Abstract
Peptide inhibitors of ethanol consumption have shown promise. The purpose of this study was to test the cyclized form of the opioid-derived dipeptide, glycyl-L-glutamine to reduce ethanol consumption after either peripheral injections or site specific injections into the nucleus accumbens (NAC) of high drinking and low drinking rats. Following I.P. cyclo-glycyl-glutamine (c-GQ), the data show a mean decrease in ethanol intake of 34.4% in P rats, and 39.4% in Sprague Dawley rats at doses between 5 and 25 mg/kg. The data show that peripherally administered c-GQ is effective in reducing ethanol consumption in both high (P) and low (SD) drinking strains of rats and suggests a therapeutic potential.
Keywords: cyclo-glycyl-glutamine, P rat, Sprague Dawley rat, alcohol intake
Introduction
Beta-endorphin (1-31) is implicated in the facilitation of ethanol intake [5, 8, 12]. However, beta-endorphin is processed into smaller peptides [7, 14] in brain sites including the nucleus accumbens (NAC). Some of these shortened forms reduce ethanol intake [18]. We have previously shown that one of these, the terminal dipeptide, glycyl-glutamine (GQ) delivered by intracerebroventricular (i.c.v.) injection [20] or directly into the nucleus accumbens (NAC) [19], significantly decreases 24 hour ethanol intake in genetic high drinking alcohol preferring (P) rats.
However, until this time, testing of GQ has been conducted only in high drinking P rats but not in low drinking strains of rats. Human studies link altered beta-endorphin responses to alcohol intake with people genetically predisposed to alcoholism [4]. Chronic use of alcohol may also reflect a central beta-endorphin deficiency which could be associated with reduced hypothalamic and pituitary beta-endorphin synthesis, release [4], and/or beta-endorphin processing to inhibitory peptides [2, 18]. If beta-endorphin neurons are altered between genetic high and low drinkers, then the response to GQ may also differ between these groups. The response to GQ was reported for a high drinking animal model [18, 19, 20]. To test the animal model for similarities with observations in the human, a low drinking strain of rats needs to be tested to determine whether GQ efficacy is unique to genetic high drinking populations or is also effective in low drinkers, and thus having applicability to a broader population.
Support for the foregoing proposition that GQ may be reduced in genetic high drinkers is based in part on the precedent in reports of cyclo-histidyl-proline (c-His-Pro). The c-His-Pro molecule is produced endogenously in lower amounts in high drinking animals [16], crosses the blood brain barrier after i.v. injection [1], and effectively reduced ethanol intake after peripheral administration [16]. Similarly for GQ, a synthesis from several reports [2, 7, 14] suggests the linear analog of the GQ peptide is also produced endogenously. In the high drinking rat this production may be genetically reduced. In at least one strain of high drinking rat, namely the AA Alko alcohol preferring rats [2], data is consistent with that notion. Specifically, alcohol stimulation of hypothalamic slices elicited the reduced release of shortened forms of beta-endorphin that are co-released with GQ, compared with the control ANA rat. Thus it is expected that GQ production is also reduced. These observations suggest an impaired production of GQ may be involved with high drinking and administration of GQ may replace the deficit, with the consequence of reduced drinking, as we previously reported following central administration of GQ [19,20]. Since GQ effectively reduced drinking there was the possibility that a cyclized GQ might also reduce drinking. If so, it may also be effective following peripheral administration, as is c-His-Pro. That proposition is supported by the action of both linear and cyclo-GQ in cardiopulmonary experiments [23] where c-GQ was shown to be effective after either peripheral or central administration, suggesting c-GQ crosses the blood brain barrier and thus has therapeutic potential.
In this report, three hypotheses were tested: 1) GQ would reduce ethanol intake in low drinking Sprague Dawley (SD) rats similar to its action in high drinking P rats. 2) c-GQ would reduce drinking similar to the action of the linear form, GQ, in the same brain site. 3) c-GQ would be effective administered peripherally in both high and low drinking strains.
2. Methods
2.1 Animals
Two strains of rats were used in this work. Male alcohol preferring (P) rats (Center for Alcohol Studies, Indiana University Medical Center, Indianapolis, IN) and male Sprague-Dawley rats (SD)(Charles River), weighing 300-350 g were maintained with food and water ad libitum under an established 12/12 h photoperiod (lights on: 0700 h). Each rat was handled daily and maintained in individual cages in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication No. 80-23, revised 1996), with all experimental protocols approved by the UMKC Animal Care and Use Committee.
2.2 Surgery
Sprague-Dawley rats and P-rats were anesthetized with 2% isoflurane (Hosperia, Lake Forest, IL) for the stereotaxic cannulation surgery. Stainless steel bilateral guide tubes (Plastics One, Roanoke VA) 2 mm center-to-center were placed with tips 1 mm above the NAC at stereotaxic coordinates AP = 1.7, L = +/-1.0, H0 = -5.5 from dura as determined from Paxinos and Watson, Atlas of Rat Brain [15]. Animals recovered from surgery on ad libitum food and water for at least 5 days before experiments.
2.3 Adaptation to Voluntary Ethanol Drinking
P rats were adapted to drinking 30% ethanol in water as previously described [11,19,20]. Briefly, P rats were individually housed in their home cage with 2 bottles of drinking solution, one water and the other an ethanol dilution. Ethanol preference was determined by presenting the following concentration of ethanol for 3 days each: 3, 5, 7, 9, 11, 13, 15, 20, and 30% ethanol. For experiments, the concentration supplied to each P rat was that at which each rat's intake was the highest g/kg. P rats typically drank more of the 30% ethanol. SD rats were adapted to drinking 10% ethanol in water by a modification of the sucrose fading method previously described [3]. Briefly, initiation was done by introducing naïve SD rats, in home cages, housed one per cage, to two drinking bottles. One bottle contained only water and the other bottle contained a sucrose-ethanol solution. The sucrose concentration was started at 3% and reduced on the following schedule: Days 1-3, 3% sucrose - 10% ethanol; Days 4-6, 2% sucrose – 10% ethanol; Days 7-9, 1% sucrose - 10% ethanol; Days 10 and beyond, 0% sucrose – 10% ethanol. Rats were continued on this latter regimen until their ethanol intake stabilized. Subsequently, experiments were begun for drug testing when daily base-line varied only 10-15%. Ethanol intakes for all rats were calculated as g of ethanol/kg of body weight (g/kg).
2.4 Injections
Central site-specific injections were delivered into the NAC using 28 ga. stainless steel injectors passed through 22 ga. bilateral guide cannulae (Plastics One, Roanoke VA). GQ (Sigma, St. Louis MO), c-GQ (Bachem, King of Prussia, PA) each at100 pmol in 1 μl each side, or saline (vehicle) treatments were pressure injected bilaterally over a period of 30 seconds using a Hamilton microliter syringe. Injectors remained in the injection site for an additional 15 seconds and were then removed and replaced by stylets. Rats were then returned to their home cages for the remainder of the experiment.
Peripheral injections were delivered by hypodermic injection from a 1 ml syringe intraperitoneally (I.P.). Injections of c-GQ or GQ were done using 25 mg/kg except where indicated otherwise. Doses were administered in approximately 0.5 ml, depending on each individual rat's body weight.
2.5 Procedures
Limited Access Protocol
SD and P rats were trained in a modification of a 2-hour limited access paradigm where a 2-bottle choice of ethanol and water were available [9, 10]. Water was available continuously. Ethanol was presented daily for 2 hours until baseline consumption was stable. Low drinking SD rats were provided 10% ethanol and high drinking P rats were provided 30% ethanol. For experiments, injections into NAC were given 20 min. before access to ethanol. Measurements of intake were done initially at 9 a.m. and again at 11 a.m.
24-h Access Protocol
Water and ethanol intake were measured daily using graduated drinking tubes (Dyets, Bethlehem, PA). Daily 24 hour intakes were measured at 9:00 to 10:00 a.m. in ml and calculated as grams ethanol per kilogram of body weight, similar to previous reports [6, 20]. For both Sprague-Dawley and P rats, intakes were recorded for 3 days before and 3 days after a central injection of either a drug or saline vehicle. Injections were delivered to NAC sites via stainless steel injectors (plastics one) connected to Hamilton microliter syringes. All injections were 1 μl bilateral and injectors remained in the injection site for an additional 15 seconds before they were removed and replaced by a stylet. Injections were given on or about 10:00 a.m. directly after the morning weighing. Body weight, water and ethanol intakes were all measured at 24 h intervals for an additional 3 days following injection.
At the end of experiments rats were administered a lethal dose of Nembutal (100 mg/kg), perfused first with phosphate buffered saline, then with 4% paraformaldehyde, and brains removed for sectioning and staining to verify cannula locations. Most animals were verified by inspection. In a few animals, 10% biotin dextran amine (BDA) (Neurotrace BDA-10,000 kit; Molecular Probes) was injected (0.2 μl at 5 min intervals for 20 min) into the drug injection site (NAC) 2 days before sacrifice as an additional verification of injection location. Tissue was sectioned at 50 microns on a freezing microtome and BDA visualization was done in free floating sections using an ABC peroxidase reagent for 2 h and the diaminobenzidine chromogen (DAB) (Vector Laboratories) resulting in the dark brown or black staining around the injection site. Sections were then mounted on slides and counterstained with cresyl violet.
2.6 Data Analysis
Data were recorded daily for body weight and ml of water and alcohol intake. Alcohol intake was calculated as g/kg. Where intake values were substantially different for baseline values, data were calculated first as the percent change for each animal, and then the group mean % change and standard error. Data normalized in this way were presented as being percent change or mean percent decrease in ethanol intake. Data were analyzed either by ANOVA and post hoc Bonferonni or by independent or dependent t-test (Minitab). P < 0.05 was accepted as significant.
3. Results
3.1 Central GQ Reduced Drinking in P and SD Rats in 2-h Limited Access Paradigm
In the first experiment, the hypothesis was tested that high drinking and low drinking strains of rats would respond to GQ in the same way. To test this hypothesis P rats were compared with SD rats in a two-hour limited access paradigm. Ethanol intake was measured for rats treated with GQ or saline injections into NAC: Figure 1 shows that mean ethanol intake in the P rats after saline treatment was 6.76 ± 0.88 g/kg. Mean ethanol intake after GQ treatment was 3.31 ± 0.9 g/kg. The difference between saline vehicle and GQ treatments were significant at P < 0.04 using an independent t-test, t = -2.75, df = 5, N = 4/group.
Figure 1. Two Hour Limited Access in P Rats.
Figure 1 shows the mean ethanol intake (g/kg) in a two hour limited access paradigm for two groups of P rats, one injected with glycyl-glutamine (GQ) (100 pmol bilaterally) into NAC and the other injected with saline vehicle 20 min before access to ethanol. Ethanol intake in GQ-treated rats was significantly less than in saline treated rats, shown by an independent t-test (* P < 0.04)
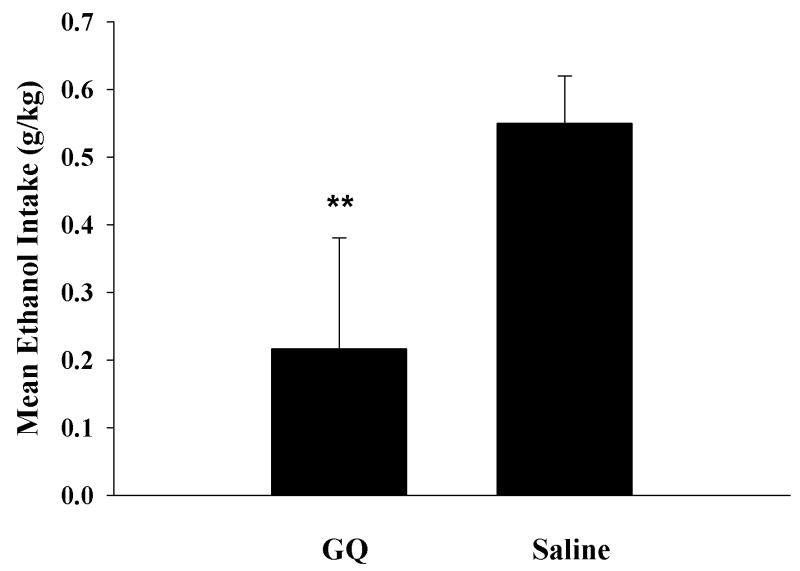
Figure 2 shows a similar result in SD rats. Mean ethanol intake in the SD rats following saline treatment was 0.55 ± 0.025 g/kg; however, after GQ treatment mean ethanol intake was 0.21 ± .067 g/kg. The saline vehicle versus GQ treatments were significantly different at P < 0.003, using an independent t-test, t = -4.76, df = 6, N = 6 GQ and 8 saline. The data clearly show that central GQ suppressed ethanol intake in both high drinking P and low drinking SD strains of rats.
Figure 2. Two Hour Limited Access in SD Rats.
Figure 2 shows mean ethanol intake (g/kg) in a two hour limited access paradigm for two groups of SD rats, one injected with glycyl-glutamine (GQ) (100 pmol bilaterally) into NAC and the other injected with saline vehicle. Ethanol intake in GQ-treated rats was significantly less than in saline treated rats, shown by an independent t-test (** P < 0.003).
3.2 Central c-GQ or GQ Reduced Drinking in P Rats
In the second experiment, the hypothesis was tested that GQ or c-GQ elicited the same suppression of ethanol intake after dipeptide administration centrally into the NAC of P rats. The two forms of the dipeptide were tested using a 24-hour 2-bottle continuous access to water and ethanol. Ethanol intake was measured for 3 days prior to injecting one of the dipeptides, and for 3 days following treatment. Figure 3 shows that mean ethanol intake before GQ treatment was 11.34 ± 0.96 g/kg, and after GQ injection was 5.7 ± 1.16 g/kg, compared with saline which was 10.37 ± 1.61 g/kg before and 9.37 ± 1.57 g/kg after injections. Saline vs. GQ treatments differed significantly on day 4 using a post hoc Bonferonni (P<0.05) after an ANOVA (F = 4.26; df = 1,14; N = 8/group; P < 0.05) (Fig 3). The saline effect was not significant. Ethanol intakes recovered towards baseline following GQ injection over the next two days.
Figure 3. 24-Hour Continuous Access in P Rats.
Figure 3 shows the mean ethanol intake (g/kg) of two groups of P rats in a 24-h continuous access paradigm. Injections were made into the NAC with GQ (100 pmol) (filled circle) or saline (open circle) immediately after the morning weighing on day 3 (arrow). Day 4 data show a significant reduction in ethanol intake after GQ but not after saline, (the Bonferonni for saline vs. GQ on day 4; * P < 0.05, after an overall significant F test from a one-way ANOVA).
Figure 4 shows that mean ethanol intake before c-GQ treatment was 8.81± 0.78 g/kg. Intake after c-GQ treatment was 5.46 ± 1.03 g/kg. Ethanol intake before saline was 10.7 ± 0.51 g/kg and after was 9.93 ± 0.72 g/kg. Evaluating the data shows c-GQ differs from saline treatment on day 4 using a post hoc Bonferonni (P<0.05) after an ANOVA (F = 6.21; df = 1,16; N = 7, c-GQ; = 11, saline; P < 0.024). The data show that both the linear- and cyclo-dipeptide treatments elicited essentially the same response, suppressing ethanol intake, following injection into the NAC. The data clearly show both forms of the drug are active in the same brain site.
Figure 4. c-GQ Injected into NAC of P Rats.
Figure 4 shows the mean ethanol intake (g/kg) of two groups of P rats injected into NAC with c-GQ (100 pmol bilaterally) (filled circle) or saline (open circle) immediately after the morning weighing on day 3 (arrow). Day 4 shows the effect. The data show a significant reduction in ethanol intake after c-GQ but not after saline, (Bonferonni for saline vs. GQ on day 4; * P < 0.05).
3.3 Peripheral c-GQ Dose Response
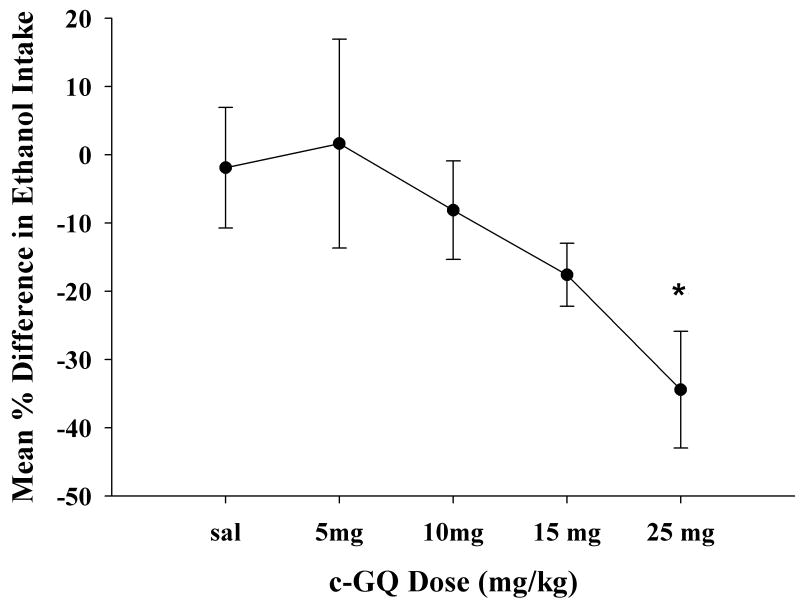
The third experiment addressed the issue of whether c-GQ is effective after peripheral administration. After demonstrating in a few animals that c-GQ was effective following peripheral administration, a dose response was determined in P rats to identify an effective peripheral dose range. The dose range tested was 0 mg/kg (saline vehicle), 5, 10, 15, and 25 mg c-GQ per kilogram of body weight (mg/kg) in a 24-h 2-bottle choice continuous access paradigm. Figure 5 shows an increasing drug effect to suppress ethanol intake that reached a maximum at 25 mg/kg which was also the lowest dose showing significance (ANOVA; F = 6.96; N = 21 for c-GQ and N = 22 for saline P<0.027). The saline vs. 25 mg/kg group means differed (Bonferonni P<0.05). The zero dose (i.e. saline) resulted in a mean fall in ethanol intake of 1.91 ± 8.84 %. At the 25 mg c-GQ dose drinking fell 34.42 ± 8.56% (mean % decrease) from 9.18 ± 0.77 before c-GQ to 6.28 ± 1.11 g/kg after c-GQ. A few animals tested at 100 mg/kg showed no further reduction of ethanol intake below that following the 25 mg/kg dose.
Figure 5. c-GQ dose response injected I.P. in P Rats.
Figure 5 shows a dose response graph for I.P. injected c-GQ (0, 5, 10, 15, and 25 mg/kg). The data, expressed as mean % difference in ethanol intake over 24 hours, show the 0 mg/kg (saline vehicle) was significantly different from the 25 mg/kg dose, and was also the lowest dose showing significance using a ANOVA followed by a post-hoc Bonferonni * P<0.05.
3.4 Peripheral c-GQ in P and SD Rats
Having determined an effective c-GQ I.P. dose, the hypothesis was tested to determine whether P and SD rats would show comparable reductions in ethanol drinking after peripheral c-GQ. Using the foregoing data (Figure 5) the 25 mg/kg dose was used for subsequent I.P. experiments. Figure 6 compares P and SD rats at the 25 mg/kg dose of c-GQ. Due to large differences in baseline intake between P and SD rats, and partly due to animal to animal variation, the data were normalized by calculating intakes as mean percent difference. The data for P rats (Figure 6) show a 34.42 ± 8.56 mean % decrease in ethanol intake following I.P. c-GQ but following saline, a 5.61 ± 8.26 % increase in ethanol intake. The comparison of c-GQ vs. saline was significant (P < 0.02; dependent t = 2.47; df = 28; (N c-GQ = 21; N saline = 22). Ethanol intake dropped from 9.18 ± 0.77 g/kg to 6.27 ± 0.70 g/kg after c-GQ and for saline controls was 9.31 ± 0.59 before and 9.33 ± 0.53 g/kg 24-h after.
Figure 6. I.P. c-GQ (25 mg/kg) or saline in P and SD rats.
Figured 6 shows the mean percent difference in 24-h ethanol intake after I.P. c-GQ or saline in P and SD rats. The data show a mean 34.4% decrease in ethanol intake of c-GQ treated P rats (dependent t-test, * P < 0.02 c-GQ vs. saline); compared with a 39.4% decrease in c-GQ treated SD rats, (dependent t-test, ** P < 0.006 c-GQ vs. saline).
The data for SD rats (Figure 6) show a 39.4 ± 0.92 mean % decrease in ethanol intake from 3.86 ± 1.3 g/kg to 2.59 ± 0.92 g/kg compared with saline vehicle controls which show a 20.5 ± 16.7 mean % increase of intakes. Comparison of c-GQ and saline data for the SD rats show a significant difference (P < 0.006; N = 8/group; dependent t = 3.88; df = 14). The response to I.P. c-GQ was proportionally the same in both high and low drinking strains of rats and significantly different from saline vehicle controls in each.
3.5 Ethanol Intake After I. P. c-GQ or GQ in P Rats
Finally, in assessing the response to c-GQ, a control was done comparing peripherally administered c-GQ with GQ. Linear and cyclo-forms of the drug were administered I.P. in P rats and the ethanol intake measured. Figure 7 shows that c-GQ reduced mean ethanol intake compared to pre-injection control, but GQ had no effect. Ethanol intake after c-GQ dropped from 12.85 ± 1.22 to 8.78 ± 1.24 g/kg and after GQ from 10.15 ± 0.66 to 10.54 ± 0.72 g/kg. The difference was significant for c-GQ using a independent t-test (** P<0.02; t = 2.34; df 14) and not significant for GQ.
Figure 7. I.P. c-GQ (25 mg/kg) or GQ (25 mg/kg) in P rats.
Figure 7 compares mean ethanol intake (g/kg) after I.P. c-GQ or I.P. GQ in P rats. The data show a decreased intake after c-GQ. The difference was significant for c-GQ using a independent t-test (** P<0.02) and not significant for GQ.
3.6 Histology of NAC Injection Sites
The injection site is shown in Figure 8 Panel A The dark brown or black stain of the biotinylated dextran amine (BDA) is in the area of the injection damage tract. The injections were slightly medial to the margin between shell and core regions of the NAC. The darkened stain from the spread of BDA appears to be half a mm or less and clearly includes the shell region where beta-endorphin staining is reported to be prevalent (12).
Figure 8. NAC Injection Site & BDA Distribution.

The biotinylated dextran amine (BDA) appears as dark brown or black stain in a cresyl violet stained section. Panels A and E are at 40 X magnification and identify landmarks in tissue sections. Panels B,D and F are 400Xs magnifications from the paired sections and show BDA at cellular levels. Panel A shows the injection site and distribution of the dark BDA stain. Panel B shows BDA in cell and neural processes. Panel C shows the dark BDA stain in fibers passing around the anterior commissure (aca) (arrow) and Panel D shows a higher magnification of these fibers. Panel E shows localization of BDA in the arcuate region and Panel F shows BDA localization in individual cells (arrows).
Panel A also shows the landmark anterior commissure and the change in morphology from the medial region (left side of the section) to the more lateral region (right side of the section is consistent with shell and core regions of NAC. The section is also consistent with the stereotaxic coordinates for the guide/injector placement (1.7 mm from bregma). The landmarks for the arcuate area are shown at the ventral 3rd ventricle (Panel E). Additionally, the higher magnification panels (B,D & F) show cellular localization of BDA in NAC and the arcuate area, and its appearance in fibers passing the anterior commissure.
3.7 Effective and Ineffective GQ Sites in NAC of P Rats
An additional experiment was done to obtain an estimate of injection site specificity. As indicated in the Methods section, guide cannulae were positioned with their lower end 1 mm dorsal to the target NAC injection site. NAC and dorsal control injection sites are illustrated in Figures 8 and 9. In this experiment, GQ injections were made at two depths in each of five P rats: one at 1 mm above the NAC target site (-5.5 mm below the dura) and a second injection 1 mm below the end of the guide cannula. Figure 10 shows there was no inhibition of drinking after injections dorsal to the NAC target injection site compared with reduced drinking after injections into the NAC target site. Specifically, after GQ injections at the dorsal site, ethanol intake remained unchanged from a mean of 10.68 ± 0.71 g/kg before injection to a mean of 10.89 ± 0.58 g/kg after. GQ injected into the NAC site was followed by a drop in ethanol intake from a value of 11.45 ± 1.55 g/kg to a mean value of 9.42 ± 1.56 g/kg or a mean percent fall of 27.02 ± 3.47%. The change in ethanol intake at the dorsal ineffective site was a 2.87 ± 4.01 % increase and was not significant. The effective vs. ineffective site responses differed significantly (P < 0.001; dependent t = 7.57; N = 5/group).
Figure 9. Effective and Ineffective Injection Sites.

Schematic diagram of Effective (NAC) (filled circle) and dorsal Ineffective control injection sites (filled triangle). (Adapted from Paxinos and Watson, 1998).
Figure 10. GQ Injections in NAC and Dorsal to NAC (100pmol).

Figure 10 compares ethanol intake (mean % Change) between the intake following GQ injections in the NAC target site (filled circle) with intake after injections 1mm dorsal (filled triangle). GQ in NAC vs. GQ dorsal to NAC, The effective vs. ineffective site responses differed significantly (*** = P < 0.001; dependent t).
3.8 Body Weight and Water Intake After GQ or Saline in P & SD Rats
To determine whether the action of GQ was specific to ethanol intake and did not effect body weight and water intake, these parameters were measured before and after injecting the dipeptide or saline into the NAC of P and SD rats. The data (Table 1) show GQ had no effect on either parameter. Similarly, c-GQ injections into the NAC had no effect on body weight or water intake nor did I.P. cGQ. Additionally, since body weights may be slower to change than intake parameters, body weights were compared for days 2-4 following I.P. c-GQ injections and showed no significant changes (P<0.76; F = 0.45; df = 4,50; N = 14). The data (Table 1) show the mean and SEM of body weight and water intake before and after injections. There was no significant difference in either parameter after GQ, c-GQ or saline.
Table 1. Body Weight and Water Intake After GQ, c-GQ or Saline.
| Weightsa (gm) |
Water
(ml) |
|||
|---|---|---|---|---|
| P Rats | ||||
| NAC GQb | ||||
| Pre-Inj | Post-Inj | Pre-Inj | Post-Inj | |
| Mean | 454.0 | 454.3 | 14.0 | 13.9 |
| SEM | 9.39 | 9.68 | 1.26 | 2.18 |
| NAC Saline | ||||
| Mean | 478.3 | 481.3 | 18.75 | 17.9 |
| SEM | 18.6 | 19.0 | 2.65 | 2.4 |
| NAC c-GQc | ||||
| Mean | 532.4 | 532.9 | 15.4 | 14.0 |
| SEM | 11.0 | 12.0 | 2.25 | 2.24 |
| I.P. cGQc | ||||
| Mean | 512.8 | 513.8 | 19.4 | 20.7 |
| SEM | 27.8 | 28.1 | 1.87 | 1.8 |
| SD Rats | ||||
| NAC GQb | ||||
| Mean | 544.9 | 548.5 | 1.25 | 2.5 |
| SEM | 15.08 | 16.44 | 0.86 | 1.7 |
| NAC Saline | ||||
| Mean | 547.2 | 546.0 | 1.0 | 0.78 |
| SEM | 15.94 | 16.3 | 0.33 | 0.43 |
All weight and water measures were taken in the morning immediately before drug or control injections and again at 24 h. Testing of weight and water using a dependent t-test comparing pre- and post- injection values were n.s.
GQ (100 pmol in 1 μl/side, bilaterally) & Saline (1μl/side, bilaterally) injected into NAC
c-GQ (100 pmol in 1 μl/side, bilaterally);c-GQ I.P. (25mg/kg).
4. Discussion
4.1 Principal Findings
The data from this study support five principal findings. First, c-GQ reduced ethanol intake after I.P. administration into both P and SD rats. Second, the linear form of the dipeptide, GQ, in NAC effectively reduced drinking in both high drinking (P) and low drinking SD (SD) strains of rats. Third, both GQ and c-GQ effectively reduced ethanol drinking proportionally the same amount after injection centrally into NAC of P rats. Fourth, the time course of GQ shows significant action two hours after injection and also at 24 h. And finally, fifth, GQ shows evidence of injection site specificity in NAC.
4.2 High and Low Drinking Strains
When considering the general applicability of a drug it is useful to demonstrate its efficacy not only in a genetic high drinking strain of rat but also in a low drinking strain. GQ was previously reported to reduce drinking in high drinking alcohol preferring (P) rats [19, 20], but had not been tested in a strain like SD rats. In the present report, the data show baseline ethanol intake values for SD rats were comparable to a previous report [9] and were lowered after GQ. Though the absolute intake values in SD rats were lower than P rat values, the proportional effect of GQ was similar in that both strains drank 30-40% less ethanol after injections into NAC. The data support the conclusion that GQ is effective in reducing ethanol intake in both normal SD and high-drinking P rats; and thus the genetic differences between the strains do not alter the response to this drug.
4.3 Central & Peripheral Administration of c-GQ
The first priority in studying c-GQ was to determine if it effectively reduced ethanol drinking. To do this, c-GQ was injected into the sites at which GQ was previously shown to be effective. Injections of c-GQ into the NAC of P rats reduced ethanol consumption to an extent comparable to the effect of the linear form of dipeptide. Thus, the NAC appears to be a site of c-GQ activity to inhibit ethanol intake.
An obvious disadvantage of the linear form of GQ is the route of administration. Although effective after site specific injection into the NAC [19], and intracerebroventricular administration [20], these routes require making a burr hole through the skull. Peripheral administration would be advantageous for possible therapeutic applications. We tested c-GQ using the I.P. route of administration and found that it also effectively reduced drinking. These data are comparable to findings of a previous cardiopulmonary study in which intra-arterial administration of c-GQ was effective in contrast to peripheral administration of the linear form [22]. Similarly, in the present study, whereas the cyclic form was effective after peripheral injection, the linear form was not. Thus it would seem, given the response to site specific and peripheral routes of administration, that c-GQ crosses from the periphery into the brain and GQ does not. Peripherally injected c-GQ thus appears to act like c-His-Pro which was shown to cross the blood brain barrier sufficiently to block ethanol induced narcosis [1]. Further, when tested in P and SD strains, I.P. c-GQ was found to be effective in both.
4.4 Two-hour Limited Access and 24-h Access
Two commonly used paradigms in studying alcoholism and drugs that alter drinking behavior are the 2-h limited access and the 24-h 2-bottle choice continuous access paradigms. The 2-h test allows one to study immediate drug effects and the 24-h test addresses longer term drug effects [6, 9, 10]. We tested for the immediate action of GQ in the 2-h test and for longer term actions in the 24-h test. The data in the present report show that GQ administered 20 min prior to placing the ethanol bottle on the home cage for 2 hours, effectively reduced ethanol intake in both P and SD rats. Similarly, GQ reduced drinking in both strains by approximately the same percentage in 24 hours. Thus the data indicate that GQ action occurs promptly and lasts for a number of hours. These findings are similar to those for naloxone [13]. This observation is also consistent with multiday effects reported previously from our laboratories using different paradigms (Simpson et al.1998). These findings are also consistent with earlier reports on other small peptides which showed long plasma half-lives and brain tissue retention times [17]. Specifically, c-Leu-Gly had a plasma half-life of 33 h and brain cerebral cortex half-life was 42 h. The half-life values correlated significantly with an animals performance in a behavioral task. The half-life for GQ has not been measured, but GQ levels 60 min after central injection showed no decrease in brain extracts (personal communication W.R. Millington). The duration of reduced drinking suggests GQ and c-GQ may have long half-life or tissue retention times sufficient to account for a 24-h or longer action.
4.5 Specificity of GQ Action
The data make it clear that GQ acts to reduce ethanol intake; however, GQ action may not be specific to ethanol intake. GQ could act in a mechanism unrelated to alcohol reward such as the thirst mechanism, or it could be acting as a result of hydrolysis to its constituent amino acids, or some other nonspecific effect. To address the first possibility we tested whether GQ is similarly effective in reducing water intake or body weight. The data for P and SD rats (Table 1) show neither parameter in either strain of rats was significantly altered by GQ or vehicle saline injections. Second, an alternative explanation for GQ action could be due to the action of one of its constituent amino acids. However, in a previous report, we showed the results of testing the constituent amino acids for action on alcohol intake [19]. Those data showed that glycine and glutamine, administered at the same 100 nmol dose used for the dipeptide, did not elicit suppression in voluntary alcohol intake. Finally, the evidence shows GQ injection site specificity in NAC. Reduced drinking resulted after injections of GQ 6.5 mm below the dura but not after injections were made 1 mm dorsal. Further, as Figure 8 shows, injector damage is defined by NAC shell & core morphology, the anterior commissure landmark, and BDA in the area of the injection damage tract. The injections were at the shell-core boundary region of the NAC. The darkened stain from the spread of BDA appears to be half a mm or less and clearly includes the shell region where beta-endorphin increases following ethanol stimulation (12). These injections are in the same sites as used in our previous work in NAC (19) and shows the same effects on alcohol drinking behavior as in the present study. Finally, following BDA injections into NAC, stain was localized in the arcuate and thus is consistent with beta-endorphin projections to NAC shell from beta-endorphin soma in the arcuate area. These data show that GQ has its action at specific sites, and that these sites are somewhat discrete. These data contribute to a growing body of evidence that the dipeptide reduces ethanol consumption by acting within specific brain sites associated with reward behaviors.
4.6 Endogenous GQ
The brain site location of GQ action suggests a possible endogenous role for GQ since the response site specificity may relate to the endogenous distribution of beta-endorphin nerve terminals. Specifically, reward sites showing both beta-endorphin nerve terminals and responsiveness to GQ include NAC, ventral tegmental area, and the central nucleus of the amygdala (GQ data not shown). The endogenous role for GQ is further supported by the finding that the dipeptide is made from post-translational cleavage of β-endorphin(1-31) and the isolation of GQ and beta-endorphin from brain tissue [14, 21]. These findings are consistent with the demonstration of PC2 enzyme cleavage of β-endorphin(1-31) into GQ and a number of 26 and 27 amino acid shortened β-endorphin peptides [7]. The tissue level of GQ was reported equal to the sum of shortened forms [21]. However, most of the shortened forms were ineffective for reducing ethanol intake in NAC [18]. β-endorphin neurons have been implicated in the modulation of ethanol intake at various brain sites [5, 8, 12] and the distribution of β-endorphin neuron terminals provide the anatomical basis [23].
Logic suggests that GQ made in β-endorphin neurons is released from β-endorphin terminals in these same sites, one of which is NAC. If this is so, and given the demonstrated action of GQ on ethanol intake, GQ may very well have an endogenous role in modulating ethanol intake in normal low drinking strains. It follows that GQ concentrations in high drinking strains might be abnormally low. This idea is consistent with peptide ratios reported in high and low drinking AA and ANA strains of rats [2]. Thus GQ may represent a new component of an endogenous regulatory system modulating ethanol drinking.
4.7 Conclusions
1) GQ reduced ethanol consumption in low drinking Sprague Dawley rats similar to its action in high drinking P rats. 2) GQ and cGQ each elicited about the same reduction in ethanol drinking following injections into the same site in NAC. 3) Peripheral injections of cGQ also reduced ethanol consumption, in both strains of rats. Thus c-GQ may have therapeutic potential, and further studies of GQ may contribute to understanding a neuronal system involved with the endogenous modulation of ethanol consumption.
Acknowledgments
The study supported by NIAAA: AA13424. The authors express their sincere appreciation to the Indiana Alcohol Research Center's Animal Production Core Colony (supported by NIAAA-funded AA07611) for supplying P rats. Dr. David Garris assisted in the preparation of the histology figure. Technical support provided by Shannon Callen is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banks WA, Kastin AJ, Akerstrom V, Jaspan JB. Radioactively iodinated cyclo(His-Pro) crosses the blood-brain barrier and reverses ethanol-induced narcosis. Am J Physiol (Endocrinol Metab 27) 1993;264:E723–E729. doi: 10.1152/ajpendo.1993.264.5.E723. [DOI] [PubMed] [Google Scholar]
- 2.DeWaele JP, Kiianmaa K, Gianoulakis C. Spontaneous and ethanol-stimulated in vitro release of beta-endorphin by the hypothalamus of AA and ANA rats. Alcohol Clin Exp Res. 1994;18:1468–1473. doi: 10.1111/j.1530-0277.1994.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 3.Files FJ, Denning CE, Hyytia P, Kiianmaa K, Samson HH. Ethanol-reinforced responding by AA and ANA rats following the sucrose-substitution initiation procedure. Alcohol Clin Exp Res. 1997;21:749–753. [PubMed] [Google Scholar]
- 4.Gianoulakis C. Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition of alcoholism. Revue de psychiatrie & de neuroscience. 2001;26:304–318. [PMC free article] [PubMed] [Google Scholar]
- 5.Herz A. Opioid reward mechanisms: a key role in drug abuse? Can J Physiol Pharmacol. 1998;76:252–258. doi: 10.1139/cjpp-76-3-252. [DOI] [PubMed] [Google Scholar]
- 6.Lankford MF, Myers RD. Opioid and 5-HT 2A receptors in alcohol drinking: Preference in HAD rats is inhibited by combination treatment with naltrexone and amperozide. Alcohol. 1996;13:53–57. doi: 10.1016/0741-8329(95)02011-x. [DOI] [PubMed] [Google Scholar]
- 7.Loh YP. Molecular mechanisms of β-endorphin biosynthesis. Biochem Pharmacol. 1992;44:843–849. doi: 10.1016/0006-2952(92)90114-x. [DOI] [PubMed] [Google Scholar]
- 8.Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of B-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharm. 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- 9.Martinetti MP, Lowery EG, Vona SR, Wichnick AN, Adler RA, Finch DG. Limited-access consumption of ascending ethanol concentrations in alcohol preferring, nonpreferring and Sprague-Dawley rats. Alcohol: Clin Exper Res. 2006;30:836–843. doi: 10.1111/j.1530-0277.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- 10.Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- 11.Myers RD. In: Methods of Psychobiology. Myers RD, editor. Academic Press; New York: 1971. [Google Scholar]
- 12.Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. The J of Neurosci. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. RC184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Braun C, Bartus RT, Crews FT. Suppression of alcohol intake by chronic naloxone treatment in P rats: Tolerance development and elevation of opiate receptor binding. Alcohol Clin Exper Res. 1999;23:1761–1771. [PubMed] [Google Scholar]
- 14.Parish DC, Smyth DG, Normanton JR, Wolstencroft JH. Glycyl-glutamine, an inhibitory neuropeptide derived from β-endorphin. Nature. 1983;306:267–270. doi: 10.1038/306267a0. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. New York: Academic Press; 1998. [Google Scholar]
- 16.Prasad C. Role of endogenous cyclo(His-Pro) in voluntary alcohol consumption by alcohol-preferring C57BL mice. Peptides. 2001;22:2113–2117. doi: 10.1016/s0196-9781(01)00570-8. [DOI] [PubMed] [Google Scholar]
- 17.Rainbow TC, Flexner JB, Flexner LB, Hoffman PL, Walter R. Distribution, survival and biological effects in mice of a behaviorally active, enzymatically stable peptide: pharmacokinetics of cyclo(Leu-Gly) and puromycin-induced amnesia. Pharmacol Biochem Behav. 1979;10:787–793. doi: 10.1016/0091-3057(79)90334-4. [DOI] [PubMed] [Google Scholar]
- 18.Resch GE. Rat and human C-terminal derivatives of β-endorphin: Actions on ethanol consumption. Alcohol Clin Exp Res. 2005 29:10A. [Google Scholar]
- 19.Resch GE, Shridharani S, Millington WR, Garris DR, Simpson CW. Glycyl-glutamine in nucleus accumbens reduces ethanol intake in alcohol preferring (P) rats. Brain Res. 2005;1058:73–81. doi: 10.1016/j.brainres.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 20.Simpson CW, Resch GE, Millington WR, Myers RD. Glycyl-L-Glutamine Injected Centrally Suppresses Alcohol Drinking in P Rats. Alcohol. 1998;16:101–107. doi: 10.1016/s0741-8329(97)00167-5. [DOI] [PubMed] [Google Scholar]
- 21.Smyth DG, Parish DC, Normanton JR, Wolstencroft JH. The C-terminal dipeptide of beta-endorphin: a neuropeptides with inhibitory activity. Life Sci. 1983;33:575–578. doi: 10.1016/0024-3205(83)90568-4. [DOI] [PubMed] [Google Scholar]
- 22.Unal CB, Owen MB, Millington WR. Cyclo-(Gly-Gln) inhibits the cardiorespiratory depression produced by beta-endorphin and morphine. Brain Res. 1997;747:52–59. doi: 10.1016/s0006-8993(96)01261-9. [DOI] [PubMed] [Google Scholar]
- 23.Watson SJ, Trujillo KA, Herman JP, Akil H. Neuroanatomical and neurochemical substrates of drug-seeking behavior: Overview and future directions. In: Goldstein A, editor. Molecular and Cellular Aspects of the Drug Addictions. New York: Springer-Verlag; 1989. pp. 29–91. [Google Scholar]