Abstract
Twist1 is a bHLH transcription factor that regulates cell proliferation, migration, and differentiation in embryonic progenitor cell populations and transformed tumor cells. While much is known about Twist1’s function in a variety of mesenchymal cell types, the role of Twist1 in endocardial cushion cells is unknown. Twist1 gain and loss of function experiments were performed in primary chicken endocardial cushion cells in order to elucidate its role in endocardial cushion development. These studies indicate that Twist1 can induce endocardial cushion cell proliferation as well as promote endocardial cushion cell migration. Furthermore, Twist1 is subject to BMP regulation and can induce expression of cell migration marker genes including Periostin, Cadherin 11, and Mmp2 while repressing markers of valve cell differentiation including Aggrecan. Previously, Tbx20 has been implicated in endocardial cushion cell proliferation and differentiation, and in the current study, Tbx20 also promotes cushion cell migration. Twist1 can induce Tbx20 expression, while Tbx20 does not affect Twist1 expression. Taken together, these data indicate a role for Twist1 upstream of Tbx20 in promoting cell proliferation and migration and repressing differentiation in endocardial cushion cells during embryonic development.
Keywords: Twist1, Tbx20, endocardial cushion development, Cadherin 11, Periostin, Mmp2, Aggrecan, Versican, cell proliferation, cell migration, siRNA, chicken
Introduction
Heart valve development is characterized by the activity of complex regulatory pathways, several of which have also been associated with adult valve disease (Bartram et al., 2001; Cripe et al., 2004; Garg et al., 2005). The onset of heart valve development is marked by the appearance of endocardial cushions in the atrioventricular (AV) canal and outflow tract of the looped heart tube (Armstrong and Bischoff, 2004). Cushion development is initiated by signaling events originating in the myocardium that cause endocardial cells to undergo an epithelial to mesenchymal transformation (EMT) and migrate into the intervening cardiac jelly (Barnett and Desgrosellier, 2003). The resulting endocardial cushions are made up of highly proliferative, migratory, undifferentiated mesenchymal cells embedded in a loose extracellular matrix (Armstrong and Bischoff, 2004; Hinton et al., 2006; Lincoln et al., 2006; Person et al., 2005; Schroeder et al., 2003; Shelton and Yutzey, 2007). As endocardial cushions remodel into mature valve leaflets, the valvular interstitial cells become less proliferative, more compartmentalized into distinct regions of the valve, and more differentiated (Hinton et al., 2006). In addition, the extracellular matrix of the valves becomes highly organized and stratified into three distinct layers (Hinton et al., 2006; Lincoln et al., 2006; Rabkin-Aikawa et al., 2005). While much is known about the events that initiate endocardial cushion development, relatively little is known about the molecular mechanisms that govern the transition from primitive endocardial cushions to mature valve leaflets.
Twist1 is a basic helix-loop-helix (bHLH) transcription factor that was first identified in Drosophila as a critical regulator of mesoderm formation (Thisse et al., 1988). Previous studies have identified roles for Twist1 in migration, differentiation, and proliferation of mesenchymal cell populations. In Twist deficient mouse models, loss of mTwist results in abnormal limb development, failure of the neural tube to close, hypoplastic branchial arches, somite abnormalities, and lethality by embryonic day 11.5 (Chen and Behringer, 1995; O'Rourke et al., 2002; Soo et al., 2002; Zuniga et al., 2002). Heterozygous hTWIST mutations in human patients have been linked to Saethre-Chotzen syndrome, a disease characterized by craniofacial abnormalities, skeletal anomalies, and limb defects (Bourgeois et al., 1998; Reardon and Winter, 1994; Zackai and Stolle, 1998). In the heart, Twist1 is expressed in the mesenchyme of developing endocardial cushions (Ma et al., 2005). However, the function of Twist1 in heart valve development has not been reported.
Tbx20, a T-box transcription factor, is highly expressed in developing endocardial cushions and continues to be expressed at lower levels in the mature mitral and tricuspid valves (Plageman and Yutzey, 2004; Shelton and Yutzey, 2007; Stennard et al., 2003; Yamagishi et al., 2004). Mutant mice with loss of Tbx20 function have hypoplastic myocardium, chamber maturation defects, and are embryonic lethal prior to the onset of endocardial cushion development (Cai et al., 2005; Singh et al., 2005; Stennard et al., 2005; Takeuchi et al., 2005). Mutations in human TBX20 are associated with defects in septation, chamber growth, and valvulogenesis (Kirk et al., 2007). In avian endocardial cushion cells, Tbx20 can promote cell proliferation and N-myc gene expression, as has also been demonstrated in maturing myocardium (Cai et al., 2005; Shelton and Yutzey, 2007). In addition, Tbx20 can promote an immature extracellular matrix in developing cushions by inducing the expression of matrix remodeling enzymes like Mmp9 and Mmp13 and repressing the expression of Aggrecan (Agg) and Versican (Vers), chondroitin sulfate proteoglycans that mark mature stratified extracellular matrix (Shelton and Yutzey, 2007). Overall, Tbx20 functions to maintain proliferative, undifferentiated, mesenchymal cushions during valve development.
Endocardial cushions are characterized by highly proliferative mesenchymal cells in a loosely organized extracellular matrix. Studies performed in chicken and mice show that endocardial cushion cells are approximately 6 times more proliferative than cells in remodeling valve leaflets (Hinton et al., 2006; Lincoln et al., 2004). Another hallmark of developing endocardial cushions is the migratory nature of the mesenchymal cells that make up the cushions. At the onset of cushion development, endothelial cells transform into migratory mesenchymal cells by becoming activated and losing cell-cell contact (Eisenberg and Markwald, 1995; Markwald et al., 1977). A role for Twist1 and Tbx20 in this aspect of endocardial cushion development has not been previously demonstrated. However, Twist1 promotes cell migration in tumor metastasis and cranial neural crest cells (Chen and Behringer, 1995; Soo et al., 2002; Yang et al., 2004), and Tbx20 promotes cell migration in cranial motor neuron cell bodies (Song et al., 2006). Cell migration is facilitated by cell adhesion proteins, matricellular factors, and remodeling enzymes including Periostin (Postn), Cadherin11 (Cad11), and Matrix Metalloproteinase 2 (Mmp2). The roles of Twist1 in regulating these migration markers or proliferation and differentiation of endocardial cushion cells have not been reported.
To investigate the role of Twist1 in the transition from endocardial cushion to remodeling valve, its expression was compared to known markers of cell proliferation, migration, and maturation in avian endocardial cushions and remodeling valves. Twist1, Tbx20, Cad11, Postn, and Mmp2 are all expressed at higher levels in endocardial cushions relative to remodeling valve leaflets. Additionally, a primary chicken endocardial cushion culture system was used to determine the role of Twist1 in cushion cell proliferation and migration. Like Tbx20, Twist1 can induce cell proliferation in endocardial cushion cells. Moreover, both Twist1 and Tbx20 can promote endocardial cushion cell migration. Furthermore, Twist1 can affect the expression of cell migration and differentiation marker genes including Cad11, Postn, Mmp2, and Agg. Finally, it was determined that Twist1 can induce the expression of Tbx20, but Tbx20 does not affect the expression of Twist1. Taken together, these studies are consistent with Twist1 acting upstream of Tbx20 to regulate aspects of endocardial cushion cell proliferation, migration, and differentiation.
Materials and methods
Chicken embryo collection
Fertilized white leghorn chicken eggs (CBT Farms, MD) were incubated at 38°C under high humidity. Embryos were collected at Hamburger Hamilton (HH) stages 25 and 36 corresponding to embryonic days 5 and 10, respectively (Hamburger and Hamilton, 1951). For histology, hearts were dissected in 1× phosphate-buffered saline (PBS) and fixed for 2 h in 4% paraformaldehyde/PBS. After fixation, embryonic tissue was dehydrated in a graded ethanol/water series (25%, 50%, 75%, 95%, 100%) and washed in xylene before being embedded in paraplast (Sigma-Aldrich) for further processing. All animal procedures were approved and performed in accordance with institutional guidelines.
In situ hybridizations
Chicken Twist1 sequence (753 bp; Genbank accession number NM 204739.1) was amplified from HH stage 30 wing cDNA using the primers 5’-GCAAGATCCAGACCCTCAAG-3’ and 5’-CTCCTCAGTGGCTCATAGGC-3’. Chicken Tbx20 sequence (820 bp; Genbank accession number AB070544) was amplified from HH stage 20 heart cDNA as previously reported (Iio et al., 2001; Plageman and Yutzey, 2004; Shelton and Yutzey, 2007). Chicken Cadherin 11 sequence (685 bp; Genbank accession number AF055342) was amplified from HH stage 34 heart cDNA using the primers 5′-AGAGCTGAAGCACGGGATAA-3′ and 5′-GCTTGTGCCGTGAGAGTGTA-3′ Chicken Periostin sequence (1001 bp; Genbank accession number NM 001030541) was amplified from HH stage 37 heart cDNA using the primers 5′-TAATGCTCTCCACCACCACA-3′ and 5′-TCTGCTGGCTTGATGATTTG -3′. Chicken Mmp2 sequence (604 bp; Genbank accession number NM 204420) was amplified from HH stage 34 heart cDNA using the primers 5’ -TGGAGGAGACTCCCATTTTG- 3’ and 5’ - GGCAGCAACCAAGAAGAGAC- 3’.
To ensure identity and specificity, all sequences were amplified by reverse transcriptase polymerase chain reaction (RT-PCR), subcloned into pGEM T-vector (Promega), and confirmed by sequencing. For each sequence, digoxigenin (DIG)-labeled antisense RNA probes were generated as previously reported (Ehrman and Yutzey, 1999; Shelton and Yutzey, 2007) with the following modifications. The Twist1, Cadherin 11, and Mmp2 probes were synthesized with SP6 polymerase from plasmids linearized with Nco I. The Periostin probe was synthesized with SP6 polymerase from a plasmid linearized with Sac II. In situ hybridization of tissue sections was performed as previously described (Shelton and Yutzey, 2007; Somi et al., 2004). Briefly, 14µm paraffin embedded chicken heart sections were mounted on Superfrost Plus microscope slides (Fisher Scientific). Sections were deparaffinized in xylene, rehydrated through an ethanol/distilled water series, and then rinsed in 1 × PBS. Sections were treated with 20µg/ml proteinase K/PBS for 6 min at 37°C. 170µl of 0.5µg/ml DIG-labeled riboprobe was used to carry out hybridizations at 70°C. Color reactions using nitroblue tetrazolium/ 5-bromo-4-chloro-3-indolyl phosphate (Roche) were allowed to develop for 8 h.
RT-PCR analysis of gene expression
Total RNA was isolated from cultures of 12 AV endocardial cushions per experimental group or 6 chicken AV canals at HH stages 25 and 36 using Trizol reagent, and cDNA was generated using SuperScript II as previously described (Shelton and Yutzey, 2007). 1µl cDNA was used for analysis by quantitative real time RT-PCR (MJ Research Opticon 2). RT-PCR reactions were performed using 20 pmol of the following primers: Twist1 (170 bp) 5’-TTCCGAATTTGCCTGTTTTT-3’ and 5’-GTTGGGTGCTTTGCTTTCAT-3’, Cadherin 11 (236 bp) 5’-GTGCCGGAGAGATCAAATGT-3’ and 5’-CCCATGTGTCCTCCCATATC-3’, Periostin (271 bp) 5’-GTGCTGTCCTGGCTACATGA-3’ and 5’-TGTGGTGGTGGAGAGCATTA-3’, Mmp2 (348 bp) 5’-CATGCGATGGGATTAGAGCA-3’ and 5’-TCATCCTGAGGGGACTCGTAG-3’. The primers used for Tbx20, Aggrecan, and GAPDH have been previously reported (Shelton and Yutzey, 2007). The identity of amplified bands was confirmed by sequencing. Gene expression levels were quantified as previously reported (Shelton and Yutzey, 2007), with the following modifications. The PCR reactions were performed as follows: 95°C, 30 sec; 50°C, 30 sec; 72°C, 30 sec; 35 cycles (Twist1), 95°C, 30 sec; 55°C, 30 sec; 72°C, 30 sec; 35 cycles (Tbx20, Mmp2), 95°C, 30 sec; 60°C, 30 sec; 72°C, 30 sec; 35 cycles (Cad11), and 95°C, 30 sec; 66°C, 30 sec; 72°C, 30 sec; 35 cycles (Postn). Fluorescence was monitored for each cycle at 72°C and gene expression levels were quantified based on a threshold cycle of detection for each amplified product calibrated to a standard curve generated for each primer pair. Each standard curve was generated using HH stage 34 whole heart cDNA and all values were normalized to GAPDH expression. Real time RT-PCR results represent four independent experiments (n=4) with reactions performed in duplicate. The calculated fold change in gene expression was determined by dividing the experimental value by the control value, which was then set to 1. Statistical significance of observed differences was calculated using a Student’s t-test (P<0.05–0.01).
Endocardial cushion cell culture
Embryonic chicken hearts were collected at HH stage 25 and pre-fused AV endocardial cushions were dissected away from the surrounding myocardium using tungsten needles. Endocardial cushions were dissected and cultured as previously reported (Shelton and Yutzey, 2007). For some experiments, recombinant human BMP2 or recombinant human Noggin was added to the culture media as previously described (Shelton and Yutzey, 2007). Cell proliferation was assessed by Bromodeoxyuridine (BrdU) incorporation as previously described (Shelton and Yutzey, 2007). Briefly, BrdU positive nuclei were identified by immunohistochemistry using a BrdU detection kit (Zymed). A 1:100 dilution of the BrdU labeling reagent in culture media was incubated with endocardial cushion cells for 1.5 h prior to fixation in 70% ethanol for 15 min at 4°C. A biotinylated mouse anti-BrdU primary antibody was used followed by a streptavadin-peroxidase conjugated secondary antibody and colorimetric detection with diaminobenzidine (DAB). Cells were then counterstained with hematoxylin. The percent of proliferating cells was calculated by dividing the number of BrdU-labeled nuclei by the total number of nuclei per microscopic field. At least 75 cells per field were counted for each treatment group. Statistical significance of observed differences was determined by Student’s t-test (P<0.01).
Recombinant adenovirus
The previously described recombinant adenovirus containing myc-epitope-tagged murine Twist1 (AdTwist1) was generously provided by M. Naski (Reinhold et al., 2006). A recombinant adenovirus containing the full length coding region of murine Tbx20 (AdTbx20) was generated as previously reported (Plageman and Yutzey, 2004; Shelton and Yutzey, 2007). After 24 h in culture, endocardial cushion cells were infected with 108 plaque forming units of the AdTwist1 virus, AdTbx20 virus, or a control virus that expressed β-galactosidase (Adβ-gal) contained in serum free media (1×M199 (Invitrogen)) as previously described (Shelton and Yutzey, 2007). Infection efficiency was determined to be greater than 90% as measured by staining with 1mg/ml X-gal(Amresco) following infection with Adβ-gal. In parallel cultures, increased expression of the murine Twist1 protein was confirmed with immunohistochemistry using a Twist1 specific antibody (Sigma) and the murine Tbx20 viral transcript was confirmed using RT-PCR.
Twist1 and Tbx20 siRNA
Two 19 nucleotide RNA duplexes corresponding to the chicken Twist1 sequence (Genbank accession number NM 204739.1) were designed and generated using BLOCK-iT™ RNAi Designer (Invitrogen). The Twist1 siRNA oligonucleotides 5’-GCAAGAUCCAGACCCUCAdTdT-3’ and 5’-UUGAGGGUCUGGAUCUUGCdTdT-3’ and 5’-CCUUCUCGGUGUGGAGAAUdTdT-3’ and 5’-AUUCUCCACACCGAGAAGGdTdT-3’ were obtained from Invitrogen. A scrambled control oligo was also generated with sequence 5’-CCGGUAAUGACACCCAAUUdTdT-3’ and 5’-AAUUGGGUGUCAUUACCGGdTdT-3’. The two Twist1-specific siRNAs were combined and a final siRNA concentration of 200nM was used with Lipofectamine 2000 (Invitrogen) to transfect cultured endocardial cushion cells as described by the manufacturer’s protocol. In addition, a 19 nucleotide RNA duplex corresponding to the chicken Tbx20 sequence (Genbank accession number AB070544) was designed and transfected as previously reported (Shelton and Yutzey, 2007). To measure the transfection efficiency, the BLOCK-iT™ Fluorescent Oligo (Invitrogen) was co-transfected along with siRNA oligos as previously reported (Shelton and Yutzey, 2007). The percent of positively transfected cells was calculated by dividing the number of fluorescently labeled nuclei by the number of total nuclei per microscopic field. The transfection efficiency was consistently greater than 75%. In three independent experiments (n=3), a total of 10 fields containing at least 75 cells per field were counted for each treatment group. The loss of Twist1 and Tbx20 mRNA expression was determined using real time RT-PCR, and loss of Twist1 and Tbx20 protein expression was confirmed by immunohistochemistry.
Immunohistochemistry
Endocardial cushion cell cultures were fixed with 4% paraformaldehyde/PBS for 30 min, washed three times in PBS/0.1%Tween 20, and treated with 3% hydrogen peroxide/PBS for 30 min. Immunohistochemistry was performed using ABC peroxidase staining kit (Pierce) according to the manufacturer’s protocol. A rabbit polyclonal antibody directed against Twist1 (Sigma) was used at a 1:800 dilution in a goat serum/PBS blocking solution (Pierce). A rabbit polyclonal antibody directed against Tbx20 (Orbigen) was used at a 1:200 dilution in blocking solution. All primary antibodies were incubated overnight at 4°C. Detection of antibody reactivity was visualized using DAB substrate (Pierce).
Cell Migration Assay
The ability of endocardial cushion cells to migrate was assayed as previously reported (Yang et al., 2006b), with the following modifications. Embryonic chicken hearts were collected at HH stage 25, and pre-fused endocardial cushions were dissected away from the surrounding myocardium and cultured as previously reported (Shelton and Yutzey, 2007). After 48 h in culture, the endocardial cushion cells were either infected with adenovirus or transfected with siRNA. Cells were incubated at 37°C for an additional 48 h and then treated with 200µl 1×trypsin-EDTA (Invitrogen) for 5 min at 37°C to detach them from the chamber slide. The cell suspensions were collected and passed through a 25G1 1/2 needle 3 times to break up cell clusters. Cells from each experimental group were counted using a hemocytometer and resuspended in supplemented media (10% fetal bovine serum, 1% penicillin/streptomycin, 1×M199 (Invitrogen)) at 1×105 cells/ml suspension. 400µl of each cell suspension was added to the top chamber of a modified Boyden chamber culture plate insert (12mm diameter, 8µm pores; Millicell) that was placed in a well of a 24 well tissue culture plate (Becton Dickinson) containing 600µl supplemented media. The tissue culture plate was incubated for 4 h at 37°C after which, cells that did not migrate through the pores of the culture plate insert were removed using a cotton swab. The remaining cells that did migrate through the pores and were adherent to the bottom of the insert were fixed in 100% MeOH for 10 min, stained with Giemsa Stain (Sigma-Aldrich), as described by the manufacturer’s protocol, and rinsed in water. The fold change in migration was calculated by dividing the number of migrated cells in the experimental groups by the number of migrated cells in the control group per microscopic field. The control value was then set to 1. In three independent experiments, a total of 10 fields containing 20–600 cells, depending on the treatment group, were counted. Significance of observed differences was determined by applying a Student’s t-test (P<0.01).
Results
Twist1 and Tbx20 are coordinately expressed with cell migration marker genes in endocardial cushions and remodeling valves
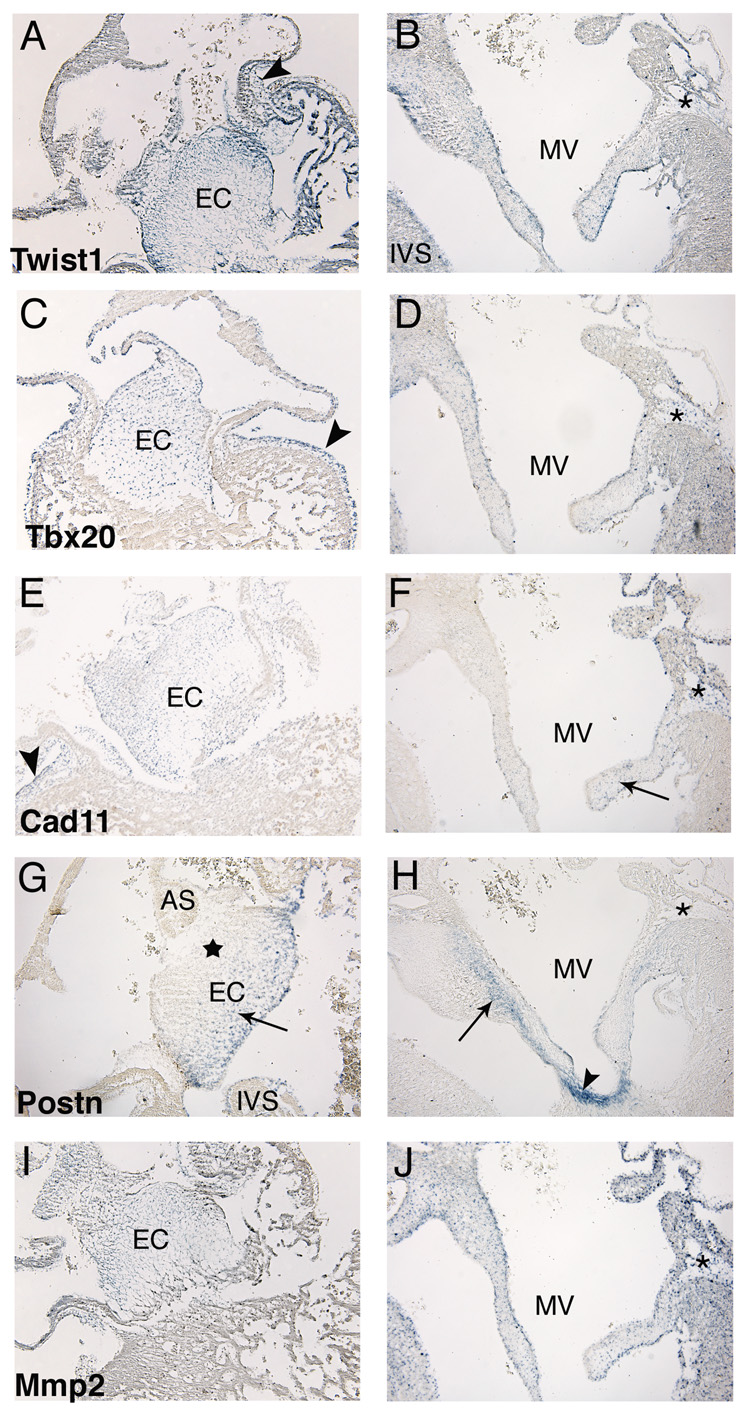
In situ hybridizations were performed on sectioned embryonic chicken hearts to localize the expression of Twist1 and Tbx20 in relation to the cell migration marker genes Cad11, Postn, and Mmp2 during the stages of endocardial cushion formation (HH stage 25) and valve remodeling (HH stage 36) in vivo. At HH stage 25, Twist1 (Fig. 1A), Tbx20 (Fig. 1C), Cad11 (Fig. 1E), and Mmp2 (Fig. 1I) are expressed throughout the entire endocardial cushion, while Postn (Fig. 1G) expression is restricted to the ventricular aspect of the endocardial cushion (arrow) and is absent from the subatrial region of the cushion (star). In addition, Twist1, Tbx20, and Cad11 expression is also evident in early epicardially derived cells (arrowheads in Fig. 1A, C, and E). In HH stage 36 remodeling mitral valves, Twist1 (Fig. 1B), Tbx20 (Fig. 1D), and Mmp2 (Fig. 1J) are expressed at low levels throughout the valve leaflet. In contrast, Cad11 (Fig. 1F) expression is reduced and restricted to the distal tips of the valve leaflet (arrow). In addition, Postn (Fig. 1H) is expressed at the intersection of the valve leaflet and the myocardium (arrow) with robust expression in the chordae tendineae and papillary muscle points of insertion (arrowhead). Furthermore, there was differential expression of these genes in the epicardially derived cells of the mural aspect of the AV junction at HH stage 36. The expression of Twist1, Tbx20, Cad11, and Mmp2 is apparent in this population of fibroblast-like mesenchymal cells (asterisks in Fig. 1B, D, F, and J), while Postn expression is not (asterisk in Fig. 1H). Together these studies show that Twist1, Tbx20, Cad11, and Mmp2 are expressed throughout the endocardial cushion, while Postn is more strongly expressed in the ventricular aspect of cushion. Later in remodeling valves, Twist1, Tbx20, Cad11, and Mmp2 are expressed at relatively lower levels throughout the valve leaflet. Postn expression is absent from the valve leaflet but strongly expressed in the intersection of the leaflet and the myocardium as well as in the chordae tendineae and papillary muscle points of insertion.
Figure 1. Twist1 and Tbx20 are coordinately expressed with cell migration marker genes in endocardial cushions and remodeling valves.
Expression of Twist1, Tbx20, Cad11, Postn, and Mmp2 was examined in sectioned HH stage 25 and HH stage 36 chicken hearts. In situ hybridizations show Twist1 (A), Tbx20 (C), Cad11 (E), and Mmp2 (I) are expressed throughout the entire endocardial cushion. In addition, Twist1, Tbx20, and Cad11 expression was also detected in early epicardially derived cells (arrowheads in panel A, C, and E). In contrast, Postn was more strongly expressed in the ventricular aspect of the cushion near the intreventricular septum (arrow in panel G) and is absent from the subatrial region of the cushion (star in panel G). In remodeling mitral valves, Twist1 (B), Tbx20 (D), and Mmp2 (J) are expressed weakly throughout the entire valve leaflet and in epicardially derived cells (asterisks in panels B, D, and J). In contrast, Cad11 expression is weak and restricted to the distal tips of the valve (arrow in panel F) but is also expressed in the epicardially derived cells (asterisk in panel F). Postn is expressed at the intersection of the valve leaflet and the myocardium (arrow in panel H) with increased expression in the chordae tendineae and the papillary muscle points of insertion (arrowhead in panel H). In contrast, Postn expression is absent in epicardially derived cells (asterisk in panel H). EC, endocardial cushion; MV, mitral valve; IVS, interventricular septum; AS, atrial septum.
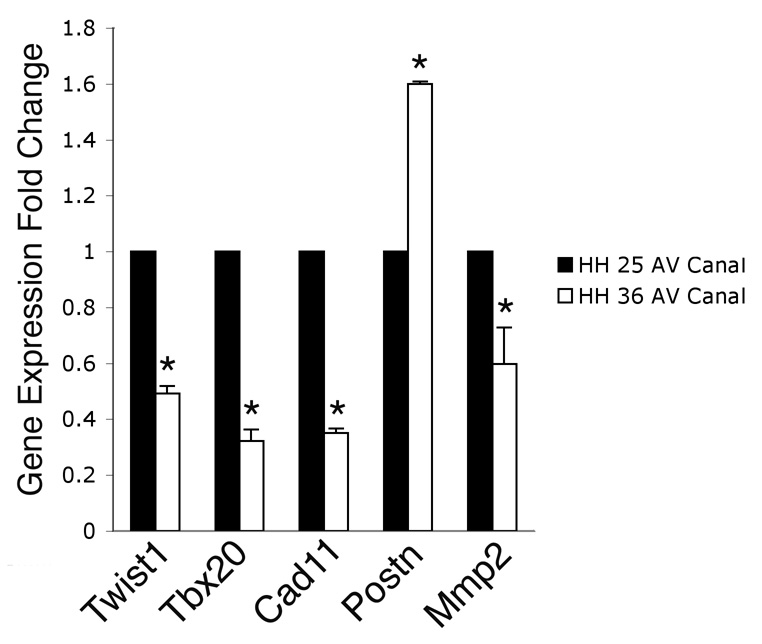
Twist1, Tbx20, Cad11, Postn, and Mmp2 expression was further quantified using RNA isolated from the AV canals of HH stage 25 and HH stage 36 chicken embryos. The expression of these genes was measured using quantitative real time RT-PCR (Fig. 2). At the endocardial cushion stage (HH stage 25), the expression of Twist1 is approximately 2-fold higher than at the remodeling valve stage (HH stage 36). Similarly, the expression of Tbx20 and Cad11 is approximately 3-fold higher in endocardial cushions relative to remodeling valves, while the expression of Mmp2 is approximately 2.5-fold higher at HH stage 25 than at HH stage 36. In contrast, the expression of Postn is 60% higher at the remodeling valve stage compared to the endocardial cushion stage. This is likely due to increased Postn expression apparent in the valve supporting structures. However, Postn expression appears to be down-regulated in the valve leaflets as determined by insitu hybridization (Fig.1H). Taken together, the insitu hybridization and real time RT-PCR data demonstrate that in vivo, Twist1 and Tbx20 are coordinately expressed at relatively high levels in endocardial cushions with the migration marker genes Cad11, Postn, and Mmp2. Furthermore, these genes remain coordinately expressed, but at relatively lower levels in remodeling valve leaflets.
Figure 2. Differential Expression of Twist1, Tbx20, and cell migration marker genes during endocardial cushion and remodeling valve stages.
Expression of Twist1, Tbx20, Cad11, Postn, and Mmp2 was examined in isolated AV canals from HH stage 25 or HH stage 36 chicken embryos using real time RT-PCR. The expression of Twist1, Tbx20, Cad11, and Mmp2 was decreased at HH stage 36 compared to the expression at HH stage 25. In contrast, the expression of Postn was increased at HH stage 36 compared to the expression at HH stage 25 consistent with increased expression in valve supporting structures. These data are representative of 4 independent real time RT-PCR experiments performed in duplicate (n=4). Statistical significance of observed differences between gene expression levels at HH stage 25 and HH stage 36 is indicated by an asterisk (P<0.05) and error bars represent standard error of the mean.
Twist1 promotes endocardial cushion cell proliferation
One of the hallmarks of heart valve development is the transition from highly proliferative endocardial cushions to less proliferative remodeling valves (Hinton et al., 2006; Lincoln et al., 2004). Twist1 has been shown to regulate cell proliferation in skull mesenchyme, somites, and branchial arches (Ishii et al., 2003; Ota et al., 2004). To determine if Twist1 plays a similar role in endocardial cushion cell proliferation, Twist1 gain and loss of function studies were performed and cell proliferation was assessed. Primary endocardial cushions free of myocardial contamination were removed from the AV canals of HH stage 25 chicken embryos and cultured as previously reported (Shelton and Yutzey, 2007).
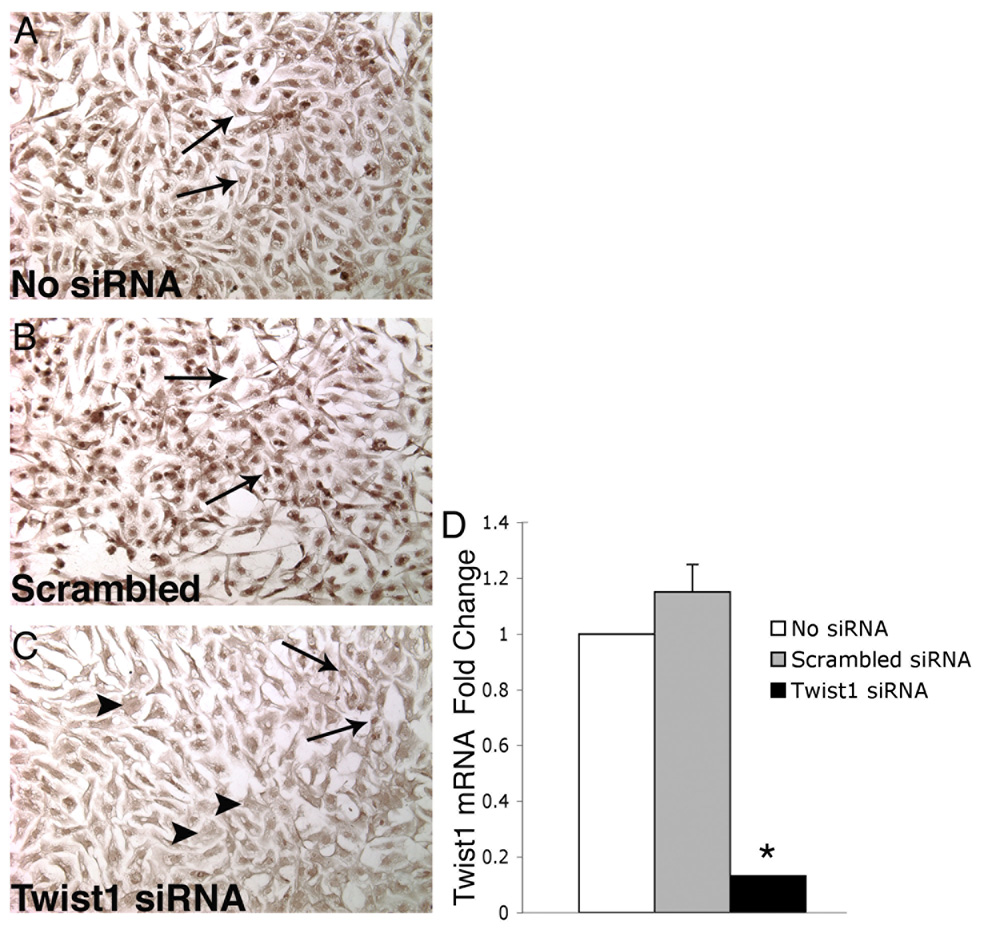
To achieve gain of Twist1 function, primary endocardial cushion cells were infected with an adenovirus that expresses murine Twist1 (AdTwist1) (Reinhold et al., 2006) or a control adenovirus that expresses β-gal (Adβ-gal). To achieve endogenous Twist1 loss of function, Twist1-specific siRNA was transfected into primary chicken endocardial cushion cells. Scrambled control siRNA was also transfected in parallel experiments. The transfection efficiency was determined to be greater than 70% (data not shown). Knockdown of Twist1 protein expression was determined using immunohistochemistry with a Twist1-specific antibody (Fig. 3A, B, C). Cells transfected with Twist1-specific siRNA (Fig. 3C) had significantly reduced nuclear staining compared to cells transfected with the scrambled control siRNA (Fig. 3B) or untransfected controls (Fig. 3A), although there were a small number of positively stained nuclei in the Twist1-specific siRNA transfected group (arrows in Fig. 3C). Additionally, transfection of Twist1-specific siRNA resulted in a greater than 80% reduction in Twist1 mRNA relative to untransfected and scrambled siRNA controls as determined by real time RT-PCR (Fig. 3D). These data indicate that primary chicken endocardial cushion cells can efficiently be transfected with sequence-specific siRNA to produce a significant loss of Twist1 function.
Figure 3. Twist1-specific siRNA transfected into primary chicken endocardial cushion cells results in decreased Twist1 protein and mRNA expression.
Twist1 protein expression was examined by immunohistochemistry with an antibody specific for Twist1 in parallel cultures of siRNA-transfected cells (A–C). Cells transfected with Twist1-specific siRNA (C) had significantly reduced nuclear staining (arrowheads) compared to controls (A, B, arrows indicate Twist1 immunopositive cells). In Twist1-specific siRNA transfected cultures, a small subset of cells remained Twist1 positive (arrows in panel C). Twist1 mRNA expression was quantified using real time RT-PCR (D). Cells transfected with Twist1-specific siRNA had an 80% reduction in Twist1 mRNA relative to untransfected and scrambled siRNA controls. The asterisk indicates a statistically significant difference from the control value and error bars represent standard error of the mean (n=3, P<0.01).
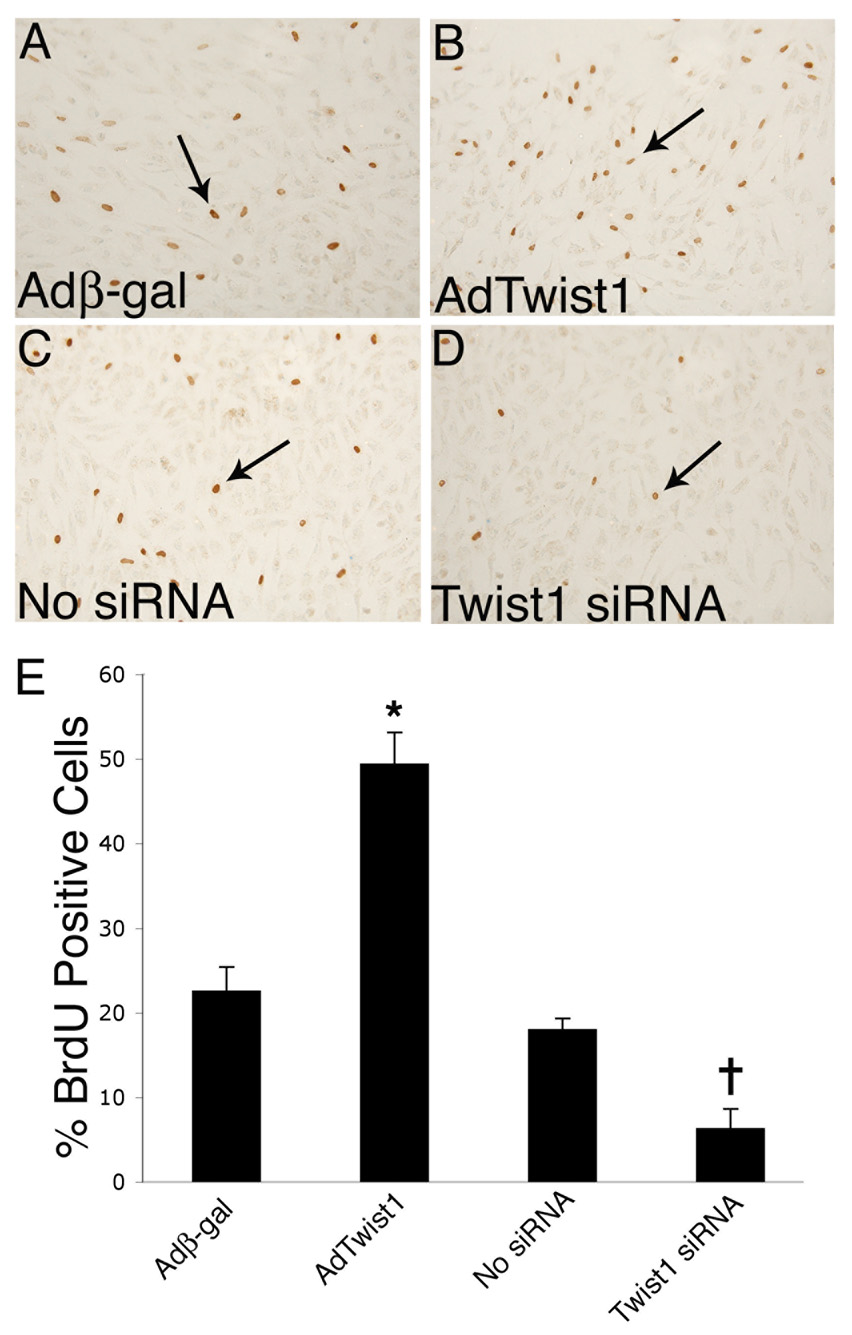
Primary endocardial cushion cell cultures were infected with AdTwist1 or Adβ-gal or transfected with Twist1-specific siRNA in order to determine the role of Twist1 in cushion cell proliferation. The number of cells in the S-phase of the cell cycle was measured by BrdU incorporation and used as an indicator of proliferation. The baseline percent of BrdU positive nuclei per total nuclei in control endocardial cushion cells infected with Adβ-gal (Fig. 4A) or untransfected control cells (Fig. 4C) was approximately 20% (Fig. 4E). Cells that were infected with AdTwist1 (Fig. 4B) were significantly more proliferative, with approximately 45% BrdU positive cells (Fig. 4E). In contrast, cells transfected with Twist1-specific siRNA (Fig. 4D) were significantly less proliferative, with approximately 7% BrdU positive cells (Fig. 4E). No significant differences in cell survival were observed for any of the experimental groups (data not shown). Taken together, these data indicate that Twist1 can promote endocardial cushion cell proliferation.
Figure 4. Twist1 promotes endocardial cushion cell proliferation.
Primary endocardial cushion cells were infected with adenoviruses expressing β-gal (Adβ-gal) or Twist1 (AdTwist1) for gain of function or transfected with Twist1-specific siRNA for loss of function. Proliferation was measured using immunohistochemistry for BrdU incorporation. Cells infected with AdTwist1 (B) exhibit increased BrdU incorporation compared to control cells infected with Adβ-gal (A) (arrows indicate positively stained cells). Cells transfected with Twist1-specific siRNA (D) have less BrdU reactivity than untransfected control cells (C). The percent of BrdU positive cells relative to total nuclei in each group is quantified (E). The asterisk represents a statistically significant difference compared to the Adβ-gal control group, while the cross represents a statistically significant difference compared to the no siRNA control group (n=3, P<0.01). Error bars represent standard error of the mean.
Tbx20 and Twist1 promote endocardial cushion cell migration
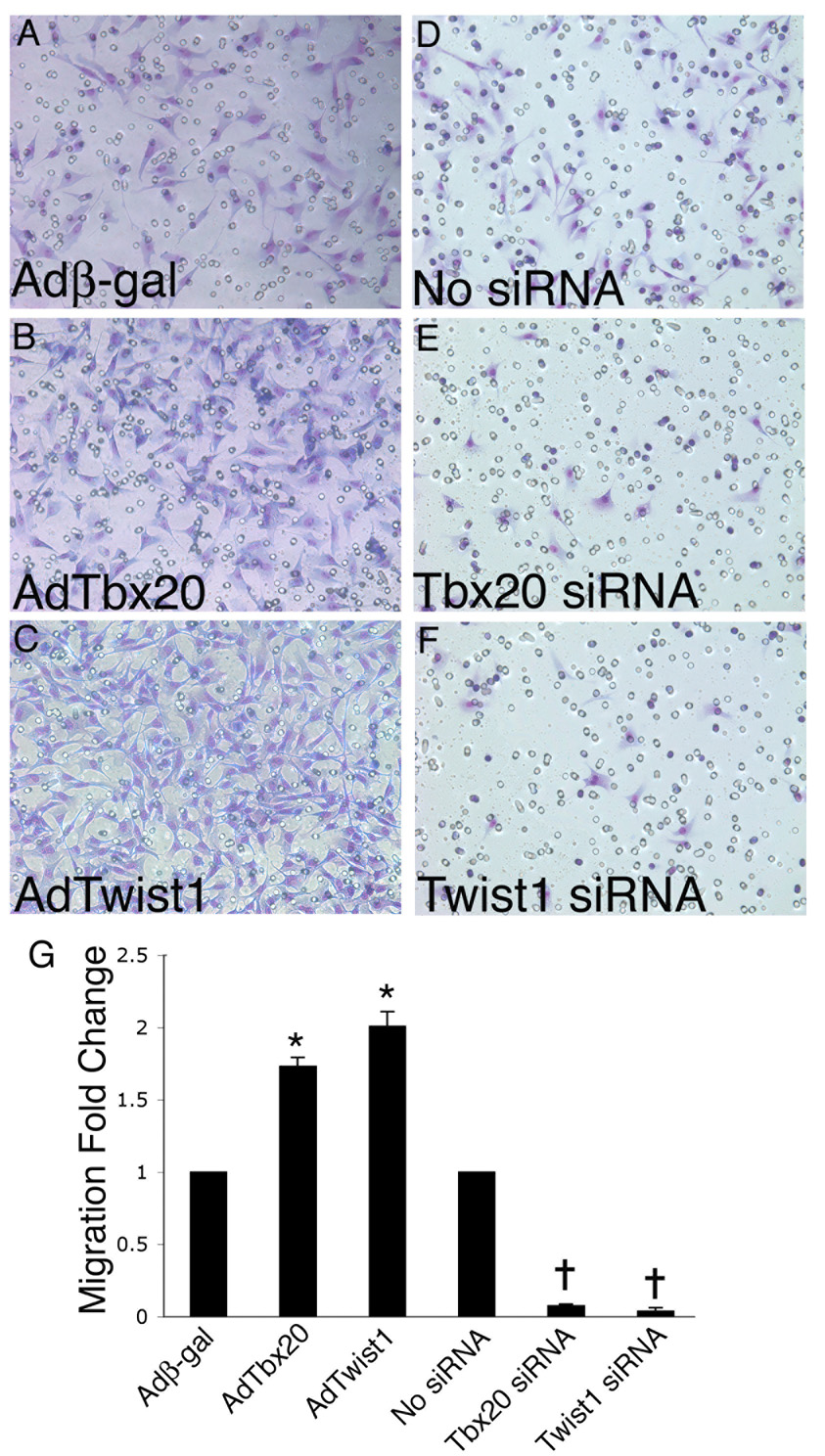
Because Tbx20 and Twist1 have both been shown to regulate cell migration in other systems (Chen and Behringer, 1995; Song et al., 2006; Soo et al., 2002; Yang et al., 2004), their role in endocardial cushion cell migration was investigated. To determine if Tbx20 and Twist1 regulate endocardial cushion cell migration, gain and loss of function experiments were performed and cell migration was assessed. Cultured endocardial cushion cells were infected with murine Tbx20 (AdTbx20), AdTwist1, or Adβ-gal adenoviruses for gain of function studies or transfected with Tbx20-specific or Twist1-specific siRNA for loss of function studies. Equal numbers of cells were then seeded on the upper chamber of a modified Boyden chamber culture plate insert containing a porous (8µm pores) membrane. Cell migration was assessed 4 hours later by counting the number of cells that migrated through the pores to the lower chamber of the insert and were adherent to the membrane. The fold change in the number of cells that migrated through the membrane was calculated by dividing the number of cells adherent to the membrane in the lower chamber for each experimental group by the number of cells adherent to the membrane in the lower chamber for the control group per microscopic field.
Cells infected with AdTbx20 (Fig. 5B) were approximately 70% more migratory (Fig. 5G) than control cells infected with Adβ-gal (Fig. 5A). Similarly, cells infected with AdTwist1 (Fig. 5C) were approximately 2 fold more migratory (Fig. 5G) than control cells infected with Adβ-gal (Fig. 5A). In contrast, cells transfected with Tbx20-specific siRNA (Fig. 5E) or Twist1-specific siRNA (Fig. 5F) were approximately 80% less migratory (Fig. 5G) than untransfected control cells (Fig. 5D). No apparent differences in cell migration were observed when untransfected control cells were compared to scrambled siRNA transfected control cells (data not shown). It should be noted that the length of time these cells were allowed to migrate (4 h) is significantly shorter than the length of time needed for these cells to undergo cell division. Therefore, observed differences can be attributed to changes in the ability of these cells to migrate and not differences in their ability to proliferate. These data are indicative of roles for Tbx20 and Twist1 in promoting endocardial cushion cell migration.
Figure 5. Tbx20 and Twist1 promote endocardial cushion cell migration.
Primary endocardial cushion cells were infected with adenoviruses expressing β-gal (Adβ-gal), Tbx20 (AdTbx20), or Twist1 (AdTwist1) for gain of function or transfected with Tbx20-specific or Twist1-specific siRNA for loss of function. Cells were stained with Giemsa Stain and migration was measured by counting the number of cells that migrated through the pores (visible in panels A–F) of a modified Boyden chamber membrane. The number of cells observed on the underside of the membrane was increased in cultures infected with AdTbx20 (B) or AdTwist1 (C) as compared to control cultures infected with Adβ-gal (A). In addition, the number of cells observed on the underside of the membrane was decreased in cultures transfected with Tbx20-specific siRNA (E) or Twist1-specific siRNA (F) as compared to untransfected control cultures (D). The fold change in the number of cells that migrated through the membrane relative to the number of control cells that migrated is quantified (G). Asterisks represent a statistically significant difference compared to the Adβ-gal control group, while crosses represent a statistically significant difference compared to the no siRNA control group (n=3, P<0.01). Error bars represent standard error of the mean.
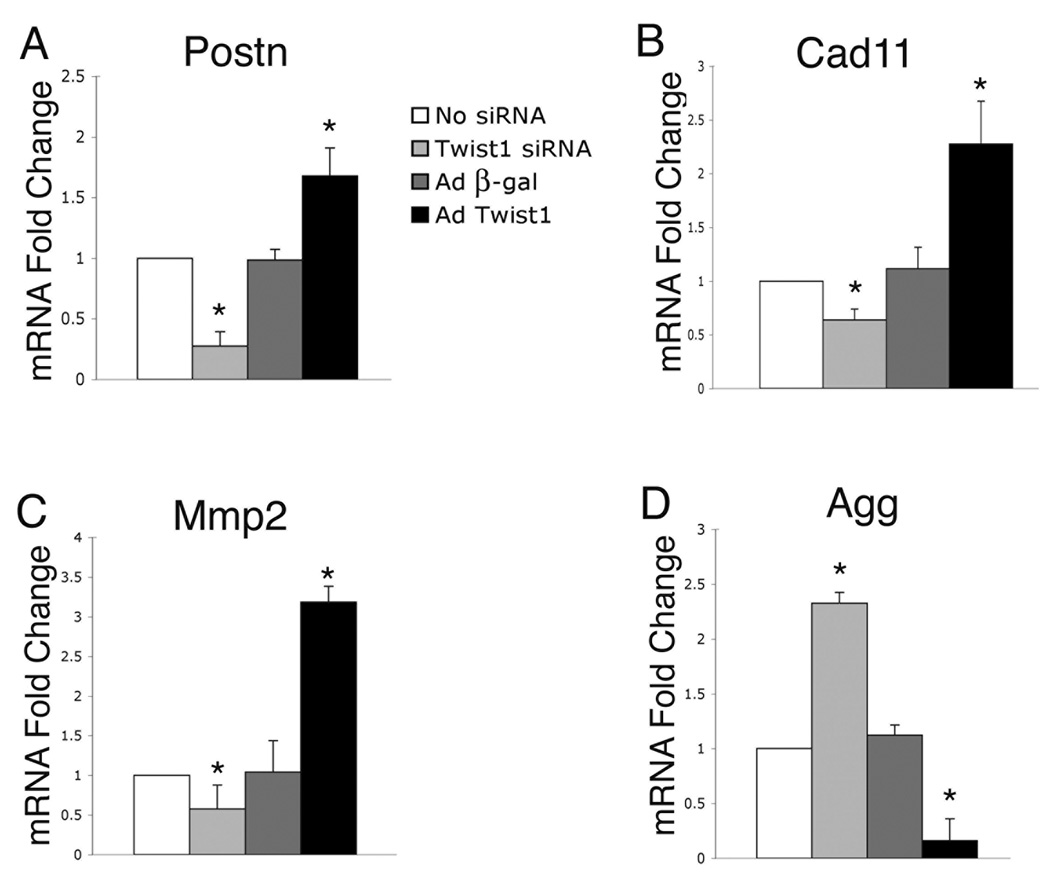
Twist1 promotes the expression of cell migration genes and inhibits differentiation marker genes in endocardial cushion cells
Genes involved in regulating aspects of cell migration and extracellular matrix organization and are coordinately expressed with Twist1 in developing endocardial cushions and are down regulated in remodeling valves (Fig. 1, Fig 2). The ability of Twist1 to affect the expression of the migration markers Postn, Cad11, and Mmp2, as well as Agg, an indicator of valve differentiation, was investigated. Primary endocardial cushion cells were infected with AdTwist1 or Adβ-gal for gain of function or transfected with Twist1-specific siRNA for loss of function. The expression of Postn, Cad11, Mmp2, and Agg was measured by quantitative real time RT-PCR. The expression of Postn in cells infected with AdTwist1 was approximately 1.5 fold higher relative to cells infected with Adβ-gal (Fig. 6A). Conversely, the expression of Postn in cells transfected with Twist1-specific siRNA was decreased by approximately 75% relative to untransfected control cells (Fig. 6A). Similarly, the expression of Cad11 in cells infected with AdTwist1 was approximately 2.3-fold higher relative to cells infected with Adβ-gal (Fig. 6B), while the expression of Cad11 in cells transfected with Twist1-specific siRNA was decreased approximately 50% relative to untransfected control cells (Fig. 6B). Additionally, the expression of Mmp2 in cells infected with AdTwist1 was approximately 3-fold higher relative to cells infected with Adβ-gal (Fig. 6C). Conversely, the expression of Mmp2 in cells transfected with Twist1-specific siRNA was decreased approximately 50% relative to untransfected control cells (Fig. 6C). Taken together, these data demonstrate that Twist1 can induce the expression of genes associated with cell migration in cultured endocardial cushion cells.
Figure 6. Altered Twist1 function affects the expression of cell migration and differentiation marker genes in endocardial cushion cells.
Primary endocardial cushion cells were infected with adenoviruses expressing β-gal (Adβ-gal) or Twist1 (AdTwist1) for gain of function or transfected with Twist1-specific siRNA for loss of function. The expression of Postn, Cad11, and Mmp2, genes associated with cell migration, and Agg, a gene associated with cushion cell differentiation, was measured by real time RT-PCR. Cells transfected with Twist1-specific siRNA had decreased Postn (A), Cad11 (B), and Mmp2 (C) expression and increased Agg (D) expression compared to untransfected control cells. In contrast, cells infected with AdTwist1 had increased Postn (A), Cad11 (B), and Mmp2 (C) expression and decreased Agg (D) expression compared to Adβ-gal infected control cells. Asterisks represent a statistically significant difference compared to the no siRNA control group (n=4, P<0.01). Error bars represent standard error of the mean.
In contrast, the expression of Agg in cells infected with AdTwist1 was decreased approximately 75% relative to cells infected with Adβ-gal (Fig. 6D), while the expression of Agg in cells transfected with Twist1-specific siRNA was approximately 2.3-fold higher relative to untransfected control cells (Fig. 6D). This is consistent with a previously published report of Twist1 repressing Agg expression in chondrocytes (Reinhold et al., 2006), and Tbx20 repressing Agg in endocardial cushion cells (Shelton and Yutzey, 2007). In addition, Twist1, like Tbx20, can repress the expression of Vers in endocardial cushion cells (data not shown) (Shelton and Yutzey, 2007). These results demonstrate that Twist1 can repress differentiation and diversified extracellular matrix gene expression in addition to promoting the expression of genes associated with cell migration.
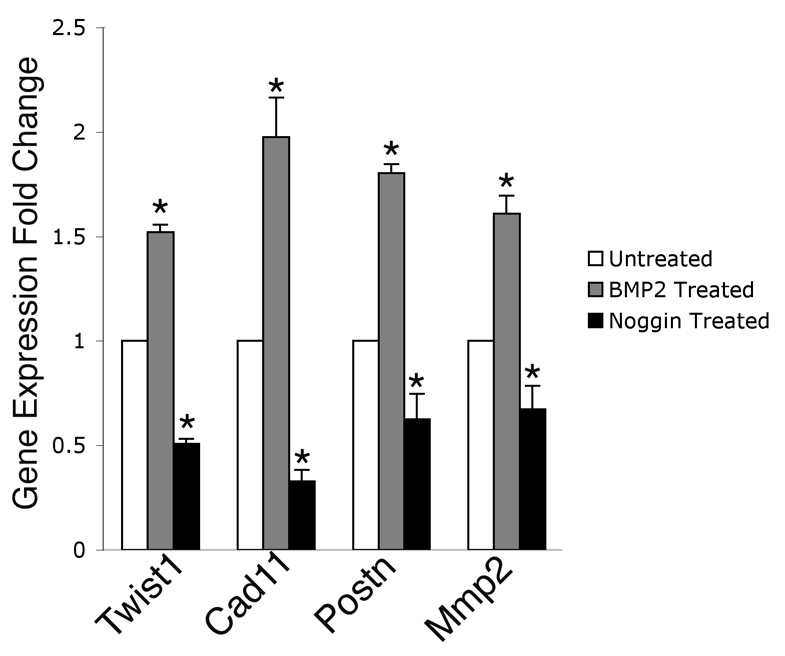
BMP2 induces Twist1, Cad11, Postn, and Mmp2 expression in endocardial cushion cells
BMP2 expression in the AV canal myocardium is essential for endocardial cushion formation (Ma et al., 2005) and BMP2 treatment induces the expression of Tbx20 in endocardial cushion cells (Shelton and Yutzey, 2007). In order to determine whether BMP2 can affect the expression of Twist1, primary endocardial cushion cells were cultured with or without the addition of recombinant human BMP2 or Noggin, a BMP inhibitor. The expression of the cell migration markers Cad11, Postn, or Mmp2 was also examined in BMP2 or Noggin treated cultures using quantitative real time RT-PCR. Addition of BMP2 induced the expression of Twist1, Cad11, Postn, and Mmp2 in endocardial cushion cells (Fig. 7). In contrast, addition of Noggin repressed the endogenous expression of Twist1, Cad11, Postn, and Mmp2 below the levels in untreated control cells. These data taken together with the Twist1 gain and loss of function analyses indicate that BMP2 can induce the expression of Twist1 and also can affect the expression of Cad11, Postn, and Mmp2, which are subject to Twist1 function in endocardial cushion cells.
Figure 7. BMP2 induces Twist1, Cad11, Postn, and Mmp2 expression in endocardial cushion cells.
Endocardial cushion cells were cultured with or without the addition of soluble recombinant BMP2 or Noggin, a BMP inhibitor. Addition of BMP2 resulted in increased Twist1, Cad11, Postn, and Mmp2 expression relative to untreated controls, while addition of Noggin attenuated that response as measured by real time RT-PCR. Asterisks represent a statistically significant difference compared to untreated control groups (n=4, P<0.01). Error bars represent standard error of the mean.
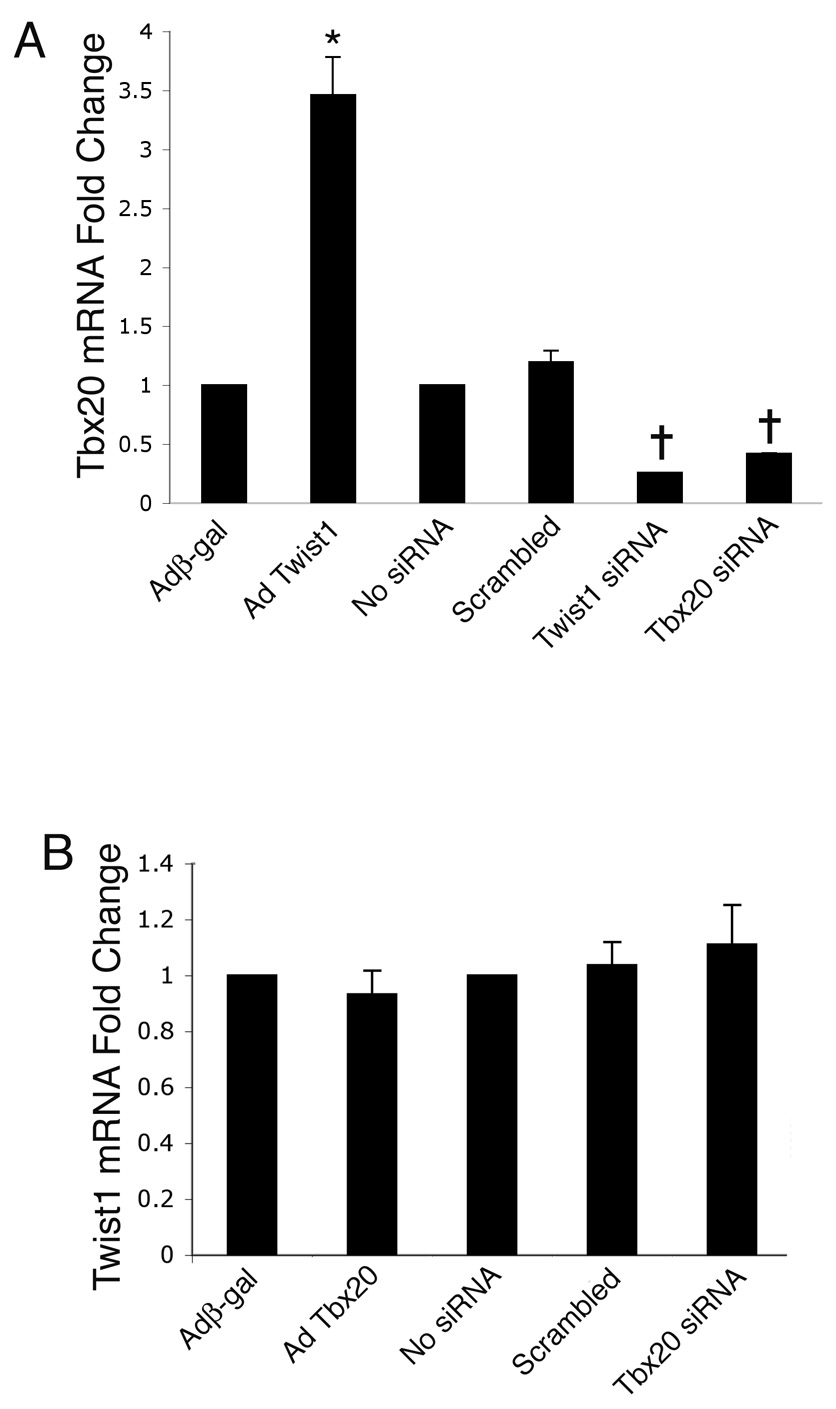
Twist1 promotes Tbx20 expression in endocardial cushion cells
We have shown that Twist1 can regulate endocardial cushion cell proliferation (Fig. 4), migration (Fig. 5), and differentiation (Fig. 6). Similarly, Tbx20 has also been shown to regulate these processes (Fig. 5) (Shelton and Yutzey, 2007). Because BMP2 can induce both Twist1 and Tbx20 in endocardial cushion cells, the hierarchical relationship between Twist1 and Tbx20 was examined. Primary endocardial cushion cells were infected with AdTwist1 or Adβ-gal for Twist1 gain of function studies or transfected with Twist1-specific siRNA or scrambled siRNA for Twist1 loss of function studies. The expression of Tbx20 was measured in these cells by quantitative real time RT-PCR. In cells infected with AdTwist1, Tbx20 expression was approximately 3.5 fold higher relative to Adβ-gal infected control cells (Fig. 8A). Additionally, in cells transfected with Twist1-specific siRNA, Tbx20 expression was decreased approximately 75% as compared to scrambled siRNA transfected and untransfected controls, and was lower than the Tbx20 expression level observed in cells transfected with Tbx20-specific siRNA (Fig. 8A). Interestingly, in situ hybridizations using probes specific for Tbx20 and Twist1 performed on HH stage 22 and 23 chicken embryos demonstrated that significant Twist1 expression precedes that of Tbx20 in the endocardial cushions (data not shown). Together these data provide evidence for Twist1 induction of Tbx20 expression in endocardial cushion cells.
Figure 8. Twist1 promotes Tbx20 expression in endocardial cushion cells.
Primary endocardial cushion cells were infected with adenoviruses expressing β-gal (Adβ-gal), Twist1 (AdTwist1), or Tbx20 (AdTbx20) or transfected with a scrambled control siRNA, Twist1-specific siRNA, or Tbx20-specific siRNA. Real time RT-PCR was used to measure the expression of Tbx20 and Twist1 on each experimental group. Cells infected with AdTwist1 had increased Tbx20 expression relative to controls, while cells transfected with Twist1-specific siRNA had decreased Tbx20 expression relative to controls (A). In contrast, cells with altered Tbx20 function showed no significant change in Twist1 expression relative to controls (B). The asterisk represents a statistically significant difference compared to the Adβ-gal control group, while the crosses represents a statistically significant difference compared to the no siRNA control group (n=4, P<0.01). Error bars represent standard error of the mean.
To determine if Tbx20 can affect Twist1 expression, primary endocardial cushion cells were infected with AdTbx20 or Adβ-gal for Tbx20 gain of function studies or transfected with Tbx20-specific siRNA or scrambled siRNA for Tbx20 loss of function studies. The expression of Twist1 was measured in these cells by quantitative real time RT-PCR. In cells with increased or decreased Tbx20 function, Twist1 expression was not significantly changed relative to the expression in control cells (Fig. 8B). Taken together, these data indicate that Twist1 is necessary and sufficient for Tbx20 expression in endocardial cushion cells, while altered Tbx20 function does not affect Twist1 mRNA expression levels in these cells. Therefore, Twist1 appears to be upstream of Tbx20 in valve progenitor cells.
Discussion
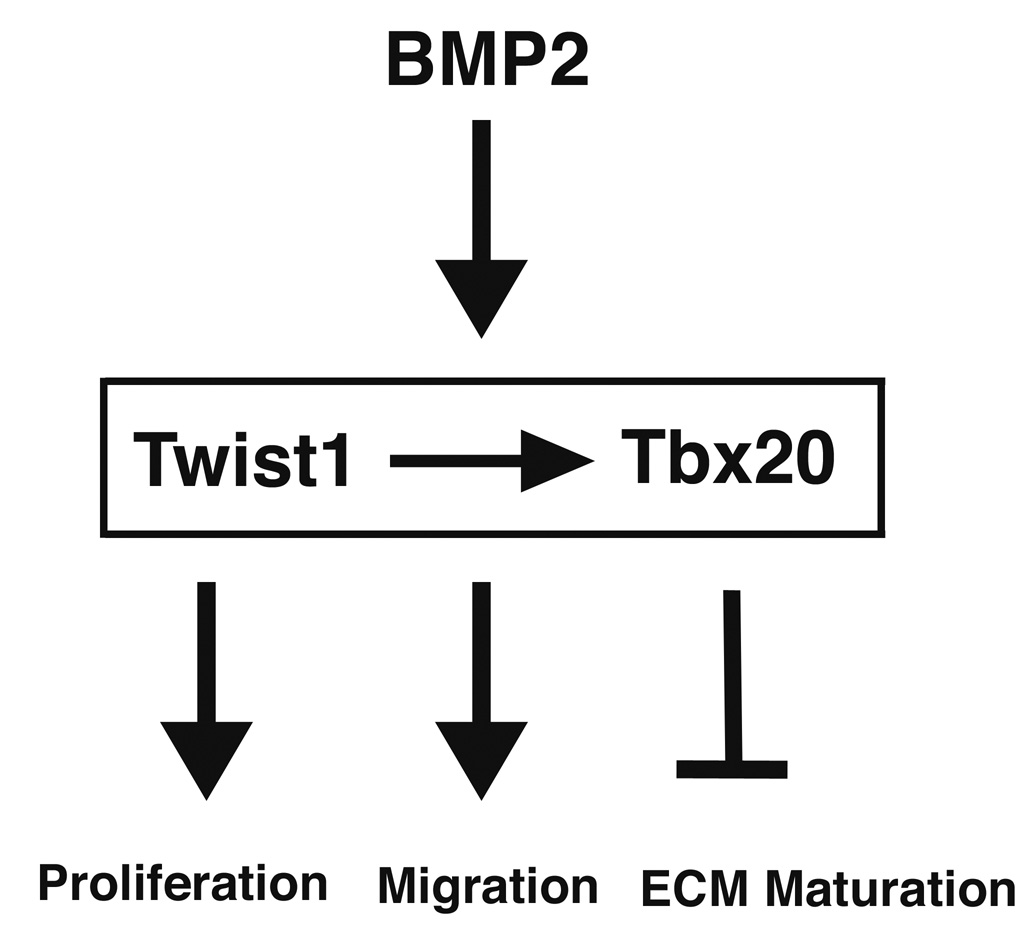
Gain and loss of function studies were performed to investigate the role of Twist1 in endocardial cushion cells. Twist1 is expressed at relatively higher levels in endocardial cushions relative to remodeling valves and is responsive to BMP signaling. This expression pattern is similar to Tbx20 expression in endocardial cushions and remodeling valves, and correlates with the expression of Cad11, Postn, and Mmp2 in these tissues. Furthermore, gain of Twist1 function can induce endocardial cushion cell proliferation and migration and repress cushion extracellular matrix organization, while loss of Twist1 function leads to reduced cell proliferation and migration and increased cushion matrix maturation. Taken together, a model for Twist1 function in endocardial cushion cells can be generated in which BMP2 induction of Twist1 induces the expression of Tbx20. High levels of Twist1 and Tbx20 in endocardial cushions can then promote the immature, proliferative, and migratory properties of the cushion mesenchyme (Fig. 9).
Figure 9. Model for Twist1 function in endocardial cushion cells.
BMP2 can induce Twist1 expression, which can promote Tbx20 expression. Twist1 and Tbx20 can then promote endocardial cushion cell proliferation and migration and repress cushion extracellular matrix organization. High levels of Twist1 and Tbx20 in endocardial cushions relative to remodeling valves is consistent with Twist1 and Tbx20 functioning to promote and maintain the proliferative, migratory, and undifferentiated nature of endocardial cushion mesenchyme.
Endocardial cushions cells are more proliferative than the interstitial cells of remodeling valves (Hinton et al., 2006; Lincoln et al., 2004). In addition, endocardial cushion cells are migratory and their proper distribution throughout the remodeling valve leaflet is essential for normal valve development (Hinton et al., 2006). Furthermore, the extracellular matrix of endocardial cushions is diffuse and unorganized, while the matrix of mature stratified valve leaflets is highly organized with increased expression of Agg and Vers in remodeling valves (Flanagan and Pandit, 2003; Hinton et al., 2006; Shelton and Yutzey, 2007). High levels of Twist1 in undifferentiated endocardial cushion cells during the time when they are most proliferative and migratory is consistent with observed Twist1 functions to promote and maintain cell proliferation and migration, while inhibiting extracellular matrix maturation. Tbx20 is also present in endocardial cushions at this time and functions in a regulatory hierarchy with Twist1 to control endocardial cushion development.
Twist1 regulates cell proliferation and migration in other developing mesenchymal cell populations as well as in transformed cells. In cultured chicken skeletal muscle satellite cells, Twist1 can promote cell proliferation while inhibiting muscle differentiation (Leshem et al., 2000). Similarly, Twist1 null mice have decreased cell proliferation and viability in somatic and branchial arch tissues (Ota et al., 2004). Furthermore, Twist1 functions in promoting cell proliferation in frontal bone skeletogenic mesenchyme (Ishii et al., 2003). Twist1 also plays a role in cell migration, another characteristic of mesenchymal cells. Studies done in Twist1 null mice demonstrate that Twist1 is required for proper neural crest cell migration in the developing forebrain (Soo et al., 2002). Moreover, in cancer cell lines, Twist1 can promote cell motility and can increase the expression of mesenchymal markers such as fibronectin and N-cadherin (Alexander et al., 2006; Yang et al., 2004). In addition, Twist1 has been shown to play an early role in tumor metastasis by inducing an epithelial to mesenchymal transition event by directly inhibiting E-cadherin expression (Yang et al., 2004; Yang et al., 2006a).
While Twist1 is involved in maintaining proliferative and migratory endocardial cushion cells in an immature extracellular matrix, no direct downstream targets of Twist1 have been identified in the heart. In osteoblasts, Twist1 can directly activate the Postn promoter by binding to the bHLH binding consensus E-box site CATGTG (Oshima et al., 2002). Similarly, we have demonstrated that Twist1 can also induce Postn expression in endocardial cushion cells (Fig. 6A), suggesting that Twist1 regulation of Postn is a common feature of osteoblasts and valve cell lineages. Interestingly, altered Tbx20 function had no affect on the expression of Postn in endocardial cushion cells (data not shown). In addition, Twist1 can induce Tbx20 expression in endocardial cushion cells, but it remains to be determined if this is a direct or indirect interaction. Twist1 is co-expressed with several T-box genes in other mesenchymal tissues such as the developing limbs (Agarwal et al., 2003; O'Rourke et al., 2002; Takeuchi et al., 1999; Tavares et al., 2001) suggesting that Twist1/T-box interactions may be a shared developmental pathway. Additionally, we have demonstrated that Twist1 can induce Cad11 expression in endocardial cushion cells (Fig.6B). While it remains to be shown whether this interaction is direct or indirect, Cad 11 has been shown to promote cell migration in vascular smooth muscle and human breast cancer cells (Feltes et al., 2002; Monahan et al., 2007) and other cadherin family members such as N-cadherin and E-cadherin are direct downstream targets of Twist1 (Alexander et al., 2006; Yang et al., 2004). Furthermore, we have shown that Twist1 can repress Agg expression in endocardial cushion cells (Fig. 6D), and this inhibitory function of Twist1 has also been demonstrated in chondrocytes (Reinhold et al., 2006). Similarly, Tbx20 can repress Agg protein and mRNA expression in endocardial cushion cells (Shelton and Yutzey, 2007). These studies support Twist1 functioning as a high-level regulatory protein in mesenchymal cell proliferation, migration, and gene expression in a variety of tissues including endocardial cushions.
Twist1 and Tbx20 are expressed together in endocardial cushions with other regulators of mesenchymal cell populations including Msx and Id family members. Twist1 has been shown to cooperate with the Nk homeobox transcription factor Msx2 to regulate mesenchymal differentiation and proliferation in the developing frontal bone (Ishii et al., 2003). Msx1 and Msx2 are also expressed in the AV canal during the stages of endocardial cushion development, and like Twist1, are subject to BMP regulation (Ma et al., 2005). In addition, it has been demonstrated that the activity of Twist1 is dependent on whether Twist1 forms homodimers or heterodimerizes with E proteins (Connerney et al., 2006). Members of the Id family of helix-loop-helix proteins lacking a basic DNA binding domain can regulate Twist1 function by binding to E proteins and sequestering them away from potential Twist1 binding partners (Massari and Murre, 2000). In developing cranial sutures, Id levels can modulate Twist1 dimerization and affect Twist1 directed regulation of Postn expression (Connerney et al., 2006). Id family members are also expressed in the developing endocardial cushions (Evans and O'Brien, 1993; Martinsen et al., 2004) where they could be affecting Twist1 function. Therefore, it is likely that complex regulation of Twist1 interacting proteins and cofactors is important for endocardial cushion maturation. Further investigations into how Twist1 interacts with factors including Msx1, Msx2, Id, and possibly T-box proteins are necessary to determine the molecular mechanisms of Twist1 function in endocardial cushions.
Heart valve disease is associated with abnormal extracellular matrix organization and disrupted valve interstitial cell compartmentalization (Bartram et al., 2001; Hinton et al., 2006; Rabkin et al., 2001). Loosely organized extracellular matrix and high levels of proliferation and migration of mesenchymal cells are also features of endocardial cushions, which contain the valve precursors in the developing embryo. Because Twist1 plays a role in cell proliferation, migration, and matrix organization in endocardial cushions and in other tissues, it is possible that a misregulation of Twist1 could lead to heart valve disease. Heterozygous hTWIST mutations in human patients have been linked to Saethre-Chotzen syndrome, a disease characterized by craniofacial abnormalities, skeletal anomalies, and limb defects (Bourgeois et al., 1998; Reardon and Winter, 1994; Zackai and Stolle, 1998). However, cardiac defects have not been reported in Twist null mice or in human patients with a Saethre-Chotzen syndrome. This could be due to functional redundancy with other Twist isoforms or due to undetected valve anomalies that do not manifest until later in life. Thus, further studies examining the expression or reexpression of Twist1 in normal and diseased adult valves may provide insight into the molecular causes and pathogenesis of heart valve disease.
Acknowledgments
The AdTwist1 adenovirus was generously provided by Dr. Michael Naski, San Antonio. In addition, we thank Heather Evans-Anderson, Michelle Combs, and Susanne Wells for technical support and scientific advice. This work was supported by an AHA Great Rivers Affiliate pre-doctoral fellowship 0715360B to ELS and NIH grant HL082716 to KEY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- Alexander NR, Tran NL, Rekapally H, Summers CE, Glackin C, Heimark RL. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res. 2006;66:3365–3369. doi: 10.1158/0008-5472.CAN-05-3401. [DOI] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003;69:58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- Bartram U, Bartelings MM, Kramer HH, Gittenberger-de Groot AC. Congenital polyvalvular disease: a review. Pediatr Cardiol. 2001;22:93–101. doi: 10.1007/s002460010169. [DOI] [PubMed] [Google Scholar]
- Bourgeois P, Bolcato-Bellemin AL, Danse JM, Bloch-Zupan A, Yoshiba K, Stoetzel C, Perrin-Schmitt F. The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre-Chotzen syndrome. Hum Mol Genet. 1998;7:945–957. doi: 10.1093/hmg/7.6.945. [DOI] [PubMed] [Google Scholar]
- Cai CL, Zhou W, Yang L, Bu L, Qyang Y, Zhang X, Li X, Rosenfeld MG, Chen J, Evans S. T-box genes coordinate regional rates of proliferation and regional specification during cardiogenesis. Development. 2005;132:2475–2487. doi: 10.1242/dev.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- Connerney J, Andreeva V, Leshem Y, Muentener C, Mercado MA, Spicer DB. Twist1 dimer selection regulates cranial suture patterning and fusion. Dev Dyn. 2006;235:1345–1357. doi: 10.1002/dvdy.20717. [DOI] [PubMed] [Google Scholar]
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–143. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Ehrman LA, Yutzey KE. Lack of regulation in the heart forming region of avian embryos. Dev Biol. 1999;207:163–175. doi: 10.1006/dbio.1998.9167. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- Evans SM, O'Brien TX. Expression of the helix-loop-helix factor Id during mouse embryonic development. Dev Biol. 1993;159:485–499. doi: 10.1006/dbio.1993.1258. [DOI] [PubMed] [Google Scholar]
- Feltes CM, Kudo A, Blaschuk O, Byers SW. An alternatively spliced cadherin-11 enhances human breast cancer cell invasion. Cancer Res. 2002;62:6688–6697. [PubMed] [Google Scholar]
- Flanagan TC, Pandit A. Living artificial heart valve alternatives: a review. Eur Cell Mater. 2003;6:28–45. doi: 10.22203/ecm.v006a04. discussion 45. [DOI] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1951;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Iio A, Koide M, Hidaka K, Morisaki T. Expression pattern of novel chick T-box gene, Tbx20. Dev Genes Evol. 2001;211:559–562. doi: 10.1007/s00427-001-0187-y. [DOI] [PubMed] [Google Scholar]
- Ishii M, Merrill AE, Chan YS, Gitelman I, Rice DP, Sucov HM, Maxson RE., Jr Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development. 2003;130:6131–6142. doi: 10.1242/dev.00793. [DOI] [PubMed] [Google Scholar]
- Kirk EP, Sunde M, Costa MW, Rankin SA, Wolstein O, Castro ML, Butler TL, Hyun C, Guo G, Otway R, Mackay JP, Waddell LB, Cole AD, Hayward C, Keogh A, Macdonald P, Griffiths L, Fatkin D, Sholler GF, Zorn AM, Feneley MP, Winlaw DS, Harvey RP. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and valvulogenesis and cardiomyopathy. Am J Hum Genet. 2007;81:280–291. doi: 10.1086/519530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y, Spicer DB, Gal-Levi R, Halevy O. Hepatocyte growth factor (HGF) inhibits skeletal muscle cell differentiation: a role for the bHLH protein twist and the cdk inhibitor p27. J Cell Physiol. 2000;184:101–109. doi: 10.1002/(SICI)1097-4652(200007)184:1<101::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230:239–250. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006;294:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am J Anat. 1977;148:85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- Martinsen BJ, Frasier AJ, Baker CV, Lohr JL. Cardiac neural crest ablation alters Id2 gene expression in the developing heart. Dev Biol. 2004;272:176–190. doi: 10.1016/j.ydbio.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan TS, Andersen ND, Panossian H, Kalish JA, Daniel S, Shrikhande GV, Ferran C, Logerfo FW. A novel function for cadherin 11/osteoblast-cadherin in vascular smooth muscle cells: modulation of cell migration and proliferation. J Vasc Surg. 2007;45:581–589. doi: 10.1016/j.jvs.2006.12.016. [DOI] [PubMed] [Google Scholar]
- O'Rourke MP, Soo K, Behringer RR, Hui CC, Tam PP. Twist plays an essential role in FGF and SHH signal transduction during mouse limb development. Dev Biol. 2002;248:143–156. doi: 10.1006/dbio.2002.0730. [DOI] [PubMed] [Google Scholar]
- Oshima A, Tanabe H, Yan T, Lowe GN, Glackin CA, Kudo A. A novel mechanism for the regulation of osteoblast differentiation: transcription of periostin, a member of the fasciclin I family, is regulated by the bHLH transcription factor, twist. J Cell Biochem. 2002;86:792–804. doi: 10.1002/jcb.10272. [DOI] [PubMed] [Google Scholar]
- Ota MS, Loebel DA, O'Rourke MP, Wong N, Tsoi B, Tam PP. Twist is required for patterning the cranial nerves and maintaining the viability of mesodermal cells. Dev Dyn. 2004;230:216–228. doi: 10.1002/dvdy.20047. [DOI] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Plageman TF, Jr, Yutzey KE. Differential expression and function of Tbx5 and Tbx20 in cardiac development. J Biol Chem. 2004;279:19026–19034. doi: 10.1074/jbc.M314041200. [DOI] [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- Rabkin-Aikawa E, Mayer JE, Jr, Schoen FJ. Heart valve regeneration. Adv Biochem Eng Biotechnol. 2005;94:141–179. doi: 10.1007/b100003. [DOI] [PubMed] [Google Scholar]
- Reardon W, Winter RM. Saethre-Chotzen syndrome. J Med Genet. 1994;31:393–396. doi: 10.1136/jmg.31.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold MI, Kapadia RM, Liao Z, Naski MC. The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. J Biol Chem. 2006;281:1381–1388. doi: 10.1074/jbc.M504875200. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81:392–406. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- Shelton EL, Yutzey KE. Tbx20 regulation of endocardial cushion cell proliferation and extracellular matrix gene expression. Dev Biol. 2007;302:376–388. doi: 10.1016/j.ydbio.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Christoffels VM, Dias JM, Trowe MO, Petry M, Schuster-Gossler K, Burger A, Ericson J, Kispert A. Tbx20 is essential for cardiac chamber differentiation and repression of Tbx2. Development. 2005;132:2697–2707. doi: 10.1242/dev.01854. [DOI] [PubMed] [Google Scholar]
- Somi S, Buffing AA, Moorman AF, Van Den Hoff MJ. Dynamic patterns of expression of BMP isoforms 2, 4, 5, 6, and 7 during chicken heart development. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:636–651. doi: 10.1002/ar.a.20031. [DOI] [PubMed] [Google Scholar]
- Song MR, Shirasaki R, Cai CL, Ruiz EC, Evans SM, Lee SK, Pfaff SL. T-Box transcription factor Tbx20 regulates a genetic program for cranial motor neuron cell body migration. Development. 2006;133:4945–4955. doi: 10.1242/dev.02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo K, O'Rourke MP, Khoo PL, Steiner KA, Wong N, Behringer RR, Tam PP. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev Biol. 2002;247:251–270. doi: 10.1006/dbio.2002.0699. [DOI] [PubMed] [Google Scholar]
- Stennard FA, Costa MW, Elliott DA, Rankin S, Haast SJ, Lai D, McDonald LP, Niederreither K, Dolle P, Bruneau BG, Zorn AM, Harvey RP. Cardiac T-box factor Tbx20 directly interacts with Nkx2-5, GATA4, and GATA5 in regulation of gene expression in the developing heart. Dev Biol. 2003;262:206–224. doi: 10.1016/s0012-1606(03)00385-3. [DOI] [PubMed] [Google Scholar]
- Stennard FA, Costa MW, Lai D, Biben C, Furtado MB, Solloway MJ, McCulley DJ, Leimena C, Preis JI, Dunwoodie SL, Elliott DE, Prall OW, Black BL, Fatkin D, Harvey RP. Murine T-box transcription factor Tbx20 acts as a repressor during heart development, and is essential for adult heart integrity, function and adaptation. Development. 2005;132:2451–2462. doi: 10.1242/dev.01799. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Koshiba-Takeuchi K, Matsumoto K, Vogel-Hopker A, Naitoh-Matsuo M, Ogura K, Takahashi N, Yasuda K, Ogura T. Tbx5 and Tbx4 genes determine the wing/leg identity of limb buds. Nature. 1999;398:810–814. doi: 10.1038/19762. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Mileikovskaia M, Koshiba-Takeuchi K, Heidt AB, Mori AD, Arruda EP, Gertsenstein M, Georges R, Davidson L, Mo R, Hui CC, Henkelman RM, Nemer M, Black BL, Nagy A, Bruneau BG. Tbx20 dose-dependently regulates transcription factor networks required for mouse heart and motoneuron development. Development. 2005;132:2463–2474. doi: 10.1242/dev.01827. [DOI] [PubMed] [Google Scholar]
- Tavares AT, Izpisuja-Belmonte JC, Rodriguez-Leon J. Developmental expression of chick twist and its regulation during limb patterning. Int J Dev Biol. 2001;45:707–713. [PubMed] [Google Scholar]
- Thisse B, Stoetzel C, Gorostiza-Thisse C, Perrin-Schmitt F. Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. Embo J. 1988;7:2175–2183. doi: 10.1002/j.1460-2075.1988.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T, Nakajima Y, Nishimatsu S, Nohno T, Ando K, Nakamura H. Expression of tbx20 RNA during chick heart development. Dev Dyn. 2004;230:576–580. doi: 10.1002/dvdy.20076. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res. 2006a;66:4549–4552. doi: 10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang L, Zheng Y. Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol Biol Cell. 2006b;17:4675–4685. doi: 10.1091/mbc.E06-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackai EH, Stolle CA. A new twist: some patients with Saethre-Chotzen syndrome have a microdeletion syndrome. Am J Hum Genet. 1998;63:1277–1281. doi: 10.1086/302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga A, Quillet R, Perrin-Schmitt F, Zeller R. Mouse Twist is required for fibroblast growth factor-mediated epithelial-mesenchymal signalling and cell survival during limb morphogenesis. Mech Dev. 2002;114:51–59. doi: 10.1016/s0925-4773(02)00048-5. [DOI] [PubMed] [Google Scholar]