Abstract
The activation of Wnt/β-catenin signalling has an important function in gastrointestinal tumorigenesis. It has been suggested that the promotion of Wnt/β-catenin activity beyond the threshold is important for carcinogenesis. We herein investigated the role of macrophages in the promotion of Wnt/β-catenin activity in gastric tumorigenesis. We found β-catenin nuclear accumulation in macrophage-infiltrated dysplastic mucosa of the K19-Wnt1 mouse stomach. Moreover, macrophage depletion in ApcΔ716 mice resulted in the suppression of intestinal tumorigenesis. These results suggested the role of macrophages in the activation of Wnt/β-catenin signalling, which thus leads to tumour development. Importantly, the conditioned medium of activated macrophages promoted Wnt/β-catenin signalling in gastric cancer cells, which was suppressed by the inhibition of tumour necrosis factor (TNF)-α. Furthermore, treatment with TNF-α induced glycogen synthase kinase 3β (GSK3β) phosphorylation, which resulted in the stabilization of β-catenin. We also found that Helicobacter infection in the K19-Wnt1 mouse stomach caused mucosal macrophage infiltration and nuclear β-catenin accumulation. These results suggest that macrophage-derived TNF-α promotes Wnt/β-catenin signalling through inhibition of GSK3β, which may contribute to tumour development in the gastric mucosa.
Keywords: gastric cancer, inflammation, macrophage, tumour necrosis factor-α, Wnt
Introduction
The canonical Wnt signalling pathway (Wnt/β-catenin pathway) operates by stabilizing β-catenin (Taketo, 2006). The phosphorylation of β-catenin by glycogen synthase kinase 3β (GSK3β) results in degradation through the ubiquitin pathway. The binding of Wnt ligands to a Frizzled receptor leads to inhibition of the β-catenin degradation complex consisting of APC, AXIN and GSK3β thereby allowing the nuclear translocation of stabilized β-catenin followed by transcriptional activation of the Wnt target genes. Wnt/β-catenin signalling has an important function in the maintenance of intestinal stem cells and progenitor cells (Korinek et al, 1998; van de Wetering et al, 2002), and its activation causes gastrointestinal tumour development (Oshima et al, 1995, 2006; Fodde et al, 2001).
The activation level of Wnt/β-catenin signalling has been reported to be a determining factor for the multiplicity of intestinal polyposis in several types of Apc gene mutant mice (Gaspar and Fodde, 2004; Li et al, 2005). Moreover, nuclear β-catenin accumulation is predominantly observed in the invasion front of colon cancer in comparison to the non-invasive tumour area (Brabletz et al, 1998). Furthermore, sensitivity of embryonic stem (ES) cells for differentiation is inhibited by increase of Wnt/β-catenin signalling activity (Kielman et al, 2002). These results, taken together, suggest that the promotion of the Wnt/β-catenin activity beyond the threshold level is required for tumorigenesis, invasion and the maintenance of cell stemness.
On the other hand, it has been established that inflammation has an important function in cancer development (Coussens and Werb, 2002). The activation of tumour necrosis factor (TNF)-α or NF-κB pathway is required for the development of hepatocellular carcinoma (Pikarsky et al, 2004) and intestinal tumours (Greten et al, 2004; Popivanova et al, 2008) through the induction of growth factors and suppression of apoptosis. Although several signalling pathways have been reported for the promotion of Wnt/β-catenin activity (Fodde and Brabletz, 2007), it has not been elucidated yet whether the inflammatory response contributes to the promotion of Wnt/β-catenin signalling during tumorigenesis.
Gastric cancer is the second most common cancer in the world and it is closely associated with Helicobacter pylori infection, which leads to chronic inflammation (Correa, 2003). Moreover, the activation of the Wnt/β-catenin signalling is found in about 30% of gastric cancer (Clements et al, 2002), suggesting that Wnt activation is one of the major causes for gastric cancer development. We recently showed that cooperation of Wnt signalling and prostaglandin E2 (PGE2) pathway causes gastric cancer development in a transgenic mouse model (Oshima et al, 2006). Because the activation of PGE2 signalling induces infiltration and activation of macrophages in the gastric mucosa (Oshima et al, 2004, 2005), these results collectively suggest that activated macrophages promote the Wnt/β-catenin signalling activity, which thus contributes to gastric cancer.
We herein show that TNF-α derived from activated macrophages promotes the Wnt/β-catenin activity in gastric cancer cells through the suppression of GSK3β. Moreover, Helicobacter infection in the Wnt1 transgenic mice resulted in macrophage infiltration and the activation of Wnt/β-catenin signalling in the gastric mucosa, which thus led to gastric tumorigenesis. These results suggest that activated macrophages in inflammatory microenvironment have an important function in gastric tumorigenesis through the promotion of the Wnt/β-catenin activity.
Results
Macrophage infiltration and β-catenin nuclear accumulation in gastric dysplasia of K19-Wnt1 transgenic mice
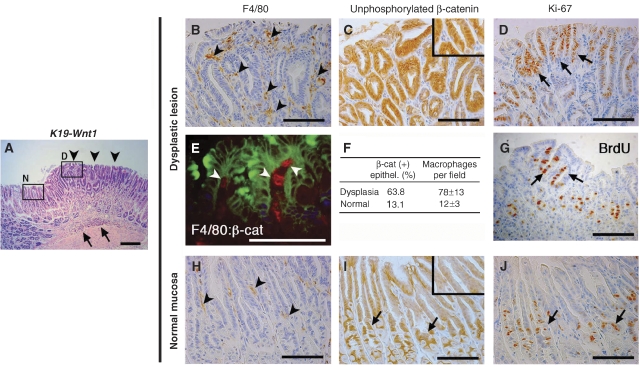
K19-Wnt1 transgenic mice expressing Wnt1 in the gastric epithelial cells develop sporadic dysplastic lesions in the glandular stomach (Oshima et al, 2006). In these dysplastic lesions, inflammatory cells infiltrated the submucosa, whereas cell infiltration was rarely detected in the adjacent normal mucosa (Figure 1A). By immunostaining, we found macrophage infiltration in the dysplastic mucosa, whereas tissue macrophages were only sparsely scattered in the normal mucosa (Figure 1B and H). We also found strong nuclear staining of β-catenin in the epithelial cells of the dysplastic mucosa (Figure 1C). Notably, β-catenin-accumulated epithelial cells were physically associated with stromal macrophages in the dysplastic lesions (Figure 1E). In contrast, mild β-catenin accumulation was found only in the cell proliferation zone of the normal mucosa (Figure 1I). We confirmed the increase in the β-catenin-positive index in the dysplastic lesion to be associated with the increased number of infiltrated macrophages (Figure 1F). Moreover, the number of Ki-67-positive proliferating cells increased in the dysplastic mucosa when compared with that in the adjacent normal region (Figure 1D and J). The mean Ki-67 labelling indices in the dysplastic lesions and normal mucosa were 43 and 15%, respectively (P=0.007). We also confirmed by BrdU incorporation analysis that cell proliferation was increased in dysplastic lesions (Figure 1G). On the basis of these results, we hypothesized that macrophage infiltration by inflammatory responses causes promotion of Wnt/β-catenin signalling activity in gastric epithelial cells, which probably leads to dysplastic changes in the gastric mucosa.
Figure 1.
Macrophage accumulation and nuclear localization of β-catenin in dysplastic lesion of the K19-Wnt1 mouse stomach. (A) Representative histology of a dysplastic lesion in the K19-Wnt1 glandular stomach (H&E staining). The arrowheads indicate the dysplastic mucosal area. The arrows indicate submucosal inflammatory infiltration. High-magnifications of boxed area (D, dysplastic lesion; and N, adjacent normal mucosa) in serial sections are shown in (B–E, G) and (H–J), respectively. Immunostaining results for macrophage marker F4/80 (B, H), β-catenin (C, I), Ki-67 (D, J), and BrdU (G) are shown. The arrowheads in (B, H) indicate mucosal macrophages. The inset in (C) indicates the nuclear localization of β-catenin in epithelial cells, whereas the inset in (I) indicates weak cytoplasmic accumulation of β-catenin. The arrows in (D, G) indicate proliferating cells that are positive for Ki-67 and BrdU, respectively. (E) Double immunofluorescence staining for F4/80 (red) and β-catenin (green) in dysplastic lesion. Arrowheads indicate macrophages. (F) The mean ratio of β-catenin-accumulated epithelial cells and the mean number of infiltrated macrophages per field in the dysplastic lesion and adjacent normal mucosa. The arrows in (I, J) indicate normal progenitor cells localized in gland neck with mild accumulation of β-catenin and positive Ki-67 staining. Bars indicate 100 μm.
Requirement of macrophages for intestinal tumour development in ApcΔ716 mice
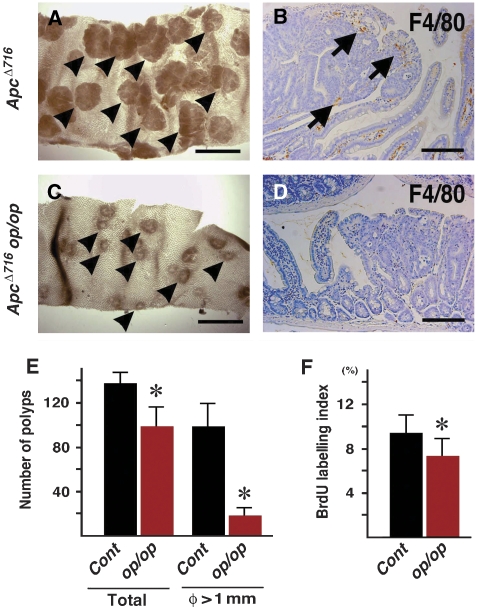
To examine whether infiltrated macrophages are required for tumorigenesis, we performed crossing experiments using op/op mice, in which the number of macrophages significantly decreased in the majority of tissues because of Csf1 mutation (Cecchini et al, 1994). We first examined whether op/op mutation suppressed macrophage infiltration in the inflamed stomach using K19-C2mE mice, which develop gastritis with heavy macrophage accumulation (Oshima et al, 2004). However, mucosal macrophages were still found in the stomach of the K19-C2mE op/op compound mice (Supplementary Figure 1). We therefore crossed op/op mice with ApcΔ716 mice, a model for intestinal polyposis caused by Wnt activation (Oshima et al, 1995). ApcΔ716 mice develop numerous polyps in entire intestinal tract (Figure 2A), and macrophages were infiltrated in the polyp stroma (Figure 2B). Interestingly, ApcΔ716 op/op compound mice showed dramatic suppression of intestinal polyposis (Figure 2C and E), and macrophages were not found in the polyp tissues (Figure 2D). Moreover, the number of polyps >1 mm significantly decreased in ApcΔ716 op/op compound mice by 80% compared with ApcΔ716 mice. Although it remains to be elucidated as to whether the loss of the CSF-1 function affects tumorigenesis, these results suggest that macrophages were required for the growth of Wnt/β-catenin-activated tumour cells. Consistently, the BrdU labelling index also decreased in ApcΔ716 op/op mouse tumours (Figure 2F).
Figure 2.
Suppression of intestinal tumorigenesis by depletion of macrophages. (A, C) Representative photographs of small intestine of ApcΔ716 mice (A) and ApcΔ716 op/op compound mutant mice (C) were taken using a dissecting microscope. The arrowheads indicate intestinal polyps. Bars indicate 2 mm. (B, D) F4/80 macrophage immunostaining of polyp tissues in ApcΔ716 (B) and ApcΔ716 op/op compound mice (D). The arrows indicate macrophages. Bars indicate 100 μm. (E) The number of total polyps and large polyps >1 mm in diameter of ApcΔ716 (Cont) and ApcΔ716 op/op compound mice (op/op) are shown as mean±s.d. (F) BrdU labelling index in polyps of ApcΔ716 (Cont) and ApcΔ716 op/op mice (op/op) are shown as mean±s.d. *P<0.05 in comparison to the control.
Fluctuation of Wnt/β-catenin signalling activity in gastric cancer cells
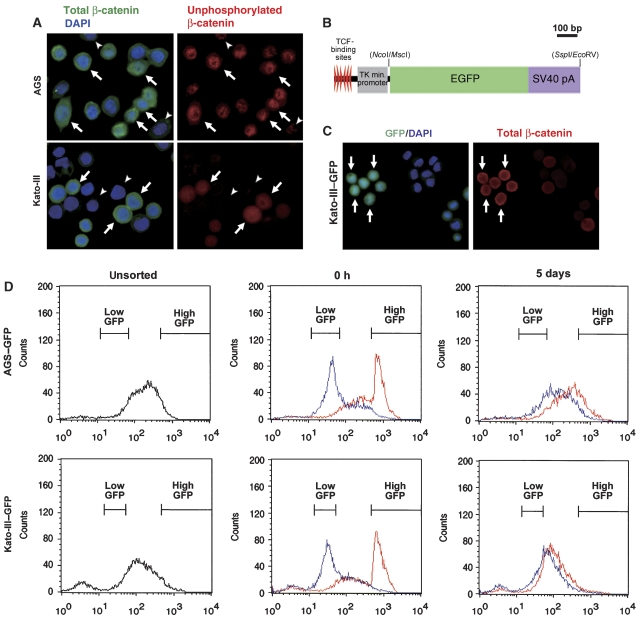
We next examined the regulation of the Wnt/β-catenin signalling activity by in vitro experiments using human gastric cancer cells, AGS and Kato-III. AGS cells harbour a heterozygous mutation in the β-catenin gene (CTNNB1) at codon 34 (Caca et al, 1999; Supplementary Figure 2), whereas CTNNB1 is amplified in Kato-III cells (Suriano et al, 2005). Consistent with the previous results (Nojima et al, 2007), both cell lines showed an elevated β-catenin/TCF transcriptional activity as detected by a TOPFLASH assay (Supplementary Figure 3). By immunocytochemistry, the cytoplasmic and nuclear accumulation of β-catenin was found in both AGS and Kato-III cells (Figure 3A, left). We also found the nuclear localization of active form of β-catenin, unphosphorylated on Ser37 and Thr41 (van Noort et al, 2002; Figure 3A, right), thus indicating the activation of Wnt/β-catenin signalling. However, several cells showed a weak β-catenin staining intensity (Figure 3A, arrowheads), suggesting that the level of Wnt/β-catenin signalling activity varies in populations.
Figure 3.
Fluctuation of the Wnt/β-catenin activity in gastric cancer cells. (A) Representative results of immunocytochemistry of AGS (top panels) and Kato-III (bottom panels) using anti-total β-catenin antibody (green) and DAPI (blue) (left panels) or anti-unphosphorylated β-catenin antibody (red) (right panels) of the same specimen. The arrows indicate cells with accumulated both total and unphosphorylated β-catenin. The arrowheads indicate cells with weak or loss of β-catenin staining. (B) Construction of TOPEGFP expression vector. EGFP cDNA was inserted between TCF-binding site/TK minimum promoter and SV40 pA signal. (C) Representative results of immunocytochemistry of Kato-III–GFP cells transiently transfected with pcDNA3-S33A-β-catenin for 72 h. GFP expression (green) with DAPI staining (blue) (left panel), and unphosphorylated β-catenin (red) of the same specimen (right panel) are shown. The arrows indicate the cell cluster with elevated GFP and unphosphorylated β-catenin levels. (D) Representative results of flow cytometry for GFP fluorescence of AGS–GFP (top) and Kato-III–GFP (bottom) cells. Flow cytometry results of the isolated high-GFP (top 5%, red line) and low-GFP (bottom 5%, blue line) populations at 0 h (centre) and 5 days (right) after isolation by cell sorting.
To examine the Wnt/β-catenin activity in the live cells, we transfected a TOPEGFP vector that expressed a green fluorescent protein (GFP) in response to β-catenin/TCF transcriptional activity (Figure 3B), and established AGS–GFP and Kato-III–GFP cell lines. We confirmed that GFP expression was induced in β-catenin accumulated cells after the transient transfection of mutant CTNNB1 (S33A) expression vector (Figure 3C). Importantly, we directly found by time-lapse video analysis that the Wnt/β-catenin activity was not constant but fluctuated in individual AGS–GFP cells (Supplementary Video 1).
To confirm the fluctuation of Wnt/β-catenin activity, we isolated by cell sorting the high-GFP (top 5%) and low-GFP (bottom 5%) populations corresponding to high and low Wnt/β-catenin signalling activity, respectively, from AGS–GFP and Kato-III–GFP cells (Figure 3D, left). Immediately after isolation, both populations showed distinct GFP intensities (Figure 3D, centre). Importantly, however, the GFP intensities of the isolated populations returned to a similar distribution to the unsorted cells after culturing for 5 days (Figure 3D, right). These results confirmed that the activation level of Wnt/β-catenin signalling fluctuates in each cell.
We next examined the level of Wnt/β-catenin activity in cell cycle-synchronized Kato-III–GFP cells. However, the ratio of a high-GFP population did not change during cell cycling, thus indicating Wnt/β-catenin fluctuation to be independent of the cell cycle status (Supplementary Figure 4).
Promotion of Wnt/β-catenin activity in gastric cancer cells by activated macrophages and TNF-α
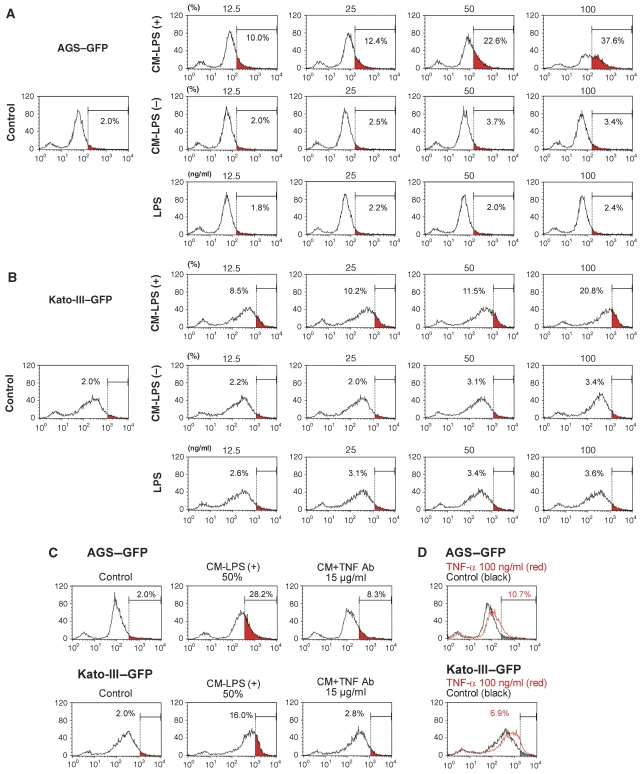
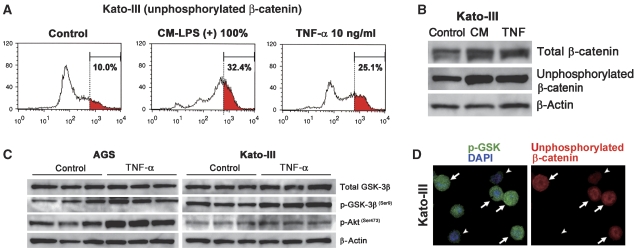
We next examined whether the activity of Wnt/β-catenin signalling is promoted by activated macrophages using the GFP reporter-transfected cells. We prepared a conditioned medium of macrophage cell line, RAW264, activated with lipopolysaccharide (LPS) for 24 h; (hereafter, CM-LPS (+)). A conditioned medium from unstimulated RAW264 cells was used as the control (CM-LPS (−)). When AGS–GFP and Kato-III–GFP cells were stimulated with CM-LPS (+), the high-GFP population (corresponding to top 2% in the untreated control cells) significantly increased in a CM concentration-dependent manner (Figure 4A and B, left and top, red area). In contrast, stimulation of the cells with CM-LPS (−) or LPS alone did not affect the Wnt/β-catenin activity in either cell line (Figure 4A and B, middle and bottom). These results indicate that the soluble factor(s) derived from activated macrophages promote the Wnt/β-catenin activity in gastric cancer cells.
Figure 4.
Enhancement of Wnt/β-catenin signalling by the activated macrophages in gastric cancer cells. (A, B) Representative results of flow cytometry of AGS–GFP (A) and Kato-III–GFP cells (B). The cells were treated with CM-LPS (+) (top), CM-LPS (−) (middle) or LPS alone (bottom) at various concentrations indicated on top of each panel. The red area in each panel indicates a population with a high GFP intensity corresponding to the top 2% of the control cells (left). The percentage of high-GFP population (red area) is indicated in each panel. (C) Representative flow cytometry of AGS–GFP (top) and Kato-III–GFP (bottom) treated with CM-LPS (+) alone (centre) or CM-LPS (+) with anti-TNF-α-neutralizing antibody (right). The percentage of a high-GFP population (red area corresponding to top 2% in control cells, left) is indicated in each panel. (D) Representative flow cytometry results of AGS–GFP (top) and Kato-III–GFP (bottom) treated with TNF-α (red lines) and untreated control (black lines). The percentage of high GFP in TNF-α-treated cells (corresponding to top 2% in control cells, grey area) is indicated in each panel.
TNF-α is a key mediator for inflammation and is produced by activated macrophages. Notably, treatment of the cells with a neutralizing antibody against TNF-α significantly suppressed the effect by CM-LPS (+) to enhance the Wnt/β-catenin activity in both cell lines (Figure 4C). Furthermore, direct treatment with TNF-α increased the level of Wnt/β-catenin activity (Figure 4D). We detected the expression of both TNF R1 and TNF R2 on AGS and Kato-III cells as well as in the primary cultured gastric epithelial cells (Supplementary Figure 5A and C and data not shown). Importantly, the blockade of either receptor by neutralizing antibody significantly suppressed the TNF-α-induced Wnt/β-catenin activation (Supplementary Figure 5B). These results suggest that TNF-α signalling through both TNF R1 and TNF R2 is thus involved in the promotion of the Wnt/β-catenin activity. By immunohistochemistry, we found most TNF-α-expressing cells in gastric tumours to be macrophages, whereas CD11c-positive dendritic cells were rarely detected (Supplementary Figure 6A). Importantly, treatment of the K19-Wnt1 mice with anti-TNF-α-neutralizing antibody resulted in a decrease of the β-catenin immunostaining intensity in the dysplastic lesions (Supplementary Figure 6B).
Moreover, we examined the Wnt-promoting effect of other inflammatory cytokines, IL-1β, IL-6 and IL-11, which have an important function in gastric tumorigenesis (Howlett et al, 2005; Fox and Wang, 2007). However, these cytokines did not promote Wnt/β-catenin activity in gastric cancer cells, although IL-1β slightly increased the high-GFP population in AGS–GFP cells (Supplementary Figure 7). These results, taken together, suggest that TNF-α is one of the important macrophage-derived factors that promote the Wnt/β-catenin activity in gastric epithelial cells and cancer cells.
Suppression of β-catenin phosphorylation by TNF-α through the inhibition of GSK3β in gastric cancer cells
By a flow cytometry analysis, we found that the level of unphosphorylated β-catenin increased significantly in Kato-III cells when cells were treated with CM-LPS (+) or TNF-α (Figure 5A). By western blotting, we confirmed the increased level of unphosphorylated β-catenin in Kato-III cells stimulated with CM-LPS (+) or TNF-α (Figure 5B). These results suggested that CM-LPS (+) or TNF-α suppressed the phosphorylation of β-catenin, resulting in Wnt/β-catenin promotion. Significantly, treatment with TNF-α resulted in an increase of GSK3β phosphorylation on Ser9 both in AGS and Kato-III cells (Figure 5C), which caused the suppression of the GSK3β activity (Cross et al, 1995). The mean relative band intensities of the phosphorylated GSK3β to the control level were 2.03±0.14 (P=0.009) and 1.68±0.31 (P=0.03) in the AGS and Kato-III cells, respectively. Interestingly, a strong immunostaining signal for phosphorylated GSK3 was found in a sub-population of Kato-III cells that also showed nuclear accumulation of β-catenin at the same time (Figure 5D). These results suggest that the promotion of Wnt/β-catenin signalling is regulated by the GSK3β phosphorylation status in gastric cancer cells. Akt is an important kinase for phosphorylation of GSK3β at Ser9 (Sharma et al, 2002). Importantly, the active form of Akt, phosphorylated on Ser473, increased significantly after TNF-α stimulation in both AGS and Kato-III cells (Figure 5C). The mean relative band intensities of the phosphorylated Akt to the control level were 2.08±0.16 (P=0.005) and 1.81±0.19 (P=0.002) in the AGS and Kato-III cells, respectively. These results suggest that TNF-α accelerates the GSK3β phosphorylation through the activation of the Akt pathway.
Figure 5.
Suppression of β-catenin phosphorylation by CM-LPS (+) or TNF-α in gastric cancer cells. (A) Representative flow cytometry results of Kato-III cells using an antibody against unphosphorylated β-catenin on Ser37 and Thr41 are shown. The cells were treated with CM-LPS (+) (centre) or TNF-α (right) for 24 h. The red area indicates population with high level of unphosphorylated β-catenin: top 10% in control cells (left). The percentage of red area is indicated in each panel. (B) Western blotting of Kato-III cells for total β-catenin (top) and unphosphorylated β-catenin (middle). The cells were treated with CM-LPS (+) (CM) or TNF-α at 10 ng/ml (TNF). β-Actin was used as an internal control (bottom). (C) Western blotting of AGS cells (left) and Kato-III cells (right) for total and phosphorylated GSK3β on Ser9, and phosphorylated Akt on Ser473. β-Actin was used as an internal control. The cells were treated with TNF-α at 100 ng/ml (TNF) in serum-free conditions. The results of three independent samples are shown for each cell line. (D) Representative immunocytochemistry for phosphorylated GSK3 (p-GSK, green) and DAPI (blue) (left) and unphosphorylated β-catenin (red) (right) of the same specimen. The arrows indicate cells with strong staining for both p-GSK and β-catenin, whereas the arrowheads indicate cells with weak staining for both.
NF-κB-independent promotion of Wnt/β-catenin activity in gastric cancer cells
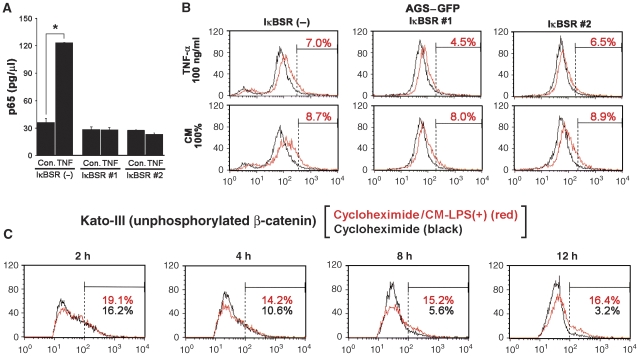
To examine whether NF-κB is involved in Wnt/β-catenin promotion by activated macrophages, we transfected IκB-superrepressor (IκBSR) expression vector into AGS–GFP cells and established two sublines, in which TNF-α-induced NF-κB activation was suppressed (Figure 6A). Notably, the TNF-α- or CM-LPS (+)-induced promotion of the Wnt/β-catenin activity did not change in either of IκBSR-transfected AGS–GFP cells (Figure 6B), thus suggesting the existence of an NF-κB-independent mechanism for the TNF-α-induced Wnt/β-catenin promotion.
Figure 6.
NF-κB-independent promotion of the Wnt/β-catenin signalling activity. (A) The level of NF-κB activity in the TNF-α-treated (TNF) or untreated control (Con.) cells (mean±s.d.). IκBSR nos. 1 and 2 are AGS–GFP-derived stable transfectants with IκBSR, whereas IκBSR (−) indicates parental AGS–GFP. (B) Representative flow cytometry results of IκBSR(−), IκBSR nos. 1 and 2 cells (left to right) treated with TNF-α (top) or CM-LPS (+) (bottom) at the indicated concentrations (red lines). The percentage of high-GFP populations (corresponding to top 2% in untreated cells, black lines) is indicated in each panel. (C) Flow cytometry results of Kato-III cells using antibody against unphosphorylated β-catenin. The cells were treated with cycloheximide alone (black line) or cycloheximide and CM-LPS (+) (red line) for 2, 4, 8 and 12 h. The percentages of the population with high level of unphosphorylated β-catenin are indicated in each panel (red, cycloheximide and CM-LPS (+); black, cycloheximide alone).
When Kato-III cells were treated with the protein synthesis inhibitor cycloheximide, the unphosphorylated β-catenin-positive population decreased gradually in an incubation time-dependent manner, probably due to the phosphorylation of β-catenin by GSK3β (Figure 6C, black lines). However, the simultaneous treatment with CM-LPS (+) significantly suppressed the cycloheximide-induced decrease of this population (Figure 6C, red lines). Therefore, it is also possible that activated macrophages suppress β-catenin phosphorylation independent of protein synthesis, which is consistent with the results that the transcription factor NF-κB pathway is not involved in the suppression of β-catenin phosphorylation.
Macrophage infiltration and Wnt/β-catenin activation in mouse gastric epithelial cells by Helicobacter infection
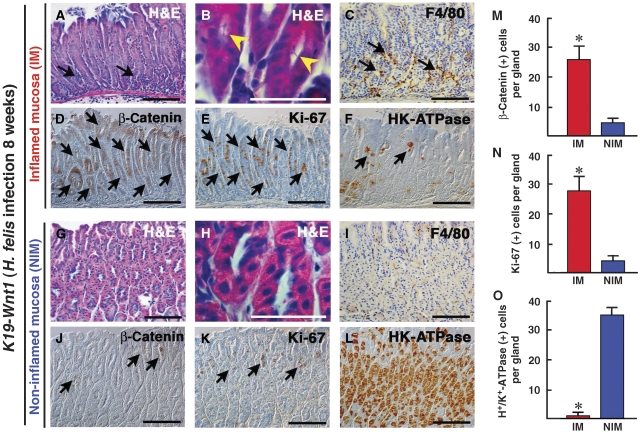
We next examined whether Wnt/β-catenin signalling is activated by infection-associated inflammation in the mouse stomach. H. felis, a close relative of H. pylori was infected into K19-Wnt1 mouse stomach. At 8 weeks after H. felis infection, the infiltration of macrophages was found in the H. felis-infected gastric mucosa (Figure 7A–C), whereas tissue macrophages were sparsely scattered in the non-infected mucosal area (Figure 7G–I). Such an inflamed mucosa in the infected K19-Wnt1 mice was only detected by histological examinations, and this inflamed mucosa was distinct from the spontaneously developed dysplastic lesions (Figure 1A). Importantly, the number of β-catenin-accumulated epithelial cells increased significantly in the infected and inflamed mucosa compared with that in the non-inflamed mucosa (Figure 7D, J and M). These results suggest that the infection-associated inflammation promotes the Wnt/β-catenin signalling activity in gastric epithelial cells in vivo. Moreover, in the inflamed mucosa, the epithelial cell proliferation detected by Ki-67 immunostaining significantly increased (Figure 7E, K and N). Furthermore, the number of H+/K+-ATPase-positive parietal cells decreased dramatically in the H. felis-infected area, thus indicating the suppression of epithelial differentiation (Figure 7F, L and O). These results, taken together, suggest that infiltrated macrophages in response to Helicobacter infection have an important function in the activation of Wnt/β-catenin signalling, thereby enhancing the proliferation and suppression of differentiation. When ApcΔ716 mice were infected with H. felis, we did not find any β-catenin-accumulated dysplastic cells in the inflamed gastric mucosa (data not shown). Therefore, it is possible that the basal activation level of Wnt/β-catenin signalling is required for its promotion.
Figure 7.
The activation of Wnt/β-catenin signalling in H. felis-infected gastric mucosa of K19-Wnt1 mice. (A–F) Representative histology of inflamed gastric mucosa (IM) of K19-Wnt1 mice infected with H. felis for 8 weeks. (G–L) Non-inflamed gastric mucosal (NIM) area of the K19-Wnt1 mice. H&E staining (A, G), high magnified H&E staining (B, H), immunostaining for F4/80 (C, I), unphosphorylated β-catenin (D, J), Ki-67 (E, K) and H+/K+-ATPase (F, L). Bars in (B, H) and other panels indicate 50 and 100 μm, respectively. The arrows in (A, C) indicate infiltrated mononuclear cells and macrophages, respectively. The yellow arrowheads in (B) indicate Helicobacter bacteria in the gland lumen, which is not found in NIM (H). The arrows in (D, E) indicate β-catenin-accumulated and proliferating epithelial cells, respectively. The arrows in (F) indicate H+/K+-ATPase-positive parietal cells that are dramatically decreased in IM compared with NIM (L). The arrows in (J, K) indicate progenitor cells with cytoplasmic β-catenin accumulation and Ki-67-positive proliferation, respectively. The mean numbers of positive cells per gland for unphosphorylated β-catenin (M), Ki-67 (N), and H+/K+-ATPase (O) in IM (red bars) and NIM (blue bars) are shown as histograms (mean±s.d.). The asterisks indicate P<0.05.
Tumour development in H. felis-infected K19-Wnt1 mouse stomach
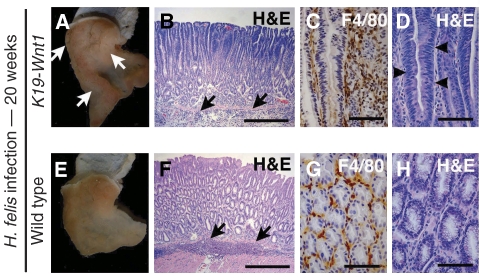
We finally examined whether H. felis infection contributes to gastric tumorigenesis in K19-Wnt1 mice. Importantly, K19-Wnt1 mice developed gastric tumours at 20 weeks after H. felis infection, whereas no tumours were found in the H. felis-infected wild-type mice (Figure 8A and E). Histologically, submucosal infiltration was found in both H. felis-infected K19-Wnt1 and wild-type mouse stomach (Figure 8B and F). Although macrophage infiltration was detected in both genotypes (Figure 8C and G), dysplastic epithelial cells were evident in the infected K19-Wnt1 tumours but not in the wild-type mouse stomach (Figure 8D and H). These results, taken together, suggest that infection-associated inflammation has an important function in gastric tumorigenesis through the promotion of the Wnt/β-catenin activity beyond the basal activation level.
Figure 8.
Tumour development in H. felis-infected K19-Wnt1 mouse stomach. K19-Wnt1 (A–D) and wild-type (E–H) mouse stomach infected with H. felis for 20 weeks. (A, E) Macroscopic photographs. The arrows in (A) indicate gastric tumours that developed in the infected K19-Wnt1 mice. H&E staining at low magnification (B, F) and high magnification (D, H). Immunostaining results for macrophage marker F4/80 (C, G). The arrows in (B, F) indicate submucosal infiltration. The arrowheads in (D) indicate dysplastic epithelial cells. Bars in (B, F) and (C, D, G, H) indicate 200 and 40 μm, respectively.
Discussion
Macrophage infiltration and cancer development, progression and stemness
The activation of Wnt/β-catenin signalling by genetic alteration, such as mutation in APC or CTNNB1, is responsible for gastrointestinal tumorigenesis. However, accumulating evidence suggests that further promotion of the Wnt/β-catenin activity has an important function in tumour growth and progression (Fodde and Brabletz, 2007). For example, increased β-catenin accumulation is found in the invasion front of colon cancer (Brabletz et al, 1998). We herein demonstrated direct evidence that the Wnt/β-catenin activity fluctuates in individual cancer cells that carry the same genetic alteration in CTNNB1. Accordingly, Wnt/β-catenin signalling can be promoted beyond the basal activated level caused by genetic alteration, and such promotion may have an important function in both tumour development and progression.
We have previously shown the induction of COX-2/PGE2 pathway to have an important function in the intestinal tumorigenesis that is triggered by Wnt/β-catenin activation (Oshima et al, 1996; Sonoshita et al, 2001). The simultaneous induction of both Wnt and PGE2 pathways is also responsible for gastric tumour development in mouse models (Oshima et al, 2006). Although PGE2 may have multiple functions for tumorigenesis, we previously found an increased PGE2 level to result in macrophage accumulation in the gastric mucosa of K19-C2mE mice (Oshima et al, 2004). Accordingly, it is possible that macrophage infiltration in dysplastic lesions of K19-Wnt1 mice is caused by the spontaneous induction of COX-2/PGE2 pathway. We herein show that Wnt/β-catenin signalling is promoted by macrophage-derived TNF-α, which contributes to gastric tumorigenesis. It is therefore possible that the induction of the COX-2/PGE2 pathway promotes Wnt/β-catenin signalling through the enhancement of macrophage infiltration, which is responsible for tumour development.
On the other hand, Wnt/β-catenin signalling is not activated in the stomach of either K19-C2mE mice or H. felis-infected ApcΔ716 mice, thus indicating that the induction of the COX-2/PGE2 pathway and subsequent macrophage infiltration are not sufficient for the promotion of Wnt/β-catenin in gastric epithelial cells. It is conceivable that the basal activation of the Wnt/β-catenin pathway by genetic alteration is required for the promotion of Wnt/β-catenin signalling.
The activation of Wnt/β-catenin pathway is also suggested to be important for stemness. Wnt/β-catenin signalling is required for the maintenance of normal intestinal stem cells (Korinek et al, 1998), and the increase in the Wnt/β-catenin activity contributes to the maintenance of stemness in ES cells (Kielman et al, 2002). Accordingly, it is possible that the increased Wnt/β-catenin activity is also important for the maintenance of cancer stem cells. Therefore, infiltrated macrophages in tumour tissues may be an important component of niche for cancer stem cells by promoting Wnt/β-catenin signalling through TNF-α pathway. This hypothesis is supported by the results that macrophages are required for the maintenance of progenitor cells in the intestinal crypt (Pull et al, 2005).
Possible pathways other than TNF-α for the promotion of the Wnt/β-catenin signalling activity
It has been shown that inflammatory cytokines, TNF-α, IL-1β, IL-6 or IL-11, have an important function in gastric tumorigenesis (Howlett et al, 2005; Fox and Wang, 2007). Among these cytokines, TNF-α is the most potent Wnt/β-catenin-promoting factor, although IL-1β slightly increases Wnt signalling in AGS cells. However, the increased level of the Wnt/β-catenin activity by CM-LPS (+) is significantly higher than that by TNF-α alone in both cell lines (Figure 4), thus suggesting the effect of other macrophage-derived factors on Wnt/β-catenin promotion. It has been reported that hepatocyte growth factor activates Wnt/β-catenin signalling through the tyrosine phosphorylation of β-catenin (Rasola et al, 2006), and platelet-derived growth factor also promotes Wnt/β-catenin signalling through suppression of β-catenin phosphorylation (Yang et al, 2006). It is therefore possible that these factor(s) contribute to Wnt/β-catenin promotion together with TNF-α in gastric tumorigenesis.
Recently, using a colon tumour mouse model, TNF R1 signalling in bone marrow-derived cells has been shown to indirectly enhance epithelial Wnt/β-catenin signalling (Popivanova et al, 2008). Accordingly, it is possible that TNF-α signalling, not only in epithelial cells but also in stromal cells, may therefore contribute to the promotion of Wnt/β-catenin signalling in cancer cells.
Promotion of Wnt/β-catenin signalling in cancer cells that retain wild-type CTNNB1
We show here that TNF-α stimulation promotes the Wnt/β-catenin signalling activity through the suppression of β-catenin phosphorylation by GSK3β. Therefore, the expression of wild-type β-catenin is required for the promotion of Wnt/β-catenin signalling in epithelial cells as well as cancer cells. Because Kato-III cells have a multicopy of the wild-type CTNNB1 (Suriano et al, 2005), TNF-α stimulation suppresses phosphorylation of these β-catenin, thus leading to the enhancement of Wnt/β-catenin signalling. On the other hand, AGS cells possess mutation in CTNNB1 close to the phosphorylation site of GSK3β (Supplementary Figure 2; Caca et al, 1999), which causes the activation of the basal Wnt/β-catenin signalling. It is therefore possible that TNF-α stimulation suppresses phosphorylation of wild-type β-catenin expressed from retained intact CTNNB1, which contributes to the promotion of the total Wnt/β-catenin activity of AGS cells. In human gastric cancer, a CTNNB1 mutation is found in about 30% of the Wnt-activated cases (Clements et al, 2002). However, either a somatic mutation or downregulation of E-cadherin (Cheng et al, 2005), secreted frizzled-related proteins (Nojima et al, 2007), or β-TrCP, a component of ubiquitin ligase complex (Kim et al, 2007) is found in gastric cancer, which leads to the basal activation of Wnt/β-catenin signalling without mutation in CTNNB1. These results, taken together, suggest that wild-type β-catenin expressed in gastric cancer cells is stabilized by inflammatory macrophages, thus resulting in the promotion of Wnt/β-catenin signalling, which contributes to cancer development, progression and the maintenance of cancer stem cells.
Accordingly, the present results suggest that the suppression of macrophage infiltration and its activation by anti-inflammatory drugs or inhibitors for PGE2 pathway is, therefore, a possible strategy for chemoprevention against gastric cancer.
Materials and methods
Animal experiments
The construction of K19-Wnt1 and K19-C2mE transgenic mice has been described previously (Oshima et al, 2004, 2006). Briefly, K19-Wnt1 mice express Wnt1, whereas K19-C2mE mice express COX-2 and mPGES-1 in gastric epithelial cells under transcriptional regulation by cytokeratin 19 (K19) gene promoter. K19-Wnt1 transgenic mice were euthanized at 30 weeks of age (n=10), and the glandular stomach was fixed with 4% paraformaldehyde and processed for histological analysis. H. felis (ATCC49179) was cultured as previously described (Oshima et al, 2004). H. felis bacteria were inoculated at 108 per mouse p.o. into K19-Wnt mice, ApcΔ716 mice (Oshima et al, 1995) and wild-type mice. The infected K19-Wnt1 mice were examined at 8 and 20 weeks after the H. felis inoculation (n=3 for each mice). The infected ApcΔ716 mice were examined at 6 weeks after infection (n=4). The localization of H. felis-infected mucosa at 8 weeks was estimated by the detection of bacteria using microscope with × 100 objective (Figure 7B). ApcΔ716 mice were crossed with op/op mice (The Jackson Laboratory) to obtain ApcΔ716 op/op compound mice (n=3) and littermate control ApcΔ716 mice (n=3). The intestine of ApcΔ716 and ApcΔ716 op/op mice was examined at 12 weeks of age, and the total number of polyps was determined using a dissecting microscope. For scoring the BrdU labelling index, the mice were injected i.p. with 200 μl of BrdU solution (Roche) and then the tissue specimens were processed for immunostaining using anti-BrdU antibody. All animal experiments were carried out according to the protocol approved by the Committee on Animal Experimentation of Kanazawa University.
Histology and immunostaining
The stomach tissues were fixed in 4% paraformaldehyde, paraffin-embedded and sectioned at 4 μm thickness. These sections were stained with H&E. Serial sections were immunostained with antibodies for Ki-67 (DakoCytomation), F4/80 (Serotec), unphosphorylated (activated) β-catenin on Ser37 and Thr41 (Upstate), H+/K+-ATPase (MBL, Nagoya, Japan) or BrdU (BD Biosciences) as the primary antibody. The staining signals were visualized using the Vectorstain Elite Kit (Vector Laboratories). The number of positive stained cells for F4/80, β-catenin, Ki-67 or H+/K+-ATPase was scored in 20 randomly selected gastric glands and the mean values were calculated. The number of BrdU-positive cells and the total number of nuclei was counted in five microscopic fields and then the mean value was calculated as the BrdU labelling index. For immunofluorescence staining, Alexa Fluor 594 donkey anti-mouse IgG or Alexa Fluor 488 anti-rat or rabbit IgG (Molecular Probes) was used as the secondary antibody.
Plasmid vector construction
For the construction of the β-catenin/TCF reporter plasmid with GFP (TOPEGFP vector), cDNA encoding EGFP was excised from pIRES2-EGFP (Clontech) and replaced with luciferase gene in TOPFLASH plasmid (Upstate). TOPEGFP reporter plasmid was transfected to cells using Effectene Transfection Reagent (Qiagen), and stable cell lines were then obtained by cell sorting. The Wnt/β-catenin activity using TOPEGFP reporter was measured by flow cytometry (see below). pcDNA3-S33A-β-catenin and pBSFI-IκBSR plasmids were kindly provided by Dr Peter Vogt at the Scripps Research Institute. pcDNA3-S33A-β-catenin was transiently transfected to Kato-III–GFP cells for 72 h to express stabilized mutant β-catenin. pBSFI-IκBSR was transfected to AGS–GFP cells to establish the sublines in which the NF-κB pathway is suppressed.
Immunocytochemistry
Gastric cancer cells grown on cover slips were fixed with 10% neutral buffered formalin and permeabilized with 0.1% Triton X-100 in PBS. Anti-total β-catenin antibody (Sigma), anti-unphosphorylated β-catenin antibody (Upstate), or anti-phosphorylated GSK3 (Cell Signaling) was used as the primary antibody, and anti-mouse IgG Alexa 594 or anti-rat IgG Alexa 488 (Molecular Probes) were used as the secondary antibody. Next, cover slips were mounted using VECTASHIELD Mounting Medium (Vector Laboratories) that contained DAPI for nuclear staining.
Cell culture and preparation of conditioned medium
Gastric cancer cell lines, AGS (ATCC) and Kato-III (Cell Resource Center for Biomedical Research, Tohoku University, Japan) were cultured in RPMI1640 medium supplemented with 10% FBS in a humidified incubator at 37°C with 5% CO2. The mouse macrophage cell line RAW264 (RIKEN BioResource Center, Tsukuba, Japan) was cultured in RPMI1640, and treated with LPS purified from Salmonella enterica serotype typhimurium (Sigma) at 100 ng/ml for 24 h. The conditioned medium, CM-LPS (+) was collected and used for the Wnt/β-catenin activation experiments. The control conditioned medium, CM-LPS (−) was prepared from non-stimulated RAW264 cell culture. For either the stimulation or inhibition of the TNF-α pathway, TNF-α (Calbiochem) or anti-mouse TNF-α-neutralizing antibody (R&D systems) was added, respectively, in the culture medium at the indicated concentration.
NF-κB ELISA assay
The nuclear extract was prepared from AGS–GFP cells or pBSFI-IκBSR-transfected clones (IκBSR nos. 1 and 2) using NE-PER Nuclear Extraction Reagents (Pierce), and the NF-κB activity was measured using an NF-κB p65 Transcription Factor Assay Kit (Active Motif). These cell lines were also used for the flow cytometry analysis.
Flow cytometry analysis
After treatment with CM-LPS (+), CM-LPS (−), LPS or TNF-α at the indicated concentrations for 48 h, AGS–GFP, Kato-III–GFP cells or pBSFI-IκBSR-transfected cells (IκBSR nos. 1 and 2) were washed with PBS containing 2% FCS and stained with 5 μg/ml propidium iodide to exclude dead cells. GFP fluorescence was examined with FACSCalibur (Becton Dickinson). A fluorescence intensity corresponding to the top 2% of the control untreated cells was judged as the high-GFP population (high-Wnt/β-catenin signalling population).
For the analysis of intracellular unphosphorylated (active) β-catenin, cells were fixed in 1–2% paraformaldehyde and permeabilized using 0.05% Triton X-100 in PBS containing 2% FCS. Next, the cells were incubated with anti-unphosphorylated β-catenin antibody on Ser37 and Thr41 (Upstate) followed by anti-mouse IgG-Alexa 488 (Molecular Probes), and examined with FACSCalibur. For the inhibition of protein translation, the cells were treated with cycloheximide (Sigma) at 0.5 μg/ml for the indicated time, and then the intracellular unphosphorylated β-catenin was measured by a FACS analysis as described above.
Western blotting analysis
For the detection of total β-catenin and unphosphorylated β-catenin, the cells were stimulated with CM-LPS (+) or TNF-α at 10 ng/ml for 24 h. For the detection of total or phosphorylated GSK3β (Ser9), and phosphorylated Akt (Ser473), the cells were cultured in serum-free conditions for 24 h followed by stimulation with TNF-α at 100 ng/ml for 24 h. Next, the cells were lysed and sonicated in lysis buffer. After centrifugation at 20 000 g, 10 μg of the supernatant protein was separated in a 10% SDS-polyacrylamide gel. Antibodies for unphosphorylated (active) β-catenin (Upstate), total β-catenin (Sigma), total GSK3β (BD Biosciences), phosphorylated GSK3 on Ser9 and 21 (Cell Signaling), and phosphorylated Akt at Ser473 (Cell Signaling) were used as the primary antibody. β-Actin was used as an internal control. The ECL detection system (Amersham Biosciences) was used to detect specific signals.
Statistical analysis
The data were analysed by the paired or unpaired t-test using Microsoft Excel (Microsoft). A value of P<0.05 was accepted as statistically significant.
Supplementary Material
Supplementary Figures
Supplementary Video 1
Supplementary Figures Legends
Supplementary Methods
Acknowledgments
We thank Manami Watanabe for her valuable technical contribution. This study was supported by Grant-in-Aid for the Third-Term Comprehensive Control Strategy for Cancer from the Ministry of Health, Labour and Welfare of Japan, Grant-in-Aid for Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Takeda Science Foundation, Japan.
References
- Brabletz T, Jung A, Hermann K, Günther K, Hohenberger W, Kirchner T (1998) Nuclear overexpression of the oncoprotein β-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract 194: 701–704 [DOI] [PubMed] [Google Scholar]
- Caca K, Kolligs FT, Ji X, Hayes M, Qian J, Yahanda A, Rimm DL, Costa J, Fearon ER (1999) β- and γ-catenin mutations, but not E-cadherin inactivation, underlie T-cell factor/lymphoid enhancer factor transcriptional deregulation in gastric and pancreatic cancer. Cell Growth Differ 10: 369–376 [PubMed] [Google Scholar]
- Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER (1994) Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120: 1357–1372 [DOI] [PubMed] [Google Scholar]
- Cheng XX, Wang ZC, Chen XY, Sun Y, Kong QY, Liu J, Li H (2005) Correlation of Wnt-2 expression and β-catenin intracellular accumulation in Chinese gastric cancers: relevance with tumour dissemination. Cancer Lett 223: 339–347 [DOI] [PubMed] [Google Scholar]
- Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM (2002) β-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 62: 3503–3506 [PubMed] [Google Scholar]
- Correa P (2003) Helicobacter pylori infection and gastric cancer. Cancer Epidemiol Biomarkers Prev 12: 238s–241s [PubMed] [Google Scholar]
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789 [DOI] [PubMed] [Google Scholar]
- Fodde R, Brabletz T (2007) Wnt/β-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol 19: 150–158 [DOI] [PubMed] [Google Scholar]
- Fodde R, Smits R, Clevers H (2001) APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer 1: 55–67 [DOI] [PubMed] [Google Scholar]
- Fox JG, Wang TC (2007) Inflammation, atrophy and gastric cancer. J Clin Invest 117: 60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar C, Fodde R (2004) APC dosage effects in tumorigenesis and stem cell differentiation. Int J Dev Biol 48: 377–386 [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M (2004) IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118: 285–296 [DOI] [PubMed] [Google Scholar]
- Howlett M, Judd LM, Jenkins B, La Gruta NL, Grail D, Ernst M, Giraud AS (2005) Differential regulation of gastric tumor growth by cytokines that signal exclusively through the coreceptor gp130. Gastroenterology 129: 1005–1018 [DOI] [PubMed] [Google Scholar]
- Kielman MF, Ridanpää M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, Taketo MM, Roberts S, Smits R, Fodde R (2002) Apc modulates embryonic stem-cell differentiation by controlling the dosage of β-catenin signaling. Nat Genet 32: 594–605 [DOI] [PubMed] [Google Scholar]
- Kim CJ, Song JH, Cho YG, Kim YS, Kim SY, Nam SW, Yoo NJ, Lee JY, Park WS (2007) Somatic mutations of the β-TrCP gene in gastric cancer. APMIS 115: 127–133 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383 [DOI] [PubMed] [Google Scholar]
- Li Q, Ishikawa TO, Oshima M, Taketo MM (2005) The threshold level of adenomatous polyposis coli protein for mouse intestinal tumorigenesis. Cancer Res 65: 8622–8627 [DOI] [PubMed] [Google Scholar]
- Nojima M, Suzuki H, Toyota M, Watanabe Y, Maruyama R, Sasaki S, Sasaki Y, Mita H, Nishikawa N, Yamaguchi K, Hirata K, Itoh F, Tokino T, Mori M, Imai K, Shinomura Y (2007) Frequent epigenetic inactivation of SFRP genes and constitutive activation of Wnt signaling in gastric cancer. Oncogene 26: 4699–4713 [DOI] [PubMed] [Google Scholar]
- Oshima H, Oshima M, Inaba K, Taketo MM (2004) Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J 23: 1669–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M (2006) Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology 131: 1086–1095 [DOI] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM (1996) Suppression of intestinal polyposis in ApcΔ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87: 803–809 [DOI] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M (1995) Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci USA 92: 4482–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Matsunaga A, Taketo MM (2005) Hyperplastic gastric tumors with spasmolytic polypeptide-expressing metaplasia caused by tumor necrosis factor-α-dependent inflammation in cyclooxygenase-2/microsomal prostaglandin E synthase-1 transgenic mice. Cancer Res 65: 9147–9151 [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y (2004) NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431: 461–466 [DOI] [PubMed] [Google Scholar]
- Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya Y, Kaneko S, Oshima M, Fujii C, Mukaida N (2008) Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118: 560–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS (2005) Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA 102: 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola A, Fassetta M, De Bacco F, D'Alessandro L, Gramaglia D, Di Renzo MF, Comoglio PM (2006) A positive feedback loop between hepatocyte growth factor receptor and β-catenin sustains colorectal cancer cell invasive growth. Oncogene 26: 1078–1087 [DOI] [PubMed] [Google Scholar]
- Sharma M, Chuang WW, Sun Z (2002) Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3β inhibition and nuclear β-catenin accumulation. J Biol Chem 277: 30935–30941 [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM (2001) Acceleration of intestinal polyposis through prostaglandin receptor EP2 in ApcΔ716 knockout mice. Nat Med 7: 1048–1051 [DOI] [PubMed] [Google Scholar]
- Suriano G, Vrcelj N, Senz J, Ferreira P, Masoudi H, Cox K, Nabais S, Lopes C, Machado JC, Seruca R, Carneiro F, Huntsman DG (2005) β-Catenin (CTNNB1) gene amplification: a new mechanism of protein overexpression in cancer. Genes Chromosomes Cancer 42: 238–246 [DOI] [PubMed] [Google Scholar]
- Taketo MM (2006) Wnt signaling and gastrointestinal tumorigenesis in mouse models. Oncogene 25: 7522–7530 [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H (2002) Wnt signaling controls the phosphorylation status of β-catenin. J Biol Chem 277: 17901–17905 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- Yang L, Lin C, Liu ZR (2006) P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from β-catenin. Cell 127: 139–155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Supplementary Video 1
Supplementary Figures Legends
Supplementary Methods