Abstract
Prenatal choline supplementation (SUP) protects adult rats against spatial memory deficits observed after excitotoxin-induced status epilepticus (SE). To examine the mechanism underlying this neuroprotection, we determined the effects of SUP on a variety of hippocampal markers known to change in response to SE and thought to underlie ensuing cognitive deficits. Adult offspring from rat dams that received either a Control or SUP diet on embryonic days 12–17 were administered saline or kainic acid (i.p.) to induce SE and were euthanized 16 days later. SUP markedly attenuated seizure-induced hippocampal neurodegeneration, dentate cell proliferation, hippocampal GFAP mRNA expression levels, prevented the loss of hippocampal GAD65 protein and mRNA expression, and altered growth factor expression patterns. SUP also enhanced pre-seizure hippocampal levels of BDNF, NGF, and IGF-1, which may confer a neuroprotective hippocampal microenvironment that dampens the neuropathological response to and/or helps facilitate recovery from SE to protect cognitive function.
Keywords: choline, kainic acid, hippocampus, seizures, bromodeoxyuridine, growth factor, glutamic acid decarboxylase, glial fibrillary acidic protein, neuroprotection
Status epilepticus (SE), a period of prolonged seizures, produces a host of plastic changes in the hippocampus that are thought to contribute to the development of temporal lobe epilepsy. SE results in substantial neuronal loss (Cavazos et al., 1994; Haas et al., 2001; Gorter et al., 2003), γ-aminobutyric acid (GABA) system alterations (e.g., Houser & Esclapez, 1996), reactive gliosis (Jorgensen et al. 1993; Niquet et al., 1994a; Kang et al., 2006), mossy fiber innervation of the dentate gyrus (Sutula et al., 1988; Ben-Ari & Represa, 1990), changes in levels of growth factors (Khrestchatisky et al., 1995; Mudo et al., 1996; Schmidt-Kastner et al., 1996; Shetty et al., 2004), and a transient increase in cell proliferation and neurogenesis (Bengzon et al., 1997; Parent et al., 1997; Scharfman et al., 2000; Hattiangady et al., 2004). These SE-induced degenerative and regenerative changes in the hippocampus are also accompanied by deficits in hippocampal-dependent learning and memory (Stafstrom et al., 1993; Liu et al., 1994; Sarkisian et al., 1997; Hort et al., 1999; Mikati et al., 2001). Understanding the relationship between the hippocampal response to SE and its cognitive consequences may lead to new insights into the treatment of SE and epilepsy.
Remarkably, dietary choline supplementation has been shown to protect rats from seizure-induced spatial memory retention deficits normally observed after SE (Yang et al., 2000; Holmes et al., 2002). Prenatal and early postnatal choline supplementation has also been shown to protect against impairments in performance on hippocampal-dependent tasks caused by aging (Meck et al., 1988, 1989; Meck & Williams, 2003; McCann et al., 2006) or neonatal alcohol exposure (Thomas et al., 2004, 2007; Wagner & Hunt, 2006). While the mechanism by which prenatal choline supplementation confers neuroprotection is not fully understood, choline is a vital nutrient important for several biological functions: acetylcholine synthesis, building biological membranes, cell signaling, and methyl donation (Blusztajn, 1998; Zeisel, 2004, 2006). Prenatal choline supplementation also enhances several features of adult hippocampal plasticity known to influence learning and memory function, such as increased baseline levels of neurogenesis, brain-derived neurotrophic factor (BDNF) (Glenn et al., 2007) and nerve growth factor (NGF) (Sandstrom et al., 2002); a reduced threshold to induce long-term potentiation (Pyapali et al., 1998; Jones et al., 1999); and enhanced depolarization-induced mitogen-activated protein kinase (MAPK) and cAMP-response element binding protein (CREB) activation (Mellott et al., 2004). Enhanced hippocampal plasticity may underlie prenatal choline supplementation’s neuroprotection of the hippocampus, rendering the hippocampus better able to withstand or recover from the deleterious effects of a neural insult.
Given that prenatal choline supplementation has been shown to protect against seizure-induced memory loss on a hippocampal-dependent task (Yang et al., 2000; Holmes et al., 2002), we hypothesized that prenatal choline supplementation alters the neurophysiological response of the hippocampus to SE. To investigate this, we used a model of excitotoxic injury to examine whether prenatal choline availability modulates a variety of markers known to change shortly after SE, including hippocampal histopathology, glutamic acid decarboxylase (GAD) expression, dentate cell proliferation, neurogenesis, astrogliosis, and growth factor content.
Materials and Methods
Animals
Twenty timed-pregnant Sprague-Dawley rats (CD strain, Charles River, Kingston, NY) were obtained on day 9 of gestation (ED9). All dams were individually housed in clear polycarbonate cages (27.9×27.9×17.8 cm) that were individually ventilated, and the colony was maintained at 21°C on a 12-h light/dark cycle with lights on at 7 a.m. Dams were fed a control diet ad libitum (AIN76-A from Dyets, American Institute of Nutrition, ICN, Nutritional Biochemical, Cleveland, Ohio; 1.1 g/kg choline chloride substituted for choline bitartrate). Prenatal diet treatments were the same as those used in studies showing memory enhancing and memory protecting effects of prenatal choline supplementation (Meck et al., 1988, 1989; Yang et al., 2000; Holmes et al., 2002; Meck & Williams, 2003). On the morning of ED11 to the morning of ED18 (ED 11–17), pregnant dams were either given ad libitum access to a control diet (n = 14) or a choline supplemented diet (n = 6). Control dams were given the AIN76-A diet containing 7.9 mmol/kg choline chloride and water sweetened with 50 mM saccharine, resulting in an average daily choline intake of 0.59 mmol/kg/day. Choline supplemented dams received the AIN76-A diet containing 7.9 mmol/kg choline chloride and water containing 25 mM choline chloride and sweetened with 50 mM saccharine, resulting in an average daily choline intake of 3.46 mmol/kg/day (approximately 6.8 times more choline than the control diet). On ED18, all dams were returned to normal drinking water. There were no significant differences in the amount of water intake, food consumed, or body weights on ED11–18 between control and choline supplemented dams (ps > .05; data not shown). After birth, offspring from the control and choline supplemented dams were toe clipped for identification and then were selected randomly and cross-fostered to dams that consumed the control diet throughout pregnancy to yield 10 pups per litter (5 males and 5 females, half from different control dams and half from supplemented dams). There were no significant differences between control and prenatally choline supplemented litter size or pup birth weights (ps > .05). On postnatal day (P) 25, pups were weaned and pair-housed with a rat of the same sex and prenatal diet condition. All offspring were given ad libitum access to the control diet through the duration of the study. Male offspring were used as subjects. All animal procedures were in compliance with the Institutional Animal Care and Use Committee of Duke University.
Induction of status epilepticus and bromodeoxyuridine injections
Status epilepticus procedures were adapted from previous reports and were based on a model of chronic temporal lobe epilepsy that yields a low mortality rate (Hellier et al., 1998; Hellier & Dudek, 1999; Hattiangady et al., 2004). Kainic acid (KA) was obtained from Ocean Produce International (Shelburne, Nova Scotia, Canada) and was dissolved in 0.9% sterile saline (Sigma, St. Louis, MO). On P60, a group of adult male control offspring (CON, n = 11) and prenatally choline supplemented offspring (SUP, n = 11) were injected with KA (2.5 mg/kg, i.p.) every hour and observations of seizure behavior were recorded according to Racine’s scale (Racine, 1972). A separate group of CON (n = 4) and SUP (n = 4) male offspring was similarly treated with hourly injections of 1 ml/kg saline. All KA-treated animals initially showed wet dog shakes and head-nodding, followed by class III (unilateral forelimb clonus), class IV (bilateral forelimb clonus with rearing), and class V (bilateral forelimb clonus with rearing and falling over) motor seizures. KA treatment continued until rats reached status epilepticus, which was defined as at least one class IV/V seizure per hour for 3 consecutive hours. If a rat displayed ≥10 class IV/V seizures in an hour, administration of the next injection was delayed to the next hour. If a rat displayed bouncing seizures after reaching status epilepticus, it was given a low dose of diazepam (2.5 mg/kg) to reduce the risk of mortality. We have observed and others have demonstrated (Scharfman et al., 2000; Pitkanen et al., 2005) that administering a low dose of diazepam following status epilepticus does not compromise the development of spontaneous epileptic motor seizures. The total KA dosage administered was titrated for each rat according to each rat’s motor seizure activity. Because it has been found that seizures, as opposed to neuronal cell death per se, lead to increases in hippocampal cell proliferation and neurogenesis (Parent et al., 1997; Tooyama et al., 2002; Smith et al., 2005) our procedures were designed such that all KA-treated rats experienced similar duration and severity of seizure activity.
After KA treatment, rats were injected subcutaneously with 5 ml of lactated Ringer’s solution (Abbott Laboratories, Chicago, IL) to prevent dehydration and were monitored closely until all seizure activity subsided. Saline-treated rats were also given 5 ml of lactated Ringer’s solution to equate their post-treatment experience to that of KA-treated rats. For the next 3 to 5 days, all rats continued to receive daily subcutaneous injections of 1–5 ml of lactated Ringer’s solution and were provided with moistened chow and slices of fresh fruit to aid recovery. Mortality rates did not differ significantly between CON rats (2/11) and SUP rats (3/11), χ2 (1, N = 22) = 0.26, p = .61.
Five days after being treated with KA or saline, all rats were administered daily injections of 5-bromo-2-deoxyuridine (BrdU; 100mg/kg/day, i.p.; Sigma, St. Louis, MO) for 10 consecutive days to label dividing cells. This injection regimen was based on past research designed to capture the impact of a variety of manipulations on cell proliferation and survival in the hippocampus (Kempermann et al., 1997; Lee, et al., 2002a; Rao et al., 2005; Glenn et al., 2007), and to parallel the time point that prenatal choline protects against seizure-induced memory deficits (Yang et al., 2000; Holmes et al., 2002).
Tissue harvesting and cresyl violet staining
Twenty-four hours after the last BrdU injection, rats were given an overdose of a ketamine/xylazine cocktail, decapitated, and brains were rapidly removed and midsagitally sectioned. The selection of this particular time point (16 days post-KA/saline treatment) was based on 1) the findings reported in Hattiangady et al. (2004) that showed robust increases in hippocampal neurogenesis 16 days following an analogous KA treatment, and 2) prenatal choline supplementation’s protection against memory deficits was observed 1–2 weeks after excitotoxin-induced status epilepticus (Yang et al., 2000; Holmes et al., 2002), which approximates the current time window we have chosen. The hippocampus from one half-brain was immediately dissected for mRNA and protein analyses and stored at −80° C until assayed. The other half-brain from each rat was immediately post-fixed in 4% paraformaldehyde for 72 hours at 4° C and then cryoprotected in a 30% sucrose solution in 1M phosphate buffer (PB). These half-brains were then sectioned coronally at 60 µm on a microtome through the rostral-caudal extent of the hippocampus and every fifth section was collected in 0.1% sodium azide in 1M PB to yield five series of 15–20 sections each. The first and second series were processed for BrdU and doublecortin (DCX) immunohistochemistry, respectively, for subsequent cell counting. Representative samples of four to five 60 µm sections that captured the rostral-caudal extent of the hippocampus were randomly selected from the third series and processed for cresyl violet staining.
Sections stained for cresyl violet were analyzed and scored for lesion severity by 2 observers blind to the treatment conditions. The following scale, adapted from Yang et al. (2000) and Holmes et al. (2002), was used to characterize each rat’s hippocampal cytopathology: a score of 0 indicated no cell loss, a score of 1 indicated minimal cell loss in CA1, CA3, and/or hilar regions, a score of 2 indicated moderate cell loss with relatively normal general cellular architecture, and a score of 3 indicated severe cell loss with considerable disruption of the general cellular architecture.
BrdU immunohistochemistry
Immunohistochemical procedures for BrdU-labeling were based on the methods of Kuhn et al. (1996). One series of free-floating sections was rinsed with tris-buffered saline (TBS: pH 7.3) followed by 30 minutes in 50% methanol and 30 minutes in 0.6% hydrogen peroxide in TBS at room temperature to reduce nonspecific staining. After rinsing again in TBS, tissue was treated for 2 hours in 50% Formamide/2x SSC (0.3 M NaCl, 0.03 M sodium citrate) at 65°C, rinsed in 2x SSC for 10 minutes, incubated in 2 N HCl for 30 minutes at 37°C, and rinsed in 0.1 M boric acid (pH 8.5) for 15 minutes. Sections were rinsed in TBS, incubated in 0.1% Triton X-100 (TTX; Sigma) and 3% normal horse serum (Vector Laboratories, Burlingame, CA) in TBS for 30 minutes at room temperature, and then incubated with the primary antibody (monoclonal mouse anti-BrdU, 1:400; Boehringer Mannheim, Indianapolis, IN) for 24 hours at 4 °C. Following this, the tissue was rinsed with TBS and incubated with the secondary antibody (biotinylated horse anti-mouse, 1:200; Vector Laboratories) for 2 hours at room temperature. The tissue was then rinsed in TBS, incubated in an avidin-biotinylated peroxidase complex (ABC, Vector Laboratories) for 1 hour at room temperature, rinsed again in TBS, and treated for peroxidase detection with diaminobenzidine (Vector Laboratories, nickel intensified) for 5 minutes. Stained sections were mounted on gelatin-coated slides, dehydrated, counterstained with cresyl violet, and coverslipped.
Doublecortin immunohistochemistry
An adjacent series of tissue sets was processed for doublecortin (DCX) immunohistochemistry. These procedures were based on the methods of Rao & Shetty (2004). Free-floating sections were rinsed in TBS, treated for 30 minutes in 50% methanol, treated for 30 minutes in 0.6% hydrogen peroxide, incubated in 0.1% TTX and 3% normal horse serum in TBS for 30 minutes at room temperature, and then incubated with the primary antibody (affinity purified polyclonal goat antibody raised against a peptide mapping at the carboxy terminus of human DCX, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA) for 4 hours at room temperature. Following this, the tissue was rinsed with TBS and incubated with the secondary antibody (biotinylated horse antigoat, 1:200; Vector Laboratories) for 2 hours at room temperature. The tissue was then rinsed in TBS, incubated in an avidin-biotinylated peroxidase complex (ABC, Vector Laboratories) for 1 hour at room temperature, rinsed again in TBS, and developed with vector grey substrate (Vector Laboratories). Stained sections were mounted on gelatin-coated slides, dehydrated, and coverslipped.
Quantification of BrdU- and DCX-labeled cells using unbiased stereology
BrdU- and DCX-labeled cells in each dentate gyrus were counted using the optical fractionator method (West, 1993, 1999; Mouton, 2002). We sampled every fifth section through the rostral-caudal extent of the dentate gyrus and used two separate sampling regions; one region encompassed the dorsal and ventral blades, including the granule cell layer and subgranular zone, and the other region included the hilus. StereoInvestigator (Microbrightfield Inc., Williston, VT) was used to sample systematically through the designated region and count numbers of labeled cells. For counting BrdU-labeled cells, we used a 40 × 40 µm counting frame and 14–41 sites per section were analyzed in 8 sections, yielding 146–232 frames per region for each rat. These parameters ensured adequate sampling of BrdU-labeled cells through the dentate gyrus. The same parameters were used for counting DCX-labeled cells with the exception of a larger counting frame (80 × 80 µm) to compensate for the more sporadic distribution of DCX-labeled cells, yielding 13–33 sites per section and 117–227 frames per region analyzed for each rat. For analysis we set an optical dissector height of 20 µm with a 2-µm guard zone and counted stained cells in each frame using a 40x objective lens. Finally, estimates of the volume of the region of dentate gyrus that was sampled for BrdU and DCX estimates were made using Cavalleri’s principle (Mouton, 2002). For each section examined, the area of the dentate gyrus was calculated by the StereoInvestigator software and was based on the boundaries of the contour tracings. Volume estimates were obtained by multiplying the section area estimates with the spacing between sampled areas. Spacing was derived by multiplying the measured, post-histology thickness of each sample by the number of sections examined.
ELISA for neurotrophic and growth factors
The entire hippocampus from the remaining half-brain from each rat was carefully dissected out, divided into 4–6 pieces along the rostral-caudal extent of the hippocampus, and half of the pieces were immediately stored at −80° C until assayed. The remaining pieces were used for reverse transcriptase PCR assays (see Reverse transcriptase PCR section). For ELISA assays, all dissected samples were first weighed individually to get their wet weights. Whole tissue extracts were prepared by adding lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% Nonidet NP-40, 10% glycerol, 2 mM 4-(2-aminoethyl)- benzenesulfonyl fluoride, 1 µg/ml leupeptin, 2 µg/ml aprotinin, 2 µg/ml pepstatin), followed by gentle sonication, incubation on ice for 15 min, and a brief centrifugation to clear. The supernatant from each sample was diluted 5 times with Dulbecco’s PBS and acidified to pH 2.6. After 15 min of incubation at room temperature, the diluted supernatants were neutralized to pH 7.6, aliquoted and frozen for subsequent measurement of brain-derived neurotrophic factor (BDNF), insulin growth-like factor-1 (IGF-1), fibroblast growth factor-2 (FGF-2), nerve growth factor (NGF), or neurotrophin-3 (NT-3) using ELISA. The above procedure was performed for all samples because a previous study indicated that acidification and subsequent neutralization with base increase the amount of detectable neurotrophins in extracts of CNS tissues (Okragly and Haak-Frendscho, 1997).
The ChemiKine™ BDNF sandwich ELISA kit (Chemicon Int., Inc.) was used to assay the BDNF levels in hippocampal lysates. BDNF levels were measured according to manufacturer’s instructions. First, sample/standard diluent was added to each well of the microplate (100 µL/well). The standards were serially diluted (1:2) from 500-0 pg/mL. Standards and samples (100 µL/well) were added to wells in duplicate and were incubated overnight at 4°C. The wells were rinsed with wash buffer and then incubated with diluted biotinylated mouse anti-BDNF monoclonal antibody (1:1000) for 2 h at room temperature. After rinsing the plate again with wash buffer, diluted streptavidin-HRP conjugate solution (1:1000) was added to wells and incubated for 1 h at room temperature. Plates were again washed and then warm TMB buffer was added. After 15 min incubation at room temperature, stop solution was added.
The Quantikine® sandwich ELISA kits (R&D Systems) were used to assay IGF-1 and FGF-2 levels in the samples. ELISAs were performed according to manufacture’s instructions. Briefly, microplates were pre-coated with the first primary antibody (monoclonal in IGF-1 and FGF-2 kits). Assay diluents were added to each well of the microplate (50 µL/well for IGF-1 assay and 100 µL/well for FGF-2 assay). Standard control samples for each neurotrophic factor were diluted serially (1:2) from either 6–0 ng/mL (IGF-1) or 32–0 pg/mL (FGF-2) or with their respective calibrator diluents and plated to two columns of wells (50 µL/well in IGF-1 assay and 100 µL/well in FGF-2 assay) designated for standard curve in every plate. The frozen ELISA samples (described above) were thawed on ice, and every sample plated in duplicate for measurement of each of the factors. Following a 2 h incubation at room temperature, wells were rinsed in wash buffer and treated with an enzyme-linked second primary antibody solution for 2 hours. The second primary antibody was a polyclonal IGF-1 antibody conjugated to HRP in IGF-1 detection kit or a monclonal FGF-2 antibody conjugated to alkaline phosphatase in FGF-2 detection kit. The wells were rinsed in wash buffer and a substrate solution was added to the wells and incubated in the dark for 30 min for IGF-1 or 45 min for FGF-2. The color reaction was stopped with 1M hydrochloric acid in the IGF-1 assay or with 2N sulfuric acid in the FGF-2 assay.
The Emax® immunoassay systems (Promega) were used to measure NGF and NT-3 in the samples. Flat-bottom 96 well plates (NUNC) were first coated with solution containing a polyclonal antibody against either NGF or NT-3 (the first primary antibody solution) prepared in carbonate coating buffer (100 µL/well, 1:1000 dilution) and incubated for 16 h at 4°C. Following a wash in TBST (Tris-buffered saline solution containing Tween 20), the coated wells were incubated with block and sample buffer (1X) for 1 h at room temperature and washed again with TBST. Standard control samples for NGF and NT-3 were diluted serially (1:2) from 250–0 pg/mL or 300–0 pg/mL, respectively, and plated in duplicate (100 µL/well). The frozen ELISA samples (described above) were thawed on ice, and every sample plated in duplicate for measurement of NGF or NT-3. Following a 6 h incubation at room temperature, wells were washed in TBST. Diluted monoclonal antibody against either NGF or NT-3 (the second primary antibody solution; 1:4000) was added to each well and incubated overnight at 4°C. The wells were washed in TBST, incubated with appropriate secondary antibody conjugated to peroxidase for 2.5 h, washed again in TBST, and treated with tetramethyl benzidine (TMB) substrate for 10 min. The chromogen reaction was stopped by adding 100 µL of 1N hydrochloric acid.
The optical density of each well was measured using the Victor3 microplate reader (PerkinElmer Life Sciences). The intensity of color was measured at a wavelength of 450 nm for all ELISAs. In order to correct for optical imperfections in the plate, readings at 540 nm were subtracted from readings at 450 nm. The standard curve was used to assess the validity of the protocol and to determine the relative concentrations of the growth factors. Values in all samples were normalized per gram of tissue assayed, and the average value for each sample was calculated separately before determining the group means.
Western blot analysis for GAD65, GAD67, and GFAP
For Western blot analysis, the lysates were prepared as described in the ELISA methods. However, a portion of the lysate was taken before dilution in Dulbecco’s PBS. The extracts were normalized for total protein and subjected to SDS-PAGE. After transfer of protein to an Immoblin P membrane (Millipore), the membrane was blocked with 5% nonfat dry milk in 1X Tris-buffered saline (TBS) containing 0.1% Tween 20 for 2 hours and then probed overnight with either anti-glial fibrillary acidic protein (GFAP) monoclonal antibody GA5 (1:1000) (Cell Signaling Technology), anti-GAD65/67 polyclonal antibody (1:1000) (Chemicon), or anti-β-actin monoclonal A5441 (1:5000; Sigma). The antibody/antigen complexes on the membranes were detected using a peroxidase-conjugated anti-mouse IgG for GFAP and β-actin (1:2000) or anti-rabbit IgG (1:5000) for GAD65/67 and visualized using the enhanced chemiluminescence method (Western Lightning, Perkin Elmer) and a Kodak Image Station 440. Digitized images of immunoblots were quantified using Kodak ID software. Protein levels were normalized with β-actin values.
Reverse transcriptase PCR
The remaining pieces of the hippocampus dissected out from the remaining half-brain from each rat were immediately homogenized in cold guanidine isothiocyanate solution, frozen on dry ice, and stored at −80°C. Total RNA was extracted from tissues by phenol and chloroform method (Chomczynski and Sacchi, 1987) and precipitated. RNA was resuspended and its quantity was determined using Quant-iT™ RiboGreen® RNA assay kit (Molecular Probes) and the Victor3 multilabel plate reader (PerkinElmer Life Sciences). Hippocampal RNA was used for reverse transcriptase (RT)-PCR using Superscript One-Step RT-PCR with Platinum Taq (Invitrogen). First strand cDNA synthesis was performed with 25 ng of total RNA, oligo dT primers and reverse transcriptase at 48°C (45 min). Primers used for PCR include β-actin (Forward: CAC AGC TGA GAG GGA AAT C, Reverse: TCA GCA ATG CCT GGG TAC), DCX (Forward: CGA TCA AAC TGG AAA CCG G, Reverse: TTT GCG TCT TGG TCG TTA CC), BDNF (Forward: TTG AGC ACG TGA TCG AAG AGC, Reverse: GTT CGG CAT TGC GAG TTC CAG), IGF-1 (Forward: GTG GAC GCT CTT CAG TTC GT, Reverse: GCT TCC TTT TCT TGT GTG TCG ATA G), FGF-2 (Forward: GGC TTC TTC CTG CGC ATC CA, Reverse: GCT CTT AGC AGA CAT TGG AAG A), NGF (Forward: TCC TTT CCT CAC TCA CCC AC, Reverse: AGG CCA CTG ACT AGG CTG AA), NT-3 (Forward: CAC CCA GAG AAC CAG AGC AG, Reverse: TCT GAA GTC AGT GCT CGG AC), GAD65 (Forward: TCT TTT CTC CTG GTG GTG CC, Reverse: CCC CAA GCA GCA TCC ACA T), GAD67 (Forward: TACGGGTTCGCACAGGTC, Reverse: CCCCAAGCAGCATCCACAT), and GFAP (Forward: ACA TCG AGA TCG CCA CCT AC, Reverse: ACA TCA CAT CCT TGT GCT CC). PCR was performed using Platinum Taq DNA polymerase with a denaturing step for 2 min at 94°C, followed by 28–40 cycles (36 cycles for β-actin; 32, DCX; 35, BDNF; 40, IGF-1; 30, FGF-2; 32, NGF; 28, NT-3; 31, GAD65; 38, GAD67; and 28, GFAP) of 1 min at 94°C, 1 min at 55°C and 2 min at 72°C, and terminated by an elongation step at 72°C for 7 min. PCR products were separated on a 10% TBE polyacrylamide gel and stained with ethidium bromide. PCR products were then visualized with the Kodak Image Station 440 (Rochester, NY) and product intensities were quantified using Kodak software. PCR product levels were normalized with β-actin values.
Statistical analyses
Numbers of BrdU-labeled and DCX-labeled cells estimated with the optical fractionator, the volumes of dentate gyrus that were estimated using Cavalleri’s principle, and protein and mRNA levels (expressed as percent of control levels) were all subjected to a 2 (Diet: CON vs. SUP) × 2 (Treatment: saline vs. KA) between-subjects ANOVA. Of particular interest were Diet × Treatment interactions. Where appropriate, a priori pairwise comparisons were used to evaluate differences between group means. A significance level of .05 was set for all statistical tests. Values are reported in the text as means ± SEM. Note that subjects contained within each experimental condition were randomly selected from different litters (n of 1/litter). Thus, we have taken the necessary precautions to be sure that our findings are not contaminated by a lack of within litter variability.
Results
Seizure activity and hippocampal histopathology after KA-induced SE
All rats treated with KA reached SE and exhibited continuous class III–IV seizures for over 3 hours. Careful observation of behavioral activity of rats receiving KA revealed that the pattern of seizure activity (progression, number, and severity of seizures) was similar for both CON and SUP rats. There were no significant differences between CON and SUP rats in the number of class III/IV/V or total number of motor seizures within the first 1-hour period of observable seizure activity (CON: class III = 0.25 ± 0.25, class IV = 7.00 ± 1.41, class V = 9.25 ± 3.61, total = 16.50 ± 4.91; SUP: class III = 4.00 ± 1.47, class IV = 10.00 ± 2.35, class V = 8.50 ± 3.52, total = 22.50 ± 6.81; all ps > .05). After exhibiting the first motor seizure, all rats entered a continuous class III–V seizure state in the second 1-hour period. To ensure that differences in seizure severity or duration did not occur beyond the 3-hr time window we used to define SE, we continued to monitor both CON and SUP rats. No differences were apparent. All rats gradually dropped out of SE within 2–3 hours of the last injection: continuous seizures subsided, followed by the emergence of 1–3 discrete Class III/IV/V seizures within a 1-hour period, and the absence of seizure activity by 5 hours after the last injection. There were also no significant differences between CON and SUP rats in the total amount of KA needed to induce SE (range = 3.75–16.25 mg/kg) or in the latency to the first motor seizure (range = 62–331 min). This variability in dose and latency to the first behavioral seizure in our rats is consistent with previous reports showing that the Sprague Dawley rats show a more variable convulsant response to KA than other rat strains (Golden et al., 1991, 1995). Thus, while the dose of KA needed to induce SE varied across rats, the SE produced was quite comparable between our CON and SUP rats.
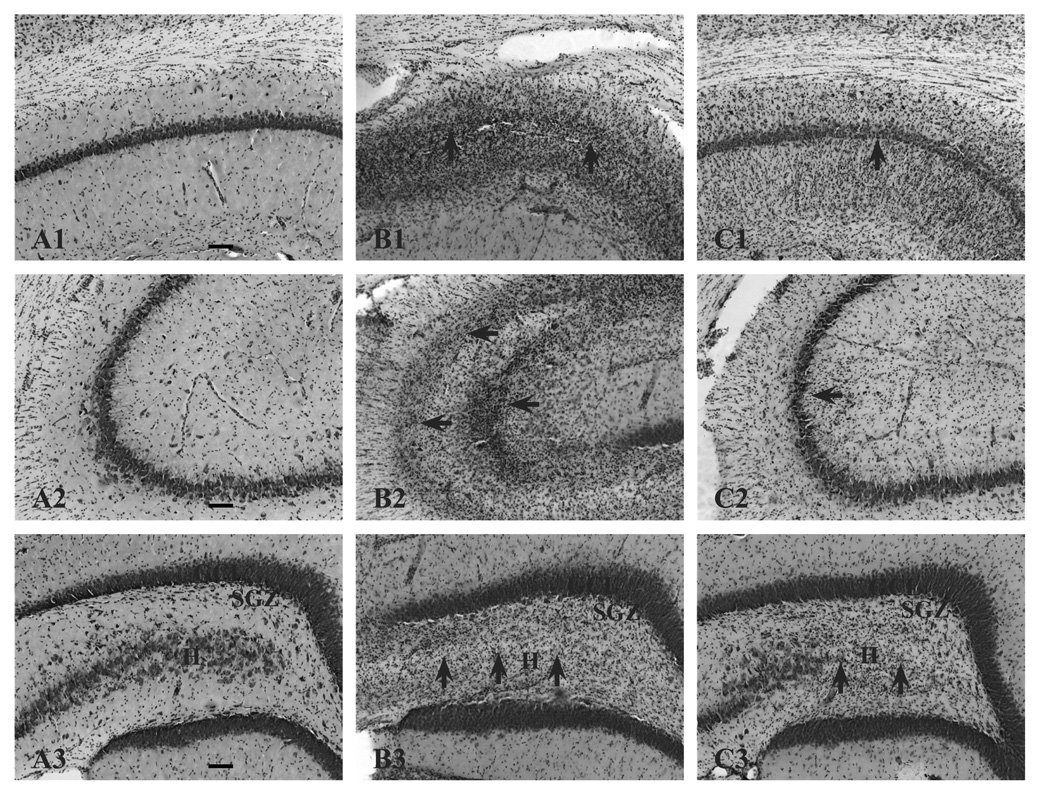
We used histopathology scores adapted from a previous report showing protection against histopathology following KA treatment as a result of prenatal choline supplementation (Holmes et al., 2002) to confirm that our KA treatment lead to similar patterns of histopathology between CON and SUP rats. Comparable to Holmes et al. (2002), we also found evidence of considerable histopathology from CON rats that experienced KA-induced SE, and this damage also appeared to be attenuated in SUP rats. Figure 1 shows photomicrographs of representative brain sections stained with cresyl violet from CON and SUP rats 16 days following KA treatment. While the granule cell layer of the dentate gyrus remained intact, areas CA1, CA3, and the hilus of KA-treated rats displayed cell loss, disrupted cytoarchitecture, and gliosis. The histopathology of the hippocampus was more severe in KA-treated CON rats when compared to KA-treated SUP rats (Fig. 1B, 1C). KA-treated SUP rats also had significantly lower histopathological scores than KA-treated CON rats (CON: 2.54 ± 0.40, SUP: 1.44 ± 0.37, t (6) = 2.01, p < .05, one-tailed). To further examine the nature of the neuropathological response to KA-induced seizures in CON and SUP rats, we quantified a number of neurobiological markers in the hippocampus known to change as a result of SE and possibly contribute to the spatial learning and memory deficits observed shortly after seizures.
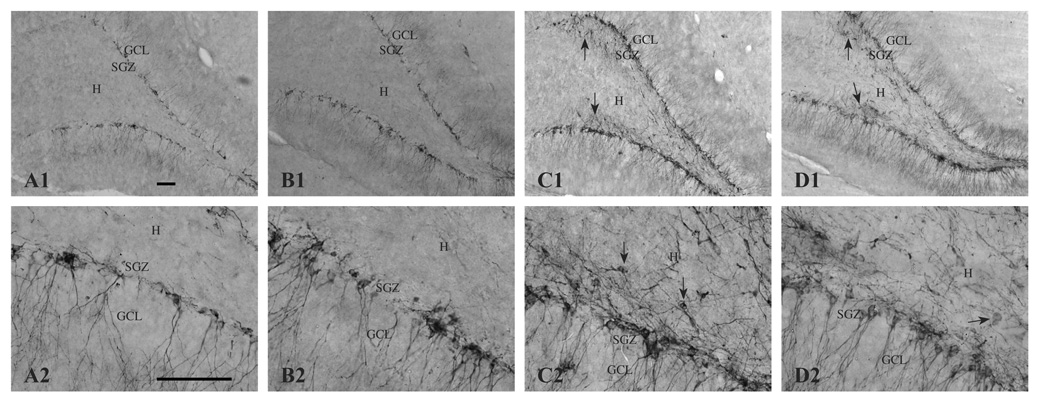
Figure 1.
Histopathology of the hippocampus 16 days following KA-induced SE in CON (B) and SUP (C) rats. Panel A depicts representative sections of CA1 (A1), CA3 (A2), and dentate gyrus (A3) regions from an intact hippocampus from a saline-treated CON rat. Saline-treated SUP rats also did not show any lesions (histology data not shown). Note more severe degeneration (cell loss, disruption of cytoarchitecture, and gliosis) in KA-treated CON rats (B1, B2, B3). Areas of damage are indicated by arrows. Dentate hilar cell loss was observed in both CON (B3) and SUP rats (C3). Photomicrographs in each set were taken with a 10x objective. Bars indicate 50 µm. GCL, granule cell layer. SGZ, subgranular zone. H, hilus.
Prenatal choline supplementation protects against SE-induced decrease in hippocampal GAD65, but not GAD67, protein and mRNA expression
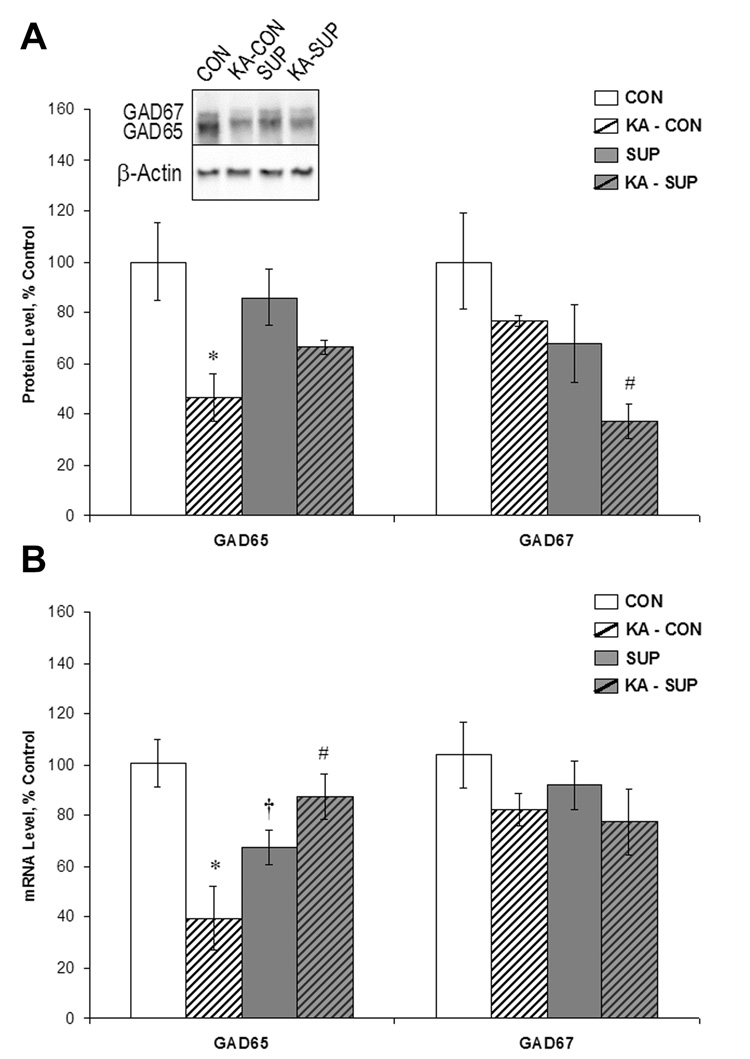
Excitotoxin-induced SE is known to cause a decrease in inhibition (Sloviter, 1987; Cornish & Wheal, 1989; Milgram et al., 1991; Kobayashi & Buckmaster, 2003) and degeneration of GABAergic neuronal function in the hippocampus (Obenaus et al., 1993; Houser & Esclapez, 1996; Morin et al., 1998; Esclapez & Houser, 1999; Shetty & Turner, 2001), which has been considered to be a significant contributor to hyperexcitability in the epileptic hippocampus. This seizure-induced disruption in GABAergic function can be measured via quantification of GAD65 and 67, enzymes important for local GABA synthesis at synaptic (GAD65) and cytoplasmic (GAD67) sites (Erlander & Tobin 1991; Esclapez et al., 1994), and may play a role in the packaging and release of GABA (Namchuk et al., 1997; Tian et al., 1999). Thus, as a marker of GABAergic function, we examined levels of hippocampal GAD65 and 67 protein (via Western blot) and mRNA in saline- and KA-treated CON and SUP rats 16 days post-SE and these data, expressed as percent of control levels, are shown in Figure 2. Analyses of mRNA levels revealed a significant Diet × Treatment interaction for GAD65 mRNA, F (1, 12) = 17.19, p < 001. Baseline GAD65 mRNA levels were 33% lower in SUP rats when compared to CON rats (p < .05; Fig. 2B). Sixteen days following SE, GAD65 mRNA levels were reduced by more than half in CON rats (p < .001), which is consistent with previous reports that show decreases in hippocampal GAD65 mRNA 3–14 days following seizures (Houser & Esclapez, 1996; Kobayashi & Buckmaster, 2003). In contrast, there was no significant difference in GAD65 mRNA levels between saline-treated and KA-treated SUP rats (Fig. 2B). Although a Diet × Treatment interaction did not reach statistical significance for GAD65 protein levels, F (1, 10) = 2.57, p = .14, planned comparisons revealed that KA-treated CON rats showed a significant decrease by more than half in GAD65 protein (p < .05) whereas GAD65 protein levels did not differ significantly between KA-treated and saline-treated SUP rats (Fig. 2A). Taken together, these data suggest a preservation of hippocampal GABAergic GAD65-containing neurons, or a more rapid recovery of GAD65 in SUP rats. In contrast, neither prenatal diet nor KA treatment had an effect on levels of GAD67 mRNA 16 days following SE (all Fs < 1), as levels of GAD67 mRNA did not significantly differ across the four conditions (Fig. 2B). Analyses of GAD67 protein levels, however, revealed that KA-induced SE led to a reduction in GAD67 protein levels in both CON rats and SUP rats, F (1, 10) = 4.78, p = .05 (Fig. 2A). SUP rats also exhibited lower levels of GAD67 protein overall, F (1, 10) = 8.43, p < .02. These findings suggest that prenatal choline supplementation’s neuroprotective effect on GAD expression 16 days following seizures may be specific to the GAD65 isoform.
Figure 2.
Comparison between CON (white bars) and SUP rats (grey bars) in GAD65 and GAD67 protein levels (A) and mRNA levels (B) (mean ± SEM percent of control levels) in the intact hippocampus (open bars) and 16 days following KA-induced SE (hatched bars). Protein levels were quantified using Western blot analysis (A). KA-treated CON rats showed a significant decrease in GAD65 protein and mRNA levels by more than half (p < .001), whereas GAD65 protein and mRNA levels did not significantly change in KA-treated SUP rats. GAD67 protein levels were reduced in both KA-treated CON and SUP rats, but GAD67 mRNA levels did not change in either group as a result of KA-induced SE. * statistically different from within-diet saline-treated group; # statistically different from KA-treated CON; † statistically different from saline-treated CON.
Prenatal choline supplementation attenuates SE-induced upregulation of hippocampal cell proliferation in the dentate gyrus
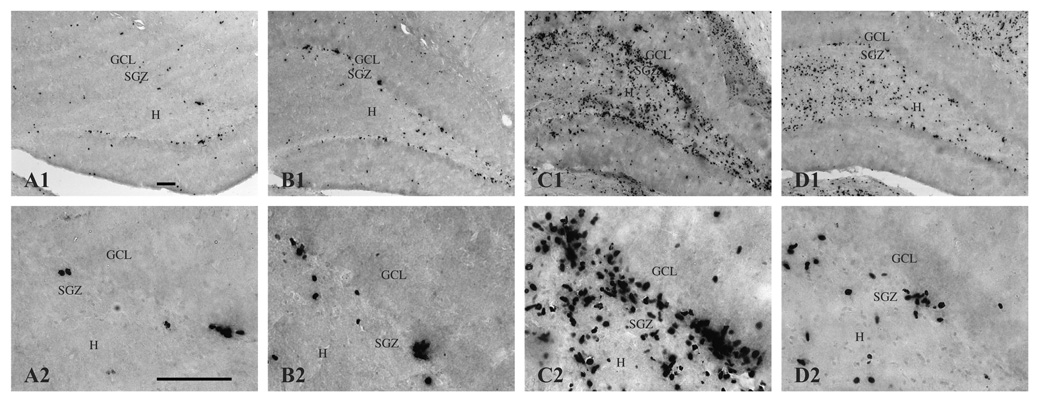
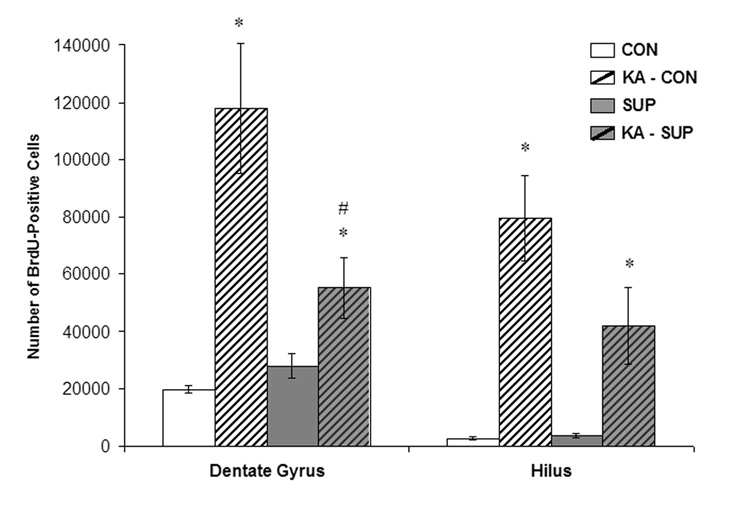
To detect changes in dentate cell proliferation as a result of KA-induced SE, we visualized cells immunopositive for the cell division marker, BrdU, administered 5–16 days post-SE to capture the time window during which savings in spatial learning and memory following excitotoxin-induced SE have been observed with prenatal choline supplementation (Yang et al., 2000; Holmes et al., 2002). The amount of cell proliferation after SE that we captured with our 10-day BrdU regimen yielded a very high density of immunostaining. Stereological procedures also require relatively thick sections for unbiased cell counting, which further contributes to our high density of BrdU labeling where many BrdU+ cells following SE were tightly clustered together and layered on top of each other, making the quantification of double labeled cells unreliable. Therefore, we quantified markers of neurogenesis and gliogenesis in the hippocampus (see additional results below). Figure 3 shows photomicrographs of BrdU labeling in the hippocampus of representative sections from CON and SUP saline- and KA-treated rats. BrdU-labeled cells were expressed throughout the rostral-caudal extent of the dentate gyrus and in both the dorsal and ventral blades. BrdU-labeled cells were evident in the subgranular zone, indicating that many of the cells were newly generated. BrdU-labeled cells were also visible in the granule cell layer, indicating that some had survived for several days and were migrating from the subgranular zone. Moreover, a vast majority of BrdU+ cells displayed morphological characteristics of normal non-pyknotic cells (e.g., round or oval nuclei that did not appear highly condensed). In KA-treated rats, a considerable number of BrdU+ cells were also present in the hilus. BrdU labeling clearly showed that while KA treatment dramatically upregulated dentate and hilar cell proliferation in CON rats, which is consistent with previous reports (Bengzon et al., 1997; Parent et al., 1997; Jessberger et al., 2005), the SE-induced increase in cell proliferation was significantly attenuated in SUP rats (Fig. 3B, 3D). This observation was confirmed by quantification of BrdU+ cells. Using unbiased stereology, estimates of the number of BrdU+ cells were generated for both the dentate gyrus and hilus for each rat and these data are shown in Figure 4. As expected, SE significantly increased the number of dentate and hilar BrdU+ cells in both CON and SUP rats (dentate gyrus: F (1, 12) = 24.73, p < .001; hilus: F (1, 12) = 32.47, p < .001). There were no main effects of Diet on dentate nor hilar cell proliferation but a significant Diet × Treatment interaction was found for dentate cell proliferation, F (1, 12) = 7.91, p < .02, which approached statistical significance for hilar cell proliferation, F (1, 12) = 3.67, p = .07). Remarkably, while CON rats exhibited a 780% increase in the total number of BrdU+ cells (dentate gyrus and hilus combined) following SE, SUP rats showed only a 207% increase. Planned comparisons revealed that KA-treated CON rats had significantly more BrdU+ cells in the dentate gyrus than KA-treated SUP rats (p < .01; Fig. 4), confirming that SE-induced cell proliferation in the dentate gyrus was markedly attenuated in SUP rats.
Figure 3.
BrdU-immunopositive cells (i.e., newly generated cells) in the dentate gyrus and hilus 16 days following saline treatment (A, CON; B, SUP) or KA-induced SE (C, CON; D, SUP). Note that the number of BrdU-labeled cells significantly increased 16 days after SE for both diet groups, but that this seizure-induced proliferative response was markedly attenuated in SUP rats (D) in comparison to CON rats (C). Photomicrographs in the top row were taken with a 10x objective and the bottom row was taken with a 40x objective. Bars indicate 50 µm. GCL, granule cell layer. SGZ, subgranular zone. H, hilus.
Figure 4.
Mean (±SEM) numbers of BrdU-labeled cells detected in the dentate gyrus and hilus of CON (white bars) and SUP (grey bars) rats 16 days following saline treatment (open bars) or KA-induced SE (hatched bars). SE significantly increased the number of BrdU-labeled cells in both CON and SUP rats (ps < .05), but this increase was significantly attenuated in SUP rats (ps < .05). * statistically different from within-diet saline-treated group; # statistically different from KA-treated CON.
To determine whether a larger overall size of structure could account for the higher numbers of new cells detected in KA-treated CON versus KA-treated SUP rats, we estimated the volume of the dentate gyrus and hilus in each rat using Cavalleri’s principle (Mouton, 2002) and did not detect any statistically significant differences between any treatment groups (Fs<1).
SE-induced hippocampal neurogenesis is not modulated by prenatal choline supplementation
To determine whether prenatal choline supplementation decreased the characteristic proliferation of new neurons after SE, we used adjacent tissue sections to visualize cells immunopositive for the microtubule-associated phosphoprotein, doublecortin (DCX), that is transiently expressed in newly born neurons that are still in the process of migrating and differentiating (Brown et al., 2003; Rao & Shetty, 2004). Others have confirmed that DCX expression is a reliable indicator of neurogenesis in the adult brain (Couillard-Depres et al., 2005). As a secondary measure, we also quantified DCX mRNA levels via RT-PCR. Figure 5 shows photomicrographs of DCX labeling in the hippocampus of representative sections from CON and SUP saline- and KA-treated rats. DCX+ cells were visible with processes in various stages of development and were evident along the subgranular zone and granule cell layer. In KA-treated rats, DCX+ cells were also detected in the hilus, which is consistent with previous studies that have shown that a portion of these newly generated neurons after SE aberrantly migrate to the hilus region (Parent et al., 1997; Hattiangady et al., 2004; Jessberger et al., 2005). In comparison to saline-treated rats many of the DCX+ neurons in KA-treated rats appeared displaced and exhibited abnormal morphological features, such as horizontally oriented cell bodies and processes (Fig. 5C, 5D).
Figure 5.
DCX-immunopositive neurons (i.e., newly generated neurons) in the dentate gyrus and hilus 16 days following saline treatment (A, CON; B, SUP) or KA-induced SE (C, CON; D, SUP). The number of DCX-labeled cells significantly increased 16 days after SE for both CON (A, C) and SUP (B, D) rats. Note that in KA-treated rats of both diet groups (C, D), DCX-positive neurons were aberrantly located in the hilus and exhibited abnormal morphological features, such as horizontally oriented cell bodies and processes (indicated by arrows). Photomicrographs in the top row were taken with a 10x objective and the bottom row was taken with a 40x objective. Bars indicate 50 µm. GCL, granule cell layer. SGZ, subgranular zone. H, hilus.
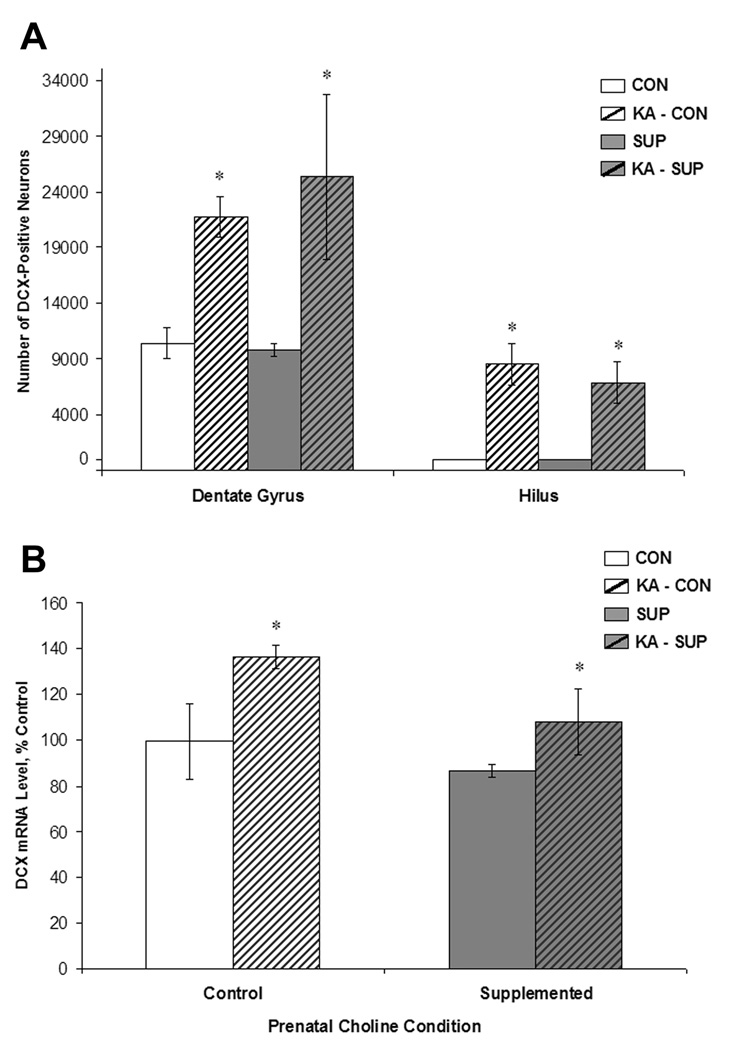
Unbiased stereological estimates of the number of DCX+ neurons in the dentate gyrus and hilus were generated for each rat and are shown in Figure 6. As expected, SE induced a dramatic increase in the number of DCX+ neurons in both CON and SUP rats (dentate gyrus: F (1, 12) = 11.93, p < .01; hilus: F (1, 12) = 34.50, p < .001). However, prenatal choline supplementation did not significantly diminish the proliferation of new neurons in response to SE; there was no significant main effect of Diet for both dentate and hilar neurogenesis or Diet × Treatment interactions (Fs < 1). There was also no significant difference in the number of DCX+ neurons between saline-treated CON and saline-treated SUP rats. SE also produced a significant increase in DCX mRNA in both CON and SUP rats (F (1, 12) = 6.49, p < .05), but prenatal choline availability did not alter baseline or SE-induced mRNA levels of DCX (Fs < 1; Fig. 6). These data revealed that unlike the pattern of BrdU immunolabeling, seizure-induced upregulation of hippocampal neurogenesis was not modified by prenatal choline availability.
Figure 6.
Mean (±SEM) numbers of DCX-labeled cells (A) and mean (±SEM) mRNA expression levels (B) detected in the dentate gyrus and hilus of CON (white bars) and SUP (grey bars) rats 16 days following saline treatment (open bars) or KA-induced SE (hatched bars). KA-induced SE significantly increased the number of DCX-labeled neurons and levels of DCX mRNA in both CON and SUP rats (ps < .05). Prenatal choline supplementation did not alter the number of DCX-labeled neurons or levels of DCX mRNA in response to KA-induced SE. * statistically different from within-diet saline-treated group.
Prenatal choline supplementation attenuates SE-induced upregulation of hippocampal GFAP protein and mRNA expression
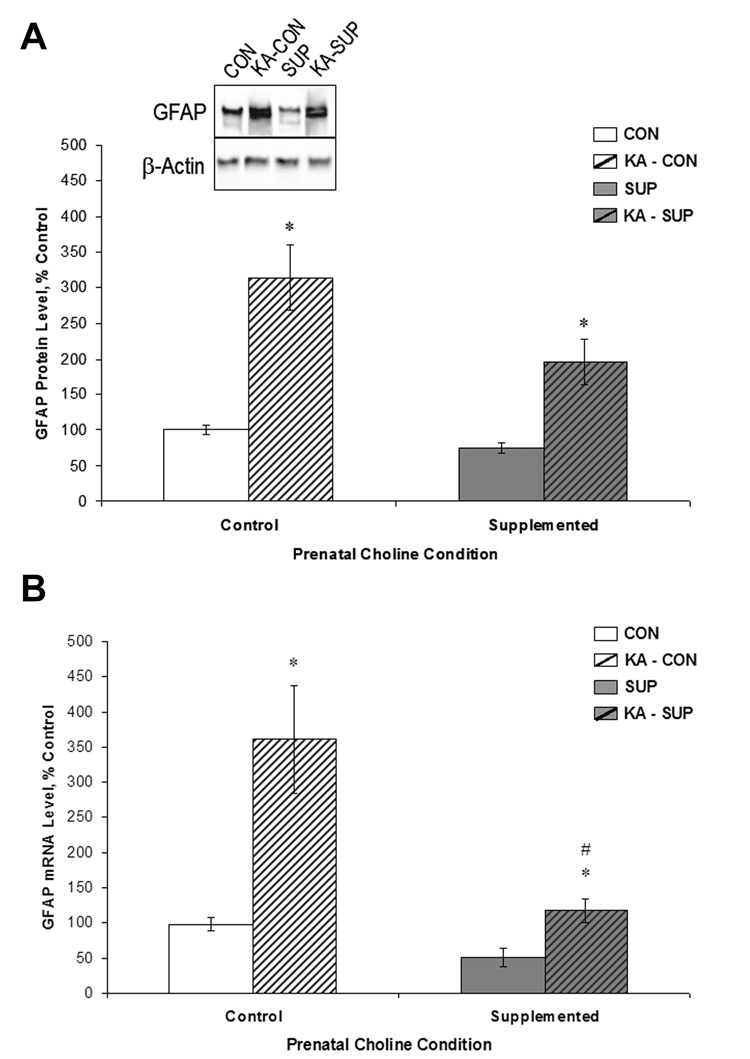
Prior work has shown that SE triggers astroglial activity as revealed by dramatic elevations of GFAP protein and mRNA expression (Jorgensen et al., 1993; Niquet et al., 1994a). GFAP is also expressed in progenitor cells that reside in the subgranular zone of the dentate gyrus and give rise to new neurons (Seri et al., 2001), which also makes GFAP a marker of interest given that SE produces robust increases in dentate cell proliferation. We quantified levels of hippocampal GFAP protein (via Western blot) and mRNA in saline- and KA-treated CON and SUP rats 16 days post-treatment and these data, expressed as percent of control levels, are shown in Figure 7. As predicted, CON and SUP rats exhibited a significant increase in GFAP protein and mRNA levels as a result of KA treatment (protein: F (1, 12) = 16.60, p < 01; mRNA: F (1, 12) = 35.37, p < .05). More importantly, there was a significant Diet × Treatment interaction for GFAP mRNA levels, F (1, 12) = 5.88, p < .05, although a Diet × Treatment interaction for GFAP protein levels did not reach statistical significance, F (1, 12) = 2.83, p = .12. Saline-treated SUP rats had significantly lower levels of hippocampal GFAP protein and mRNA than CON rats (ps < .05; Fig. 7). However, SE-induced increases in GFAP mRNA were significantly reduced in KA-treated SUP rats; mRNA levels rose 3.5-fold in CON rats, but only doubled in SUP rats (Fig. 7B, ps ≤ .02). A similar, but not significant, pattern of change was observed for GFAP protein levels: a 3.1-fold increase following KA in CON rats, but only a 2.6-fold increase in SUP rats (Fig. 7A, p = .07). Collectively, these data suggest that SE-induced upregulation in GFAP levels may be attenuated in SUP rats.
Figure 7.
Comparison between CON (white bars) and SUP rats (grey bars) in GFAP protein levels (A) and mRNA levels (B) (mean ± SEM percent of control levels) in the intact hippocampus (open bars) and 16 days following KA-induced SE (hatched bars). GFAP protein levels were quantified using Western blot analysis (A). SE significantly increased GFAP protein and mRNA levels in both CON and SUP rats (ps < .05), but this increase was significantly attenuated in SUP rats. * statistically different from within-diet saline-treated group; # statistically different from KA-treated CON.
Growth factor expression in the intact and KA-lesioned hippocampus as a function of prenatal choline availability
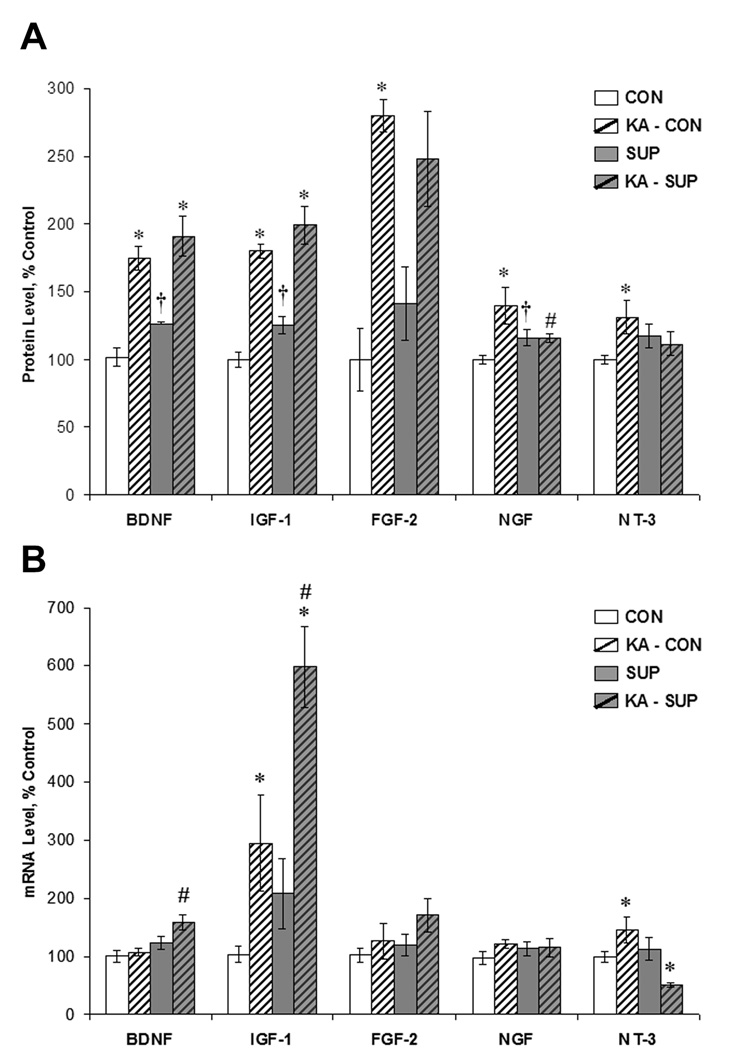
The expression of growth factors is thought to be an important mediator of hippocampal neurogenesis (Cameron et al. 1998; Anderson et al., 2002; Lee et al., 2002a; 2002b) and some factors are known to change in response to status epilepticus (Gall & Isackson, 1989; Gall et al., 1991; Mudo et al., 1996; Schmidt-Kastner et al., 1996; Katoh-Semba et al., 1999; Shetty et al., 2004). We therefore quantified hippocampal protein levels (via ELISA) and mRNA levels (via RT-PCR) of the following growth factors known to influence neurogenesis: BDNF, IGF-1, FGF-2, NGF, and NT-3. Figure 8 shows the relative protein levels and mRNA levels for each factor in CON and SUP saline- and KA-treated rats 16 days post-treatment (protein levels expressed as percent of control levels because protein concentrations varied greatly between growth factors).
Figure 8.
Comparison between CON (white bars) and SUP (grey bars) rats in growth factor protein (A) and mRNA (B) levels (mean ± SEM percent of control levels) in the intact hippocampus (open bars) and 16 days following KA-induced SE (hatched bars). Protein levels were quantified using ELISA. SE led to a significant increase in BDNF, IGF-1, FGF-2, NGF, and NT-3 protein levels in CON rats, but a significant increase in only BDNF and IGF-1 protein levels was noted for SUP rats. Baseline protein levels of BDNF, IGF-1, and NGF were significantly higher in SUP rats. SUP rats also exhibited significant increases in BDNF and IGF-1 mRNA and a decrease in NT-3 mRNA 16 d following SE, whereas CON rats showed SE-induced increases in IGF-1 and NT-3 mRNA. * statistically different from within-diet saline-treated group; # statistically different from KA-treated CON; † statistically different from saline-treated CON.
Because we were ultimately interested in the potential effect of prenatal choline availability on hippocampal growth factor content in baseline conditions and in response to SE, we conducted planned comparisons between saline-treated CON and saline-treated SUP rats, as well as between saline-treated and KA-treated rats within each diet condition. In adult rats that did not experience seizures, protein levels of BDNF (p < .001), IGF-1 (p < .03), and NGF (p < .05) were significantly higher in prenatal SUP rats than saline-treated CON rats. Although differences between saline-treated CON and SUP rats in protein levels of FGF-2 (p = .29) and NT-3 (p = .13) failed to reach statistical significance, the respective means are in a similar direction (Fig. 8A). Collectively, these results suggest that SUP rats have a higher baseline hippocampal protein content of several neurotrophic factors.
As expected, SE significantly increased protein levels of BDNF (p < .001), IGF-1 (p < .001), FGF-2 (p < .01), NGF (p < .01), and NT-3 (p < .05) in CON rats. In contrast, SUP rats exhibited a significant increase only in BDNF (p < .001) and IGF-1 (p < .001) in response to SE (Fig. 8A). There was a similar trend for a SE-induced increase in FGF-2 in SUP rats (p = .06). It is important to note that because protein levels of BDNF and IGF-1 were significantly elevated in saline-treated SUP rats, the increase in these neurotrophic factor levels following SE in supplemented rats (51% increase in BDNF; 59% increase in IGF-1) was not as large as it was in CON rats (BDNF: 72% increase; IGF-1: 81% increase). Together, these data suggest that prenatal choline supplementation either prevents or attenuates protein expression of some growth factors in response to SE.
We also quantified via RT-PCR the relative hippocampal mRNA levels of each growth factor (Fig. 8B). These data revealed a slightly different pattern of results than that of protein levels. While baseline mRNA levels were not significantly altered by prenatal choline supplementation (p’s > .05), prenatal choline availability had striking effects on SE-induced alterations in BDNF, IGF-1, and NT-3. BDNF mRNA levels were significantly elevated in KA-treated SUP rats in comparison to KA-treated CON rats (p < .01); SUP rats had a 29% increase in BDNF mRNA levels following SE whereas CON rats showed virtually no change in BDNF mRNA levels. Similarly, IGF-1 mRNA levels were also significantly higher in KA-treated SUP rats than in KA-treated CON rats (p < .05). There was an unexpected Diet × Treatment interaction for NT-3 mRNA levels where CON rats showed an increase in NT-3 mRNA following SE, whereas the opposite pattern was revealed for SUP rats, F (1, 12) = 11.75, p < .01 (Fig. 8B). These data suggest that in addition to affecting levels of growth factor protein expression, prenatal choline availability also modulates levels of growth factor mRNA following SE.
Discussion
We have shown that increased availability of choline during early development produced a long-lasting change in the fetal brain that led to a dramatically reduced hippocampal response 16 days following KA-induced SE. Compared to control rats, adult offspring of dams that were fed a choline-enriched diet for just 1 week, mid-gestation, responded to SE with: 1) reduced hippocampal histopathology, 2) robust protection against loss of GAD65 protein and mRNA expression, 3) marked attenuation of dentate cell proliferation, 4) dramatic reduction of GFAP mRNA expression (with a tendency for GFAP protein to be reduced as well), and 5) an altered pattern of growth factor expression. These data are particularly important because prior work has shown a savings in hippocampal-dependent learning and memory at a similar time point following excitotoxin-induced SE in prenatally choline-supplemented rats (Yang et al., 2000; Holmes et al., 2002), suggesting that increased choline availability to the fetus may alter neural development such that the adult brain is protected from SE-induced damage and cognitive impairment. While these data do not rule out the possibility that choline supplements may have neuroprotective actions at other periods, we know that choline has profound memory-enhancing effects if it is supplemented on ED12–17 or PD15–30, but not at other developmental or adult timeframes (Meck & Williams, 2003).
Consistent with previous findings (Holmes et al., 2002), we report that prenatally choline supplemented rats (SUP) showed reduced cell loss and gliosis in CA1, CA3, and hilus compared to control-fed rats even though all rats experienced the same severity and duration of seizure activity. While widespread neurodegeneration of the hippocampus is a notorious consequence of prolonged seizures (Niquet et al., 1994a; Fujikawa et al., 2000; Kotloski et al., 2002), GABAergic neurons are particularly vulnerable (Obenaus et al., 1993; Houser & Esclapez, 1996; Esclapez & Houser, 1999; Shetty & Turner, 2001). Thus, our finding that prenatal choline supplementation prevented the loss of hippocampal GAD65 protein and mRNA (though not GAD67 response to SE) is particularly interesting. Our findings may represent a protection of GABAergic neurons following SE, although loss of functional inhibition may not be due to a loss of inhibitory neurons per se (Sloviter, 1987; Davenport et al., 1990; Esclapez & Houser, 1999). Rather, a loss in GAD65 expression may contribute to a disruption in GABAergic function, as mice deficient in GAD65 are susceptible to epileptic seizures (Kash et al., 1997). Alternatively, our SUP rats may show an early restorative upregulation in GAD65 production. Indeed, Houser and Esclapez (1996) have found that remaining GABAergic hippocampal neurons upregulate GAD65 mRNA 2–4 months, but not 3–14 days, following seizure-induced neuronal damage. Future studies are needed to determine if the savings seen in our SUP rats are in numbers of GABAergic neurons or in altered recovery of GAD expression or both.
SE produces a robust reactive astroglial response in the hippocampus, yet our data reveal that seizure-induced increases in GFAP mRNA and dentate cell proliferation were significantly reduced in SUP rats. There was also a tendency for GFAP protein levels and hilar cell proliferation levels to be attenuated as well. SE-induced reactive astrogliosis plays a significant role in the aberrant synaptic remodeling of the hippocampus thought to underlie the development of epilepsy (Jorgensen et al., 1993; Niquet et al., 1994a, 1994b; Represa et al., 1995). Reactive astrocytes that proliferate in response to seizures express several genes that promote neurite outgrowth (Represa et al., 1993; Niquet et al., 1994a, 1994b; Stringer, 1996) and the increase in the expression of these genes coincides with both the emergence of mossy fiber sprouting and the period when both GFAP and hippocampal cell proliferation are elevated (Khrestchatisky et al., 1995; Represa et al., 1995; Bengzon et al., 1997; Parent et al., 1997; Borges et al., 2006). An attenuation of seizure-induced astrogliosis in SUP rats may thus indicate a reduced progression of epilepsy following SE.
We did not detect any differences between diet groups in dentate and hilar neurogenesis following SE. Previous work has shown that dentate neuronal and astroglial proliferation following SE likely arises from independent progenitor pools: inhibiting hippocampal neurogenesis before SE does not prevent astrogliosis or mossy fiber sprouting (Parent et al., 1999). Thus, prenatal choline supplementation may have a preferential effect on just one type of proliferative hippocampal response following SE. Although we did not detect changes in the number of DCX+ dentate and hilar neurons following seizures as a function of prenatal choline availability, it remains to be determined whether prenatal choline supplementation alters specific properties (e.g., migration pattern, morphology, neuronal functionality) of newborn neurons following seizures that are hypothesized to contribute to the development of epilepsy (Scharfman, 2004; Scharfman & Gray, 2007).
In order to further investigate the possible mechanisms of prenatal choline-induced neuroprotection, we examined expression of neurotrophic factors that are known to support hippocampal plasticity and are regulated by seizure activity. It is well established that 1 hour to 4 days after SE, hippocampal NGF and BDNF are transiently increased while NT-3 levels are downregulated (e.g., Gall et al., 1991; Rocamora et al., 1994; Marcinkiewicz et al., 1997; Shetty et al., 2004). Less is known about growth factor responses beyond one week after SE, but hippocampal NGF and NT-3 protein are still upregulated 45 days following SE while BDNF returns to baseline (Shetty et al., 2003). Our study adds to this timeline by demonstrating large increases in hippocampal BDNF, IGF-1, FGF-2, NGF, and NT-3 protein 16 days post-SE in control-fed rats. Thus, the hippocampal growth factor response to SE may be characterized by periods of elevation and/or reduction in levels that are specific for each factor. Levels of BDNF and IGF-1 protein were significantly elevated in both SUP and CON rats following KA-treatment, although the percent increase in these growth factors following SE compared to baseline levels was reduced in the SUP rats. These data may suggest that the response of these growth factors to SE is attenuated, or that some ceiling prevents further increases in the SUP rats. The latter hypothesis seems somewhat unlikely because we find that the NGF and NT-3 protein response to SE is completely attenuated in the KA-treated SUP rats compared to KA-treated CON rats, suggesting that prenatal choline supplementation reduces the dramatic elevation of several growth factors following SE. Previous work has shown that blocking NGF’s actions prior to kindling both retards kindling and prevents mossy fiber sprouting (Rashid et al., 1995; Van der Zee et al., 1995); therefore, the attenuated NGF response to KA-induced in SUP rats may confer protection against mossy fiber sprouting. Additionally, BDNF and IGF-1 hippocampal mRNA following SE were greater in SUP than CON rats, while NT-3 mRNA was lower in SUP rats following SE. This difference between protein and mRNA responses to SE may occur because mRNA is present within the intrinsic cells of the tissue, whereas protein may also be additionally contributed by the axons and terminals of the afferent projections to the hippocampus. Whether the muted response of some growth factors to SE in our supplemented rats underlies our finding of reduced cell proliferation following SE remains to be determined.
What may be key to understanding the neuroprotection conferred by prenatal choline supplementation that we report here, as well as the choline-induced protection against memory deficits following seizures reported previously (Yang et al., 2000; Holmes et al., 2002), is an altered hippocampal microenvironment in SUP rats prior to SE. SUP rats showed enhanced baseline levels of several growth/neurotrophic factors (BDNF, NGF, IGF-1) in the hippocampus, compared to CON rats. This finding extends our previous demonstrations of higher BDNF protein in 8-month old (Glenn et al., 2007) and NGF protein in 20- and 90-day old (Sandstrom et al., 2002) female offspring of choline-supplemented dams. Infusions of BDNF or bFGF into the hippocampus prior to SE (Liu et al., 1993; Liu & Holmes, 1997; Reibel et al., 2000) as well as exercise (Setkowicz & Mazur, 2006), which stimulates BDNF, NGF, and IGF-1 (Gomez-Pinilla et al., 1998; Cotman & Berchtold, 2002), have been shown to ameliorate the progression of excitotoxin-induced SE. Prior exercise also mitigates learning and memory deficits shortly after KA-induced seizures (Gobbo & O’Mara, 2005). These data support our view that higher baseline growth factor levels in our SUP rats prior to SE may confer a neuroprotective hippocampal microenvironment that would make SUP rats more resilient to the deleterious effects of seizures. The larger growth factor pool in the SUP hippocampus may be due to several factors. Prenatal choline supplemented rats may have enhanced cholinergic neurotransmission (Blusztajn et al., 1998) and increased responsiveness to cholinergic stimulation (Montoya et al., 2000), factors that are known to upregulate hippocampal BDNF and NGF expression (Lindefors et al., 1992; Knipper et al., 1994). Prenatal choline supplementation also enhances the phosphorylation of CREB (Mellott et al., 2004), and CREB-related factors regulate BDNF transcription (Tao et al., 1998).
Although we did not detect a significant increase in baseline dentate neurogenesis with prenatal choline supplementation in our young males, previous work from our laboratory has demonstrated higher baseline neurogenesis in 8-month old SUP female rats compared to age-matched controls (Glenn et al., 2007). Our inability to detect differences in baseline neurogenesis in the present study may be due to a high baseline rate of neurogenesis in the 2-month old rat (Kuhn et al., 1996; Rao et al., 2006), which may mask any effects of prenatal choline.
The mechanism by which choline supplementation to the embryo causes long-lasting changes in the adult brain and behavior is not yet fully understood, but one likely mechanism relates to choline’s role as a methyl donor (via its conversion to betaine) used for DNA methylation. Prenatal choline availability during ED12–18 determines ED 18 brain betaine concentration (Garner et al., 1995), alters both global and gene-specific DNA methylation (Niculescu et al., 2004, 2006; Kovacheva et al., in press), and leads to changes in adult expression of genes relevant for hippocampal plasticity (Mellott et al., 2007). Enhanced hippocampal plasticity likely plays a significant role in both the memory-enhancing effects of prenatal choline supplementation (Meck & Williams, 2003; McCann et al., 2006) and its neuroprotective actions. Much like our prenatal choline supplemented adult rats, juvenile rats that experience SE also show resistance to seizure-induced hippocampal cell loss, disinhibition, and mossy fiber sprouting (Sperber et al., 1991; Haas et al., 2001), and exhibit savings in spatial learning and memory (Stafstrom et al., 1993; Sarkisian et al., 1997) when compared to adult rats that experience SE. The retention of juvenile-like neuroplasticity and response to seizures into adulthood may contribute to prenatal choline supplementation’s preservation of cognitive function in the face of a neural insult.
Our findings may have important clinical implications. While this study examined only the neuroprotective effects of prenatal choline supplementation, some reported neuroprotective actions of choline appear to be postnatal (Thomas et al., 2007); thus, infants at risk for seizures might benefit from added choline to formula or choline supplementation to mother’s diet. These data also suggest that prenatal choline deficiency may make offspring more vulnerable to the deleterious consequences of seizures, though this remains to be determined. While estimates of normal human consumption of choline is about 425–550 mg/day, intake by pregnant woman is frequently less than half this amount (Zeisel, 2004; 2006), and menopausal status and some genetic polymorphisms of choline metabolism may even further compromise choline availability in humans (da Costa et al., 2006), suggesting that our findings may be of clinical importance to women who may be more vulnerable to choline deficiency.
Acknowledgements
We would like to thank Ashok Shetty for his helpful comments, Ed Dudek for offering guidance on experimental procedures, Jennifer Fraser, Liz Kirby, and Ashley Tsang for assistance with data collection; and Lauren Williamson for her editorial guidance. This work was supported by AG009525 to C.L.W. and J.K.B. and AG025052 to MJG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Represa A. Brief seizure episodes induce long-term potentiation and mossy fiber sprouting in the hippocampus. Trends Neurosci. 1990;13:312–318. doi: 10.1016/0166-2236(90)90135-w. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Cermak JM, Holler T, Jackson DA. Imprinting of hippocampal metabolism of choline by its availability during gestation: implications for cholinergic neurotransmission. J Physiol Paris. 1998;92:199–203. doi: 10.1016/s0928-4257(98)80010-7. [DOI] [PubMed] [Google Scholar]
- Borges K, McDermott D, Irier H, Smith Y, Dingledine R. Degeneration and proliferation of astrocytes in the mouse dentate gyrus after pilocarpine-induced status epilepticus. Exp Neurol. 2006;201:416–427. doi: 10.1016/j.expneurol.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cornish SM, Wheal HV. Long-term loss of paired pulse inhibition in the kainic acid-lesioned hippocampus of the rat. Neuroscience. 1989;28:563–571. doi: 10.1016/0306-4522(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Davenport CJ, Brown WJ, Babb TL. GABAergic neurons are spared after intrahippocampal kainate in the rat. Epilepsy Res. 1990;5:28–42. doi: 10.1016/0920-1211(90)90063-2. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16:215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Houser CR. Up-regulation of GAD65 and GAD67 in remaining hippocampal GABA neurons in a model of temporal lobe epilepsy. J Comp Neurol. 1999;412:488–505. [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa DG, Shinmei SS, Cai B. Kainic acid-induced seizures produce necrotic, not apoptotic, neurons with internucleosomal DNA cleavage: implications for programmed cell death mechanisms. Neuroscience. 2000;98:41–53. doi: 10.1016/s0306-4522(00)00085-3. [DOI] [PubMed] [Google Scholar]
- Gall C, Murray K, Isackson PJ. Kainic acid-induced seizures stimulate increased expression of nerve growth factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 1991;9:113–123. doi: 10.1016/0169-328x(91)90136-l. [DOI] [PubMed] [Google Scholar]
- Gall CM, Isackson PJ. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- Garner SC, Mar MH, Zeisel SH. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J Nutr. 1995;125:2851–2858. doi: 10.1093/jn/125.11.2851. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo OL, O'Mara SM. Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factor. Behav Brain Res. 2005;159:21–26. doi: 10.1016/j.bbr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Golden GT, Smith GG, Ferraro TN, Reyes PF. Rat strain and age differences in kainic acid induced seizures. Epilepsy Res. 1995;20:151–159. doi: 10.1016/0920-1211(94)00079-c. [DOI] [PubMed] [Google Scholar]
- Golden GT, Smith GG, Ferraro TN, Reyes PF, Kulp JK, Fariello RG. Strain differences in convulsive response to the excitotoxin kainic acid. Neuroreport. 1991;2:141–144. doi: 10.1097/00001756-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, So V, Kesslak JP. Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience. 1998;85:53–61. doi: 10.1016/s0306-4522(97)00576-9. [DOI] [PubMed] [Google Scholar]
- Gorter JA, Goncalves Pereira PM, van Vliet EA, Aronica E, Lopes da Silva FH, Lucassen PJ. Neuronal cell death in a rat model for mesial temporal lobe epilepsy is induced by the initial status epilepticus and not by later repeated spontaneous seizures. Epilepsia. 2003;44:647–658. doi: 10.1046/j.1528-1157.2003.53902.x. [DOI] [PubMed] [Google Scholar]
- Haas KZ SE, Opanashuk LA, Stanton PK, Moshe SL. Resistance of Immature Hippocampus to Morphologic and Physiologic Alterations Following Status Epilepticus or Kindling. Hippocampus. 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Spontaneous motor seizures of rats with kainate-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999;35:47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48:3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Hort J, Brozek G, Mares P, Langmeier M, Komarek V. Cognitive functions after pilocarpine-induced status epilepticus: changes during silent period precede appearance of spontaneous recurrent seizures. Epilepsia. 1999;40:1177–1183. doi: 10.1111/j.1528-1157.1999.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Romer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol. 2005;196:342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Jones JP, Meck WH, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res Dev Brain Res. 1999;118:159–167. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Jorgensen MB, Finsen BR, Jensen MB, Castellano B, Diemer NH, Zimmer J. Microglial and astroglial reactions to ischemic and kainic acid-induced lesions of the adult rat hippocampus. Exp Neurol. 1993;120:70–88. doi: 10.1006/exnr.1993.1041. [DOI] [PubMed] [Google Scholar]
- Kang TC, Kim DS, Kwak SE, Kim JE, Won MH, Kim DW, Choi SY, Kwon OS. Epileptogenic roles of astroglial death and regeneration in the dentate gyrus of experimental temporal lobe epilepsy. Glia. 2006;54:258–271. doi: 10.1002/glia.20380. [DOI] [PubMed] [Google Scholar]
- Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, Baekkeskov S. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Inaguma Y, Ito H, Kato K. Brain-derived neurotrophic factor, nerve growth and neurotrophin-3 selected regions of the rat brain following kainic acid-induced seizure activity. Neurosci Res. 1999;35:19–29. doi: 10.1016/s0168-0102(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Khrestchatisky M, Ferhat L, Charton G, Bernard A, Pollard H, Represa A, Ben-Ari Y. Molecular correlates between reactive and developmental plasticity in the rat hippocampus. J Neurobiol. 1995;26:426–436. doi: 10.1002/neu.480260314. [DOI] [PubMed] [Google Scholar]
- Knipper M, da Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994;6:668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloski R, Lynch M, Lauersdorf S, Sutula T. Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. Prog Brain Res. 2002;135:95–110. doi: 10.1016/S0079-6123(02)35010-6. [DOI] [PubMed] [Google Scholar]
- Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J Biol Chem. 2007;282:31777–31788. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Ernfors P, Falkenberg T, Persson H. Septal cholinergic afferents regulate expression of brain-derived neurotrophic factor and beta-nerve growth factor mRNA in rat hippocampus. Exp Brain Res. 1992;88:78–90. doi: 10.1007/BF02259130. [DOI] [PubMed] [Google Scholar]
- Liu Z, D'Amore PA, Mikati M, Gatt A, Holmes GL. Neuroprotective effect of chronic infusion of basic fibroblast growth factor on seizure-associated hippocampal damage. Brain Res. 1993;626:335–338. doi: 10.1016/0006-8993(93)90598-h. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gatt A, Werner SJ, Mikati MA, Holmes GL. Long-term behavioral deficits following pilocarpine seizures in immature rats. Epilepsy Res. 1994;19:191–204. doi: 10.1016/0920-1211(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Liu Z, Holmes GL. Basic fibroblast growth factor is highly neuroprotective against seizure-induced long-term behavioural deficits. Neuroscience. 1997;76:1129–1138. doi: 10.1016/s0306-4522(96)00412-5. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz M, Nagao T, Day R, Seidah NG, Chretien M, Avoli M. Pilocarpine-induced seizures are accompanied by a transient elevation in the messenger RNA expression of the prohormone convertase PC1 in rat hippocampus: comparison with nerve growth factor and brain-derived neurotrophic factor expression. Neuroscience. 1997;76:425–439. doi: 10.1016/s0306-4522(96)00318-1. [DOI] [PubMed] [Google Scholar]
- McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30:696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Mellott TJ, Follettie MT, Diesl V, Hill AA, Lopez-Coviella I, Blusztajn JK. Prenatal choline availability modulates hippocampal and cerebral cortical gene expression. Faseb J. 2007;21:1311–1323. doi: 10.1096/fj.06-6597com. [DOI] [PubMed] [Google Scholar]
- Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. Faseb J. 2004;18:545–547. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- Mikati MA, Tarif S, Lteif L, Jawad MA. Time sequence and types of memory deficits after experimental status epilepticus. Epilepsy Res. 2001;43:97–101. doi: 10.1016/s0920-1211(00)00187-x. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Yearwood T, Khurgel M, Ivy GO, Racine R. Changes in inhibitory processes in the hippocampus following recurrent seizures induced by systemic administration of kainic acid. Brain Res. 1991;551:236–246. doi: 10.1016/0006-8993(91)90938-r. [DOI] [PubMed] [Google Scholar]
- Montoya DA, White AM, Williams CL, Blusztajn JK, Meck WH, Swartzwelder HS. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res Dev Brain Res. 2000;123:25–32. doi: 10.1016/s0165-3806(00)00075-4. [DOI] [PubMed] [Google Scholar]
- Morin F, Beaulieu C, Lacaille JC. Selective loss of GABA neurons in area CA1 of the rat hippocampus after intraventricular kainate. Epilepsy Res. 1998;32:363–369. doi: 10.1016/s0920-1211(98)00033-3. [DOI] [PubMed] [Google Scholar]
- Mouton P. Principles and practices of unbiased stereology. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- Mudo G, Jiang XH, Timmusk T, Bindoni M, Belluardo N. Change in neurotrophins and their receptor mRNAs in the rat forebrain after status epilepticus induced by pilocarpine. Epilepsia. 1996;37:198–207. doi: 10.1111/j.1528-1157.1996.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Namchuk M, Lindsay L, Turck CW, Kanaani J, Baekkeskov S. Phosphorylation of serine residues 3, 6, 10, and 13 distinguishes membrane anchored from soluble glutamic acid decarboxylase 65 and is restricted to glutamic acid decarboxylase 65alpha. J Biol Chem. 1997;272:1548–1557. doi: 10.1074/jbc.272.3.1548. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. Faseb J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89:1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niquet J, Ben-Ari Y, Represa A. Glial reaction after seizure induced hippocampal lesion: immunohistochemical characterization of proliferating glial cells. J Neurocytol. 1994;23:641–656. doi: 10.1007/BF01191558. [DOI] [PubMed] [Google Scholar]
- Niquet J, Jorquera I, Ben-Ari Y, Represa A. Proliferative astrocytes may express fibronectin-like protein in the hippocampus of epileptic rats. Neurosci Lett. 1994;180:13–16. doi: 10.1016/0304-3940(94)90902-4. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okragly AJ, Haak-Frendscho M. An acid-treatment method for the enhanced detection of GDNF in biological samples. Exp Neurol. 1997;145:592–596. doi: 10.1006/exnr.1997.6500. [DOI] [PubMed] [Google Scholar]
- Parent JM, Tada E, Fike JR, Lowenstein DH. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci. 1999;19:4508–4519. doi: 10.1523/JNEUROSCI.19-11-04508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J. Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res. 2005;63:27–42. doi: 10.1016/j.eplepsyres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol. 1998;79:1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Rashid K, Van der Zee CE, Ross GM, Chapman CA, Stanisz J, Riopelle RJ, Racine RJ, Fahnestock M. A nerve growth factor peptide retards seizure development and inhibits neuronal sprouting in a rat model of epilepsy. Proc Natl Acad Sci U S A. 1995;92:9495–9499. doi: 10.1073/pnas.92.21.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibel S, Larmet Y, Le BT, Carnahan J, Marescaux C, Depaulis A. Brain-derived neurotrophic factor delays hippocampal kindling in the rat. Neuroscience. 2000;100:777–788. doi: 10.1016/s0306-4522(00)00351-1. [DOI] [PubMed] [Google Scholar]
- Represa A, Niquet J, Charriaut-Marlangue C, Ben-Ari Y. Reactive astrocytes in the kainic acid-damage hippocampus have the phenotypic features of type-2 astrocytes. J Neurocytol. 1993;22:299–310. doi: 10.1007/BF01187128. [DOI] [PubMed] [Google Scholar]
- Represa A, Niquet J, Pollard H, Ben-Ari Y. Cell death, gliosis, and synaptic remodeling in the hippocampus of epileptic rats. J Neurobiol. 1995;26:413–425. doi: 10.1002/neu.480260313. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Massieu L, Boddeke HW, Palacios JM, Mengod G. Differential regulation of the expression of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNAs in adult rat brain after intrahippocampal injection of quinolinic acid. Brain Res Mol Brain Res. 1994;26:89–98. doi: 10.1016/0169-328x(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002;947:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR, Tandon P, Liu Z, Yang Y, Hori A, Holmes GL, Stafstrom CE. Multiple kainic acid seizures in the immature and adult brain: ictal manifestations and longterm effects on learning and memory. Epilepsia. 1997;38:1157–1166. doi: 10.1111/j.1528-1157.1997.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Functional implications of seizure-induced neurogenesis. Adv Exp Med Biol. 2004;548:192–212. doi: 10.1007/978-1-4757-6376-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Gray WP. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia. 2007;48 Suppl 2:33–41. doi: 10.1111/j.1528-1167.2007.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Humpel C, Wetmore C, Olson L. Cellular hybridization for BDNF, trkB, and NGF mRNAs and BDNF-immunoreactivity in rat forebrain after pilocarpine-induced status epilepticus. Exp Brain Res. 1996;107:331–347. doi: 10.1007/BF00230416. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setkowicz Z, Mazur A. Physical training decreases susceptibility to subsequent pilocarpine-induced seizures in the rat. Epilepsy Res. 2006;71:142–148. doi: 10.1016/j.eplepsyres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Glutamic acid decarboxylase-67-positive hippocampal interneurons undergo a permanent reduction in number following kainic acid-induced degeneration of ca3 pyramidal neurons. Exp Neurol. 2001;169:276–297. doi: 10.1006/exnr.2001.7668. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Shetty GA. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem. 2003;87:147–159. doi: 10.1046/j.1471-4159.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Smith PD, McLean KJ, Murphy MA, Turnley AM, Cook MJ. Seizures, not hippocampal neuronal death, provoke neurogenesis in a mouse rapid electrical amygdala kindling model of seizures. Neuroscience. 2005;136:405–415. doi: 10.1016/j.neuroscience.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Haas KZ, Stanton PK, Moshe SL. Resistance of the immature hippocampus to seizure-induced synaptic reorganization. Brain Res Dev Brain Res. 1991;60:88–93. doi: 10.1016/0165-3806(91)90158-f. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Chronopoulos A, Thurber S, Thompson JL, Holmes GL. Age-dependent cognitive and behavioral deficits after kainic acid seizures. Epilepsia. 1993;34:420–432. doi: 10.1111/j.1528-1157.1993.tb02582.x. [DOI] [PubMed] [Google Scholar]
- Stringer JL. Repeated seizures increase GFAP and vimentin in the hippocampus. Brain Res. 1996;717:147–153. doi: 10.1016/0006-8993(96)00059-5. [DOI] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O'Bryan KA, O'Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci U S A. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooyama I, Bellier JP, Park M, Minnasch P, Uemura S, Hisano T, Iwami M, Aimi Y, Yasuhara O, Kimura H. Morphologic study of neuronal death, glial activation, and progenitor cell division in the hippocampus of rat models of epilepsy. Epilepsia. 2002;43 Suppl 9:39–43. doi: 10.1046/j.1528-1157.43.s.9.10.x. [DOI] [PubMed] [Google Scholar]
- Van der Zee CE, Rashid K, Le K, Moore KA, Stanisz J, Diamond J, Racine RJ, Fahnestock M. Intraventricular administration of antibodies to nerve growth factor retards kindling and blocks mossy fiber sprouting in adult rats. J Neurosci. 1995;15:5316–5323. doi: 10.1523/JNEUROSCI.15-07-05316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu Z, Cermak JM, Tandon P, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK, Holmes GL. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. J Neurosci. 2000;20:RC109. doi: 10.1523/JNEUROSCI.20-22-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Nutritional importance of choline for brain development. J Am Coll Nutr. 2004;23:621S–626S. doi: 10.1080/07315724.2004.10719433. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]