SYNOPSIS
Objectives
The capacity to properly address the worldwide incidence of infectious diseases lies in the ability to detect, prevent, and effectively treat these infections. Therefore, identifying and analyzing inhibitory agents are worthwhile endeavors in an era when few new classes of effective antimicrobials have been developed. The use of geological nanomaterials to heal skin infections has been evident since the earliest recorded history, and specific clay minerals may prove valuable in the treatment of bacterial diseases, including infections for which there are no effective antibiotics, such as Buruli ulcer and multi-drug resistant infections.
Methods
We have subjected two iron-rich clay minerals, which have previously been used to treat Buruli ulcer patients, to broth culture testing of antibiotic-susceptible and -resistant pathogenic bacteria to assess the feasibility of using clay minerals as therapeutic agents.
Results
One specific mineral, CsAg02, demonstrated bactericidal activity against pathogenic Escherichia coli, extended-spectrum β-lactamase (ESBL) E. coli, S. enterica serovar Typhimurium, Pseudomonas aeruginosa, and Mycobacterium marinum and a combined bacteriostatic/bactericidal effect against Staphylococcus aureus, penicillin-resistant S. aureus (PRSA), methicillin-resistant S. aureus (MRSA), and Mycobacterium smegmatis, while another mineral with similar structure and bulk crystal chemistry, CsAr02, had no effect on or enhanced bacterial growth. The <0.2 μm fraction of CsAg02 and CsAg02 heated to 200°C or 550°C retained bactericidal activity, while cation-exchanged CsAg02 and CsAg02 heated to 900°C no longer killed E. coli.
Conclusions
Our results indicate that specific mineral products have intrinsic, heat-stable antibacterial properties, which could provide an inexpensive treatment against numerous human bacterial infections.
Keywords: infections, nanominerals, therapeutics, natural, bactericidal, bacteriostatic
Introduction
Medicinal and therapeutic use of mineral products has impacted human health for thousands of years, and pure clay minerals, such as smectite and illite, are nanomaterials of geological origin. The intentional consumption of earth materials, such as clays, by humans and animals is known as geophagy, a complex behavior, largely attributed to religious beliefs, cultural practices, psychological disorders, cosmetics, dietary/nutritional needs, and medicinal benefits.1-3 Early research focused on the extraordinary adsorptive properties of clay minerals and the health benefits recognized in aiding digestive processes and cleansing and protecting the skin.4, 5 Due to the small particle size (<2.0 μm), these natural geological products have a vast surface area (hundreds of square meter per gram of clay) with high concentrations of ions and compounds located on the surfaces. Despite the clear, beneficial effects on human health related to ridding the body of foreign substances, few studies have investigated the antibacterial properties of clay minerals.6-10 Moreover, to our knowledge, there have been no published scientific reports that examine the antibacterial activities of clay minerals on a broad-spectrum panel of bacterial pathogens, including antibiotic-resistant strains, that infect and cause disease in humans.
Documented use of the two clay minerals described in this study as a therapeutic treatment of Buruli ulcer11 suggests that these natural nanomaterials have significant effects on infectious bacteria and wound healing. In 2001, a French humanitarian working in the Ivory Coast of Africa began treating children with Buruli ulcer with the two clay minerals described herein. Within days of initiating treatment with clay poultices, the therapeutic properties of the clay minerals were demonstrated with the initiation of rapid, non-surgical debridement of the destroyed tissue. Extended treatment with the clay minerals resulted in continued debridement of the ulcer, tissue regeneration, and wound healing. After several months of daily clay applications, the Buruli ulcer wounds healed with soft, supple scarring and return of normal motor function.9, 11 These therapeutic observations are highly relevant since antibiotic treatment is only effective for pre-ulcerative lesions and has generally been unsuccessful with the ulcerative form of Buruli ulcer disease.12 Currently, the only accepted treatment of an advanced Mycobacterium ulcerans infection, the causative agent of Buruli ulcer disease, is surgical excision of the ulcerative lesion along with extended healthy tissue to prevent persistent subcutaneous infection.13 This costly and dangerous treatment often leads to significant loss of tissues and possible permanent disability. To begin understanding how these two mineral products were effective at healing patients infected with M. ulcerans, we have initiated antimicrobial susceptibility testing of several human bacterial pathogens.
Here we report an assessment of the broad-spectrum antibacterial properties of two different clay minerals: CsAg02, an iron-rich smectite and illite clay mineral enriched with magnesium and potassium, and CsAr02, an iron-rich smectite and illite clay mineral enriched with calcium.9 To assess the usefulness of these nanominerals as antibacterial agents, we tested a range of human bacterial pathogens, including Pseudomonas aeruginosa, extended-spectrum β-lactamase (ESBL) E. coli, methicillin-resistant Staphylococcus aureus (MRSA) and Mycobacterium marinum. M. marinum is genetically closely related to M. ulcerans and is a cutaneous pathogen that can cause nodular and ulcerated skin lesions and can invade deeper structures including synovia, bursae, and bone.14 M. marinum infection generally occurs when traumatized skin of an extremity is exposed to contaminated aquariums, salt water, or marine animals.14
Our in vitro microbiological investigations indicate that the two clay minerals, while similar in major phases and bulk chemistry9, have striking and opposite effects on bacterial populations, ranging from enhanced microbial growth to complete growth inhibition. This research documents the therapeutic effect of the clay minerals previously used to treat Buruli ulcer patients9, 11 and represents initial investigations aimed at identifying the mechanism by which particular clay nanomaterials exhibit antibacterial behavior. Our goal is to identify new inhibitory agents in an era when bacterial antibiotic resistance continues to challenge human health and the availability of new antimicrobial compounds is limited.
Materials and methods
Bacterial strains
The following bacterial strains, obtained from the American Type Culture Collection (ATCC), were used as target antibiotic-susceptible organisms in these studies: E. coli ATCC 25922, S. enterica serovar Typhimurium ATCC 14028, P. aeruginosa ATCC 27853, S. aureus ATCC 29213, M. smegmatis ATCC 19420, and M. marinum ATCC 927. With the exception of the mycobacterial strains, the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) recommends these bacteria as quality control strains for laboratory testing of antimicrobials.15 Upon receipt, all bacterial cultures were grown in the appropriate liquid medium (described below) and stored at −70°C prior to use. ESBL E. coli ATCC 51446 was obtained from Sonora Quest Laboratories (Tempe, AZ, USA) and confirmed by MicroScan Negative MIC Panel Type 30 (Dade Behring, West Sacramento, CA, USA) testing to be resistant to be resistant to 11 antibiotics (Table 1). Penicillin-resistant S. aureus (PRSA) was obtained from the ASU School of Life Sciences microbiological culture collection, subjected to minimum inhibitory concentration disc diffusion susceptibility testing by Sonora Quest Laboratories (Tempe, AZ, USA), and confirmed to be resistant to penicillin (data not shown). MRSA was obtained from Sonora Quest Laboratories (Tempe, AZ, USA) and confirmed by MicroScan Positive MIC Panel Type 20A (Dade Behring, West Sacramento, CA, USA) testing to be resistant to 10 antibiotics (Table 2).
Table 1.
Antimicrobial susceptibility patterns of the ESBL E. coli ATCC 51446 strain used in the CsAg02 and CsAr02 antimicrobial assays. The susceptible concentration of the ESBL E. coli strain and the qualitative susceptibility interpretation for each antibiotic are shown.
| Antimicrobial Agent | Concentration (mg/L) | Qualitative Susceptibility* |
|---|---|---|
| Amikacin | 16 | Susceptible, ≤16 |
| Amoxicillin/K Clavulanate | ≤8/4 | Susceptible, ≤8/4 |
| Ampicillin/Sulbactam | >16/8 | Resistant, >16/8 |
| Ampicillin | >16 | Resistant, >16 |
| Aztreonam | 8 | Susceptible, ≤8 |
| Cefazolin | >16 | Resistant, >16 |
| Cefepime | >16 | Resistant, >16 |
| Cefoxitin | ≤8 | Susceptible, ≤8 |
| Cefotaxime | >32 | Resistant, >32 |
| Cefotetan | ≤4 | Susceptible, ≤16 |
| Ceftazidime | 8 | Susceptible, ≤8 |
| Ceftizoxime | >32 | Resistant, >32 |
| Ceftriaxone | >32 | Resistant, >32 |
| Cefuroxime | >16 | Resistant, >16 |
| Cefalotin | >16 | Resistant, >16 |
| Chloramphenicol | ≤8 | Susceptible, ≤8 |
| Gatifloxacin | ≤2 | Susceptible, ≤2 |
| Gentamicin | 4 | Susceptible, ≤4 |
| Levofloxacin | ≤2 | Susceptible, ≤2 |
| Meropenem | ≤1 | Susceptible, ≤4 |
| Nitrofurantoin | ≤32 | Susceptible, ≤32 |
| Piperacillin | >64 | Resistant, >64 |
| Piperacillin/Tazobactam | ≤8 | Susceptible, ≤8 |
| Tetracycline | >8 | Resistant, >8 |
| Ticarcillin/K Clavulanate | 32 | Intermediate, 32−64 |
| Tobramycin | 8 | Intermediate, 8 |
| Trimethoprim/Sulfamethoxazole | ≤2/38 | Susceptible, ≤2/38 |
The endpoint of susceptibility interpretation is indicated for each antibiotic.
Table 2.
Antimicrobial susceptibility patterns of the MRSA strain used in the CsAg02 and CsAr02 antimicrobial assays. The susceptible concentration of the MRSA strain and the qualitative susceptibility interpretation for each antibiotic are shown.
| Antimicrobial Agent | Concentration (mg/L) | Qualitative Susceptibility* |
|---|---|---|
| Amoxicillin/K Clavulanate | >4/2 | Resistant, >4/2 |
| Ampicillin | >8 | Resistant, >8 |
| Ampicillin/Sulbactam | 16/8 | Susceptible, ≤16/8 |
| Cefazolin | 16 | Intermediate, 16 |
| Chloramphenicol | 8 | Susceptible, ≤8 |
| Ciprofloxacin | >2 | Resistant, >2 |
| Clindamycin | 0.5 | Susceptible, ≤0.5 |
| Erythromycin | >4 | Resistant, >4 |
| Gatifloxacin | 4 | Resistant, >2 |
| Gentamicin | 2 | Susceptible, ≤4 |
| Imipenem | 2 | Susceptible, ≤4 |
| Levofloxacin | >4 | Resistant, ≥4 |
| Linezolid | 4 | Susceptible, ≤4 |
| Moxifloxacin | 2 | Resistant, ≥2 |
| Nitrofurantoin | ≤32 | Susceptible, ≤32 |
| Penicillin | >8 | Resistant, >0.25 |
| Piperacillin/Tazobactam | >8 | Resistant, >8 |
| Oxacillin | >2 | Resistant, >2 |
| Rifampin | ≤1 | Susceptible, ≤1 |
| Quinupristin/Dalfopristin | ≤0.25 | Susceptible, ≤1 |
| Tetracycline | ≤1 | Susceptible, ≤4 |
| Trimethoprim/Sulfamethoxazole | 5/9.5 | Susceptible, 2/38 |
| Vancomycin | ≤2 | Susceptible, ≤2 |
The endpoint of susceptibility interpretation is indicated for each antibiotic.
Bacterial growth media and growth conditions
E. coli, S. enterica serovar Typhimurium, and P. aeruginosa were cultured using Luria Bertani (LB) broth or agar, Nutrient broth or agar, and Trypticase Soy broth or agar, respectively, at 37°C. S. aureus strains were cultured using Trypticase Soy broth or agar at 37°C. Bacterial cultures of M. smegmatis and M. marinum were grown in supplemented Middlebrook 7H9 broth [Middlebrook 7H9 broth supplemented with 10% OADC (oleic acid, albumin, dextrose and catalase), 0.2% glycerol, and 0.05% Tween 80] at 37°C and 30°C, respectively.16 Viable cell counting of mycobacteria was performed on supplemented Middlebrook 7H10 agar (Middlebrook 7H10 supplemented with 10% OADC) after 48 h of growth at 37°C for M. smegmatis and after 5 d of growth at 30°C for M. marinum. For assays described below, all bacteria were cultured with gentle rotary mixing to ensure contact with the clay minerals and to prevent sedimentation.
Clay minerals
The CsAg02 and CsAr02 minerals used in this study were both supplied by Brunet de Courssou from the batches used in the Buruli ulcer clinics located in western Africa.11 The clays were subjected to factory processing, which included drying (below 80°C) and grinding, before use. X-ray diffraction analyses (limits of detection <5%) of the French clay samples indicate that they are composed predominantly of smectite and illite minerals. The bulk CsAg02 clay is composed of 24% Fe-illite and 50% Fe-smectite, while the bulk CsAr02 clay is composed of 27% Fe-illite, 30% Fe-smectite, 3% kaolinite, and 3% chlorite.9 The surface areas of CsAg02 and CsAr02 are 115.4 m2/g and 90.5 m2/g, respectively9, which provides a potentially large reactive area for ion exchange. Major element analyses of the clay sized fraction (< 2.0 μm) of CsAg02 and CsAr02 showed that both are iron-rich (∼ 6% wt.)9, while other clays obtained from the supplier of CsAg02 have lower iron content (data not shown). Levels of trace elements, including silver, arsenic, and lead, in the clay minerals were all below minimum inhibitory concentrations and will be reported elsewhere. Before use in any susceptibility testing, all mineral samples were sterilized by autoclaving at 121°C for 1 h.
Particle size fractionation of clay minerals
Since clay samples are not pure clay minerals and can contain numerous detrital minerals, such as quartz, feldspar, and carbonates, clay-sized particles of <2.0 μm in diameter were collected and separated by particle size. After a series of washes and ultrasonication of a clay suspension in distilled-deionized water to remove chloride salts, the clay sample suspension (at room temperature) was centrifuged in 50 mL conical tubes in a swinging arm rotor with an axial distance of 15 cm from the center axis to the bottom of the tube. According to Stokes Law, a centrifuge speed of 750 rpm for 3.3 min will settle particles >2.0 μm as long as the water temperature, viscosity, and settling distance are fixed.17 The suspended material, which contains <2.0 μm particles, were centrifuged at 2,000 rpm for 1.8 min to settle the 1.0−2.0 μm particles. For purification, this process was performed in triplicate. After collecting the 1.0−2.0 μm particles, the remaining suspension containing <1.0 μm particles was centrifuged for 29 min at 2,500 rpm to settle the 0.2−1.0 μm particles. The remaining <0.2 μm particles, in the resulting suspension, were dried at 60°C or less. All fractionated and collected CsAg02 clay particles were dried, crushed with a mortar and pestle, and sterilized by autoclaving at 121°C for 1 h.
Cation-exchanged minerals
To remove the natural exchangeable cations, CsAg02 (1 g) was saturated with 1M KCl (25 mL) and shaken for 24 h. The clay was then washed and dialysed to remove Cl−, leaving K+ ions in the exchangeable sites of the clay. Potassium-exchanged CsAg02 was dried, crushed with a mortar and pestle, and sterilized by autoclaving at 121°C for 1 h.
Thermal treatment of clay minerals
To gradually destroy the water and hydroxyl bonds in the mineral structure, separate aliquots of CsAg02 were heated to 200°C and 550°C for 24 h. Heating to 200°C dehydrates the interlayer of smectite18, while heating to 550°C dehydroxylates most iron-rich clays19. Finally, heating to 900°C for 24 h destroys the clay structure completely, leaving only oxide components of the clay. For this procedure, 1−2 g of clay was placed in a porcelain crucible, fitted with a porcelain lid, and heated to either 200°C, 550°C, or 900°C in a muffle furnace. All thermal treatments were performed in air to generate a highly oxidizing environment. Although microorganisms would be destroyed by thermal treatments, the heated CsAg02 minerals were subsequently sterilized by autoclaving at 121°C for 1 h before performing antimicrobial assays.
Antimicrobial assays
Bacterial strains were grown overnight and diluted with fresh medium to achieve an approximate density of 1 × 107 cfu/400 μL. To confirm the initial bacterial counts, serially-diluted bacterial cultures were plated on the appropriate agar plates and enumerated. After dilution, sterilized clay minerals (200 mg) were introduced into 400 μL of media containing the initial pathogen inoculum to achieve a consistency similar to the hydrated clay poultices used to treat Buruli ulcer patients. The bacteria – mineral mixtures were incubated with constant rotary agitation for the appropriate times and temperatures, as described above. Positive controls for growth of bacteria in the absence of clay minerals were included in each series of independent experiments. To ensure that the clay samples were sterilized after autoclaving and maintained sterilization during storage, negative control growth experiments with clay minerals in LB broth were performed several times throughout the course of the study. Incubation of the bacteria – clay mixtures were performed as follows: E. coli, S. enterica serovar Typhimurium, P. aeruginosa, S. aureus, and MRSA were incubated 24 h at 37°C, M. smegmatis was incubated 48 h at 37°C, and M. marinum was incubated 5 d at 30°C. After incubation, mixtures were subjected to successive 10-fold serial dilutions in the appropriate medium, mixed with a vortex shaker to ensure dispersion, and quantitatively cultured in duplicate onto agar plates to determine the number of viable bacteria. Additionally, 100 μL of the bacteria – clay suspension was directly plated onto agar plates to assess the bacterial viability in undiluted samples. At least three independent antimicrobial assays with specific clay minerals and specific bacterial strains were performed. Viable cell counts are expressed as log10 cfu per 400 μL.
Statistical analysis
Statistical analysis was performed using Prism 4 (GraphPad Software, San Diego, CA, USA) and was calculated using a two-tailed paired Student t test. A P value of <0.05 was considered statistically significant.
Results
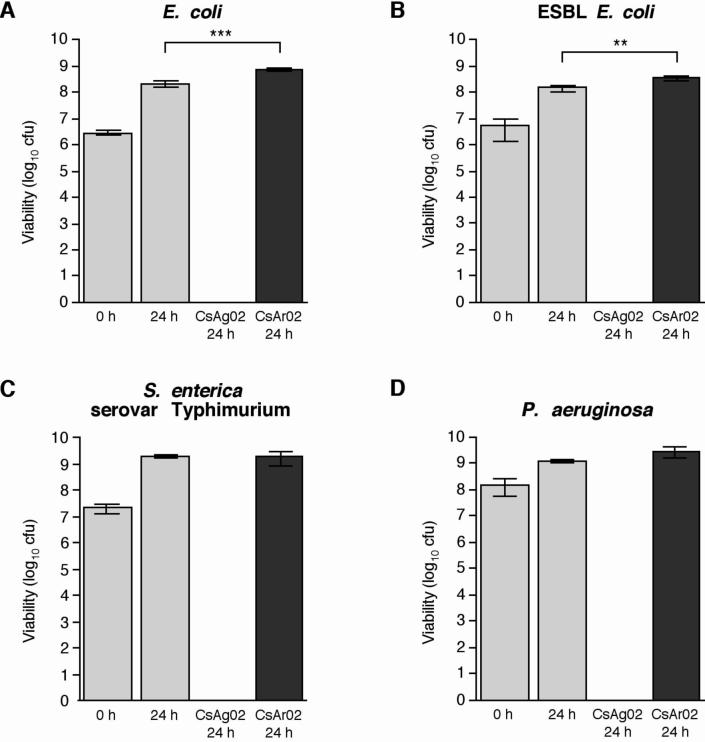
To assess the effect of the CsAg02 and CsAr02 minerals on the growth of clinically-relevant Gram-negative bacteria, susceptibility testing of E. coli ATCC 25922, ESBL E. coli ATCC 51446, S. enterica serovar Typhimurium ATCC 14028, and P. aeruginosa ATCC 27853 was performed in liquid cultures. To achieve poultice consistencies similar to those used to treat Buruli ulcer patients, initial bacterial cultures (∼107 bacteria/400 μL) were mixed with 200 mg of clay minerals and incubated at 37°C on a rotating drum for 24 hours. After incubation, the bacteria – clay mixtures were diluted and plated onto agar to determine the number of viable bacteria (Fig. 1). Based on these susceptibility experiments performed in triplicate, incubation with the CsAg02 clay minerals resulted in complete killing of several antibiotic-sensitive Gram-negative bacteria: E. coli (Fig. 1A), S. enterica serovar Typhimurium (Fig. 1C), and P. aeruginosa (Fig. 1D). Most notably, CsAg02 demonstrated a bactericidal effect against ESBL E. coli (Fig. 1B), which is resistant to 11 of the 27 tested antibiotics (Table 1). In contrast, in the presence of the CsAr02 clay minerals, growth of the susceptible and resistant E. coli strains was significantly enhanced (Fig. 1A and 1B) while growth of S. enterica serovar Typhimurium and P. aeruginosa growth was not significantly different compared to bacterial growth in media alone (Fig. 1C and 1D). No microbial growth was evident in minerals that were subjected to sterilization before use in the antimicrobial susceptibility assays (data not shown).
Fig. 1.
Bactericidal activity of CsAg02 against susceptible and resistant Gram-negative pathogens: (A) E. coli ATCC 25922, (B) ESBL E. coli ATCC 51446, (C) Salmonella enterica serovar Typhimurium ATCC 14028, and (D) P. aeruginosa ATCC 27853. The reported values represent the average and standard deviation of at least three independent experiments. Statistical significance (paired t test) of Cs-inoculated 24 h bacterial growth compared to standard 24 h bacterial growth: ***, P = 0.0006; **, P = 0.007.
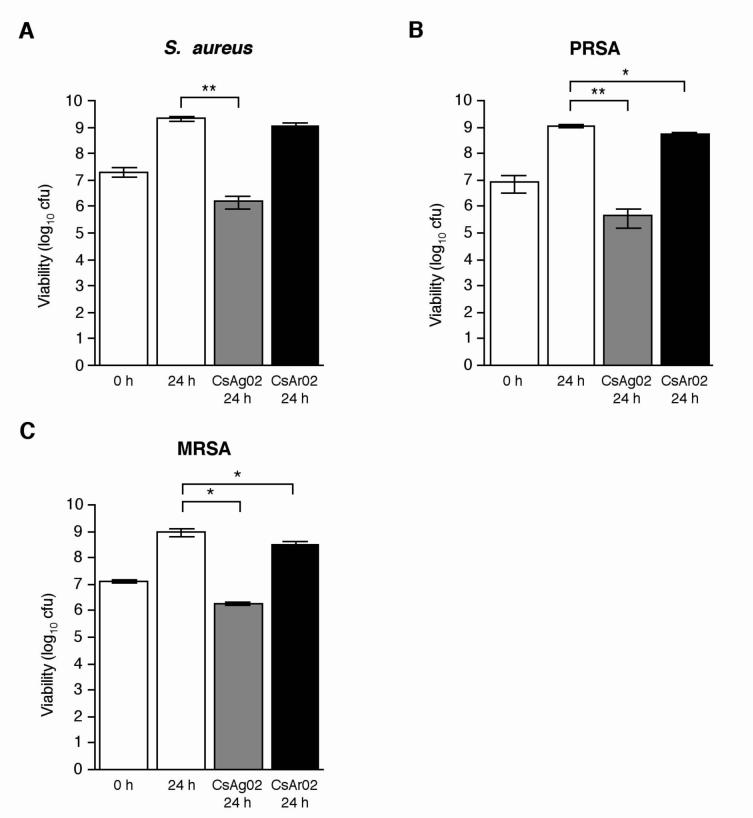
Susceptibility testing with S. aureus ATCC 29213 was performed as described above to assess the potential broad-spectrum inhibitory activity of CsAg02. In addition, to assess the inhibitory activity of CsAg02 against antibiotic-resistant S. aureus, a single antibiotic-resistant strain, PRSA, and a multidrug-resistant strain, MRSA, were also subjected to susceptibility testing. The MRSA strain exhibits resistance to 10 of the 23 tested antibiotics, including methicillin (oxacillin) (Table 2). Incubation with CsAg02 minerals resulted in partial growth inhibition of all three S. aureus strains while growth was unaffected in the presence of CsAr02 minerals (Fig. 2). In contrast to the complete bactericidal effect demonstrated with Gram-negative bacteria, incubation with CsAg02 appears to have more of a bacteriostatic effect on the S. aureus strains. Compared to the initial S. aureus cfu, viability of the susceptible S. aureus strain and the PRSA and MRSA strains after 24 h was decreased approximately 10-fold. The comparable effect on antibiotic-susceptible S. aureus and MRSA indicates that the mechanism of CsAg02 growth inhibition is unique and, more importantly, is unrelated to the mode of action of β-lactam, macrolide, and fluoroquinolone antibiotics. These results are of significant clinical relevance considering the serious health implications of MRSA infections.
Fig. 2.
Effects of CsAg02 and CsAr02 on S. aureus ATCC 29213 (A), PRSA (B), and MRSA (C) growth. Average values and standard deviations from three independent experiments are shown. Statistical significance (paired t test) of Cs-inoculated 24 h bacterial growth compared to standard 24 h bacterial growth: **, P < 0.004; *, P < 0.05.
To assess the effect of the CsAr02 and CsAg02 clay minerals on the growth of nonpathogenic and pathogenic mycobacterial strains, susceptibility testing of M. smegmatis ATCC 19420 and M. marinum ATCC 927 was performed. Initial mycobacterial cultures (∼107 bacteria/400 μL) were incubated with 200 mg of bulk clay at 37°C on a rotating drum for 48 hours (M. smegmatis) or 5 days (M. marinum). After incubation, the bacteria – clay mixtures were diluted and plated onto supplemented Middlebrook 7H10 agar to determine the number of viable bacteria. Similar to the bactericidal effects demonstrated upon Gram-negative bacteria (Fig. 1), incubation with CsAg02 resulted in complete growth inhibition of M. marinum (Fig. 3B). Unlike the bactericidal effect upon M. marinum, M. smegmatis growth was reduced 1,000-fold in comparison to cultures grown without minerals for 48 h (Fig. 3A). Incubation with CsAr02 clay did not significantly affect M. smegmatis growth, while M. marinum growth was decreased approximately 50-fold in the presence of CsAr02 (Fig. 3). Notably, the CsAg02 clay was able to completely kill the pathogenic mycobacterial strain, M. marinum, most closely related to M. ulcerans, and significantly reduce the growth of the nonpathogenic M. smegmatis, while CsAr02 clay partially inhibited M. marinum growth, but had no effect on M. smegmatis growth. The bactericidal effect of CsAg02 and bacteriostatic effect of CsAr02 on M. marinum growth (Fig. 3B) strongly suggests that these minerals will have a detrimental effect on the growth of genetically-similar M. ulcerans, as indicated by the topical application of these two minerals to effect a cure for Buruli ulcer patients.11 These results are especially promising considering that there is no therapeutic cure for the ulcerative form of Buruli ulcer disease.
Fig. 3.
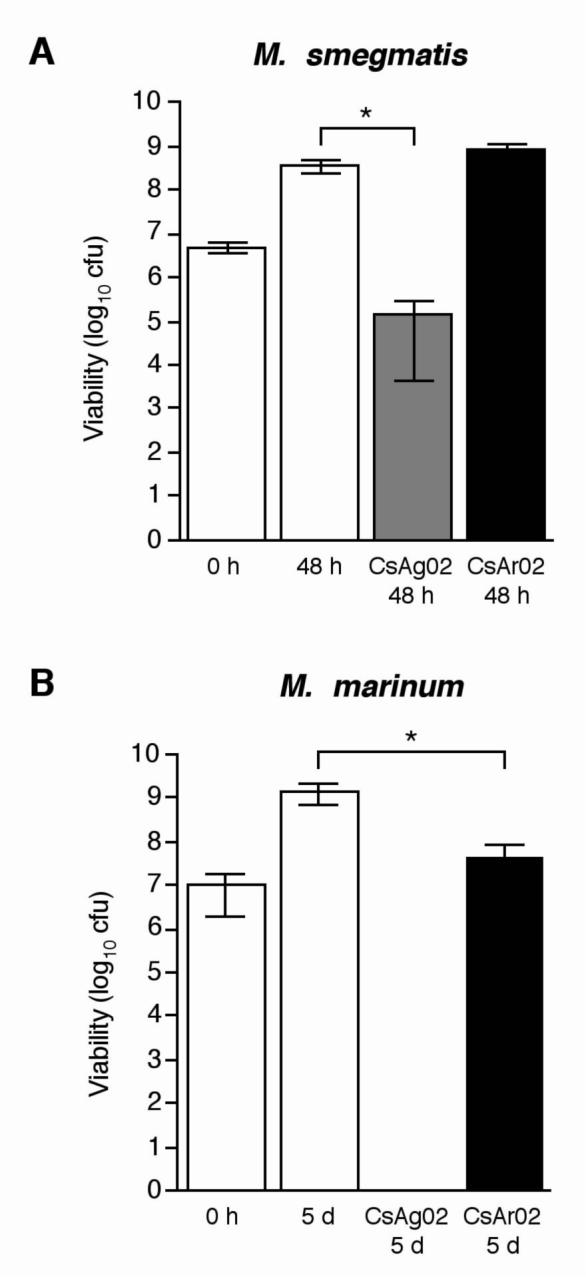
Effects of CsAg02 and CsAr02 on mycobacterial growth. For antimicrobial susceptibility testing, M. smegmatis ATCC 19420 (A) and M. marinum ATCC 927 (B) were grown in the presence of CsAg02 or CsAr02 for 48 h at 37°C and 5 d at 30°C, respectively. The reported values represent the average and standard deviation of at least three independent experiments. Statistical significance (paired t test) of Cs-inoculated bacterial growth compared to standard bacterial growth: *, P < 0.05.
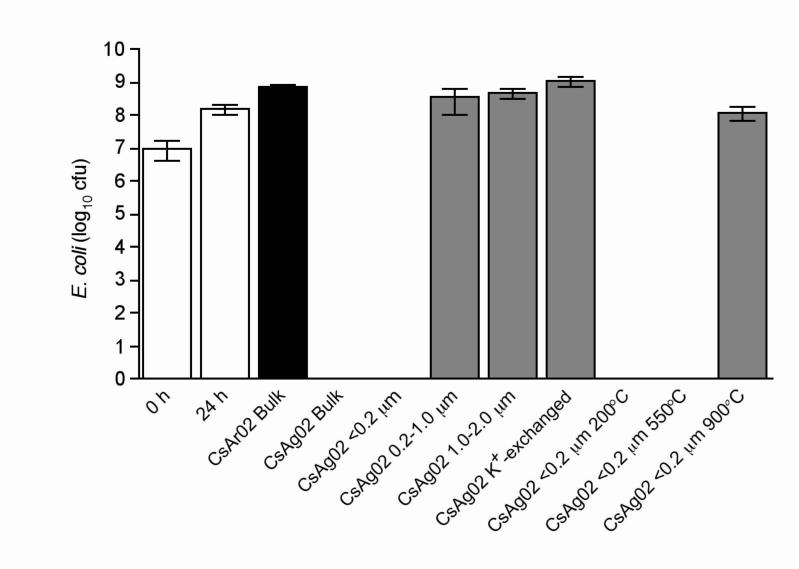
To eliminate the potential that a non-clay mineral in the bulk clay sample is responsible for killing bacteria and to determine if the crystal structure of the minerals is important in the antibacterial effectiveness, we separated the clay minerals by size fractions. By differentially centrifuging the mineral particles, we concentrated most of the non-clay minerals in the coarse size fraction (1−2 μm), while the smaller size fractions were progressively enriched in pure clay minerals. To determine if the relative surface area of mineral crystals was an important parameter for antibacterial activity, various crystal size fractions of CsAg02 (<0.2 μm, 0.2−1.0 μm, and 1.0−2.0 μm) were tested against E. coli (Fig. 4). Results indicate that the finest size fraction (<0.2 μm), which makes up the greatest percentage of the bulk sample, is bactericidal, while the larger CsAg02 size fractions showed no statistically significant effect on E. coli growth (Fig. 4).
Fig. 4.
Effects of CsAg02 fractionation, K+-exchange, and thermal treatment on E. coli growth. E. coli ATCC 25922 was grown in the absence of clay minerals (white bars) for 24 h, in the presence of bulk CsAr02 (black bar), and in the presence of fractionated, K+-exchanged, or thermally-treated CsAg02 (grey bars) for 24 h. The reported values represent the average and standard deviation of at least three independent experiments.
Standard cation exchange was performed on CsAg02 where the natural exchange sites (primarily interlayer ions in the expandable smectite mineral) were replaced by K+.17 Incubation of E. coli with K+-exchanged CsAg02 resulted in complete loss of bactericidal activity (Fig. 4). These results indicate that a chemical exchange from CsAg02 is responsible for the antibacterial activity and that surface properties of the clay have no direct effect on bacterial growth. Similar to CsAr02, K+-exchanged CsAg02 enhanced the growth of E. coli (Fig. 4).
Clay minerals can be heated to gradually remove or destroy different bonds in the mineral structure.20 By progressively destroying different bonds and vaporizing mineral elements in thermal reactions, we may determine if variable clay structures and/or chemical characteristics of the mineral play a role in the antibacterial effectiveness of CsAg02. Heating the clay to 200°C sterilizes the sample, dehydrates the minerals, and collapses the mineral interlayers, while mineral dehydroxylation and decomposition and combustion of organic matter occurs after heating to 550°C.20, 21 Heating the clay to 900°C destroys its silicate structure, oxidizes the clay components, and releases many elements that are volatile at lower temperatures.20 Since autoclaving the CsAg02 clay to achieve sterilization does not affect its antibacterial activity, it was not unexpected to determine that heating the CsAg02 <0.2 μm fraction to 200°C was not detrimental to its bactericidal properties (Fig. 4). Heating the CsAg02 <0.2 μm fraction to 550°C also resulted in retention of its bactericidal activity on E. coli (Fig. 4), indicating that CsAg02 organic compounds and elements bound to the hydroxyls are not associated with antibacterial activity. Upon exposure to 900°C-heated CsAg02, E. coli growth was unaffected and was similar to control cultures lacking clay minerals (Fig. 4). Since CsAg02 no longer kills after heating to 900°C, the remaining oxides, non-volatile elements in their oxidized forms, and increased surface area are not associated with the antibacterial property.
Discussion
Worldwide, the number of antibiotic-resistant pathogenic bacteria has substantially increased within the past 50 years.22 These alarming trends, reduced drug discovery and development productivity23, and the emergence and increasing incidence of antibiotic-resistant infections, such as S. aureus, Streptococcus pneumoniae, Enterococcus faecalis, and Mycobacterium tuberculosis24-28, indicate a progressive need to identify and analyze new antibacterial agents.
Numerous mineral products are currently used for therapeutic purposes in the pharmaceutical and cosmetic industries. For example, smectite minerals adhere to the gastrointestinal mucosa to adsorb and rid the body of dissolved toxins, bacteria, and viruses, while kaolinite and palygorskite are primarily used as antidiarrheal agents and to soothe the digestive tract.4 In addition, kaolinite and smectite clay minerals are hallmark additives used by the cosmetic industry in topical applications to serve as skin protectants and to absorb skin secretions.4 The data obtained in this study, along with biosafety studies with animals and controlled human trials, will be essential in supporting and validating the use of specific minerals or derived products as inexpensive antibacterial agents. Analyses of the chemical interaction at the mineral – bacterial interface are ongoing and will be pertinent in understanding the mechanism by which bacterial growth inhibition occurs.
Buruli ulcer is recognized by the WHO as a global health threat and recent observations showing that the clay minerals used in this study were effective at healing necrotic Buruli ulcer disease 11 prompted our investigations of these geological nanomaterials. The use of clay minerals in the treatment and healing of Buruli ulcer lesions represents great promise for the development of an inexpensive cure for many skin diseases and topical infections. Our aim is to evaluate the effect of these minerals on M. ulcerans, but initial investigations have been directed toward assessing the broad-spectrum antibacterial properties of specific minerals against several bacterial pathogens.
CsAg02 and CsAr02 are geological materials, primarily composed of the minerals, smectite and illite, which contain variable elemental constituents and adsorbed ions, and have been used to treat children with Buruli ulcer.11 The CsAg02 mineral exhibits bactericidal activity against E. coli, ESBL E. coli, S. enterica serovar Typhimurium, P. aeruginosa, and M. marinum and significantly reduces growth of S. aureus, PRSA, MRSA, and nonpathogenic M. smegmatis approximately 1,000-fold compared to cultures grown without added mineral products. We have demonstrated that the CsAg02 inhibitory effect occurs in liquid culture medium, while a similar mineral, CsAr02, displays enhanced or no effect on bacterial viability in liquid culture. To our knowledge, these experiments represent precedential investigations of a novel geological nanomaterial that displays broad-spectrum antibacterial effects against human pathogens, including antibiotic-resistant strains.
Clay minerals are layered substances comprised of sheets of silicate tetrahedra (SiO4) and octahedra (containing Al, Mg, Fe). Smectite and illite, the primary minerals in CsAg02 and CsAr02, are similar in structure (2:1 layer clays) but the silicate layers of the smectite are separated by an expandable interlayer region containing water, cations, and molecules. If the interlayer region is collapsed, due to a high attraction between the silicate sheets with cations (primarily K+) balancing the charge, then the mineral is illite. The interlayer surfaces of smectite are negatively charged due to substitution of Al3+ for Si4+ in some of the tetrahedral sites and Mg2+ for Al3+ (for example) in octahedral sites. Therefore, cations are most commonly attracted to the interlayer, with K+, Na+, Ca2+, and NH4+ being preferred and forming weak surface bonds. The edges of the crystals can have a positive charge and attract anions such as hydroxyls, phosphates, or sulfates.29 Water can enter the interlayer and expand the clay structure to accommodate additional compounds, including neutral and negatively charged species. Illite has the same structure as smectite, but has a higher layer charge, which attracts and holds interlayer cations (particularly K+) tightly. Such interlayer cations are not easily exchanged with solutions, but are ‘fixed-cations’.17 Because of the special quality of smectite for incorporating various ions, the surface can be either hydrophilic or hydrophobic depending on the charge and available solutes. A hydrophobic surface is inherently organophyllic and could harbor an organic substance that is lethal to bacteria. If the mineral surface is hydrophilic, it might compete with bacteria for cations that are essential nutrients for metabolic function or release a toxic inorganic substance that either inhibits a particular metabolic function or precipitates on the cell wall.
Enhanced growth of bacteria upon incubation with clay minerals, as demonstrated with CsAr02, is not an uncommon occurrence, as microorganisms inherently require numerous trace elements to facilitate growth. Montmorillonite, a variety of 2:1 layer smectite, and kaolinite, a 1:1 layer clay composed of one tetrahedral layer and one octahedral layer, can stimulate bacterial growth and promote biofilm formation to serve as important bioremediation substances.30 For example, Shewanella oneidensis respires structural ferric iron bound by smectite and uses it as the sole electron acceptor for bacterial growth.31 Additionally, clay minerals may serve to protect environmental bacteria from UV irradiation or toxic substances.32 By providing structural support and organic or inorganic nutrient acquisition via its high cationic exchange capacity, clay minerals, particularly montmorillonite, may protect bacteria and serve as a minimal nutritional sphere for bacterial proliferation.33
Since cation exchange eliminated the antibacterial activity of CsAg02, we have analyzed the exchange solution by inductively coupled plasma mass spectrometry (ICP-MS) to assess the abundance of elements in the exchange solution relative to the total amount in the clay (L.W. Williams and S.E. Haydel, unpublished results). The most significantly abundant elements removed by cation exchange are silicon (30% of total), barium (35% of total), and strontium (45% of total) (L.W. Williams and S.E. Haydel, unpublished results). Since a silicon concentration of greater than 5,000 mg/L (178 mM) is toxic for E. coli34, the levels of silicon in the CsAg02 exchange solution (96 mg/L; 3.42 mM) are not likely to be important for bactericidal activity. High levels of barium (144 mg/L; 1.05 mM) and strontium (154 mg/L; 1.76 mM) in the CsAg02 exchange solution could be of interest since these elements potentially could be acquired by cation uptake systems, inhibit transport mechanisms, or substitute as enzymatic cofactors in bacterial cells to interfere with normal physiological processes.35-39 Although the CsAr02 exchange solution contains slightly lower levels of barium (119 mg/L) (L.W. Williams and S.E. Haydel, unpublished results), E. coli cultures incubated in the presence of high levels of barium (144 mg/L) grew normally (data not shown). Thus far, no single element identified in the CsAg02 cation exchange solution was greatly different than the concentration identified in the CsAr02 exchange solution or was determined to be solely responsible for E. coli toxicity (L.W. Williams and S.E. Haydel, unpublished results). Therefore, the possibility exists that the antibacterial activity could be attributed to a combination of elements and/or chemical compounds that work in concert to mediate toxicity.
The uniqueness of the French clay minerals is evident considering that it has previously been used to treat children afflicted with Buruli ulcer9, 11 and shown to kill or significantly decrease the growth of both antibiotic-susceptible and antibiotic-resistant human bacterial pathogens. Moreover, of the six independent clay samples collected from the French supplier and tested against various bacteria (data not shown), only CsAg02 displayed antibacterial effects. Analyses of the various trace element components, exchanged ions, and surface properties of the clays are in progress. However, there is not a single component of the CsAg02 clay (e.g., transition metals) that stands out as an obvious antibacterial agent, so it may be a fortuitous combination of factors (multiple components) responsible for the inhibitory property. Further work is in progress to identify additional antibacterial nanominerals, to isolate chemicals released from the antimicrobial clays, and to simulate the effect using synthetic materials.
Clay minerals can affect bacterial metabolism indirectly by altering the physicochemical properties of a specific environment or directly through surface interactions.40 Although we demonstrate that the nanoparticle-sized fraction (<200 nm) of CsAg02 retains bactericidal activities against E. coli, cation-exchanged CsAg02 completely loses its antibacterial effectiveness. Therefore, we hypothesize that the physicochemical properties of hydrated CsAg02 indirectly kill bacteria by generating an unfavorable environment. Since the physicochemical properties of iron-rich smectite can be greatly affected by the structural Fe oxidation state41, determining the oxidation state of Fe in the CsAg02 nanoparticle-sized fraction, which contains primarily iron-rich illite and smectite minerals9, is important. Thermal inactivation of the antibacterial properties when CsAg02 is heated to 900°C could indicate that the change in oxidation state of inherent elements, including Fe which is highly oxidized at 900°C, or the loss of vaporized elements removes the critical components of the antibacterial mineral.
Reactive oxygen species, including oxygen ions, free radicals, and peroxides, are by-products of aerobic bacterial metabolism with demonstrated toxic effects on bacteria.42 Mediated by the Fenton reaction, hydroxyl radicals are formed by the reduction of hydrogen peroxide and ferrous iron42, 43 and cause oxidative damage to bacterial DNA, proteins, and lipids44-48. Furthermore, numerous transition metals, which are inherent in clay minerals, can also participate in Fenton-like reactions to produce hydroxyl radicals.49 The combination of elevated levels of reduced iron in CsAg02 and excessive free radical production in the presence of oxygen could cause oxidative stress and damage to bacterial cells, resulting in death.50, 51 Most importantly, Kohanski et al.52 recently demonstrated that three major classes of bactericidal antibiotics stimulate production of hydroxyl radicals via Fenton chemistry to contribute to bacterial cell death. However, regarding CsAg02, additional experiments are necessary to assess the effect that varying redox environments and chemical speciation changes have on microbial viability.
During the past 25 years, approximately 70% of newly discovered drugs introduced in the U.S. have been derived from natural products.23 Our discovery that natural geological minerals harbor antibacterial properties should provide impetus for exploring terrestrial sources for the presence of novel therapeutic compounds. Combining the availability of natural bioactive resources with powerful combinatorial chemistry optimization methodologies could result in the development of new antibacterial agents to fight existing antibiotic-resistant infections and diseases for which there are no known therapeutic agents, such as advanced M. ulcerans infections.
Acknowledgements
We thank the ASU College of Liberal Arts and Sciences for supporting travel to France for sample collection and geologic field investigations, Sonora Quest Laboratories (Tempe, AZ, USA) for providing quality control bacterial strains and for performing the antimicrobial susceptibility panels, and Dr. Thierry Ferrand for supplying additional clay samples. Thierry Brunet de Courssou is responsible for alerting us to the humanitarian efforts and clinical observations of his mother, Line Brunet de Courssou, who passed away in 2006. We appreciate her hard work and careful observations of the effect of clay minerals on the wounds of Buruli ulcer patients in western Africa.
Funding This research was supported by Public Health Service grant AT003618 from the National Institutes of Health to L.B.W. and S.E.H. and an ASU research initiatives grant to S.E.H.
Footnotes
Transparency declarations None to declare.
References
- 1.Abrahams PW, Parsons JA. Geophagy in the tropics: a literature review. Geogr J. 1996;162:63–72. [Google Scholar]
- 2.Aufreiter S, Hancock RGV, Mahaney WC, et al. Geochemistry and mineraology of soils eaten by humans. Int J Food Sci Nutr. 1997;48:293–305. [Google Scholar]
- 3.Hunter JM. Geophagy in Africa and in the United States. Geogr Rev. 1973;63:170–95. [Google Scholar]
- 4.Carretero MI. Clay minerals and their beneficial effects upon human health. A review. Appl Clay Sci. 2002;21:155–63. [Google Scholar]
- 5.Viseras C, Lopez-Galindo A. Pharmaceutical applications of some spanish clays (sepiolite, palygorskite, bentonite): some preformulation studies. Appl Clay Sci. 1999;14:69–82. [Google Scholar]
- 6.Herrera P, Burghardt RC, Phillips TD. Adsorption of Salmonella enteritidis by cetylpyridinium-exchanged montmorillonite clays. Vet Microbiol. 2000;74:259–72. doi: 10.1016/s0378-1135(00)00157-7. [DOI] [PubMed] [Google Scholar]
- 7.Hu CH, Xu ZR, Xia MS. Antibacterial effect of Cu2+-exchanged montmorillonite on Aeromonas hydrophila and discussion on its mechanism. Vet Microbiol. 2005;109:83–8. doi: 10.1016/j.vetmic.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Tong G, Yulong M, Peng G, et al. Antibacterial effects of the Cu(II)-exchanged montmorillonite on Escherichia coli K88 and Salmonella choleraesuis. Vet Microbiol. 2005;105:113–22. doi: 10.1016/j.vetmic.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Williams LB, Holland M, Eberl DD, et al. Killer clays! Natural antibacterial clay minerals. Mineral Soc Bull. 2004;139:3–8. [Google Scholar]
- 10.Wilson MJ. Clay mineralogical and related characteristics of geophagic materials. J Chem Ecol. 2003;29:1525–47. doi: 10.1023/a:1024262411676. [DOI] [PubMed] [Google Scholar]
- 11.Brunet de Courrsou L. Study Group Report on Buruli Ulcer Treatment with Clay.. 5th WHO Advisory Group Meeting on Buruli Ulcer; Geneva, Switzerland. 2002. [Google Scholar]
- 12.van der Werf TS, van der Graaf WT, Tappero JW, et al. Mycobacterium ulcerans infection. Lancet. 1999;354:1013–8. doi: 10.1016/S0140-6736(99)01156-3. [DOI] [PubMed] [Google Scholar]
- 13.Weir E. Buruli ulcer: the third most common mycobacterial infection. Can Med Assoc J. 2002;166:1691. [PMC free article] [PubMed] [Google Scholar]
- 14.Edelstein H. Mycobacterium marinum skin infections. Report of 31 cases and review of the literature. Arch Intern Med. 1994;154:1359–64. doi: 10.1001/archinte.154.12.1359. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards . Performance standards for antimicrobial susceptibility testing–14th edition: Approved standard M100-S15. NCCLS; Wayne, PA, USA: 2002. [Google Scholar]
- 16.Aubry A, Jarlier V, Escolano S, et al. Antibiotic susceptibility pattern of Mycobacterium marinum. Antimicrob Agents Chemother. 2000;44:3133–6. doi: 10.1128/aac.44.11.3133-3136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson ML. Soil chemical analysis--Advanced course. University of Wisconsin; Madison, WI: 1974. [Google Scholar]
- 18.Moore DM, Reynolds RC. X-ray diffraction and the identification and analysis of clay minerals. 2nd edition. Oxford University Press; New York: 1997. [Google Scholar]
- 19.Frost RL, Ruan H, Kloprogge JT, et al. Dehydration and dehydroxylation of nontronites and ferruginous smectite. Thermochimica Acta. 2000;346:63–72. [Google Scholar]
- 20.Giese RF. Differential scanning calorimetry of clay minerals and their intercalates. In: Stucki JW, Bish DL, editors. Thermal analysis in clay science. Clay Minerals Society; Chantilly, VA: 1990. pp. 10–48. [Google Scholar]
- 21.Guggenheim S, Koster van Groos AF. Baseline studies of the clay minerals society source clays: thermal analysis. Clay Clay Miner. 2001;49:433–43. [Google Scholar]
- 22.Andersson DI, Levin BR. The biological cost of antibiotic resistance. Curr Opin Microbiol. 1999;2:489–93. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 23.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 24.Diederen BM, Kluytmans JA. The emergence of infections with community-associated methicillin resistant Staphylococcus aureus. J Infect. 2006;52:157–68. doi: 10.1016/j.jinf.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Menichetti F. Current and emerging serious Gram-positive infections. Clin Microbiol Infect. 2005;11:22–8. doi: 10.1111/j.1469-0691.2005.01138.x. [DOI] [PubMed] [Google Scholar]
- 26.Shah PM. The need for new therapeutic agents: what is the pipeline? Clin Microbiol Infect. 2005;11:36–42. doi: 10.1111/j.1469-0691.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma R, Sharma CL, Kapoor B. Antibacterial resistance: current problems and possible solutions. Indian J Med Sci. 2005;59:120–9. [PubMed] [Google Scholar]
- 28.Zetola N, Francis JS, Nuermberger EL, et al. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–86. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 29.Brindley GW, Brown GC. Crystal structures of clay minerals and their X-ray identification. Mineralogical Society; London: 1980. [Google Scholar]
- 30.Chaerun SK, Tazaki K, Asada R, et al. Interaction between clay minerals and hydrocarbon-utilizing indigenous microorganisms in high concentrations of heavy oil: implications for bioremediation. Clay Miner. 2005;40:105–14. [Google Scholar]
- 31.Kostka JE, Dalton DD, Skelton H, et al. Growth of iron(III)-reducing bacteria on clay minerals as the sole electron acceptor and comparison of growth yields on a variety of oxidized iron forms. Appl Environ Microbiol. 2002;68:6256–62. doi: 10.1128/AEM.68.12.6256-6262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bitton G, Henis Y, Lahav N. Effect of several clay minerals and humic acid on the survival of Klebsiella aerogenes exposed to ultraviolet irradiation. Appl Microbiol. 1972;23:870–4. doi: 10.1128/am.23.5.870-874.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lünsdorf H, Erb RW, Abraham WR, et al. 'Clay hutches': a novel interaction between bacteria and clay minerals. Environ Microbiol. 2000;2:161–8. doi: 10.1046/j.1462-2920.2000.00086.x. [DOI] [PubMed] [Google Scholar]
- 34.Adams LK, Lyon DY, McIntosh A, et al. Comparative toxicity of nano-scale TiO2, SiO2 and ZnO water suspensions. Water Sci Technol. 2006;54:327–34. doi: 10.2166/wst.2006.891. [DOI] [PubMed] [Google Scholar]
- 35.Cuzic S, Hartmann RK. Studies on Escherichia coli RNase P RNA with Zn2+ as the catalytic cofactor. Nucleic Acids Res. 2005;33:2464–74. doi: 10.1093/nar/gki540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estrela C, Sydney GB, Bammann LL, et al. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J. 1994;6:85–90. [PubMed] [Google Scholar]
- 37.Snijder HJ, Dijkstra BW. Bacterial phospholipase A: structure and function of an integral membrane phospholipase. Biochim Biophys Acta. 2000;1488:91–101. doi: 10.1016/s1388-1981(00)00113-x. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya T, Rosen BP. Characterization of an active transport system for calcium in inverted membrane vesicles of Escherichia coli. J Biol Chem. 1975;250:7687–92. [PubMed] [Google Scholar]
- 39.Wackett LP, Dodge AG, Ellis LB. Microbial genomics and the periodic table. Appl Environ Microbiol. 2004;70:647–55. doi: 10.1128/AEM.70.2.647-655.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stotzky G. Influence of soil mineral colloids on metabolic processes, growth, adhesion, and ecology of microbes and viruses. In: Huang PM, Schnitzer M, editors. Interactions of soil minerals with natural organics and microbes. Soil Society of America, Inc.; Wisconsin: 1986. pp. 305–427. [Google Scholar]
- 41.Stucki JW, Bailey GW, Gan H. Oxidation-reduction mechanisms of iron-bearing phyllosilicates. Appl Clay Sci. 1996;10:417–30. [Google Scholar]
- 42.Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–9. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 43.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–2. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 44.Beauchamp C, Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970;245:4641–6. [PubMed] [Google Scholar]
- 45.McCord JM, Day ED., Jr. Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978;86:139–42. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- 46.Moody CS, Hassan HM. Mutagenicity of oxygen free radicals. Proc Natl Acad Sci USA. 1982;79:2855–9. doi: 10.1073/pnas.79.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenberg JT, Demple B. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR-mutants. EMBO J. 1988;7:2611–7. doi: 10.1002/j.1460-2075.1988.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storz G, Christman MF, Sies H, et al. Spontaneous mutagenesis and oxidative damage to DNA in Salmonella typhimurium. Proc Natl Acad Sci USA. 1987;84:8917–21. doi: 10.1073/pnas.84.24.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.HaMai D, Bondy SC, Becaria A, et al. The chemistry of transition metals in relation to their potential role in neurodegenerative processes. Curr Top Med Chem. 2001;1:541–51. doi: 10.2174/1568026013394796. [DOI] [PubMed] [Google Scholar]
- 50.Keyer KImlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci USA. 1996;93:13635–40. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Touati D. Iron and oxidative stress in bacteria. Arch Biochem Biophys. 2000;373:1–6. doi: 10.1006/abbi.1999.1518. [DOI] [PubMed] [Google Scholar]
- 52.Kohanski MA, Dwyer DJ, Hayete B, et al. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]