Abstract
Decision making in a social group displays two unique features. First, humans and other animals routinely alter their behaviors in response to changes in their physical and social environment. As a result, the outcomes of decisions that depend on the behaviors of multiple decision makers are difficult to predict, and this requires highly adaptive decision-making strategies. Second, decision makers may have other-regarding preferences and therefore choose their actions to improve or reduce the well-beings of others. Recently, many neurobiological studies have exploited game theory to probe the neural basis of decision making, and found that these unique features of social decision making might be reflected in the functions of brain areas involved in reward evaluation and reinforcement learning. Molecular genetic studies have also begun to identify genetic mechanisms for personal traits related to reinforcement learning and complex social decision making, further illuminating the biological basis of social behavior.
Introduction
The problem of decision making is challenging, because the future outcomes from a particular action are seldom fully predictable. Therefore, decision makers must always take uncertainty into consideration when they make choices1. In addition, such action-outcome relationships can change frequently, requiring adaptive decision-making strategies that depend on the observed outcomes of their previous choices2. Accordingly, neurobiological studies on decision making have focused on the brain mechanisms for mediating the effect of uncertainty and improving the decision-making strategies by trial and error. Such studies have found that signals related to reward magnitude and probability are widespread in the brain and often modulated by the active process of decision making3–9 (see other papers in this issue). Some of these brain areas might be also involved in updating the preference and strategies of decision makers10–14.
Compared to solitary animals, animals living in a large social group face many unique challenges and opportunities, as reflected in various cognitive abilities in social domain, such as communication and other prosocial behaviors15. This review focuses on the neural basis of socially interactive decision making in humans and other primates. The basic building blocks of decision making that underlie the process of learning and valuation also play important roles for decision making in social contexts. However, interactions among multiple decision makers in a social group display some new features. First, behaviors of humans and animals can change frequently, as they seek to maximize their self-interests according to the information available from their environment. This makes it difficult to predict the outcomes of a decision-maker’s actions and to choose optimal actions accordingly. As a result, more sophisticated learning algorithms might be required for social decision making16,17. Second, social interactions open the possibility of competition and cooperation. Humans and animals indeed act not only to maximize their own self-interest, but sometimes also to increase or decrease the well-beings of others around them. These unique aspects of social decision making are reflected in the activity of brain areas involved in learning and valuation.
Game theory and social preference
A good starting point for studies of social decision making is game theory18. In its original formulation, game theory seeks to find the strategies that a group of decision makers will converge on, as they try to maximize their own payoffs. Nash equilibrium refers to a set of such strategies from which no individual players can increase their payoffs by changing their strategies unilaterally19. In a two-player competitive game known as the matching pennies (Fig 1a), for example, each player can choose between two alternative options, such as the head and tail of a coin. One of the players wins if both players choose the same option, and loses otherwise. For the matching pennies game with a symmetrical payoff matrix as shown in Figure 1a, the Nash equilibrium is to choose both options with the same probabilities. Any other strategy can be exploited by the opponent and therefore reduces the expected payoff. A large number of studies in both humans and non-human primates found, however, that for competitive games, such as matching pennies, the predictions based on Nash equilibrium are often systematically violated17,20,21. As discussed below, this might be due to various learning algorithms used by the decision makers to improve the outcomes of their choices iteratively.
Figure 1.
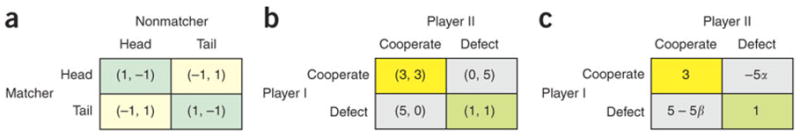
Payoff matrix for the games of matching pennies (a) and prisoner’s dilemma (b,c). a. A pair of numbers within each parenthesis indicate the payoffs to the matcher and non-matcher, respectively, for the matching pennies game. b. A pair of numbers within the parenthesis indicate the payoffs to the players I and II, respectively, for the prisoner’s dilemma game. The yellow and green rectangles correspond to mutual cooperation and mutual defection, respectively. c. The player I’s utility function adjusted according to the model of inequality aversion. The values of α and β indicate the sensitivity to the disadvantageous and advantageous inequality. For β > .0.4, mutual cooperation becomes a Nash equilibrium.
How game theory can be used to investigate the question of cooperation and altruism can be illustrated by the well known game of the prisoner’s dilemma. Two players participate in this game, and each can choose between cooperation and defection. Each player receives a higher payoff by defecting, regardless of whether the other player chooses to cooperate or defect, but the payoff to each player is higher for mutual cooperation than for mutual defection, hence creating a dilemma (Fig 1b). If this game is played only once, and the players care only about their own payoffs, both players should defect, which corresponds to the Nash equilibrium for this game. In reality as well as in laboratory experiments, however, both of these assumptions are frequently violated. Games can be played repeatedly, often among the same set of players. This makes it possible for some players to train others and force them to deviate from the equilibrium predictions for one-shot games. In addition, humans often cooperate in the prisoner’s dilemma games, regardless of whether the game is one-shot or repeated22. Therefore, for humans, decision making in social contexts may not be entirely driven by their self-interest, but at least partially by their preferences regarding the well-beings of other individuals. In fact, cooperation and altruistic behaviors abound in human societies23, and might also exist in other non-human primates24–26. Theoretical studies show that multiple mechanisms, such as kin selection, direct and indirect reciprocity, and group selection, can increase the fitness of cooperators and thus sustain cooperation23,27. Ironically, costly punishment of defectors or free-riders, often referred to as altruistic punishment, also provides an effective means to deter defection23, 28–30.
In economics, the subjective desirability of a particular choice is quantified by its utility function. Although the notion of utility is often linked to the state of the decision-maker’s personal wealth, when people take into consideration the well-beings of other individuals, the utility function can be expanded to incorporate social preference. For example, Fehr and Schmidt31 proposed that the utility function can be modified by the decision maker’s aversion to inequality. For two-player games, the first player’s utility, U1(x), for the payoff to the two players x=[x1 x2], can be defined as follows.
where ID = max{x2 − x1, 0} and IA = max{x1 − x2, 0} refer to the inequalities that are disadvantageous and advantageous to the first player, respectively. The coefficients α and β indicate sensitivities to disadvantageous and advantageous inequalities, respectively, and it is assumed that β ≤ α and 0 ≥ β < 1. Therefore, for a given payoff to the first decision maker, x1, U1(x) is maximal when x1=x2, giving rise to the preference for equality. When the monetary payoff in the prisoner’s dilemma is replaced by this utility function with the value of β sufficiently large, mutual cooperation and mutual defection both become Nash equilibria32 (Fig 1c). When this occurs, a player will cooperate as long as he or she believes that the other player will cooperate as well.
Evidence for altruistic social preference and aversion to inequality has been also found in other experimental games, such as dictator game, ultimatum game, and trust game17,32, and their possible neural substrates have been examined in several studies33. In a dictator game, the dictator receives a fixed amount of money and donates a part of it to the recipient. This ends the game, so there is no opportunity for the recipient to retaliate. Any amount of donation reduces the payoff to the dictator, so the amount of money donated by the dictator provides a measure of altruism. During dictator games, people tend to donate on average about 25% of their money17. An ultimatum game is similar to the dictator game in that one of the players (proposer) offers a proportion of the money given to the recipient, who now has the opportunity to reject the offer. If the offer is rejected, neither player receives any money. The average offer in ultimatum games is about 40%, and since this is significantly higher than in the dictator game, it implies that the proposers are motivated to avoid the potential rejection17. Indeed, during the ultimatum game, the recipients reject the offers below 20% about half the time. Another important element in social interaction is captured by a trust game, in which one of the players (investor) invests a proportion of his or her money. This money then is multiplied, often tripled, and transferred to the other player (trustee). The trustee then decides how much of this transferred money would be returned to the investor. The amount of money invested by the investor measures the trust the investor has on the trustee, and the amount of repayment reflects the trustee’s trustworthiness. In other words, trust games quantify the effect of any moral obligations the trustee might feel towards the investor. Empirically, the investor tends to invest roughly a half of his or her money, and the trustee tends to repay the amount comparable to the original investment17.
Studies on experimental games in non-human primates can provide important insights into the evolutionary origins of social preference displayed by human decision makers. For example, when chimpanzees were tested in a reduced form of the ultimatum game in which the proposer’s choice was binary and between two different pre-set offers, they tended to choose the options that maximize their self-interest, both as proposers and recipients34. Therefore, even though chimpanzees and other non-human primates display altruistic behaviors, fairness plays much more important role in social decision making for humans.
Learning in social decision making
When a group of players play the same game repeatedly, some players might try to train other players. For example, the recipient in an ultimatum game might reject some offers, not as a result of aversion to inequality, but in order to increase his or her long-term payoff by penalizing a greedy proposer. To better isolate the effect of social preference, therefore, many of the behavioral studies on experimental games do not allow their subjects to interact with the same partners repeatedly. In reality, however, learning plays an important role, since people and animals interact with the same individuals repeatedly.
Reinforcement learning theory2 formalizes the problem faced by a decision maker trying to discover optimal strategies in an unfamiliar environment (Fig 2). This theory has been successfully applied to the environment that includes multiple decision-makers17,20,21,35,36. In reinforcement learning theory, the sum of future rewards expected from a particular action in a particular state of the environment is referred to as value function. Future rewards are often exponentially discounted so that immediate rewards contribute more to the value functions. Similar to utility functions in economics, value functions determine the actions chosen by the decision makers. In addition, the difference between the reward predicted from the value functions and the actual reward is referred to as reward prediction error. In simple or direct reinforcement learning algorithms, value functions are updated only for the chosen actions and only when there is a reward prediction error2.
Figure 2.
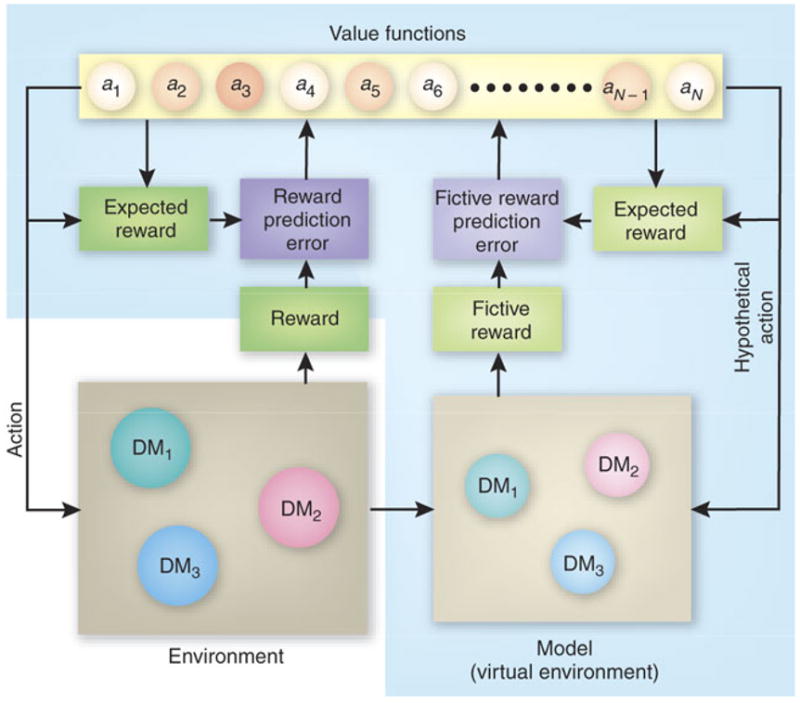
A model-based reinforcement learning model applied to social decision making. The decision maker receives reward according to his or her own action and those of other decision makers (DM) in the environment, and updates the value functions according to the reward prediction error. In addition, the decision maker updates his or her model of the environment, including the predicted actions of other decision makers. The fictive reward prediction errors (fRPE) resulting from such model simulations also influence the value functions.
Although reward has a powerful effect on choice behavior, decision makers receive many other signals from their environment. For example, they may discover, after their choices, how much reward they could have received had they chosen a different action. When such hypothetical payoffs or fictive rewards differ from the rewards expected from the current value functions, the resultant error signals, referred to as fictive reward prediction error37 or regret38, can be used to update the value functions of corresponding actions. Such fictive reward prediction errors can indeed influence the decision maker’s subsequent behaviors during financial decision making37. In model-based reinforcement learning algorithms, fictive reward signals can be generated from various types of simulations or inferences based on the decision maker’s model or knowledge of the environment. These fictive reward signals might play a crucial role in social decision making, when the simulated environment includes other decision makers (DM in Fig 2).
In game theory, estimating the payoffs from alternative strategies based on the expected actions of other players is referred to as belief learning16,17. For example, imagine that you have observed that a particular decision maker tended to apply the strategy of tit-for-tat during a repeated prisoner’s dilemma game. By simulating hypothetical interactions with such a player, you can update the value functions for cooperation and defection and might discover that in the long run, cooperation with this player would produce a higher average payoff than defection. Belief learning and other model-based reinforcement learning algorithms can also update the value functions for multiple actions simultaneously. So far, studies on competitive games in humans and other primates have failed to provide strong evidence for such model-based reinforcement learning or belief learning20,36,39,40. In contrast, both theoretical and empirical studies have shown that the reputation and moral characters of individual players influence the likelihood and level of cooperation41–43. For example, a player who has donated frequently in the past is more likely to receive donations when such information is publicly available42. Similarly, people tend to invest more money as investors in trust games when they face individuals with positive moral qualities43. Therefore, belief learning models might account for how images of individual players are propagated.
Neural basis of reinforcement learning and valuation
During the last decade, reinforcement learning theory has become a dominant paradigm for studying the neural basis of decision making (see other articles in this issue)44. In particular, single-neuron recording studies in non-human primates found that midbrain dopamine neurons encode reward prediction errors10,45. Dopamine neurons also decrease their activity when the expected reward is delayed46 or omitted10,47. In addition, neurons modulating their activity according to rewards and value functions have been identified in many areas in the primate brain, including the amygdala48, the basal ganglia49–51, the posterior parietal cortex3,52,53, the lateral prefrontal cortex54–56, the medial frontal cortex57–59, and the orbitofrontal cortex60–62. Nevertheless, how these signals related to value functions in multiple areas are updated by real and fictive reward error signals and influence action selection is still largely unknown63.
Neuroimaging studies in human subjects have also found signals related to expected reward in multiple brain areas, such as the amygdala, the striatum, the insula, and the orbitofrontal cortex64–66. Non-invasive nature of neuroimaging makes it possible to investigate the neural mechanisms for complex financial and social decision making in humans. On the other hand, the signals measured in neuroimaging studies, such as blood-oxygen-level-dependent (BOLD) signals, reflect the activity of individual neurons only indirectly. In particular, BOLD signals in functional magnetic resonance imaging (fMRI) experiments might reflect inputs to a given brain area more closely than its outputs67. Comparison of the results obtained from single-neuron recording and fMRI studies must take into consideration such methodological differences.
Neural correlates of social decision making
Socially interactive decision making tends to be dynamic and the process of discovering an optimal strategy can be further complicated by the fact that decision makers often act according to their other-regarding preferences. Nevertheless, the basic neural processes involved in outcome evaluation and reinforcement learning might be generally applicable, regardless of whether the outcome of choice is determined socially or not. For example, single-neuron recording studies have found that neurons in the dorsolateral prefrontal cortex of rhesus monkeys often encode the signals related to the animal’s previous choice and its outcome conjunctively not only during a memory saccade task68 but also in a computer-simulated matching pennies task56. Neurons in the posterior parietal cortex also modulate their activity according to the expected reward or its utility during both a foraging task52 and a computer-simulated competitive game53. Similarly, imaging studies have found that many brain areas implicated in reward evaluation and reinforcement learning, such as the striatum, insula, and orbitofrontal cortex, are also recruited during social decision making (Fig 3). However, as described below, activity in these brain areas during social decision making is also influenced by factors that are unique to social interactions.
Figure 3.
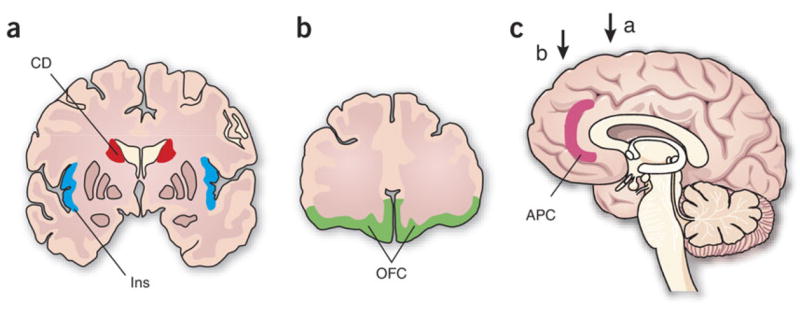
Brain areas involved in social decision making. a and b. Coronal sections of the human brain showing the caudate nucleus (CD), the insula (Ins), and the orbitofrontal cortex (OFC). c. Sagittal section showing the anterior paracingulate cortex (APC).
One of the areas that play a key role in socially interactive decision making is the striatum. During decision making without any social interactions, activity in the striatum is influenced by both real and fictive reward prediction errors11,12,37. Reward prediction errors during social decision making also lead to activity changes in the striatum. For example, during the prisoner’s dilemma game, cooperation results in a positive BOLD response in the ventral striatum, when this was reciprocated by the partner, but produces a negative BOLD response in the same areas when the cooperation was not reciprocated69,70. In addition, the caudate nucleus of the trustee in a repeated trust game displays activity correlated with the reputation of the investor71. Interestingly, when the investors in trust games receive detailed descriptions of the trustees’ positive moral characters, they tend to invest money more frequently. Moreover, the activity in the caudate nucleus of the investor related to the decision of the trustee is attenuated or abolished when the investor relies on the information about the trustee’s moral character43.
As described above, theoretical and behavioral studies have shown that altruistic punishment of unfair behaviors promotes cooperation. Neuroimaging studies have provided an important insight into the neural mechanisms for producing such costly punishing acts. For example, during the ultimatum game, unfair offers produce stronger activation in the recipient’s anterior insula, when they are rejected than when they are accepted72. Since the insula is involved in evaluation of various negative emotional states, such as disgust73, its activation during the ultimatum game might reflect negative emotions associated with unfair offers. In addition, the investors who have the option of punishing unfair trustees during the trust game often apply costly punishment74. Such punishment may have some hedonic value to the investors, since activity in the caudate nucleus of the investor was correlated with the magnitude of punishment and increased only when this punishment was effective. By comparing the proposer’s brain activity during the ultimatum game and dictator game, a recent study has also found that the dorsolateral prefrontal cortex, lateral orbitofrontal cortex, and caudate nucleus play an important role in evaluating the threat of potential punishment75.
Inequality aversion can give rise to not only altruistic punishment of norm violators but also charitable donation. It has been found that the mesolimbic dopamine system, including the ventral tegmental area and the striatum, is activated by both personal monetary reward and the decisions to donate money to charity76. In contrast, the activity of the lateral orbitofrontal cortex increased when the decision makers opposed the charitable organization by refusing donations. Activity in the caudate nucleus and ventral striatum increased with the amount of money donated to a charity, even when this was mandatory77. However, the activity in both of these areas was higher when the donation was voluntary.
Although fairness norms have strong influence on social decision making, what is considered fair is likely to depend on various contextual factors, such as the sense of entitlement78 and the need for competitive interactions with other players79. Similarly, when two participants play the same task and receive the monetary reward for correct answers, the activity in the ventral striatum increased with the amount of money paid to the subject but decreased with the amount of money earned by its partner80. In other words, when the subjects are evaluated and rewarded by the same criterion, the activity in the ventral striatum was more closely related to the subject’s relative payment compared to the partner’s payment than the absolute personal payment of the subject. This raises the possibility that the striatal response to the reward received by others might change depending on whether a particular social interaction is perceived as competition or cooperation. Indeed, during a board game in which the subjects were required to interact with each other competitively or cooperatively, a number of brain areas were activated differentially depending on the nature of interaction81. For example, compared to competition, cooperation resulted in stronger activation in the anterior frontal cortex and medial orbitofrontal cortex. However, whether and how these cortical areas influence the striatal activity related to social preference is currently not known.
Social decision making frequently requires theory of mind, namely, the ability to predict the actions of other players based on their knowledge and intentions82,83. Many of neuroimaging studies on experimental games have found that social interactions with human players produce stronger activations in several brain areas, often in the anterior paracingulate cortex (Fig 3c), than similar interactions with computer players84–86. Assuming that more sophisticated inferences are used to deal with human players than with computer players, such findings might provide some clues on the cortical areas specialized for theory of mind. Accordingly, it has been suggested that the anterior paracingulate cortex might play an important role in representing mental states of others82, 84–8. Using the trust game, a recent study has also identified a unique role of the cingulate cortex in representing the information about the agent responsible for a particular outcome89. The cortical network involved in theory of mind and perception of agency is, however, still not well characterized, and is likely to involve additional areas. For example, the posterior superior temporal cortex has been implicated in perception of agency83,86,88, and its activity is correlated with the subject’s tendency for altruistic behavior90.
Genetic variations in social decision making
The fitness value of many social behaviors, such as cooperation with genetically unrelated individuals, often depends on various environmental conditions, including the prevalence of individuals with the same behavioral traits. Thus, individual traits related to social decision making could remain heterogeneous in the population, because the selective forces favoring different traits might be balanced91. Indeed, studies on experimental games commonly reveal substantial individual variability in the behaviors of decision makers, and neuroimaging studies on social behaviors found that activity in several brain areas, such as the striatum and insula, is correlated with the decision maker’s tendency to display altruistic behaviors69,73,74,75. Some of this variability might be due to genetic factors. For example, the minimum acceptable offer during an ultimatum game is more similar between monozygotic twins than between dizygotic twins92.
The genetic mechanisms regulating the synaptic transmission for dopamine and serotonin might underlie individual differences in behaviors and neural circuitries implicated in reinforcement learning and therefore also contribute to the individual variability in social decision making. Among the genes related to dopamine functions, dopamine receptor D2 (DRD2) gene has received much attention. For example, DRD2 polymorphism, such as Taq 1A and C957T, influences how efficiently the decision makers can modify their choice behaviors according to the negative consequences of their previous actions93,94. Taq 1A polymorphism also influenced the magnitude of fMRI signals related to negative feedback93. In contrast, polymorphism in the dopamine- and cAMP-regulated phosphoprotein of molecular weight 32 kDa (DARPP-32) influences the rate of learning based on positive outcomes, whereas Val/Met substitution in catechol-O-methyltransferase (COMT) might influence the ability to adjust the choice behavior rapidly on a trial-by-trial basis by modulating the dopamine level in the prefrontal cortex56,94. Social decision making might be also influenced by the genes involved in the serotonin metabolism, such as serotonin (5-HT) transporter-linked polymorphism (5-HTTLPR)95,96. For example, rhesus monkeys carrying only the short variant of rhesus 5-HTTLPR displayed reduced abilities to switch in object discrimination reversal learning and displayed a higher level of aggression97. Little is known, however, about the neurophysiological changes associated with such genetic variability that might underlie behavioral changes in social decision making. It should be also emphasized that any effects of genetic variability on such complex behaviors as social decision making are likely to involve interactions among multiple genes and among genes and environment96,98.
Conclusion
Social decision making represents one of the most complex animal behaviors, and it oftenrequires the animals to recognize the intentions of other animals correctly and to adjust their behavioral strategies rapidly. In addition, humans can cooperate or compete with one another, and institutional and other contextual factors influence the extent to which humans would sacrifice their personal gains in order to increase or decrease the well-beings of others. As demonstrated by a number of recent studies, the neural basis of such complex social decision making can be investigated quantitatively by applying game theory. These studies have found that the key brain areas involved reinforcement learning, such as the striatum and orbitofrontal cortex, also underlie choices made in social settings. In addition, although it was not focused in this article, the influence of specific hormones on social behavior has been demonstrated. For example, a high level of testosterone increases the likelihood that the recipient would reject relatively low offers during the ultimatum game99, and oxytocin increases the amount of money transferred by the investor during the trust game100. Nevertheless, our current knowledge of neural mechanisms for social decision making is still limited. This will improve as we understand better the genetic and neurophysiological basis of information processing in the brain’s reward system.
Acknowledgments
I am grateful to Michael Frank for helpful discussions. This research was supported by the National Institute of Health (MH073246 and DA024855).
References
- 1.Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–92. [Google Scholar]
- 2.Sutton RS, Barton AG. Reinforcement learning: an introduction. MIT Press; Cambridge, Massachusetts, USA: 1998. [Google Scholar]
- 3.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–38. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 4.Brieter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functionalimaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 5.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 6.Glimcher PW, Rustichini A. Neuroeconomics: the consilience of brain and decision. Science. 2004;306:447–52. doi: 10.1126/science.1102566. [DOI] [PubMed] [Google Scholar]
- 7.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 9.Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006;51:381–90. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 11.O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–37. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 12.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–46. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 13.O’Doherty J, et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–54. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 14.Tricomi EZ, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–92. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 15.Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–79. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- 16.Fudenberg D, Levine DK. The theory of learning in games. MIT Press; Cambridge, Massachusetts, USA: 1998. [Google Scholar]
- 17.Camerer CF. Behavioral game theory: experiments in strategic interaction. Princeton Univ. Press; Princeton, New Jersey, USA: 2003. [Google Scholar]
- 18.von Neumann J, Morgenstern O. Theory of games and economic behavior. Princeton Univ. Press; Princeton, New Jersey, USA: 1944. [Google Scholar]
- 19.Nash JF. Equilibrium points in n-person games. Proc Natl Acad Sci USA. 1950;36:48–9. doi: 10.1073/pnas.36.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erev I, Roth AE. Predicting how people play games: reinforcement learning in experimental games with unique, mixed strategy equilibria. Am Econ Rev. 1998;88:848–81. [Google Scholar]
- 21.Lee D, Conroy ML, McGreevy BP, Barraclough DJ. Reinforcement learning and decision making in monkeys during a competitive game. Cogn Brain Res. 2004;22:45–5. doi: 10.1016/j.cogbrainres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Sally D. Conversation and cooperation in social dilemmas. Ration Soc. 1995;7:58–92. [Google Scholar]
- 23.Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425:785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 24.Hauser MD, Chen MK, Chen F, Chuang E. Give unto others: genetically unrelated cotton-top tamarin monkeys preferentially give food to those who altruistically give food back. Proc R Soc Lond B. 2003;270:2363–2370. doi: 10.1098/rspb.2003.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warneken F, Tomasello M. Altruistic helping in human infants and young chimpanzees. Science. 2006;311:1301–1303. doi: 10.1126/science.1121448. [DOI] [PubMed] [Google Scholar]
- 26.de Waal FBM. Putting the altruism back into altruism: the evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 27.Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314:1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clutton-Brock TH, Parker GA. Punishment in animal societies. Nature. 1995;373:209–216. doi: 10.1038/373209a0. [DOI] [PubMed] [Google Scholar]
- 29.Fehr E, Gächter S. Altruistic punishment in humans. Nature. 2002;415:137–140. doi: 10.1038/415137a. [DOI] [PubMed] [Google Scholar]
- 30.Boyd R, Gintis H, Bowles S, Richerson PJ. The evolution of altruistic punishment. Proc Natl Acad Sci USA. 2003;100:3531–3535. doi: 10.1073/pnas.0630443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehr E, Schmidt KM. A theory of fairness, competition, and cooperation. Q J Econ. 1999;114:817–868. [Google Scholar]
- 32.Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn Sci. 2007;11:419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Sanfey AG. Social decision-making: insights from game theory and neuroscience. Science. 2007;318:598–602. doi: 10.1126/science.1142996. [DOI] [PubMed] [Google Scholar]
- 34.Jensen K, Call J, Tomasello M. Chimpanzees are rational maximizers in an ultimatum game. Science. 2007;318:107–109. doi: 10.1126/science.1145850. [DOI] [PubMed] [Google Scholar]
- 35.Sandholm TW, Crites RH. Multiagent reinforcement learning in the iterated prisoner’s dilemma. Biosystems. 1996;37:147–166. doi: 10.1016/0303-2647(95)01551-5. [DOI] [PubMed] [Google Scholar]
- 36.Lee D, McGreevy BP, Barraclough DJ. Learning and decision making in monkeys during a rock-paper-scissors game. Cogn Brain Res. 2005;25:416–430. doi: 10.1016/j.cogbrainres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Lohrenz T, McCabe K, Camerer CF, Montague PR. Neural signature of fictive learning signals in a sequential investment task. Proc Natl Acad Sci USA. 2007;104:9493–9498. doi: 10.1073/pnas.0608842104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coricelli G, Dolan RJ, Sirigu A. Brain, emotion and decision making: the paradigmatic example of regret. Trends Cogn Sci. 2007;11:258–265. doi: 10.1016/j.tics.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Mookherjee D, Sopher B. Learning and decision costs in experimental constant sum games. Games Econ Behav. 1997;19:97–132. [Google Scholar]
- 40.Feltovich N. Reinforcement learning vs. belief-based learning models in experimental asymmetric-information games. Econometrica. 2000;68:605–641. [Google Scholar]
- 41.Nowak MA, Sigmund K. Evolution of indirect reciprocity by image scoring. Nature. 1998;393:573–577. doi: 10.1038/31225. [DOI] [PubMed] [Google Scholar]
- 42.Wedekind C, Milinski M. Cooperation through image scoring in humans. Science. 2000;288:850–852. doi: 10.1126/science.288.5467.850. [DOI] [PubMed] [Google Scholar]
- 43.Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nat Neurosci. 2005;8:1611–1618. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- 44.Kawato M, Samejima K. Efficient reinforcement learning: computational theories, neuroscience and robotics. Curr Opin Neurobiol. 2007;17:205–212. doi: 10.1016/j.conb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 46.Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci. 2007;10:1615–1624. doi: 10.1038/nn2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- 50.Cromwell HC, Schultz W. Effects of expectations for different reward magnitude on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- 51.Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310:1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- 52.Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304:1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- 53.Dorris MC, Glimcher PW. Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron. 2004;44:365–378. doi: 10.1016/j.neuron.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- 55.Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24:415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- 56.Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- 57.Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- 58.Seo H, Lee D. Temporal filtering of reward signals in the dorsal anterior cingulate cortex during a mixed-strategy game. J Neurosci. 2007;27:8366–8377. doi: 10.1523/JNEUROSCI.2369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sohn JW, Lee D. Order-dependent modulation of directional signals in the supplementary and presupplementary motor areas. J Neurosci. 2007;27:13655–13666. doi: 10.1523/JNEUROSCI.2982-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 61.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 62.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee D, Rushworth MFS, Walton ME, Watanabe M, Sakagami M. Functional specialization of the primate frontal cortex during decision making. J Neurosci. 27:8170–8173. doi: 10.1523/JNEUROSCI.1561-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Doherty JP. Reward representation and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 65.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 66.Montague PR, King-Casas BK, Cohen JD. Imaging valuation models in human choice. Annu Rev Neurosci. 2006;29:417–448. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- 67.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 68.Tsujimoto S, Sawaguchi T. Neuronal representation of response-outcome in the primate prefrontal cortex. Cereb Cortex. 2004;14:47–55. doi: 10.1093/cercor/bhg090. [DOI] [PubMed] [Google Scholar]
- 69.Rilling JK, et al. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 70.Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohern JD. Opposing BOLD responses to reciprocated and unreciprocated altruism in putative reward pathways. Neuroreport. 2004;15:2539–2543. doi: 10.1097/00001756-200411150-00022. [DOI] [PubMed] [Google Scholar]
- 71.King-Casas B, et al. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- 72.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision making in the ultimatum game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 73.Phillips ML, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- 74.de Quervain DJF, et al. The neural basis of altruistic punishment. Science. 2004;305:1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- 75.Spitzer M, Fischbacher U, Herrnberger B, Grön G, Fehr E. The neural signature of social norm compliance. Neuron. 2007;56:185–196. doi: 10.1016/j.neuron.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 76.Moll J, et al. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- 78.Hoffman E, McCabe K, Shachat K, Smith V. Preferences, property rights, and anonymity in bargaining games. Games Econ Behav. 1994;7:346–380. [Google Scholar]
- 79.Schotter A, Weiss A, Zapater I. Fairness and survival in ultimatum and dictatorship games. J Econ Behav Organ. 1996;31:37–56. [Google Scholar]
- 80.Fliessbach K, et al. Social comparison affects reward-related brain activity in the human ventral striatum. Science. 2007;318:1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- 81.Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage. 2004;23:744–751. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallagher HL, Frith CD. Functional imaging oftheory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 83.Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 84.McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proc Natl Acad Sci USA. 2001;98:11832–11835. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gallagher HL, Jack AI, Roepstorff A, Frith CD. Imaging the intentional stance in a competitive game. Neuroimage. 2002;16:814–821. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- 86.Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. The neural correlates of theory of mind with interpersonal interactions. Neuroimage. 2004;22:1694–1703. doi: 10.1016/j.neuroimage.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 87.Bhatt M, Camerer CF. Self-referential thinking and equilibrium as states of mind in games: fMRI evidence. Games Econ Behav. 2005;52:424–459. [Google Scholar]
- 88.Fukui H, et al. The neural basis of social tactics: an fMRI study. Neuroimage. 2006;32:913–920. doi: 10.1016/j.neuroimage.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 89.Tomlin D, et al. Agent-specific responses in the cingulate cortex during economic. Science. 2006;312:1047–1050. doi: 10.1126/science.1125596. [DOI] [PubMed] [Google Scholar]
- 90.Tankersley D, Stowe CJ, Huettel SA. Altruism is associated with an increased neural response to agency. Nat Neurosci. 2007;10:150–151. doi: 10.1038/nn1833. [DOI] [PubMed] [Google Scholar]
- 91.Penke L, Denissen JJA, Miller GF. The evolutionary genetics of personality. Eur J Pers. 2007;21:549–587. [Google Scholar]
- 92.Wallace B, Cesarini D, Lichtenstein P, Johannesson M. Heritability of ultimatum game responder behavior. Proc Natl Acad Sci USA. 2007;104:15631–15634. doi: 10.1073/pnas.0706642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klein TA, et al. Genetically determined differences in learning from errors. Science. 2007;318:1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- 94.Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci USA. 2007;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 96.Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- 97.Izquierdo A, Newman TK, Higley JD, Murray EA. Genetic modulation of cognitive flexibility and socioemotional behavior in rhesus monkeys. Proc Natl Acad Sci USA. 2007;104:14128–14133. doi: 10.1073/pnas.0706583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yacubian J, et al. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci USA. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burnham TC. High-testosterone men reject low ultimatum game offers. Proc R Soc B. 2007;274:2327–2330. doi: 10.1098/rspb.2007.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]


