Abstract
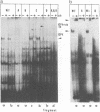
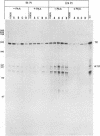
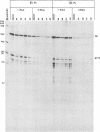
A previous report (P. Mavromara-Nazos and B. Roizman, Proc. Natl. Acad. Sci. USA 86:4071-4075, 1989) demonstrated that substitution of sequences of the thymidine kinase (tk) gene, a beta gene, extending from -16 to +51 with sequences extending from -12 to +104 of the gamma 2 UL 49.5 gene in viral recombinant R3820 conferred upon the chimeric gene gamma 2 attributes in the context of the viral genome in a productive infection. The UL49.5 gene sequences extending from -179 to +104 contain four DNA binding sites for the major regulatory protein ICP4. Of these sites, two map between nucleotides +20 and +80 within the sequence which confers gamma 2 regulation upon the chimeric gene. To determine the role of these ICP4 binding sites in conferring the gamma 2 gene attributes, sequences comprising the two ICP4 binding sites were mutagenized and used to reconstruct the R3820 recombinant virus. In addition, a new recombinant virus (R8023) was constructed in which tk sequences extending from -240 to +51 were replaced with wild-type or mutated sequences contained between nucleotides -179 to +104 of the UL 49.5 gene. Vero cells infected with the recombinant viruses in the presence or absence of phosphonoacetate, a specific inhibitor of viral DNA synthesis, were then tested for accumulation of tk RNA by using an RNase protection assay. The results indicate that in the recombinant R3820, a mutation which destroyed one of the two UL49.5 ICP4 DNA binding sites significantly reduced the accumulation of tk RNA at both early and late times after infection. The effect of this mutation was less pronounced in cells infected with the R8023 virus, whose chimeric tk gene contains the two upstream UL49.5 ICP4 binding sites. None of the mutations affected the sensitivity of the chimeric genes to phosphonoacetate. The mutated site appears to be involved in the accumulation of RNA.
Full text
PDF


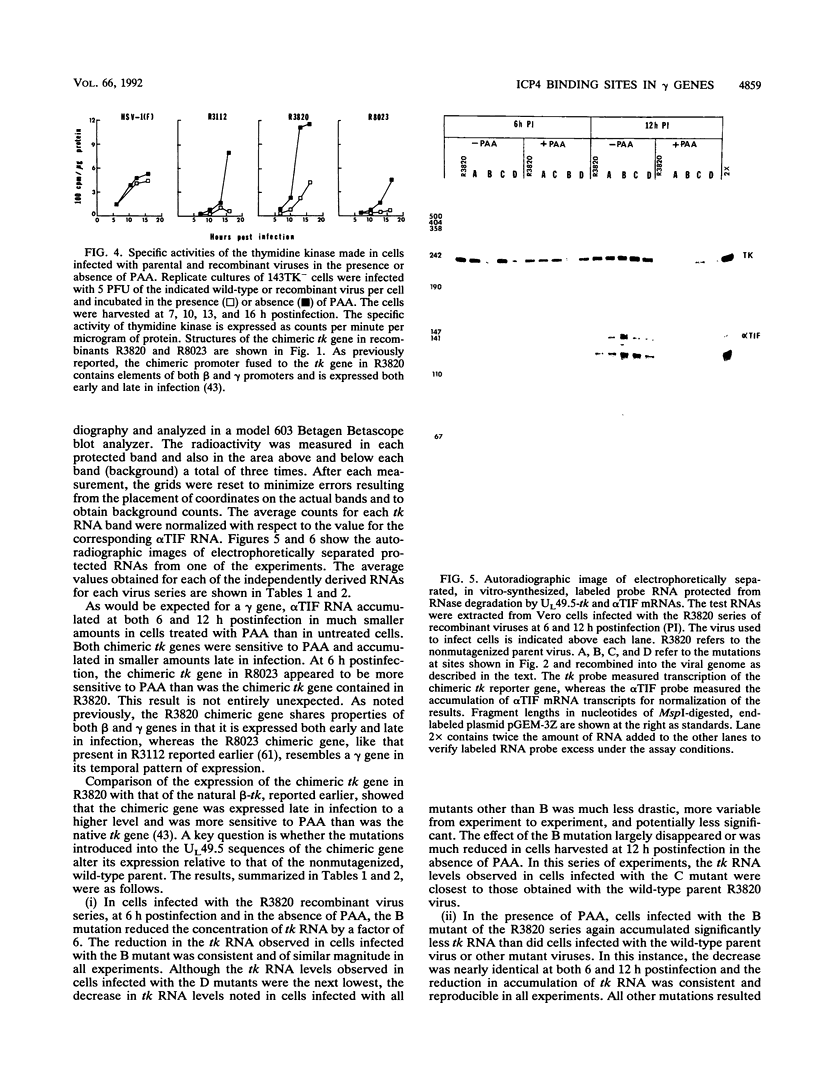
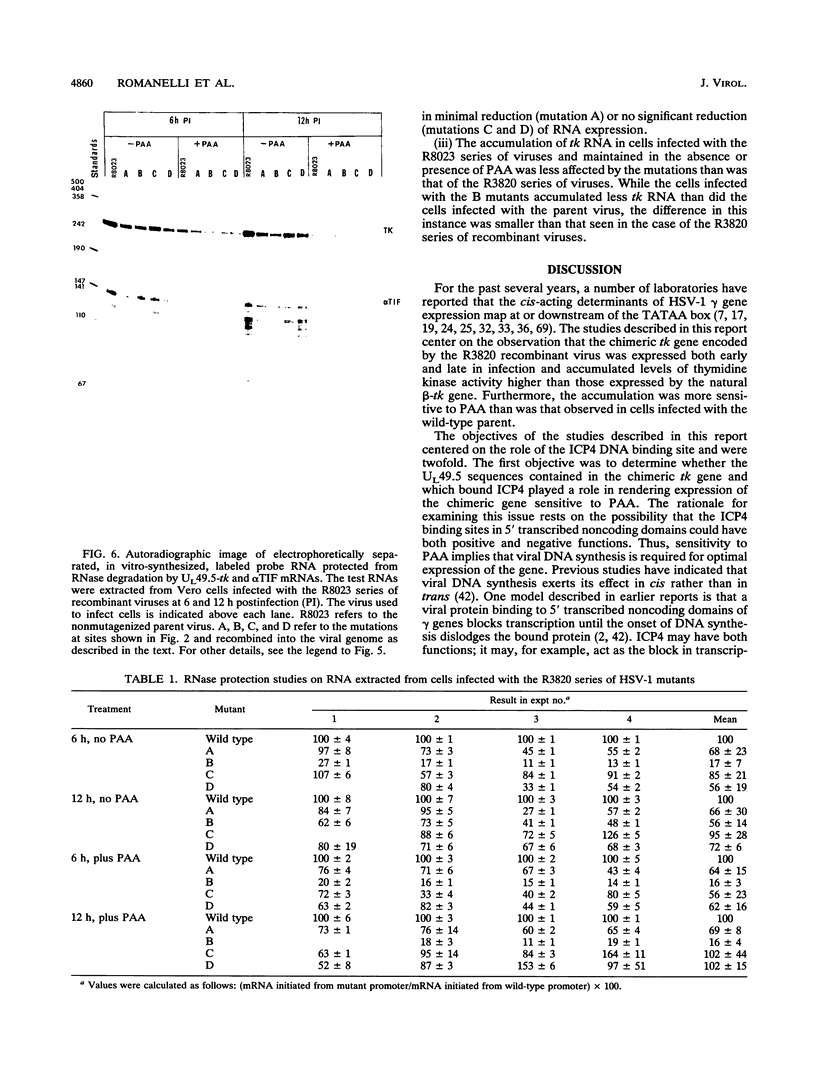
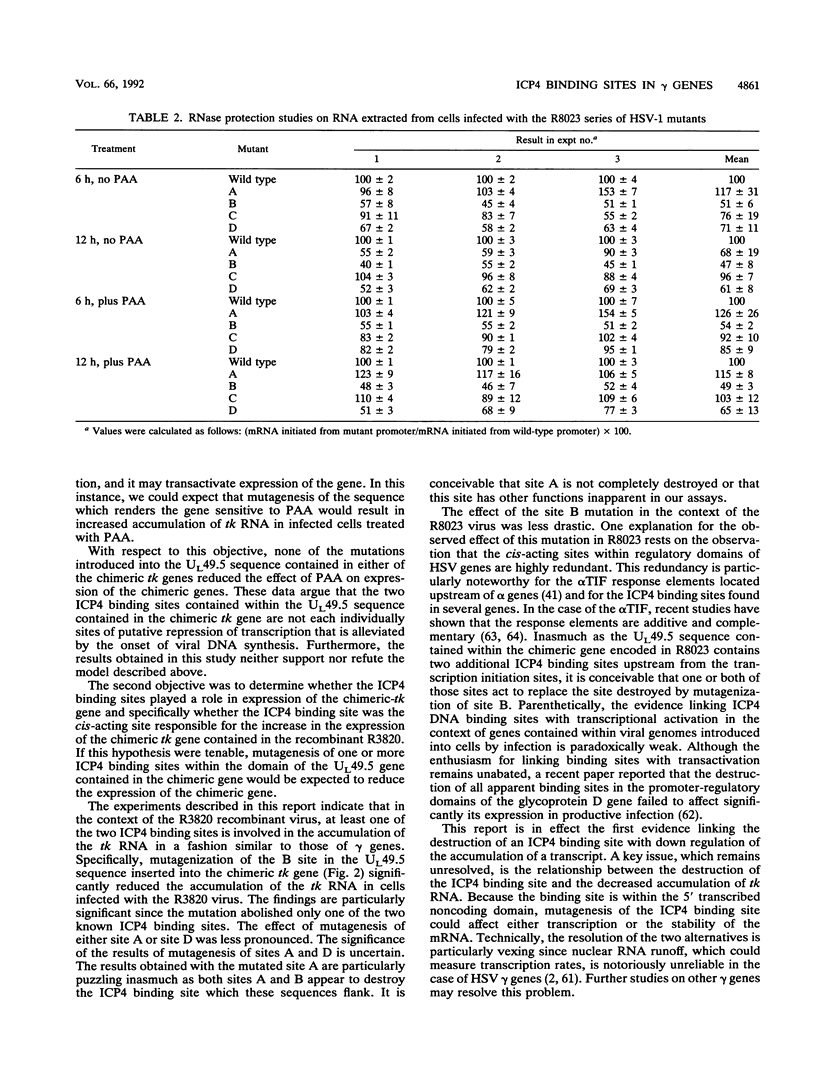


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Braun D. K., Pereira L., Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984 Oct;52(1):108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenakis M., Campadelli-Fiume G., Roizman B. Regulation of glycoprotein D synthesis: does alpha 4, the major regulatory protein of herpes simplex virus 1, regulate late genes both positively and negatively? J Virol. 1988 Jan;62(1):148–158. doi: 10.1128/jvi.62.1.148-158.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenakis M., Hubenthal-Voss J., Campadelli-Fiume G., Pereira L., Roizman B. Construction and properties of a cell line constitutively expressing the herpes simplex virus glycoprotein B dependent on functional alpha 4 protein synthesis. J Virol. 1986 Nov;60(2):674–682. doi: 10.1128/jvi.60.2.674-682.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. E., Roizman B. The unique sequence of the herpes simplex virus 1 L component contains an additional translated open reading frame designated UL49.5. J Virol. 1992 Jan;66(1):562–566. doi: 10.1128/jvi.66.1.562-566.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterson W., Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983 May;46(2):371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard P., Faber S., Wilcox K. W., Pizer L. I. Herpes simplex virus immediate early infected-cell polypeptide 4 binds to DNA and promotes transcription. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4016–4020. doi: 10.1073/pnas.83.11.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair E. D., Blair C. C., Wagner E. K. Herpes simplex virus virion stimulatory protein mRNA leader contains sequence elements which increase both virus-induced transcription and mRNA stability. J Virol. 1987 Aug;61(8):2499–2508. doi: 10.1128/jvi.61.8.2499-2508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. E., Palfreyman J. W., Preston C. M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984 Nov 25;180(1):1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., McCarthy A. M., Schaffer P. A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985 Nov;56(2):558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol Cell Biol. 1985 Aug;5(8):1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Activities of herpes simplex virus type 1 (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 1987 Jun 11;15(11):4491–4511. doi: 10.1093/nar/15.11.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988 Mar;62(3):732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato J. A., Spitzner J. R., Muller M. T. A predictive model for DNA recognition by the herpes simplex virus protein ICP4. J Mol Biol. 1991 Jun 5;219(3):451–470. doi: 10.1016/0022-2836(91)90186-a. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. DNA sequence elements required for regulated expression of the HSV-1 glycoprotein D gene lie within 83 bp of the RNA capsites. Nucleic Acids Res. 1983 Oct 11;11(19):6647–6666. doi: 10.1093/nar/11.19.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber S. W., Wilcox K. W. Association of the herpes simplex virus regulatory protein ICP4 with specific nucleotide sequences in DNA. Nucleic Acids Res. 1986 Aug 11;14(15):6067–6083. doi: 10.1093/nar/14.15.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan W. M., Papavassiliou A. G., Rice M., Hecht L. B., Silverstein S., Wagner E. K. Analysis of the herpes simplex virus type 1 promoter controlling the expression of UL38, a true late gene involved in capsid assembly. J Virol. 1991 Feb;65(2):769–786. doi: 10.1128/jvi.65.2.769-786.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman I. H., Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L. M., Draper K. G., Frink R. J., Costa R. H., Wagner E. K. Herpes simplex virus mRNA species mapping in EcoRI fragment I. J Virol. 1982 Aug;43(2):594–607. doi: 10.1128/jvi.43.2.594-607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L. E., Anderson K. P., Shipman C., Jr, Wagner E. K. Viral DNA synthesis is required for the efficient expression of specific herpes simplex virus type 1 mRNA species. Virology. 1980 Feb;101(1):10–24. doi: 10.1016/0042-6822(80)90479-1. [DOI] [PubMed] [Google Scholar]
- Homa F. L., Glorioso J. C., Levine M. A specific 15-bp TATA box promoter element is required for expression of a herpes simplex virus type 1 late gene. Genes Dev. 1988 Jan;2(1):40–53. doi: 10.1101/gad.2.1.40. [DOI] [PubMed] [Google Scholar]
- Homa F. L., Otal T. M., Glorioso J. C., Levine M. Transcriptional control signals of a herpes simplex virus type 1 late (gamma 2) gene lie within bases -34 to +124 relative to the 5' terminus of the mRNA. Mol Cell Biol. 1986 Nov;6(11):3652–3666. doi: 10.1128/mcb.6.11.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubenthal-Voss J., Houghten R. A., Pereira L., Roizman B. Mapping of functional and antigenic domains of the alpha 4 protein of herpes simplex virus 1. J Virol. 1988 Feb;62(2):454–462. doi: 10.1128/jvi.62.2.454-462.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Everett R. D. DNA replication is required for abundant expression of a plasmid-borne late US11 gene of herpes simplex virus type 1. Nucleic Acids Res. 1986 May 12;14(9):3609–3625. doi: 10.1093/nar/14.9.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Everett R. D. The control of herpes simplex virus type-1 late gene transcription: a 'TATA-box'/cap site region is sufficient for fully efficient regulated activity. Nucleic Acids Res. 1986 Nov 11;14(21):8247–8264. doi: 10.1093/nar/14.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., MacLean C., Marsden H. S., Dalziel R. G., Everett R. D. The product of gene US11 of herpes simplex virus type 1 is expressed as a true late gene. J Gen Virol. 1986 May;67(Pt 5):871–883. doi: 10.1099/0022-1317-67-5-871. [DOI] [PubMed] [Google Scholar]
- Jones P. C., Roizman B. Regulation of herpesvirus macromolecular synthesis. VIII. The transcription program consists of three phases during which both extent of transcription and accumulation of RNA in the cytoplasm are regulated. J Virol. 1979 Aug;31(2):299–314. doi: 10.1128/jvi.31.2.299-314.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattar-Cooley P., Wilcox K. W. Characterization of the DNA-binding properties of herpes simplex virus regulatory protein ICP4. J Virol. 1989 Feb;63(2):696–704. doi: 10.1128/jvi.63.2.696-704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler P. K., Duncan J., Keith B. D., Hupel T., Smiley J. R. Regulation of herpes simplex virus true late gene expression: sequences downstream from the US11 TATA box inhibit expression from an unreplicated template. J Virol. 1991 Dec;65(12):6749–6760. doi: 10.1128/jvi.65.12.6749-6760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Ruyechan W. T., Roizman B., Halliburton I. W. Molecular genetics of herpes simplex virus: demonstration of regions of obligatory and nonobligatory identity within diploid regions of the genome by sequence replacement and insertion. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3896–3900. doi: 10.1073/pnas.75.8.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie T. M., Roizman B. DNA-binding site of major regulatory protein alpha 4 specifically associated with promoter-regulatory domains of alpha genes of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4700–4704. doi: 10.1073/pnas.83.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M., Buttyan R., Spear P. G. Herpes simplex virus gene expression in transformed cells. I. Regulation of the viral thymidine kinase gene in transformed L cells by products of superinfecting virus. J Virol. 1976 Nov;20(2):413–424. doi: 10.1128/jvi.20.2.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Generation of an inverting herpes simplex virus 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the alpha 47 gene. J Virol. 1986 May;58(2):583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Differentiation between alpha promoter and regulator regions of herpes simplex virus 1: the functional domains and sequence of a movable alpha regulator. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4917–4921. doi: 10.1073/pnas.79.16.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavromara-Nazos P., Roizman B. Activation of herpes simplex virus 1 gamma 2 genes by viral DNA replication. Virology. 1987 Dec;161(2):593–598. doi: 10.1016/0042-6822(87)90156-5. [DOI] [PubMed] [Google Scholar]
- Mavromara-Nazos P., Roizman B. Delineation of regulatory domains of early (beta) and late (gamma 2) genes by construction of chimeric genes expressed in herpes simplex virus 1 genomes. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4071–4075. doi: 10.1073/pnas.86.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight J. L., Pellett P. E., Jenkins F. J., Roizman B. Characterization and nucleotide sequence of two herpes simplex virus 1 genes whose products modulate alpha-trans-inducing factor-dependent activation of alpha genes. J Virol. 1987 Apr;61(4):992–1001. doi: 10.1128/jvi.61.4.992-1001.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N., Roizman B. Binding of the herpes simplex virus major regulatory protein to viral DNA. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9808–9812. doi: 10.1073/pnas.86.24.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N., Spector D., Mavromara-Nazos P., Kristie T. M., Roizman B. The DNA-binding properties of the major regulatory protein alpha 4 of herpes simplex viruses. Science. 1988 Mar 25;239(4847):1531–1534. doi: 10.1126/science.2832940. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987 Mar;61(3):858–865. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Comparison of upstream sequence requirements for positive and negative regulation of a herpes simplex virus immediate-early gene by three virus-encoded trans-acting factors. J Virol. 1987 Jan;61(1):190–199. doi: 10.1128/jvi.61.1.190-199.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J Virol. 1985 Mar;53(3):751–760. doi: 10.1128/jvi.53.3.751-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J Virol. 1985 Dec;56(3):723–733. doi: 10.1128/jvi.56.3.723-733.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson T., Everett R. D. The regions of the herpes simplex virus type 1 immediate early protein Vmw175 required for site specific DNA binding closely correspond to those involved in transcriptional regulation. Nucleic Acids Res. 1988 Dec 9;16(23):11005–11025. doi: 10.1093/nar/16.23.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., McKnight J. L., Jenkins F. J., Roizman B. Nucleotide sequence and predicted amino acid sequence of a protein encoded in a small herpes simplex virus DNA fragment capable of trans-inducing alpha genes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5870–5874. doi: 10.1073/pnas.82.17.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Everett R. D., Tedder D. G., Elliott M., Litman B. Nucleotides within both proximal and distal parts of the consensus sequence are important for specific DNA recognition by the herpes simplex virus regulatory protein ICP4. Nucleic Acids Res. 1991 Feb 11;19(3):477–483. doi: 10.1093/nar/19.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Roberts M. S., Boundy A., O'Hare P., Pizzorno M. C., Ciufo D. M., Hayward G. S. Direct correlation between a negative autoregulatory response element at the cap site of the herpes simplex virus type 1 IE175 (alpha 4) promoter and a specific binding site for the IE175 (ICP4) protein. J Virol. 1988 Nov;62(11):4307–4320. doi: 10.1128/jvi.62.11.4307-4320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Adachi A., Urakawa T., Booth T. F., Thomas C. P. Identification of bluetongue virus VP6 protein as a nucleic acid-binding protein and the localization of VP6 in virus-infected vertebrate cells. J Virol. 1990 Jan;64(1):1–8. doi: 10.1128/jvi.64.1.1-8.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M., Homa F. L., Glorioso J. C., Levine M. Regulation of the herpes simplex virus type 1 late (gamma 2) glycoprotein C gene: sequences between base pairs -34 to +29 control transient expression and responsiveness to transactivation by the products of the immediate early (alpha) 4 and 0 genes. Nucleic Acids Res. 1987 Apr 10;15(7):3097–3111. doi: 10.1093/nar/15.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard A. A., DeLuca N. A. A second-site revertant of a defective herpes simplex virus ICP4 protein with restored regulatory activities and impaired DNA-binding properties. J Virol. 1991 Feb;65(2):787–795. doi: 10.1128/jvi.65.2.787-795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard A. A., Imbalzano A. N., DeLuca N. A. Separation of primary structural components conferring autoregulation, transactivation, and DNA-binding properties to the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1989 Sep;63(9):3714–3728. doi: 10.1128/jvi.63.9.3714-3728.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Roizman B. gamma 2-Thymidine kinase chimeras are identically transcribed but regulated a gamma 2 genes in herpes simplex virus genomes and as beta genes in cell genomes. Mol Cell Biol. 1985 Mar;5(3):518–528. doi: 10.1128/mcb.5.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. R., Johnson D. C., Pizer L. I., Everett R. D. The ICP4 binding sites in the herpes simplex virus type 1 glycoprotein D (gD) promoter are not essential for efficient gD transcription during virus infection. J Virol. 1992 Feb;66(2):623–631. doi: 10.1128/jvi.66.2.623-631.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D., Purves F., Roizman B. Mutational analysis of the promoter region of the alpha 27 gene of herpes simplex virus 1 within the context of the viral genome. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5268–5272. doi: 10.1073/pnas.87.14.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D., Purves F., Roizman B. Role of alpha-transinducing factor (VP16) in the induction of alpha genes within the context of viral genomes. J Virol. 1991 Jul;65(7):3504–3513. doi: 10.1128/jvi.65.7.3504-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder D. G., Everett R. D., Wilcox K. W., Beard P., Pizer L. I. ICP4-binding sites in the promoter and coding regions of the herpes simplex virus gD gene contribute to activation of in vitro transcription by ICP4. J Virol. 1989 Jun;63(6):2510–2520. doi: 10.1128/jvi.63.6.2510-2520.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder D. G., Pizer L. I. Role for DNA-protein interaction in activation of the herpes simplex virus glycoprotein D gene. J Virol. 1988 Dec;62(12):4661–4672. doi: 10.1128/jvi.62.12.4661-4672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980 May 29;285(5763):329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- Weinheimer S. P., McKnight S. L. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J Mol Biol. 1987 Jun 20;195(4):819–833. doi: 10.1016/0022-2836(87)90487-6. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Narayanan P. R. Expression of the herpes simplex virus type 1 glycoprotein C gene requires sequences in the 5' noncoding region of the gene. J Virol. 1990 Jan;64(1):445–449. doi: 10.1128/jvi.64.1.445-449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]