-ir axons are unlikely to arise from regions without nuclear, neuronal AR
A second method was used to identify potential regions that give rise to AR-ir axons. Intraventricular injections of colchicine, an agent that disrupts microtubules and, therefore, fast axonal transport, causes proteins destined for transport to accumulate within the cell body. This technique has been used by other laboratories to successfully identify novel populations of cells that normally produce estrogen receptors exclusively for transport, such as the mediodorsal nucleus of the thalamus (Blaustein and Olster, 1993; Moga et al., 2000).
In our experiments, six adult male rats each were assigned to one of three groups, including sham control, colchicine only, or colchicine + testosterone propionate (TP). The colchicine + TP group was added because the hypothalamic-pituitary-gonadal axis relies on an intact transport system (Khar, et al., 1979), and colchicine may have disrupted hypothalamic function, leading to alterations in hormone levels. This was an important consideration because gonadectomy had previously been shown to reduce the number of AR-ir axons (DonCarlos et al., 2003). Animals were anesthetized with sodium pentobarbital (50mg/ml, 42mg/kg, ip) and given a subcutaneous hormone implant (TP or placebo, 21 day release pellet, 0.5mg TP total or vehicle, Innovative Research). Stereotaxic injections into the lateral ventricle were performed as described above for ibotenic acid lesions, using a 25μl Hamilton syringe and a 5μl injection (10μg/μl) of colchicine (Sigma) or vehicle. Animals were monitored carefully for overt signs of illness, and sacrificed by perfusion fixation three days after surgery. Brains were removed, sectioned at 40 microns, and a one in three series was processed for AR localization as previously described (DonCarlos et al., 2003), including standard controls. All animal protocols had received approval from the Loyola University Medical Center Institutional Animal Care and Use Committee.
All sections were qualitatively analyzed for the presence of a novel population of AR containing neurons. The number of AR-immunoreactive neurons was also semi-quantitatively analyzed in five sections per brain for the dorsal CA1 region of the hippocampus (n=5 colchicine, n=5 control) on the side opposite the colchicine injection and two sections per brain for the dorsal raphe (n=3 colchicine, n=3 control) using a MicroBrightfield NeuroLucida system. Coronal sections from the perirhinal cortex, piriform cortex, entorhinal cortex, and amygdala were analyzed for axon density as well, again using the stereological sampling method employed by the StereoInvestigator software (MicroBrightField) as described above. All counts were made by an observer blinded to the treatment groups.
No novel cell groups were observed to contain nuclear AR-ir in neurons following disruption of axonal transport; that is, colchicine treatment did not result in an accumulation of AR in cell groups that did not contain nuclear AR in the sham control groups. This leads to the conclusion that extranuclear AR is anterogradely transported from cells that also contain abundant somal/nuclear AR. Furthermore, colchicine treatment, when combined with TP, did not alter the number of AR-ir nuclei in a putative source of AR-ir axons, the dorsal CA1 region of the hippocampus (data expressed as mean +/− SEM number of AR-ir nuclei per 40 micron section; control animals, 4959 +/− 1239, colchicine + TP treated animals, 4941+/− 1050) nor did it affect the number of AR-ir nuclei in a second region, the dorsal raphe nucleus, (mean number of AR-ir nuclei per 40 micron section, control animals, 1354 +/− 146, colchicine +TP treated animals 1379 +/− 102). On the other hand, colchicine treatment greatly diminished or, in animals without testosterone supplementation, completely extinguished the staining of AR-ir neurons in hypothalamic and septal nuclei suggesting that the colchicine increased turnover or decreased synthesis of AR in these cell groups without an accompanying accumulation of protein targeted for transport.
Evidence for transport of AR through the axon
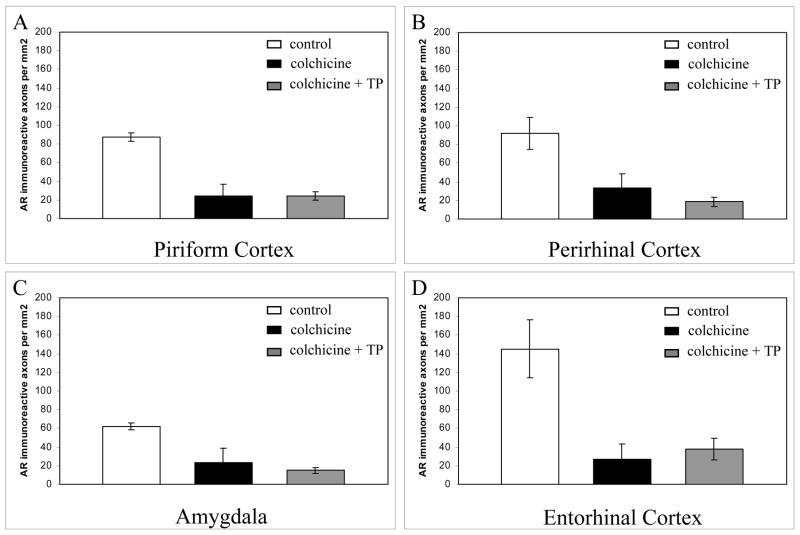
As mentioned before, a consequence of colchicine-induced disruption of microtubules is the prevention of fast axonal transport. In contrast with the lack of an effect on AR-ir nuclei in the regions examined, disruption of microtubules with colchicine reduced the density of AR-ir axons in the perirhinal cortex, piriform cortex, entorhinal cortex, and the amygdala by 70 to 80 % (Figure 5). AR-ir axon numbers were equally decreased in colchicine-treated animals that received testosterone propionate supplements compared with those that received colchicine but no androgen replacement. Therefore, the colchicine-induced reduction in AR-ir axon density in cortical regions appears to be due to the disruption of axonal transport, and not a function of a reduction in testosterone levels.
Figure 5. Colchicine-induced microtubule disruption reduced the density of AR-ir axons in the A) piriform cortex, B) perirhinal cortex, C) amygdala, and D) entorhinal cortex of male rats.
Control = intracerebroventricular (i.c.v.) vehicle with placebo pellet implant. Colchicine = i.c.v. colchicine with placebo pellet implant. Colchicine + TP = i.c.v. colchicine with testosterone propionate implant. Error bars represent standard error of the mean.
Finally, although colchicine is a potent neurotoxin in some cell groups, such as the granule cells of the dentate gyrus and cerebellum, it spares neurons of the cerebral cortex and of the CA1 region (Goldschmidt and Steward, 1980; Steward, et al., 1984; Ikegaya and Matsuki, 2002), which appears to give rise to the AR-ir projections. It is therefore unlikely that the reductions in AR-ir axon density following colchicine were to a loss of the cells that produce extranuclear AR or to a loss of synaptic targets within the cerebral cortex.
Possible functional role of AR-ir axons in androgen-mediated behaviors and pathologies attributed to the CA1
The real question is what does extranuclear AR do at the cellular and at the organismal level? The effects of rapid actions of androgens on physiology and behavior, and non-classical mechanisms of AR in neuroprotection, are reviewed in other contributions in this special issue of Hormones and Behavior. Therefore, the comments here will be restricted primarily, although not exclusively, to what role extranuclear AR and rapid effects of androgens might have in generalized hippocampal function.
The identification of the CA1 region of the hippocampus as the putative origin of the AR-ir axons, in addition to the documented behaviors and pathologies that respond to androgens, introduces many possible roles for AR within these axons, some of which are discussed in other articles within this issue. The hippocampus is central to learning and memory and relies on its interconnections with the amygdala and temporal cortical regions ( Knowles, 1992; Chrobak, et al., 2000; Alonso, 2002). Spatial and attentional memory, which are enhanced in males compared with females in some respects (Postma, et al., 2004; Sandstrom, et al., 2006), but not others (Williams, et al., 1990), involve the interconnection between the hippocampus and the entorhinal cortex (Chrobak et al., 2000; Bannerman, et al., 2001). Studies in testicular feminized (Tfm) mice and gonadectomized rats have recently attributed this difference to the actions of androgens, most likely acting through ARs (Frye, et al., 2004; Jones and Watson, 2005; Rizk, et al., 2005).
Damage to the hippocampus and its interconnections with the temporal region of the cortex and amygdala are central to the pathology of Alzheimer’s disease (Corsellis, 1977; Perl and Pendlebury, 1986); senile plaques and neurofibrillary tangles are detected in the hippocampus and entorhinal cortex early in the disease process (Ohm, et al., 2002). Although estrogen is neuroprotective in many models of neurodegeneration and injury and may impact the progression of Alzheimer’s disease (Garcia-Segura, et al., 2001), evidence continues to accumulate that androgens are a critical factor in neuroprotection as well. Clinical studies have demonstrated that both men and women with Alzheimer’s disease have lower levels of free testosterone and higher levels of sex hormone binding globulin than age-matched controls, implicating low testosterone as a risk factor for the disease (Paoletti, et al., 2004). Estradiol levels in these same patients were higher than controls, suggesting that low estrogen levels were either not a significant factor in disease onset or posed an additional risk factor (Paoletti et al., 2004). A final piece of clinical evidence for an action of androgens on hippocampal function involves apolipoprotein E4, a lipoprotein variant that increases the risk for developing Alzheimer’s disease and induces cognitive decline (Mayeux, et al., 1993). Androgens attenuate the cognitive deficits associated with the apolipoprotein E4 genotype (Hammond, et al., 2001; Raber, et al., 2002 and see this issue for a review), possibly via non-nuclear actions.
Basic science studies have also demonstrated that androgens are potent neuroprotective agents in the hippocampus and cortex (Bialek, et al., 2004). Gonadectomy of male rats increases the size of excitotoxic lesions of the hippocampal formation (Azcoitia, et al., 1999; Ramsden, et al., 2003); both testosterone and dihydrotestosterone attenuate the cell loss that follows the lesion (Ramsden et al., 2003). Furthermore, testosterone, acting via AR, is neuroprotective in primary cortical neurons exposed to serum deprivation (Hammond, et al., 2001) and in primary hippocampal neurons exposed to amyloid beta (Pike, 2001).
This last reference is one of a series of reports on the impact of androgens and AR in neuroprotection that importantly illuminate the possible role of extranuclear AR in neuronal function. Pike and colleagues (reviewed in this issue) treated primary hippocampal cultures or AR-expressing cell lines with androgens (either T or DHT) and exposed the cultures to toxins that produce apoptotic cell death. Although the protection does not require genomic activation, but is rather due to rapid, non-genomic effects of androgens, nevertheless, it does require AR. In this paradigm, agents that act as antagonists to transcriptional effects of AR, flutamide and cyproterone acetate, act instead as agonists, in that each is capable of promoting cell survival to the same extent as androgens, although when combined with androgen, the effect is not additive. On the other hand, flutamide and cyproterone acetate do block androgen-induced neuroprotection in other neuronal cells, including cerebellar granule cells (Ahlbom, et al., 2001; Hammond et al., 2001) and the effects appear to depend on genomic mechanisms. There are numerous possible explanations for this, but among these are that extranuclear AR is present in hippocampal CA1 axons, but absent in cerebellar granule cells (according to our light microscopic studies), thus, the extranuclear AR may mediate neuroprotection in the hippocampus alone.
What does extranuclear AR do at the cellular level?
The cellular function of extranuclear AR within axons is unknown, but several major possibilities exist. The first possibility, as suggested by Wagner et al., for extranuclear ER in axons (Wagner et al., 1998) is that AR retains its function as a transcription factor but acts on mitochondrial DNA instead of nuclear DNA. AR has been localized to mitochondria in sperm (Solakidi, et al., 2005), and mitochondrial DNA does contain several genes with androgen response elements in the promoter regions. For example, mitochondrial aspartate aminotransferase and cytochrome c both contain androgen response elements in their gene promoters and their protein levels are modulated by androgens (Cornwall, et al., 1992; Juang, et al., 1995). Additionally, estrogen receptors and other steroid receptors can modulate mitochondrial genes and can translocate to the mitochondria upon ligand binding (Demonacos, et al., 1996; Pedram, et al., 2006). If extranuclear AR activates transcription of mitochondrial genes, then this would not be expected to have a particularly rapid effect, but instead would require the usual time for transcription and translation to take place.
Another possible role for extranuclear AR within axons is that the receptors activate signal transduction pathways. Androgens rapidly activate the MAPK and PI3 kinase pathways in numerous cell types, including hippocampal neurons (Nguyen, et al., 2005), prostate cancer cells (Steinsapir, et al., 1991; Peterziel, et al., 1999), epithelial cells (Baron, et al., 2004; Castoria, et al., 2004), skeletal muscle cells (Estrada, et al., 2003), Sertoli cells (Cheng, et al., 2002; Fix, et al., 2004), osteoblasts (Kang, et al., 2004), oocytes (Lange, et al., 2007) and in a glial cell line (Gatson, et al., 2006), and in some cases it is known that the responses require the classical AR acting outside the nucleus. That the nuclear actions of the classical receptor are not necessary for activation of kinase pathways was demonstrated definitively by studies on Xenopus laevis or Rana pipiens oocytes in which nuclei were removed but androgens nevertheless activated MAPK (reviewed in Lange et al., 2007). The effects of androgens on signaling cascades and the response of a cell to insult can be quite different depending on whether the androgen is a native, membrane permeable androgen, or an androgen rendered membrane-impermeable by binding to a membrane-impermeable protein ( Nguyen et al., 2005; Gatson et al., 2006; Gatson and Singh, 2007). Rapid activation of signal transduction pathways by steroid hormone receptors is well documented for many other members of the steroid receptor family, including estrogen receptors (Razandi, et al., 1999; Singh, et al., 1999; Kuroki, et al., 2000; Abraham, et al., 2004; Alexaki, et al., 2006; Guo, et al., 2006 ), progesterone receptors (Bagowski, et al., 2001) and vitamin D receptors (Ma, et al., 2006). In addition, new forms of these receptors that are related to the classical nuclear receptors (Toran-Allerand, et al., 2002) or completely unrelated proteins that belong to the G-protein coupled receptor family ( Lieberherr and Grosse, 1994; Zhu, et al., 2003; Thomas, et al., 2006; Brailoiu, et al., 2007) have been postulated to mediate the rapid actions of steroid hormones, and increasing evidence suggests that the cellular effects of steroid hormones involves integration of the rapid activation of signaling cascades by steroids with the transcriptional activation that occurs more slowly in the nucleus ( Levin, 2005; Estrada, et al., 2006; Hammes and Levin, 2007).
Studies of AR dynamics have shown that unliganded ARs are primarily cytoplasmic, moving into the nuclear compartment within 15–60 min of ligand binding, and back to the cytoplasm for recycling. Therefore, extranuclear ARs in axons and axon terminals may represent a repository of ARs that are available for retrograde transport to the nucleus should the need arise; that, in essence, these are ARs eventually destined for a role in transcription but are in storage. However, anterograde and retrograde axonal transport are energy-intensive processes that typically target cellular proteins to active functional zones, rather than distant storage areas. Moreover, if the purpose of transporting steroid receptors into axons, dendritic spines or glial processes is essentially for storage, it might be expected that the putative storage sites would be similar for all steroid receptors, but this is not the case. On the other hand, although gonadectomy for one week decreases axonal AR in male rats, and our initial observations of axonal AR was made in animals that had been treated with testosterone or dihydrotestosterone one hour before sacrifice, we have not performed time course experiments to test whether exogenous T given to intact or short term gonadectomized animals alters axonal AR content over the course of several hours or days. Therefore, although we think it unlikely that ARs in axons represent a distant pool of receptors in storage for possible slow translocation to the nucleus, this idea merits consideration and solid experimental testing.
What is the role of extranuclear AR at the organismal level?
A question that arises is, if androgens have rapid actions, why? Androgen levels rise rapidly in response to social cues--in humans as well as animals-- observations that the media have publicized widely; there are many examples of this phenomenon, and only a few will be mentioned here for illustrative purposes (others are reviewed elsewhere in this issue). For example, testosterone levels were found to be higher in football/soccer fans whose team had just won, compared to fans on the losing side (Bernhardt, et al., 1998). In men with high baseline testosterone levels, viewing excerpts from a movie with themes of male dominance and power (The Godfather II) further increased salivary testosterone levels within 45 minutes of the movie, with the reverse occurring in women with high baseline testosterone levels (Schultheiss et al., 2004). Social interactions profoundly alter androgen levels in fish; simply observing intermale aggression from behind a one-way mirror elevates urinary testosterone levels within 30 minutes (Oliveira, et al., 2001). In contrast, androgen levels were not affected in individuals who attacked themselves in a mirror, resulting in an even match with no winner or loser, even though the number of attacks increased with time (Oliveira, et al., 2005). Therefore, the rise in androgens is not related to aggressive/attack activity per se, but to the social cues the aggression communicates. In this regard, it is relevant that, in rodents, androgen exposure can result in associative learning in a conditioned place preference paradigm and is reinforcing in self-administration paradigms (reviewed in Sato, et al., this issue), because these types of learning typically require close temporal pairing of the conditioned and unconditioned stimulus. Although the rapid elevations in androgens undoubtedly activate genomic events, is seems unlikely that the long interval between higher androgen levels and the outcome would lead to associative learning or reinforcement. Therefore, a rapid, non-genomic effect, perhaps exerted through activation of extranuclear AR and signaling cascades, might be the more likely route for such learning to take place. If AR-ir axons in the cortex do indeed arise from hippocampal CA1 pyramidal neurons as we propose, then the extranuclear AR is situated in the key hippocampal outflow pathway that pairs environmental cues (in particular, place) with experience.
In conclusion, the studies reviewed in this paper indicate that in addition to its role as a nuclear transcription factor, AR may be involved in the regulation of rapid membrane or cytoplasmic signaling in axons and dendrites of specific neuronal populations in the CNS. AR appears to be transported from the perikaryon to the axonal compartment by fast axonal transport. AR localization in axons is abundant in the cerebral cortex and amygdala and the origin of these axons appears to be the dorsal CA1 region of the hippocampus. Axonal AR may mediate androgenic modulation of neuronal activity in the hippocampus, cerebral cortex and amygdala and participate in rapid hormonal modulation of synaptic plasticity, cognition and behavior.
Acknowledgments
The authors thank Andrew Plodkowski, M.D., Rachel Baskin, Julie Kelso, and Betty Lorenz. Supported by a Woodrow Wilson Dissertation Fellowship in Women’s Health and a Schmitt Foundation Fellowship to S.S., and by NIH MH62588, MH69995 and the Banes Foundation to LLDC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145(7):3055–61. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Research. 2001;892(2):255–62. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Alexaki VI, Charalampopoulos I, Kampa M, Nifli AP, Hatzoglou A, Gravanis A, Castanas E. Activation of membrane estrogen receptors induce pro-survival kinases. The Journal of Steroid Biochemistry and Molecular Biology. 2006;98(2–3):97–110. doi: 10.1016/j.jsbmb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Alonso MV, Monica RM, Depino Amaicha M, Mello e Souza Tadeu, Pereira Patricia, Szapiro German, Viola Haydee, Pitossi Fernando, Izquierdo Ivan, Medina Jorge H. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12(4):551–60. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Audet MA, Doucet G, Oleskevich S, Descarries L. Quantified regional and laminar distribution of the noradrenaline innervation in the anterior half of the adult rat cerebral cortex. Journal of Comparative Neurology. 1988;274(3):307–18. doi: 10.1002/cne.902740302. [DOI] [PubMed] [Google Scholar]
- Azcoitia Ii, Fernandez-Galaz C, Sierra A, Garcia-Segura LM. Gonadal hormones affect neuronal vulnerability to excitotoxin-induced degeneration. Journal of Neurocytology. 1999;28(9):699–710. doi: 10.1023/a:1007025219044. [DOI] [PubMed] [Google Scholar]
- Bagowski CP, Myers JW, Ferrell JE., Jr The classical progesterone receptor associates with p42 MAPK and is involved in phosphatidylinositol 3-kinase signaling in Xenopus oocytes. Journal of Biological Chemistry. 2001;276(40):37708–14. doi: 10.1074/jbc.M104582200. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Lemaire M, Wilbrecht L, Jarrard L, Iversen SD, Rawlins JN, Good MA. The role of the entorhinal cortex in two forms of spatial learning and memory. Experimental Brain Research. 2001;141(3):281–303. doi: 10.1007/s002210100868. [DOI] [PubMed] [Google Scholar]
- Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. Journal of Biological Chemistry. 2004;279(15):14579–86. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- Bernhardt PJM, Dabbs J, Fielden J, Lutter C. Testosterone changes during vicarious experiences of winning and losing among fans at sporting events. Physiology and Behavior. 1998;65:59–62. doi: 10.1016/s0031-9384(98)00147-4. [DOI] [PubMed] [Google Scholar]
- Bialek M, Zaremba P, Borowicz KK, Czuczwar SJ. Neuroprotective role of testosterone in the nervous system. Polish Journal of Pharmacology. 2004;56(5):509–18. [PubMed] [Google Scholar]
- Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131(1):281–90. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Olster DH. Colchicine-induced accumulation of estrogen receptor and progestin receptor immunoreactivity in atypical areas in guinea-pig brain. Journal of Neuroendocrinology. 1993;5(1):63–70. doi: 10.1111/j.1365-2826.1993.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun S, Brailoiu G, Mizuo K, Sklar L, Oprea T, Prossnitz E, Dun N. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. Journal of Endocrinology. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6(2):81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. Journal of Comparative Neurology. 1992;324(2):143–79. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Castoria G, Lombardi M, Barone MV, Bilancio A, Di Domenico M, De Falco A, Varricchio L, Bottero D, Nanayakkara M, Migliaccio A, Auricchio F. Rapid signalling pathway activation by androgens in epithelial and stromal cells. Steroids. 2004;69(8–9):517–22. doi: 10.1016/j.steroids.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. Journal of Comparative Neurology. 2006;497(1):101–14. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Research Reviews. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Yu Z, Zhou D, Mattson MP. Phosphatidylinositol-3-kinase-Akt kinase and p42/p44 mitogen-activated protein kinases mediate neurotrophic and excitoprotective actions of a secreted form of amyloid precursor protein. Experimental Neurology. 2002;175(2):407–14. doi: 10.1006/exnr.2002.7920. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Lorincz A, Buzsaki G. Physiological patterns in the hippocampo-entorhinal cortex system. Hippocampus. 2000;10(4):457–65. doi: 10.1002/1098-1063(2000)10:4<457::AID-HIPO12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Orgebin-Crist MC, Hann SR. Differential expression of the mouse mitochondrial genes and the mitochondrial RNA-processing endoribonuclease RNA by androgens. Molecular Endocrinology. 1992;6(7):1032–42. doi: 10.1210/mend.6.7.1508219. [DOI] [PubMed] [Google Scholar]
- Corsellis JA. Observations on the neuropathology of dementia. Age & Ageing. 1977:20–9. doi: 10.1093/ageing/6.suppl.20. [DOI] [PubMed] [Google Scholar]
- Crews D, Traina V, Wetzel FT, Muller C. Hormonal control of male reproductive behavior in the lizard, Anolis carolinensis: role of testosterone, dihydrotestosterone, and estradiol. Endocrinology. 1978;103(5):1814–21. doi: 10.1210/endo-103-5-1814. [DOI] [PubMed] [Google Scholar]
- Deacon TW, Eichenbaum H, Rosenberg P, Eckmann KW. Afferent connections of the perirhinal cortex in the rat. Journal of Comparative Neurology. 1983;220(2):168–90. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- Demonacos C, Karayanni N, Hatzoglou E, Tsiriyiotis C, Spandidos D, Sekeris C. Mitochondrial genes as sites of primary action of steroid hormones. Steroids. 1996;61:226–232. doi: 10.1016/0039-128x(96)00019-0. [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987;21(3):807–24. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Garcia-Ovejero D, Sarkey S, Garcia-Segura LM, Azcoitia I. Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology. 2003;144(8):3632–8. doi: 10.1210/en.2002-0105. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Sarkey S, Lorenz B, Azcoitia I, Garcia-Ovejero D, Huppenbauer C, Garcia-Segura LM. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience. 2006;138(3):801–7. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Dufy B, Vincent JD, Fleury H, Du Pasquier P, Gourdji D, Tixier-Vidal A. Membrane effects of thyrotropin-releasing hormone and estrogen shown by intracellular recording from pituitary cells. Science. 1979;204(4392):509–11. doi: 10.1126/science.107590. [DOI] [PubMed] [Google Scholar]
- Estrada M, Espinosa A, Muller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144(8):3586–97. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. Journal of Cellular Science. 2006;119(4):733–43. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. Journal of Comparative Neurology. 1978;180(3):509–32. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(30):10919–24. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Teyler TJ. 17-alpha-Estradiol and 17-beta-estradiol in hippocampus. Brain Research Bulletin. 1983;10(6):735–9. doi: 10.1016/0361-9230(83)90206-x. [DOI] [PubMed] [Google Scholar]
- Frye C, Park D, Tanaka M, Rosellini R, Svare B. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference. Psychoneuroendocrinology. 2001;26:731–750. doi: 10.1016/s0306-4530(01)00027-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL, Seliga AM, Wawrzycki JM. 5[alpha]-reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendocrinology. 2004;29(8):1019–27. doi: 10.1016/j.psyneuen.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Progress in Neurobiology. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Gaspar ML, Meo T, Bourgarel P, Guenet JL, Tosi M. A single base deletion in the Tfm androgen receptor gene creates a short-lived messenger RNA that directs internal translation initiation. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(19):8606–10. doi: 10.1073/pnas.88.19.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatson J, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028–2034. doi: 10.1210/en.2005-1395. [DOI] [PubMed] [Google Scholar]
- Gatson J, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–2464. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- Goldschmidt RB, Steward O. Preferential neurotoxicity of colchicine for granule cells of the dentate gyrus of the adult rat. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(5):3047–51. doi: 10.1073/pnas.77.5.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith SK, Joyce JN. Dopamine D2 receptor expression in hippocampus and parahippocampal cortex of rat, cat, and human in relation to tyrosine hydroxylase-immunoreactive fibers. Hippocampus. 1994;4(3):354–73. doi: 10.1002/hipo.450040318. [DOI] [PubMed] [Google Scholar]
- Guo RX, Wei LH, Tu Z, Sun PM, Wang JL, Zhao D, Li XP, Tang JM. 17[beta]-Estradiol activates PI3K/Akt signaling pathway by estrogen receptor (ER)-dependent and ER-independent mechanisms in endometrial cancer cells. The Journal of Steroid Biochemistry and Molecular Biology. 2006;99(1):9–18. doi: 10.1016/j.jsbmb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Hammes S, Levin E. Extranuclear steroid receptors: nature and action. Endocr Rev. 2007 doi: 10.1210/er.2007-0022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hammond CB. Menopause and hormone replacement therapy: an overview. Obstet Gynecol. 1996;87(2 Suppl):2S–15S. doi: 10.1016/0029-7844(95)00429-7. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. Journal of Neurochemistry. 2001;77(5):1319–26. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. Journal of Neuroscience. 2007;27(8):2102–11. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Research. 1991;19(9):2373–8. doi: 10.1093/nar/19.9.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuett W, Qian H. A stochastic model of oscillatory blood testosterone levels. Bull Math Biol. 2006;68:1383–99. doi: 10.1007/s11538-006-9098-4. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Matsuki N. Regionally selective neurotoxicity of NMDA and colchicine is independent of hippocampal neural circuitry. Neuroscience. 2002;113(2):253–6. doi: 10.1016/s0306-4522(02)00217-8. [DOI] [PubMed] [Google Scholar]
- Jelks KB, Wylie R, Floyd CL, McAllister AK, Wise P. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-alpha. Journal of Neuroscience. 2007;27(26):6903–13. doi: 10.1523/JNEUROSCI.0909-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. Journal of Neuroscience. 2000;20(18):6974–82. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GA, Curtis DR, De Groat WC, Duggan AW. Central actions of ibotenic acid and muscimol. Biochemical Pharmacology. 1968;17(12):2488–9. doi: 10.1016/0006-2952(68)90141-x. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiology and Behavior. 2005;85(2):135–41. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Juang HH, Costello LC, Franklin RB. Androgen modulation of multiple transcription start sites of the mitochondrial aspartate aminotransferase gene in rat prostate. Journal of Biological Chemistry. 1995;270(21):12629–34. doi: 10.1074/jbc.270.21.12629. [DOI] [PubMed] [Google Scholar]
- Kang HY, Cho CL, Huang KL, Wang JC, Hu YC, Lin HK, Chang C, Huang KE. Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. Journal of Bone and Mineral Research. 2004;19(7):1181–90. doi: 10.1359/JBMR.040306. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses mu-opioid- and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. Journal of Neuroscience. 1992;12(7):2745–50. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The effects of microelectrophoretically applied estrogen, cortisol and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of the female rat. Experimental Brain Research. 1977;30(1):53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- Khar A, Kunert-Radek J, Jutisz M. Involvement of microtubule and microfilament system in the GnRH-induced release of gonadotropins by rat anterior pituitary cells in culture. FEBS Letters. 1979;104(2):410–4. doi: 10.1016/0014-5793(79)80864-9. [DOI] [PubMed] [Google Scholar]
- Knowles WD. Normal anatomy and neurophysiology of the hippocampal formation. Journal of Clinical Neurophysiology. 1992;9(2):252–63. [PubMed] [Google Scholar]
- Kowianski P, Lipowska M, Morys J. The piriform cortex and the endopiriform nucleus in the rat reveal generally similar pattern of connections. Folia Morphologica (Warszawa) 1999;58(1):9–19. [PubMed] [Google Scholar]
- Kritzer MF. Long-term gonadectomy affects the density of tyrosine hydroxylase- but not dopamine-beta-hydroxylase-, choline acetyltransferase- or serotonin-immunoreactive axons in the medial prefrontal cortices of adult male rats. Cerebral Cortex. 2003;13(3):282–96. doi: 10.1093/cercor/13.3.282. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. European Journal of Pharmacology. 2000;400(2–3):205–9. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Lange C, Gioeli D, Hammes S, Marker P. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annual Review of Physiologyl. 2007;69:171–199. doi: 10.1146/annurev.physiol.69.031905.160319. [DOI] [PubMed] [Google Scholar]
- Levin E. Integration of the extranuclear and nuclear actions of estrogen. Molecular Endocrinology. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberherr M, Grosse B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. Journal of Biological Chemistry. 1994;269(10):7217–23. [PubMed] [Google Scholar]
- Lorenz B, Garcia-Segura LM, DonCarlos LL. Cellular phenotype of androgen receptor-immunoreactive nuclei in the developing and adult rat brain. Journal of Comparative Neurology. 2005;492(4):456–68. doi: 10.1002/cne.20763. [DOI] [PubMed] [Google Scholar]
- Ma Y, Yu WD, Kong RX, Trump DL, Johnson CS. Role of nongenomic activation of phosphatidylinositol 3-Kinase/Akt and mitogen-sctivated protein kinase/extracellular signal-regulated kinase kinase/extracellularsignal-regulated kinase 1/2 pathways in 1,25D3-mediated apoptosis in squamous cell carcinoma cells. Cancer Research. 2006;66(16):8131–8. doi: 10.1158/0008-5472.CAN-06-1333. [DOI] [PubMed] [Google Scholar]
- Markowska A, Bakke HK, Walther B, Ursin H. Comparison of electrolytic and ibotenic acid lesions in the lateral hypothalamus. Brain Research. 1985;328(2):313–23. doi: 10.1016/0006-8993(85)91043-1. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Ottman R, Tatemichi TK, Tang MX, Maestre G, Ngai C, Tycko B, Ginsberg H. The apolipoprotein epsilon 4 allele in patients with Alzheimer’s disease. Annals of Neurology. 1993;34(5):752–4. doi: 10.1002/ana.410340527. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. Journal of Comparative Neurolology. 2005;491(2):81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, Hernandez FJ, Herrick SP, Pierce JP, Iadecola C, Drake CT. Cellular and subcellular localization of androgen receptor immunoreactivity relative to C1 adrenergic neurons in the rostral ventrolateral medulla of male and female rats. Synapse. 2007;61(5):268–78. doi: 10.1002/syn.20370. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. Journal of Comparative Neurolology. 2005;491(2):81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. Journal of Comparative Neurology. 2001;429(3):355–71. [PubMed] [Google Scholar]
- Moga MM, Geib BM, Zhou D, Prins GS. Androgen receptor-immunoreactivity in the forebrain of the Eastern Fence lizard (Sceloporus undulatus) Brain Research. 2000;879(1–2):174–82. doi: 10.1016/s0006-8993(00)02771-2. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science. 1986;233(4760):226–8. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- Nguyen TVV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: Role in neuroprotection. Journal of Neurochemistry. 2005;94(6):1639–51. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- Ohm TG, Munch S, Schonheit B, Zarski R, Nitsch R. Transneuronally altered dendritic processing of tangle-free neurons in Alzheimer’s disease. Acta Neuropathologica (Berlin) 2002;103(5):437–43. doi: 10.1007/s00401-001-0486-4. [DOI] [PubMed] [Google Scholar]
- Oliveira R, Carneiro L, Canário A. Behavioural endocrinology: no hormonal response in tied fights. Nature. 2005;437:207–208. doi: 10.1038/437207a. [DOI] [PubMed] [Google Scholar]
- Oliveira R, Lopes M, Carneiro L, Canário A. Watching fights raises fish hormone levels. Nature. 2001;409:475. doi: 10.1038/35054128. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. Journal of Comparative Neurology. 1982;205(1):30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Congia S, Lello S, Tedde D, Orru M, Pistis M, Pilloni M, Zedda P, Loddo A, Melis GB. Low androgenization index in elderly women and elderly men with Alzheimer’s disease. Neurology. 2004;62(2):301–303. doi: 10.1212/01.wnl.0000094199.60829.f5. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Molecular Biology of the Cell. 2006;17(5):2125–37. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl DP, Pendlebury WW. Neuropathology of dementia. Neurologic Clinics. 1986;4(2):355–68. [PubMed] [Google Scholar]
- Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18(46):6322–9. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Research. 2001;919(1):160–5. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Postma A, Jager G, Kessels RPC, Koppeschaar HPF, van Honk J. Sex differences for selective forms of spatial memory. Brain and Cognition. 2004;54(1):24–34. doi: 10.1016/s0278-2626(03)00238-0. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. Journal of Neuroscience. 2002;22(12):5204–9. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122(3):573–8. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Molecular Endocrinology. 1999;13(2):307–19. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Rizk A, Robertson J, Raber J. Behavioral performance of tfm mice supports the beneficial role of androgen receptors in spatial learning and memory. Brain Research. 2005;1034(1–2):132–8. doi: 10.1016/j.brainres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rosen G, O’Bryant E, Matthews J, Zacharewski T, Wade J. Distribution of androgen receptor mRNA expression and immunoreactivity in the brain of the green anole lizard. Journal of Neuroendocrinology. 2002;14(1):19–28. doi: 10.1046/j.0007-1331.2001.00735.x. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Hormones and Behavior. 2006;50(1):18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. Journal of Comparative Neurology. 1984;222(3):313–42. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- Schmidt BM, Gerdes D, Feuring M, Falkenstein E, Christ M, Wehling M. Rapid, nongenomic steroid actions: A new age? Frontiers in Neuroendocrinology. 2000;21(1):57–94. doi: 10.1006/frne.1999.0189. [DOI] [PubMed] [Google Scholar]
- Schultheiss O, MM MW, Stanton S. Effects of affiliation and power motivation arousal on salivary progesterone and testosterone. Hormones and Behavior. 2004;46:592–599. doi: 10.1016/j.yhbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Silverman A, DonCarlos L, Morrell J. Ultrastructural characteristics of estrogen receptor-containing (ER) neurons in the ventrolateral nucleus (VL) of the female guinea pig. Journal of Neuroendocrinology. 1991;3:623–634. doi: 10.1111/j.1365-2826.1991.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. Journal of Neuroscience. 1999;19(4):1179–88. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Sex steroid effects on extrahypothalamic CNS. II. Progesterone, alone and in combination with estrogen, modulates cerebellar responses to amino acid neurotransmitters. Brain Research. 1987;422(1):52–62. doi: 10.1016/0006-8993(87)90539-7. [DOI] [PubMed] [Google Scholar]
- Smith SS, Woodward DJ, Chapin JK. Sex steroids modulate motor-correlated increases in cerebellar discharge. Brain Research. 1989;476(2):307–16. doi: 10.1016/0006-8993(89)91251-1. [DOI] [PubMed] [Google Scholar]
- Solakidi S, Psarra A, Nikolaropoulos S, Sekeris C. Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece. Human Reproduction. 2005;20:3481–3487. doi: 10.1093/humrep/dei267. [DOI] [PubMed] [Google Scholar]
- Steinsapir J, Socci R, Reinach P. Effects of androgen on intracellular calcium of LNCaP cells. Biochemical and Biophysical Research Communications. 1991;179(1):90–6. doi: 10.1016/0006-291x(91)91338-d. [DOI] [PubMed] [Google Scholar]
- Steward O, Goldschmidt RB, Sutula T. Neurotoxicity of colchicine and other tubulin-binding agents: a selective vulnerability of certain neurons to the disruption of microtubules. Life Sciences. 1984;35(1):43–51. doi: 10.1016/0024-3205(84)90150-4. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130(1):151–63. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dressing G, Pang Y, Berg H, Tubbs C, Benninghoff A, Doughty K. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids. 2006;71:310–316. doi: 10.1016/j.steroids.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. Journal of Neurochemistry. 1994;62(5):1750–6. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Tokarz RR. Hormonal regulation of male reproductive behavior in the lizard Anolis sagrei: a test of the aromatization hypothesis. Hormones and Behavior. 1986;20(3):364–77. doi: 10.1016/0018-506x(86)90044-9. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. Journal of Neuroscience. 2002;22(19):8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. Journal of Comparative Neurology. 1991;313(4):643–68. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. Journal of Comparative Neurology. 1999;407(4):555–82. [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Albrecht D. Reciprocal connections of the hippocampal area CA1, the lateral nucleus of the amygdala and cortical areas in a combined horizontal slice preparation. Neuroscience Research. 2002;44(1):91–100. doi: 10.1016/s0168-0102(02)00092-5. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Silverman AJ, Morrell JI. Evidence for estrogen receptor in cell nuclei and axon terminals within the lateral habenula of the rat: regulation during pregnancy. Journal of Comparative Neurology. 1998;392(3):330–42. [PubMed] [Google Scholar]
- Williams C, AM AB, Meck W. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behavioral Neuroscience. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10(4):398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17 beta-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Research. 1991;543(1):148–52. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. Journal of Neuroscience. 1992;12(8):3217–25. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. Anabolic-androgenic steroid dependence? Insights from animals and humans. 2007 doi: 10.1016/j.yfrne.2007.12.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. From the Cover: Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2231–6. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]