Abstract
Post-traumatic stress disorder (PTSD) is a severe anxiety disorder that may develop after experiencing or witnessing a traumatic event. Recent clinical evidence has suggested the involvement of neurodegenerative pathology in the illness, particularly with brain imaging studies revealing a marked reduction in hippocampal volume. Of greater significance is that these anatomical changes appear to be positively correlated with the degree of cognitive deficit noted in these patients. Stress-induced increases in plasma cortisol have been implicated in this apparent atrophy. Although not definitive, clinical studies have observed a marked suppression of plasma cortisol in PTSD. The basis for hippocampal neurodegeneration and cognitive decline therefore remains unclear. Stress and glucocorticoids increase glutamate release, which is recognized as an important mediator of glucocorticoid-induced neurotoxicity. Recent preclinical studies have also noted that glutamate and nitric oxide (NO) play a causal role in anxiety-related behaviors. Because of the prominent role of NO in neuronal toxicity, cellular memory processes, and as a neuromodulator, nitrergic pathways may have an important role in stress-related hippocampal degenerative pathology and cognitive deficits seen in patients with PTSD. This paper reviews the preclinical evidence for involvement of the NO-pathway in PTSD, and emphasizes studies that have addressed these issues using time-dependent sensitization – a putative animal model of PTSD.
Keywords: PTSD, nitric oxide, glutamate, GABA, glucocorticoids, NOS, stress, time-dependent sensitization (TDS)
Introduction
Classified as an anxiety disorder, post-traumatic stress disorder (PTSD) is characterized by hyperarousal, avoidance, and various amnesic symptoms caused by exposure to a severe traumatic event (APA 1994) (Table 1). By definition PTSD occurs in the aftermath of exposure to trauma, but there is growing awareness of the importance of multiple exposures to trauma in predicting the onset and severity of this disorder (Brewin 2001; Maes et al 2001). Nevertheless, the more severe the initial trauma and the more intense the acute stress symptoms, the higher is the risk for developing PTSD (Gore and Richards 2002).
Table 1.
Main symptoms of PTSD
|
Data adapted from APA (1994).
Abbreviations: PTSD, post-traumatic stress disorder.
The most characteristic features of PTSD are pneumonic in nature (APA 1994) and include amnesia, flashbacks, fragmentation of memories (Elzinga and Bremner 2002), and an abnormal startle response; the latter reflecting an inability to properly integrate memories (van der Kolk 1994). In lieu of the evidence for degeneration of the hippocampus in patients with PTSD, a dysfunctional hippocampus may represent the anatomic basis for the fragmentation of memory. Although glucocorticoids have received the greatest attention with regards to the possible mechanisms involved in hippocampal shrinkage (McEwen 1999; Sapolsky 2000b), their role, as well as other molecules involved in cellular resilience, requires more stringent evaluation.
Recent preclinical studies have found that stress exerts significant effects on nitric oxide synthase (NOS) activity, while clinical trials have emphasized the role of gamma-amino butyric acid (GABA)-glutamate balance as a putative neurobiological target in the treatment of PTSD. This paper reviews the role of GABA and glutamate in stress, especially the preclinical evidence for involvement of the nitric oxide (NO)-pathway in PTSD, and studies that have addressed these issues using time-dependent sensitization – a putative animal model of PTSD. From this point of view, we address their role as protagonists of neuronal degeneration and atrophy evident in neuroimaging studies of patients with PTSD, and how this may unfold into new avenues of treatment.
Anatomy and neurobiology of PTSD
Brain areas accepted as critical in mediating the stress response are the hippocampus and prefrontal cortex. These areas are in turn affected by the stress response. Imaging studies in PTSD patients have demonstrated volume reductions in the hippocampus (Bremner 1999; Elzinga and Bremner 2002), while structural changes, as well as functional deficits have also been observed in the medial prefrontal cortex in PTSD (Bremner 2002; Elzinga and Bremner 2002). Proper functioning of the hippocampus is necessary for explicit or declarative memory. Damage to the hippocampus resulting from stress not only causes problems in dealing with memories relating to past stressful experiences, but also impairs new learning (Bremner 1999; Elzinga and Bremner 2002). The prefrontal cortex, on the other hand, plays an important role in the process of fear conditioning, specifically with regard to extinction of conditioned fear responses (Le Doux 1998; Hamann 2001). In this regard, the medial prefrontal cortical areas modulate fear responding through inhibitory connections with the amygdala (Le Doux 1998), which in turn plays a crucial role in fear conditioning (Akirav et al 2001).
Recent studies suggest that structural abnormalities in PTSD may reflect either pre-trauma vulnerability or a consequence of trauma exposure (Gilbertson et al 2002). Furthermore, while increased plasma cortisol levels occur immediately after or during stress (Yehuda et al 1990), clinical studies of cortisol in PTSD have met with mixed results. Thus, investigators have documented plasma cortisol as unchanged (Baker et al 1999), elevated (Liberzon et al 1999), and suppressed (Yehuda et al 2000). Given these diverse results, the causal role for glucocorticoids in hippocampal neurodegeneration and cognitive decline in patients with PTSD remains unclear.
A key issue in the pathogenesis of PTSD seems to be within associative learning and other behavioral processes mediated by the hippocampus. These processes involve the glutamate N-methyl-D-aspartate (NMDA) receptors (Heresco-Levy and Javitt 1998) and the NO signaling pathway (O’Dell et al 1994; McLeod et al 2001). Moreover, the synaptic localization of NOS and the soluble guanylyl cyclase (McLeod et al 2001) facilitates the functioning of NO as a retrograde messenger – and thereby serves as a possible modulator of long-term potentiation (LTP) (Burette et al 2002).
LTP represents a form of synaptic learning that is known to exist in the hippocampus, striatum, and neocortex, and has been proposed as a cellular model for some forms of learning and memory. LTP describes a prolonged increase in the size of the postsynaptic response to a presynaptic stimulus of given strength. Activation of NMDA receptors are obligatory for the induction of the type of LTP that occurs in the hippocampus CA1 region. These events are initiated by Ca2+ flow through open, unblocked NMDA channels that lead to alterations in the strength of interneuronal synaptic connectivity (Bliss and Collingridge 1993; Bloom 2001). In the hippocampus, LTP is most evident in the dentate gyrus granule cells where glutamate activation of both AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) and NMDA receptors (Vallance and Collier 1994) are required.
It is interesting that prolonged loss of consciousness following terrifying events appears to protect against the development of PTSD (Adler 1993). Although coma is not yet fully understood, it may involve disruption of glutamatergic pathways (O’Brien and Nutt 1998). Similarly, central nervous system (CNS) suppressants such as ethanol and benzodiazepines exert some of their effect by suppressing glutamatergic function, these frequently being abused by patients with PTSD, possibly assisting in preventing the reemergence of previously established memories (Collingridge and Bliss 1995). The activation of neuronal NO synthase (nNOS) and other Ca2+ -dependant enzymes may account for many of the beneficial as well as deleterious affects associated with NMDA receptor activation, including neuronal development (Garthwaite 1991), learning, and memory (Iga et al 1993), but also cell death and neurodegeneration (Dawson VL and Dawson TM 1995).
Role of sensitization and kindling in PTSD
Models such as fear conditioning and kindling have been central to our understanding of fear circuitry, particularly through the processing of fear-relevant information between the amygdala, locus coeruleus, hippocampus, and thalamus. The association between stressful life events and the onset and chronicity of psychiatric illness dates back to Kraepelin (1921) who proposed that in depression, the illness may become increasingly autonomous and less reliant on environmental adversity. This mechanism may hold for most psychiatric illnesses that are characterized by a progressive worsening over time. The kindling phenomena was first described by Goddard et al (1969) who noted that repeated subthreshold electrical stimulation leads to full-blown seizures. After sufficient repetition the seizures begin to occur spontaneously. The kindling hypothesis therefore has particular relevance for viewing how synapses react to environmental stressors, and represents one way to integrate a range of data describing the neurophysiology and neurochemistry of the onset, long-term development, and treatment responsiveness of PTSD. The primary neural substrate of kindling involves glutamate and NMDA receptor activation, with inhibitory GABA pathways exerting essentially a permissive role on the kindling action of glutamate (Post and Weiss 1998). This evidence not only emphasizes the involvement of excitatory glutamatergic and inhibitory GABA’ergic pathways in the neuropathology of PTSD (Chambers et al 1999; Vaiva et al 2004), but also the process of sensitization and kindling in the neuro-development of the disorder.
Time-dependent sensitization (TDS): an animal model of PTSD
Animal models of PTSD have utilized intense stressors, aversive challenges, and situational reminders of a traumatic stress in an attempt to model long-term effects on behavioral, autonomic, and hormonal responses seen in humans with PTSD (Uys et al 2003). As alluded to earlier, the concept of sensitization is an important concept in the neuro-development of PTSD and is emphasized by repeated trauma over time by stress–restress in the TDS model.
In this model, animals are exposed to a single session of prolonged stress (eg, 2 h restraint followed by a 20 min forced swim, followed by exposure to ether or halothane vapors). The animals are allowed to recover for a week and are then subjected to a brief restress on day 7 (30 min of restraint stress or 20 min swim stress), the rationale being that the frequency of exposure to situational reminders contributes to the maintenance over time of fear-related behavioral disturbances. The model has proven to resemble the clinical situation with accurate face validity (how well the model resembles the psychiatric condition), construct validity (how well the model is consistent with theoretical rationale), and predictive validity (how the model responds to the drugs used in humans with PTSD).
Face validity
Stress-restress evokes a pronounced acoustic startle response (Kahn and Liberzon 2004), as well as spatial memory deficits together with lowered plasma corticosterone (Harvey, Naciti, et al 2003), consistent with clinical findings in PTSD. The TDS model emphasizes the role of prior trauma in predicting subsequent dysfunction, allows for the study of bidirectional expression of symptoms (enhanced or reduced responsiveness to environmental stimuli), and provides credible intra-subject variation (Yehuda and Antelman 1993). These represent important phenomenological and biological correlates of PTSD.
Construct validity
The model has proved valuable for studying hypothalamic-pituitary-adrenal (HPA) abnormalities relevant to PTSD (Yehuda and Antelman 1993), in that animals subjected to TDS display the enhanced sensitivity to negative glucocorticoid feedback that is often characteristic of PTSD (Liberzon et al 1997; Liberzon, Lopez, et al 1999; Harvey, Naciti, et al 2003). Of particular importance is that stress–restress leads to changes in hippocampal serotonin (5HT) 5HT1A and prefrontal cortex 5HT2A receptors (Harvey, Naciti, et al 2003), which are brain areas that are intimately involved in memory and stress responsiveness. These data also correlate positively with the accepted role of 5HT in the pathology of the disease and its responsiveness to serotonin reuptake inhibitors (SRIs) (Argypolous et al 2000). In the context of this review, TDS stress also evokes distinct changes in GABA and glutamate receptors (Harvey et al 2004).
Predictive validity
The effects of TDS-induced stress on spatial memory are attenuated by the 5HT reuptake inhibitor, fluoxetine (Harvey et al 2005), and by the steroid synthesis inhibitor, ketoconazole (Brand et al 2004). These data correlate positively with the putative causal role of serotonin and glucocorticoids in PTSD-associated memory changes.
How may glucocorticoids be involved in the biobehavioral pathology of PTSD?
Exposure to an acute stressful event leads to a profound release of glucocorticoids from the adrenal cortex (Bremner 1999). Important to note is that glucocorticoids have bidirectional effects on memory function. While hippocampal damage is associated with exposure to excessive levels of glucocorticoids (Bremner et al 1999), too low levels of glucocorticoids are also detrimental for normal hippocampal function (de Kloet et al 1999).
Repeated administration of corticosterone decreases 5-HT1A-mediated inhibition of the hippocampus (Karten et al 1999) through a process that occurs at the level of protein synthesis (Bijak et al 2001), resulting in reduced 5-HT1A receptor expression (Montgomery et al 2001). Consequently, stress-induced corticosterone release immediately after stress will make the hippocampus more vulnerable to the laying down of long-term emotion-driven memories.
In both PTSD and TDS stress, increased sensitivity of hippocampal glucocorticoid receptors (Yehuda 1998) and release of glutamate may heighten the vulnerability of the hippocampus to atrophy, even in the absence of higher cortisol levels (Sapolsky 2000b). Indeed, marked changes in glutamate NMDA receptors have been observed following TDS stress (Harvey et al 2004). Furthermore, high levels of corticosterone induce a rapid and non-genomic prolongation of NMDA receptor-mediated Ca2+ elevation in cultured rat hippocampal neurons (Takahashi et al 2002), while stress-mediated changes in hippocampal spine densities are NMDA receptor dependent (Shors et al 2004). These effects will further compromise hippocampal function, resulting in decreased spatial memory performance and reduced hippocampal volume as noted in PTSD patients.
Dysfunctional glucocorticoid-glutamate activity, described above, will lay the foundation for an impaired ability of neurons to survive coincident insults, particularly worsening neurotoxicity evoked by adverse conditions such as seizures, hypoxia-ischemia, metabolic poisons, hypoglycemia, oxidative stress, and exposure to excessive glucocorticoids (Sapolsky 2000b). The profound effect of glucocorticoids on hippocampal volume is clearly illustrated in patients with Cushing’s disorder, with smaller hippocampal volumes being reported in these patients (Starkman et al 1992). Although the hippocampus normally regulates glucocorticoid release through inhibitory effects on the HPA axis (Bremner 1999; Bremner et al 1999), hippocampal damage would result in disruption of this negative feedback loop, increasing the exposure of the hippocampus to glucocorticoid toxicity (Sapolsky 2000a, 2000b). However, abnormally low glucocorticoid levels are also associated with reduced hippocampal function (de Kloet et al 1999). For example, adrenalectomy results in a significant reduction in LTP, while exposure to stress results in a further reduction in LTP (Shors et al 1990).
The role of glucocorticoids in the neuropathology and development of PTSD can therefore not be underestimated. However, establishing its exact contribution is difficult considering the varied results obtained in clinical studies (Baker et al 1999; Liberzon, Abelson, et al 1999; Yehuda et al 2000). Nevertheless, of particular interest is that the paradoxical and rather unexpected observation of reduced cortisol in PTSD is closely emulated by TDS stress in animals (Liberzon et al 1997; Liberzon, Lopez et al 1999; Harvey, Naciti, et al 2003). These data suggest that a decreased cortisol level is conducive to decreased memory performance (Harvey, Naciti, et al 2003). The association between hypocortisolemia and neurodegeneration appears contradictory. However, despite the implications for glucocorticoid-induced hippocampal damage in stress and anxiety disorders, they may not be solely responsible for the hippocampal damage and subsequent memory deficits observed in PTSD.
Although the massive release of glucocorticoids during and immediately following exposure to trauma may result in hippocampal atrophy (Starkman et al 1992; Bremner 1999; Sapolsky 2000b), PTSD is a disorder that develops and worsens over time (Bremner 1999). It is thus possible that massive glucocorticoid secretion due to acute trauma exposure is only the first step in a cascade of events leading to later neurodegeneration and hippocampal atrophy with subsequent memory deficits. This cascade may involve kindling and sensitization as well as a host of molecular messengers, both neuroprotective and neurotoxic in nature, that will ultimately determine the progressive deterioration of neuronal function eventually culminating in the behavioral pathology typical of PTSD. Although various possible candidates could be discussed, for the purpose of this review we will maintain our focus on the glutamate-NO-pathway and the effects of TDS stress.
Glutamate-NMDA receptor pathway
Alterations of glutamatergic and NMDA receptor functions have been proposed to play a role in the etiology of PTSD in humans (van der Kolk 1994; Dawson VL and Dawson TM 1995; Chambers et al 1999). The NMDA receptor is involved in the normal processes of memory encoding while overstimulation of the NMDA receptor leads to the formation of strongly ingrained emotional memories via excessive mobilization of free cytosolic Ca2+. However, high levels of NMDA-Ca2+ activity are toxic to cells (McCaslin and Oh 1995) and, much like cortisol and memory described earlier, a delicate balance is needed for optimal neuronal function that does not hold any threat to neuronal survival.
Both stress and glucocorticoids have been found to increase glutamate concentrations in the hippocampal synapse, acknowledged as a prime mediator of glucocorticoid-induced neurotoxicity (Sapolsky 2000b). The extreme neurotoxic potential of glutamate is well recognized in Alzheimer’s disease (Louzada et al 2004), schizophrenia (Heresco-Levy 1999), and possibly affective illnesses (Harvey 1996). One of the cardinal symptoms of Alzheimer’s disease is cognitive impairment and loss of mnemonic function, and has its origin in excessive glutamate activity (Dawson VL and Dawson TM 1995). PTSD, similarly, is characterized by a loss of cognitive abilities with evidence for increased glutamatergic activity (Chambers et al 1999). In support of this, a recent pilot study by Heresco-Levy and colleagues (2002) report on the clinical evidence for efficacy of D-cycloserine, a partial agonist at the glycine regulatory site on the NMDA receptor, in the treatment of PTSD.
Stress and glucocorticoids not only increase glutamate concentrations in the hippocampus, but glucocorticoids also selectively increase glutamate accumulation in response to excitotoxic insults in this brain region (Sapolsky 2000a). Thus, hippocampal damage resulting from the effects of increased levels of glucocorticoids due to trauma exposure will further elevate levels of glutamate, thus potentiating the neurotoxic process. It is therefore clear that, while increased levels of glucocorticoids may initiate hippocampal damage, it also activates other neurotoxic pathways that may drive neuronal damage over a protracted period after the traumatic event.
Recently, various animal studies have begun to tease out the role of glutamate during severe stress. For example, mice lacking a fully functional glutamate NMDA receptor are less sensitive to stress induced by the elevated plus-maze, light-dark box, and forced swimming tests (Miyamoto et al 2002). Using the TDS model, Kahn and Liberzon (2004) describe the inhibitory effect of the glutamate receptor inhibitor, topiramate, on an exaggerated acoustic startle response induced by TDS stress. Focusing more on the long-term sequelae of acute severe stress, we noted that TDS stress evokes a profound effect on glutamate receptors in the hippocampus of rats three weeks after stress exposure (Harvey et al 2004). In the latter study, TDS stress induced a significant down-regulation of hippocampal NMDA receptors. Despite the indication that overstimulation of the NMDA receptor might explain the neurodegeneration observed in PTSD, preclinical studies have found that inhibition of glutamate reuptake, resulting in increased glutamate levels, leads to a decrease in NMDA receptor density (Cebers et al 1999). This is suggested as a possible neuroprotective mechanism to counteract NMDA receptor overstimulation (Naskar and Dreyer 2001).
Nitric oxide and its relevance to stress and PTSD
Nitric oxide (NO) pathway
Glutamatergic stimulation of NMDA receptors activates a number of enzymes including NOS, cyclooxygenases, proteases, lipases, and protein kinases by evoking a long-lasting (100 millisecond) Ca2+-ion influx. In the presence of calmodulin, Ca2+ activates NOS, which converts the amino-acid L-arginine to Nω-hydroxy-L-arginine, which is further converted to NO and L-citrulline (Nathan 1992; Knowles and Moncada 1994). A small gaseous molecule (MW 30Da) with a biological half-life of minutes, NO is rapidly degraded to nitrites and nitrates. However, its great lipid solubility affords it the unique ability to move quickly within and between cells.
NOS exist in three different isoforms that are either constitutive or inducible (Table 2). The activity of the constitutive NOS depends on Ca2+ and calmodulin, whereas the inducible NOS is independent of Ca2+. Endothelial eNOS is mainly located in the cell membrane, neuronal nNOS in neuronal cells, while inducible iNOS is located in macrophages and glial cells (Nathan 1992). All NOS isoforms are dependant on NADPH (β-nicotinamide adenine dinucleotide phosphate) and calmodulin. In iNOS, calmodulin is present in a tightly bound form, such that iNOS produces NO in a sustained manner in the presence of adequate substrate (Marletta 1993). Calcium-calmodulin binds to the constitutive enzyme in a reversible manner, but binds irreversibly to the inducible enzyme, so that neurons and endothelial cells containing the constitutive enzyme produce receptor-regulated pulses of NO, while the inducible enzyme in macrophages and microglia produces sustained levels of NO in response to cytokines that are not regulated by receptors (McCaslin and Oh 1995).
Table 2.
NOS isoforms
| Neural (n NOS, type 1) |
Inducible (iNOS, type 2) |
Endothelial (eNOS, type 3) |
|
|---|---|---|---|
| Cells first identified in | Neurons | Macrophages | Endothelium |
| Other cells expressing | Myocytes | Astrocytes | Neurons |
| Astrocytes | Microglia | ||
| Intracellular localization | Soluble or membrane bound | Soluble or membrane bound | Largely membrane bound |
| Ca2+ dependency | Activity depends on elevated Ca2+ | Activity is independent of elevated Ca2+ | Activity depends on elevated Ca2+ |
| Expression | Constitutive inducible under certain circumstances, eg, trauma | Inducible | Constitutive |
| Amounts of NO released | Small, pulses | Large, continuous | Small, pulses |
| Proposed function | Regulation | Host defense | Regulation |
| Activators | Glutamate | Lipopolysaccharide | Acetylcholine |
| Noradrenaline |
Data adapted from Yun et al (1997) and Moncada et al (1997).
Soluble guanylate cyclase represents the most important NO receptor in the brain (Dawson VL and Dawson TM 1995), with its activation by NO following glutamate-mediated activation of the NMDA receptor leading to an increase in the second messenger – cyclic guanosine monophosphate (cGMP) (Fedele et al 2001). Important neuronal effects of cGMP include activation of G-kinase, activation or inhibition of phosphodiesterase and subsequent effects on cyclic adenosine monophosphate (cAMP), effects on ion channels and G-proteins, and neurotransmitter release (Harvey 1996; Prast and Philippu 2001). All these actions exert a significant effect on neuronal function. The effects of cGMP are terminated by the phosphodiesterase (PDE) family, among which PDE 3 and PDE 5 are considered specific for cGMP (Soderling and Beavo 2000).
NO news or good news
NO may possess both neuroprotective and neurodestructive properties (Dawson VL and Dawson TM 1995; McCaslin and Oh 1995). By virtue of its unpaired electron, NO may promote the formation of free radicals and has been linked to various neurodegenerative processes (Ischiropoulos and Beckham 2003). However, it has also been reported to exert a protective action in neurodegenerative disorders (Robb and Connor 2002). Concerning the former, nitrosylation, DNA damage, lipid peroxidation, and energy depletion may contribute to NO-mediated neurotoxicity (Cuzzocrea et al 2001), while its antioxidant properties (Wink et al 1999) and ability to suppress expression of iNOS (Colasanti and Suzuki 2000) may contribute a neuroprotective effect. Moreover, by inhibiting its own synthesis (Griscavage et al 1995) and by down-regulating the NMDA receptor by S-nitrosylation at the redox-sensitive site on the receptor (Tanaka et al 1993; McCaslin and Oh 1995), NO will negatively influence its own synthesis and activation. The importance of NO-cGMP in the pathology and treatment of neuropsychiatric illness is clear in the literature, eg, depression (Harvey, McEwen, et al 2003), schizophrenia (Baba et al 2004), bipolar disorder (Yanik et al 2004), and AIDS dementia (Vincent et al 1999).
Stress and NO
Several findings indicate that NO plays an important role in anxiety-related disorders. High concentrations of NOS are found in brain regions involved in the modulation of anxiety and defensive behavior (Vincent and Kimura 1992), and exposure to stressful stimuli has been found to induce the activation of NO-producing neurons in the amygdala, hypothalamus, peri-aquaductal grey, and pedunculopontine tegmental nucleus (Krukoff and Khalili 1997). It is therefore not surprising that behavioral and pharmacological studies have associated dysfunctional NMDA-NO-cGMP activity with anxiety states. Consequently, NMDA receptor antagonists (Padovan et al 2000), guanylate cyclase inhibitors (Eroglu and Caglayan 1997; Heiberg et al 2002), and NOS inhibitors (Volke, Wegener, Bourin, et al 2003) all demonstrate significant anxiolytic properties in animal models of anxiety. Moreover, inhibition of cGMP breakdown by coadministration of the cGMP-selective PDE inhibitor, sildenafil, causes anxiogenic effects in the mouse elevated plus maze (Volke, Wegener, Vasar 2003; Kurt et al 2004).
Interestingly, antidepressants that have proved useful in treating PTSD also inhibit NOS in the hippocampus (Wegener et al 2003) as well as modulate NMDA receptors (Skolnik 1999), thus further confirming the potential role of this pathway in the pathology and pharmacology of anxiety and stress-related disorders. It is in this context noteworthy that several in vitro studies have shown that activation of postsynaptic 5-HT2A/2C receptors decrease cGMP levels generated in response to NMDA administration (Marcoli et al 1997; Maura et al 2000). The inhibitory action of the 5HT2A receptor on cGMP may afford a means whereby 5HT and SRIs may treat PTSD.
Stress increases glutamate (Magarinos and McEwen 1995) and 5HT release (Joseph and Kennett 1983; Vahabzadeh and Fillenz 1994) in the hippocampus, with the subsequent activation of 5HT2A receptors (Vaidya et al 1999). The result is the genesis of an anxiety state as well as down-regulation of brain-derived neurotrophic factor (BDNF) expression (Vaidya et al 1999). The latter event will adversely affect neuronal function and integrity, such that the biobehavioral and neurodegenerative changes that occur in PTSD are mediated by glutamate but driven by excessive 5HT. In support of this, the 5HT reuptake enhancer, tianeptine, reverses stress-induced decreases in hippocampal neurogenesis (Czeh et al 2001), while central depletion of 5HT prevents TDS stress-induced hippocampal 5HT1A receptor changes (Harvey et al 2005).
Studies in animals using the TDS model describe an immediate elevation in NOS activity that is sustained for a period of 3 weeks post-TDS stress (Table 3). This protracted elevation in NO-production in the hippocampus is driven primarily by the iNOS isoform (Harvey et al 2004). Similarly, increased expression of nNOS (De Oliviera et al 2000) and iNOS (Madrigal et al 2001, 2003) has been noted to occur in limbic brain regions following acute restraint stress in rats.
Table 3.
Kinetic data for hippocampal NOS in control animals, in animals immediately after the acute stressors (day 1; acute), after the re-stress (day 7), and on day 21 post stress (ps).
| Group | Vmax (mean ± sem) |
Km (mean ± sem) |
N |
|---|---|---|---|
| Control | 6.18 ± 3.15 | 3.77 ± 2.46 | 15 |
| Acute | 12.04 ± 1.43a | 3.54 ± 2.10 | 8 |
| Re-stress | 12.35 ± 6.85a | 3.17 ± 1.80 | 6 |
| Day 21 ps | 16.86 ± 6.76a | 3.16 ± 1.16 | 20 |
Data adapted from Harvey et al (2004) and Oosthuizen F (2003 unpub).
p < 0.05 compared with control (Dunnetts test).
Of particular significance is that TDS stress-induced iNOS activation is inhibited by the glucocorticoid synthesis inhibitor, ketoconazole (Harvey et al 2004). Moreover, ketoconazole has been shown to inhibit development of anxious behavior in rats exposed to stressors (Cohen et al 2000). In this manner, NO contributes to the neuro-degenerative process initiated by an acute increase in glucocorticoids. Thus, while an initial activation of constitutive NOS may follow acute stress (De Oliveira et al 2000), iNOS appears to play a more prominent role during chronic stress (Homayoun et al 2002). Since PTSD represents a form of chronic, repeated stress due to flashbacks and re-experiencing, this is of major significance considering that iNOS-activity is increased over a sustained period post stress. iNOS in neighboring astrocytes and glia produce NO in a sustained manner, thus producing increased levels of NO, resulting in the formation of long-lived oxidant species such as peroxynitrite, and culminating in local neuronal damage and hippocampal shrinkage, as observed in patients with PTSD.
Inhibition of TDS-induced activation of iNOS with ketoconazole also brings a strong association with stress-induced release of cortisol. iNOS is activated by cytokines (Dawson VL and Dawson TM 1995) – pivotal modulators of inflammatory processes (Campbell et al 2003). Psychological stress in humans is associated with increased secretion of proinflammatory cytokines such as interleukin-6 (Maes et al 1999). High levels of cerebrospinal fluid interleukin-6 have been measured in patients with PTSD (Baker et al 2001). Interleukin-6 secretion is suppressed by glucocorticoids (Baker et al 2001) such that under conditions when glucocorticoid levels are low in PTSD, this may allow an unopposed increase in interleukin-6 secretion, with subsequent increase in iNOS activation.
Since NO is able to down-regulate its own synthesis both directly on NOS (Griscavage et al 1995) and via down-regulation of the NMDA receptor (McCaslin and Oh 1995), these neuroprotective mechanisms may comprise a possible protective role for NO in the initial stages post stress. Neuronal NOS may, however, be more sensitive than iNOS to the above inhibiting action of NO (Griscavage et al 1995). This suggests that overproduction of NO in the hippocampus following TDS stress more likely is the result of iNOS induction and not activation of nNOS. Indeed, activation of NOS by TDS stress is selectively blocked by the selective iNOS inhibitor, aminoguanidine and not the selective nNOS inhibitor, 7-nitroindazole (Harvey et al 2004). The decrease in NMDA receptor density following TDS stress (Harvey et al 2004) further underlines the involvement of iNOS and not nNOS, since iNOS activation is not regulated by neuronal receptors (McCaslin and Oh 1995).
Acute versus chronic stress and dysregulation of GABA-glutamate balance
The natural development of PTSD is dependent on various comorbid factors, including the nature of the prior trauma and intra-subject variation. Of course a question relating to the natural development of PTSD post stress is why various people exposed to the same stressor respond differently, some developing PTSD and others not (McNally 1998). This suggests that a natural protective mechanism is in place to prevent excessive release of neurodestructive forces after a severely stressful event and which may not be adequate in patients that eventually develop the disorder. The mechanism that may be involved here, and how it maintains or loses its efficacy in regulating the normal response to trauma, is an important consideration.
NO via nNOS is increased after acute stress (Krukoff and Khalilli 1997; De Oliviera et al 2000) where NO acts predominantly as a neuromodulator by decreasing glutamate release, NO release, NOS activity, and increasing GABA release (see Figure 1 for detailed description) (Harvey 1996; Prast and Philippu 2001). Under these conditions, it will have a beneficial effect on memory consolidation (McLeod et al 2001). Thus, this acute increase in NO may underlie a normal physiological and protective role for NO in the early stages of stress. However, under conditions of protracted reexposure to stressful stimuli, as is the case after TDS stress, a different dynamic may be involved that predicts a gradual loss of homeostatic regulatory mechanisms controlling the process of consolidating or extinguishing trauma-related memories and culminating in iNOS activation (see Figure 2 for detailed description). On the positive side, TDS stress-induced NOS activation has been found to result in a decrease in NMDA receptor density (Harvey et al 2004) that will adversely affect glutamate-driven LTP and consequent memory formation. This reactive response may also represent a form of neuroprotection as well as attenuation of trauma-related memories.
Figure 1.
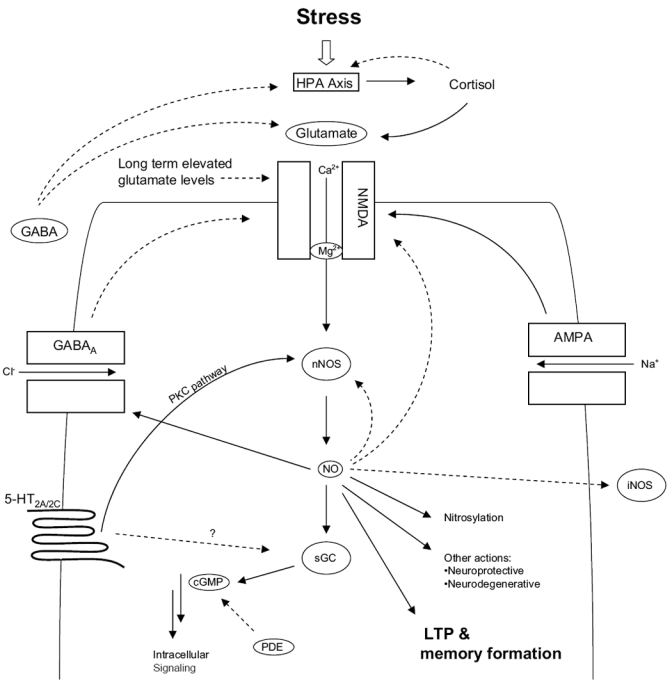
Role of nNOS in TDS stress. Immediately after an acute stress, NO is released via the action of adrenal glucocorticoids and the sequential release of glutamate in the hippocampus, activation of NMDA receptors, and the influx of Ca2+ leading to the activation of nNOS. 5HT released as a consequence of stress acts on 5HT2 receptors activating constitutive nNOS via the protein kinase C (PKC) pathway. NO then exerts various neuromodulatory effects (Prast and Phillippu 2001) as well as promotes the cellular processes of plasticity and memory either via itself, or by the synthesis of its second messenger, cGMP. However, NO prevents this cascade from progressing indefinitely by inhibiting its own synthesis (nNOS), inhibiting NMDA receptor function, promoting GABA release that further attenuates glutamate activity and by inhibiting the expression of iNOS. 5HT also inhibits guanylate cyclase-cGMP, thereby also effectively down-regulating the NO-pathway. Raised glucocorticoids inhibit cytokine release preventing any possible mobilization of iNOS. Shortly after the immediate response to a stressful event, glucorticoids then shut down the cortisol response by feedback inhibition of the HPA axis. Solid lines indicate activation; broken lines indicate inhibition. (Abbreviations are listed at the end of the paper.)
Figure 2.
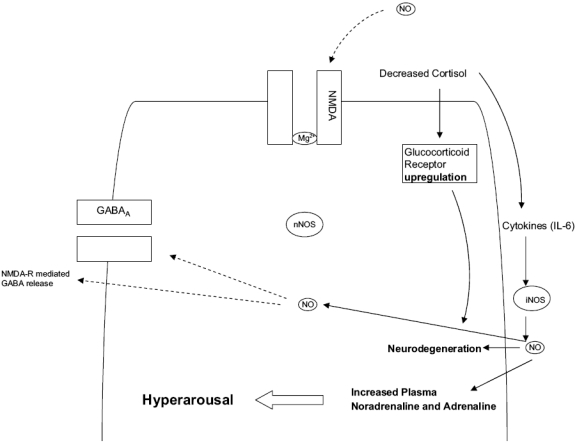
Role of iNOS in TDS stress. Under conditions of prolonged, recurrent stress, glucocorticoids fail to shut down the stress response leading to overt release of catecholamines and indolamines. In addition, a reactive hyperresponsiveness of the HPA axis ensues resulting in gross inhibition of glucorticoid release resulting in hypocortisolemia and disinhibition of cytokine expression. The result is an increased synthesis of IL-6 and subsequent expression of iNOS in local glia and astrocytes. The latter begins producing cell-toxic levels of NO for a protracted period, while an up-regulated state of the glucocorticoid receptor will increase susceptibility to cell toxic events, with profound consequences for the hippocampus. In an attempt to shut off excessive excitotoxic activity, NMDA receptors are down-regulated, leading to deficits in critical NMDA-driven cellular events such as neuroplasticity. Moreover, excessive NO inhibits GABA release thus destabilizing critical GABA glutamate balance. Activated iNOS in the CNS can also promote sympatho-adrenal outflow, complementing that already induced by raised CRF, thereby further increasing plasma catecholamines, worsening the state of hyperarousal already present. Solid lines indicate activation; broken lines indicate inhibition.
Another key player involved in cellular resilience is GABA. It is of major significance that TDS stress-induced activation of hippocampal iNOS and the down-regulation of NMDA receptors described above are associated with a significant attenuation of total hippocampal GABA (Harvey et al 2004). GABA occupies a critical role in inhibiting glutamatergic transmission via presynaptic GABA-B heteroreceptors (Yamada et al 1999). Further, swim stress-induced GABA release in the hippocampus is potentiated by NO (Engelman et al 2002), suggesting an important protective mechanism to curb excessive glutamate-NOS activation through actions on GABA release (Figure 1). Acute stress increases GABA (Engelmann et al 2002), possibly an immediate response to quench the excitotoxic burst of glutamate. However, it would appear that under conditions of repeated trauma, as with TDS, this protective effect of GABA is lost. Within this same context, it is pertinent to note that NO also inhibits NMDA receptor-mediated GABA release (Moller et al 1995), such that raised NO depletes neuronal GABA thus leaving the excitotoxic effects of glutamate unopposed (Figure 2).
GABA pathways play an important role in regulating normal affective state (Shiah and Yatham 1998), while also forming an integral part of the stress response. If GABA does indeed play a neuroprotective role in the central nervous system by opposing excitotoxic insults resulting from glutamate, what is the status of GABA in PTSD, and why does GABA not protect against neurotoxic insults resulting in hippocampal damage and memory deficits seen in PTSD?
GABA has been studied in PTSD to a limited degree, although a recent paper has indicated that post-trauma GABA plasma levels are low and may represent a predictive factor in the development of acute post-traumatic stress disorder (Vaiva et al 2004). In fact, people with panic disorder also appear to present with abnormally low GABA levels (Goddard et al 2004). The latter clinical data are reminiscent of that obtained in animals subjected to TDS (Harvey et al 2004). Thus, under conditions of chronic, recurrent stress, the role of GABA under these conditions either changes or becomes depleted and is no longer able to function optimally as expected. Untreated, PTSD will get progressively worse, with escalating GABA-glutamate imbalance with profound neurodegenerative consequences.
A unifying hypothesis
Acute stress is therefore associated with the release of glucocorticoids, as well as 5HT, glutamate, and NO in the hippocampus. The immediate increase in glucocorticoids acts on hippocampal glucocorticoid receptors, normally unresponsive to low circulating levels of cortisol, while glutamate activates hippocampal NMDA receptors (see Figure 3 for detailed description). These combined responses act to facilitate learning and the laying down of trauma-related memories. Continued activation of hippocampal glucocorticoid and NMDA receptors over a protracted period, as may occur through flashbacks and reexperiencing, will result in the excessive consolidation of trauma memories. The associated release of GABA after acute trauma, together with feedback inhibition of cortisol on the HPA axis, represents the normal homeostatic response that essentially counters the abnormal laying down of these memories. In addition, acute release of NO via nNOS and NMDA receptor activation down-regulates the NMDA receptor, while localized release of 5HT will attenuate the mobilization of Ca2+, NO, and cGMP (Marcoli et al 1997; Maura et al 2000). This will also prevent excessive emotional memory consolidation (see Figure 1). Together, these processes represent an immediate response to trauma and persons that spontaneously remit. However, in patients subjected to severe emotional trauma, and who later develop PTSD, parts of or all of these mechanisms may be compromised so that the acute stress response continues indefinitely, eventually developing into a chronic, recurring stress response.
Figure 3.
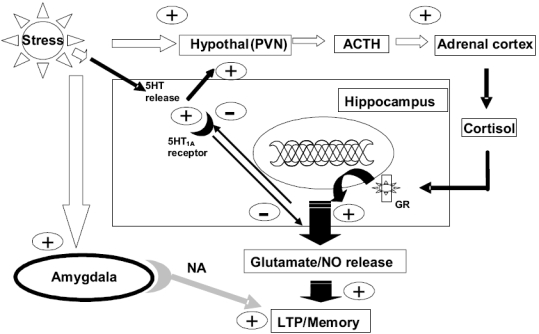
Neuroanatomy of memory and TDS stress. Acute moderate to high stress activates serotonergic neurons in the hippocampus to release 5HT. 5HT then activates postsynaptic 5HT1A receptors that inhibit the process of hippocampal LTP, dependent on glutamate-NO mechanisms, and effectively suppress the encoding of excessive emotional memory. However, 5HT will also stimulate the release of corticotrophin (ACTH) from the hypothalamus, resulting in the secretion of glucocorticoids. Activation of the glucocorticoid receptor in the hippocampus inhibits the expression of 5HT1A receptors, thereby preventing 5HT1A receptors from inhibiting LTP-mediated hippocampal memory formation, while the subsequent release of glutamate evoked by glucocorticoids will further promote inappropriate memory consolidation. As the stressor increases in intensity, or becomes recurrent, the amygdala is activated, which further activates hypothalamic glucocorticoid release and reinforces hippocampal LTP via noradrenergic mechanisms, while the absence of adequate 5HT1A-mediated suppression of hippocampal LTP, together with excessive glutamatergic activity, all combine to promote the consolidation of emotional-driven memories.
Although studies of cortisol levels in PTSD have produced mixed results, clinical findings suggest that low cortisol levels in PTSD may constitute a vulnerability marker related to chronic PTSD in addition to being a state-related characteristic of acute/chronic PTSD (Yehuda et al 2000). A major function of cortisol may be to contain stress-activated reactions, with the HPA response ultimately being terminated by the negative feedback inhibition of cortisol at pituitary, hypothalamus, and extra-hypothalamic brain sites (Yehuda et al 2000). However, suppressed cortisol that has been evident in PTSD may possibly be mediated by a gradual hypersensitization of the HPA axis in the aftermath of the initial stressors (Yehuda et al 2000). This phenomenon has also been described in animals subjected to TDS stress (Liberzon et al 1997; Harvey, Naciti, et al 2003). In chronic PTSD, low cortisol levels are noted in the presence of high catecholamine levels (Yehuda et al 1997), and it has since been suggested that individuals who develop PTSD respond to a traumatic event by failing to release sufficient levels of cortisol for a long enough period of time to shut down sympathetic nervous system responses to stress (Yehuda and Harvey 1997), especially increased release of noradrenaline and 5HT (Figure 3). Afferent NA’ergic neurons from the amygdala innervating the hippocampus will facilitate LTP and the laying down of emotional memories (Figure 3).
Increased sensitivity of hippocampal glucocorticoid receptors in PTSD (Yehuda 1998) together with the release of glutamate heighten the vulnerability of the hippocampus to atrophy even in the absence of higher cortisol levels (Sapolsky 2000b). In addition, stress-induced release of 5HT in the hippocampus (Linthorst et al 2002; Hajos-Korcsok et al 2003) may further provoke glutamate release in the absence of hypercortisolemia (McEwen 1997). At this point, hypersensitization of the HPA axis has occurred resulting in hypocortisolemia. The resultant disinhibition of cytokines due to the lack of adequate cortisol release favors the release of proinflammatory cytokines, particularly IL-6, and the increased expression of iNOS and COX (Figure 2). With repeated trauma, NO originally produced as a neuroprotectant from constitutive NOS now takes on its malignant alter ego courtesy of iNOS. Of significance is that iNOS, and the activation of cyclooxygenase, may be responsible for the central activation of sympathoadrenal outflow induced by corticotrophin (CRF) (Okada et al 2003). Since CRF is already raised in PTSD (Sautter et al 2003), this will aggravate the peripheral manifestations of PTSD, including hyperarousal (Figure 2). The end scenario is the protracted release of cell- and neurotoxic levels of NO, culminating in neuronal degeneration, hippocampal shrinkage, and the behavioral and emotional sequelae that characterizes PTSD.
Clinical implications
The above theoretical depiction of how NO may influence the stress axis, and in this manner determine whether the body reacts adversely or not to a severe stressor, is speculative at this time. Nevertheless, a small clinical study in acute PTSD has hinted of the possible involvement of NO in PTSD (Yeh et al 2002), while evidence using the TDS model of PTSD has revealed some distinct parallels with this hypothesis.
Given the possible causal role of the NO-pathway in PTSD, how may one address the question of adequate treatment of the disorder? Viewing the preclinical evidence presented by the TDS studies, a particularly attractive approach would be the use of the steroid synthesis inhibitor KCZ in the immediate post-stress period. While mefipristone has recently been used in a clinical study in depression (Belanoff et al 2002), this approach has not been explored in PTSD. The idea of using this class of drug is, however, gaining increased recognition as a possible avenue for future research in anxiety and stress disorders (Strohle 2003).
Apart from its antiglucocorticoid properties, KCZ may offer something unique. Like mefipristone and metyrapone, KCZ is a well established pharmacological tool used to inhibit adrenal steroid synthesis by binding the heme iron of cytochrome P450 isoforms, mediating steroid hydroxylation reactions (Wolff et al 1993). TDS studies (Brand et al 2004; Harvey et al 2004) indicate that early increases in glucocorticoids immediately after the initial stressor may be critical for the protracted effects of repeated trauma on spatial memory, 5HT receptors, and NOS activity.
An interesting observation was the ability of KCZ to increase the dissociation constant (Km) of hippocampal NOS. This observation strongly suggests that KCZ may also be affecting NOS directly and independently of released glucocorticoids by binding to a critical site on the enzyme. Indeed, NOS bears striking homology to CYP450 (Bredt et al 1991), while KCZ binding to the heme structure within NOS has been found to reduce the maximal velocity of the enzyme (Wolff et al 1993). Moreover, KCZ is a competitive inhibitor of the obligatory NOS cofactor, calmodulin, thereby preventing formation of NO (Wolff et al 1993). Given the pharmacological evidence for the involvement of iNOS in TDS stress (Harvey et al 2004), it is of particular interest that KCZ inhibits iNOS expression (Baroni et al 1999). This strongly suggests that KCZ is able to reverse the increase in NOS activity evoked by stress-restress and, furthermore, also posits a possible dual mode of action for KCZ in preventing trauma-related sequelae in PTSD by suppressing the immediate release of glucocorticoids, blocking hypersensitive glucocorticoid receptors and/or preventing any subsequent NO-driven neuronal events.
Considering the important role that the SRIs have in the treatment of PTSD, it is of interest that patients suffering from depression have an increased level of nitrogen oxides (NOx) compared with healthy controls (Finkel et al 1996; Suzuki et al 2001), and that this is reduced following treatment with paroxetine (Finkel et al 1996). Since SRIs have been found to inhibit NOS activity in key limbic brain areas as measured by microdialysis (Wegener et al 2003), these findings indirectly suggest the pharmacological relevance of NOS inhibition with paroxetine during the treatment of depression. Similar proof-of-concept studies have not been undertaken in PTSD. Nevertheless, the aforementioned studies in depression not only emphasizes the role of NOS as a putative new target in the treatment of anxiety and stress-related disorders, but also associates current treatment modalities for PTSD with clinically relevant actions on the NO pathway.
In conclusion, animal studies employing TDS stress provide evidence that NO has a definite role in the pathophysiology of repeated trauma, particularly the induction of iNOS and the protracted release of cell-toxic amounts of NO. TDS-induced hypocortisolemia may set the scene for increased IL-6 release that will promote iNOS expression. Moreover, TDS evokes profound changes in limbic GABA-glutamate function that, together with the elevated NO levels, may underlie the neurodegenerative symptoms evident in PTSD, including hippocampal shrinkage and various memory changes, such as reexperiencing, cognitive disturbances, and amnesia.
Acknowledgments
This work has been supported by the South African Medical Research Council (BHH), the National Research Foundation (grant nr 2053203) (BHH), the Danish Medical Research Council (grant nr 22-02-0509) (GW), and the Lundbeck Foundation (grant nr 158/02) (GW).
Abbreviations
- PTSD
post-traumatic stress disorder
- NO
nitric oxide
- TDS
time-dependent sensitization
- NOS
nitric oxide synthase
- GABA
gamma-amino butyric acid
- NMDA
N-methyl-D-aspartate
- LTP
long-term potentiation
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- CNS
central nervous system
- nNOS
neuronal NO synthase
- HPA
hypothalamic-pituitary-adrenal
- 5HT
hippocampal serotonin
- SRIs
serotonin reuptake inhibitors
- NADPH
β-nicotinamide adenine dinucleotide phosphate
- cGMP
cyclic guanosine monophosphate
- cAMP
cyclic adenosine monophosphate
- CRF
corticotrophin-releasing hormone/factor
- ACTH
corticotrophin
- NOx
nitrogen oxides
- PDE
phosphodiesterase
- sGC
soluble guanylyl cyclase
- PVN
paraventricular nucleus
References
- Adler A. Neuropsychiatric complications in victims of Boston’s Coconut Grove disaster. J Am Med Assoc. 1993;123:1098–101. [Google Scholar]
- Agrypolous SV, Sandford JJ, Nutt DJ. The psychobiology of anxiolytic drugs. Part 2: Pharmacological treatments of anxiety. Pharmacol Therap. 2000;88:213–27. doi: 10.1016/s0163-7258(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Akirav I, Sandi C, Richter-Levin G. Differential activation of hippocampus and amygdala following spatial learning under stress. Eur J Neurosci. 2001;14:719–25. doi: 10.1046/j.0953-816x.2001.01687.x. [DOI] [PubMed] [Google Scholar]
- [APA] American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV) 4. Washington: APA; 1994. [Google Scholar]
- Baba H, Suzuki T, Arai H, et al. Expression of nNOS and soluble guanylate cyclase in schizophrenic brain. Neuroreport. 2004;15:677–80. doi: 10.1097/00001756-200403220-00020. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–17. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–8. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Baroni A, Ruocco V, De Paolis P, et al. Ketoconazole inhibits lipopolysaccacharide-induced activation of the nitric oxide synthase gene in the murine macrophage cell line J774. Arch Dermatol Res. 1999;291:54–8. doi: 10.1007/s004030050383. [DOI] [PubMed] [Google Scholar]
- Belanoff JK, Rothschild AJ, Cassidy F, et al. An open label trial of C-1073 (mifepristone) for psychotic major depression. Biol Psychiatry. 2002;52:386–92. doi: 10.1016/s0006-3223(02)01432-4. [DOI] [PubMed] [Google Scholar]
- Bijak M, Zahorodna A, Tokarski K. Opposite effects of antidepressants and corticosterone on the sensitivity of hippocampal CA1 neurons to 5-HT1A and 5-HT4 receptor activation. Naunyn-Schmied Archiv Pharmacol. 2001;363:491–8. doi: 10.1007/s002100000389. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bloom FE. Neurotransmission and the central nervous system. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman and Gillman’s the pharmacological basis of therapeutics. 10. New York: McGraw-Hill; 2001. pp. 293–320. [Google Scholar]
- Brand L, Naciti C, Stein DJ, et al. Cognitive dysfunction and serotonin receptor changes evoked by stress-restress are reversed by the steroid synthesis inhibitor, ketoconazole. Paper presented at the 24th Collegium Internationale Neuropsychopharmacologicum (CINP) Congress; Jun 20–24; Paris, France. 2004. [Google Scholar]
- Bredt DS, Hwang PH, Glatt C, et al. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P450 reductase. Nature. 1991;351:714–18. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Neuroimaging studies in post traumatic stress disorder. Curr Psychiatry Rep. 2002;4:254–63. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, et al. Neural correlates of exposure to traumatic pictures and sounds in Vietnam combat veterans with and without post-traumatic stress disorder: a positron emission topography study. Biol Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behav Res Ther. 2001;39:373–93. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Burette A, Zabel U, Weinberg RJ, et al. Synaptic localization of nitric oxide synthase and soluble guanylyl cyclase in the hippocampus. J Neurosci. 2002;22:8961–70. doi: 10.1523/JNEUROSCI.22-20-08961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IK, Roberts LJ, Wicks IP. Molecular targets in immune-mediated diseases: the case of tumour necrosis factor and rheumatoid arthritis. Immunol Cell Biol. 2003;81:354–66. doi: 10.1046/j.0818-9641.2003.01185.x. [DOI] [PubMed] [Google Scholar]
- Cebers G, Cebere A, Wagner A, et al. Prolonged inhibition of glutamate re-uptake down-regulates NMDA receptor functions in cultures cerebellar granule cells. J Neurochem. 1999;72:2181–90. doi: 10.1046/j.1471-4159.1999.0722181.x. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Bremner JD, Moghaddam B, et al. Glutamate and post-traumatic stress disorder: towards a psychobiology of dissociation. Semin Clin Neuropsychiatry. 1999;4:274–81. doi: 10.153/SCNP00400274. [DOI] [PubMed] [Google Scholar]
- Cohen H, Benjamin J, Kaplan Z, et al. Administration of high-dose ketoconazole, an inhibitor of steroid synthesis, prevents posttraumatic anxiety in an animal model. Eur Neuropsychopharmacol. 2000;10:429–35. doi: 10.1016/s0924-977x(00)00105-x. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–6. [PubMed] [Google Scholar]
- Colasanti M, Suzuki H. The dual personality of NO. Trends Pharmacol Sci. 2000;21:249–52. doi: 10.1016/s0165-6147(00)01499-1. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Riley DP, Caputi AP, et al. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–59. [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Physiological and toxicological actions of nitric oxide in the central nervous system. In: Ignarro L, Murad F, editors. Nitric oxide: biochemistry, molecular biology, and therapeutic implications. London: Academic Pr; 1995. pp. 323–30. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–6. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- De Oliviera RM, Del Bel EA, Mamede-Rosa ML, et al. Expression of neuronal nitric oxide synthase mRNA in stress-related brain regions after restraint stress. Neurosci Lett. 2000;289:123–6. doi: 10.1016/s0304-3940(00)01287-8. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affec Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wolf G, Horn TFW. Release patterns of excitatory and inhibitory amino acids within the hypothalamic supraoptic nucleus in response to direct nitric oxide administration during forced swimming in rats. Neurosci Lett. 2002;324:252–4. doi: 10.1016/s0304-3940(02)00211-2. [DOI] [PubMed] [Google Scholar]
- Eroglu L, Caglayan B. Anxiolytic and antidepressant properties of methylene blue in animal models. Pharmacol Res. 1997;36:381–5. doi: 10.1006/phrs.1997.0245. [DOI] [PubMed] [Google Scholar]
- Fedele E, Marchi M, Raiteri M. In vivo NO/cGMP signalling in the hippocampus. Neurochem Res. 2001;26:1069–78. doi: 10.1023/a:1012309223236. [DOI] [PubMed] [Google Scholar]
- Finkel MS, Laghrissi-Thode F, Pollock BG, et al. Paroxetine is a novel nitric oxide synthase inhibitor. Psychopharmacol Bull. 1996;32:653–8. [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991;14:60–7. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci. 2002;5:1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Rothman DL, et al. Family psychopathology and magnitude of reductions in occipital cortex GABA levels in panic disorder. Neuropsychopharmacology. 2004;29:639–40. doi: 10.1038/sj.npp.1300374. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Gore TA, Richards G. Posttraumatic stress disorder [online] 2002 Accessed Feb 2002. URL: http://www.emedicine.com.
- Griscavage JM, Hobbs AJ, Ignarro LJ. Negative modulation of NOS by NO and nitroso compounds. In: Ignarro L, Murad F, editors. Advances in pharmacology. Volume 34. Nitric oxide: biochemistry, molecular biology, and therapeutic implications. London: Academic Pr; 1995. pp. 215–34. [DOI] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cog Sci. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hajos-Korcsok E, Robinson DD, Yu JH, et al. Rapid habituation of hippocampal serotonin and norepinephrine release and anxiety-related behaviors, but not plasma corticosterone levels, to repeated footshock stress in rats. Pharmacol Biochem Behav. 2003;74:609–16. doi: 10.1016/s0091-3057(02)01047-x. [DOI] [PubMed] [Google Scholar]
- Harvey BH. Affective disorders and nitric oxide: a role in pathways to relapse and refractoriness? Hum Psychopharmacol. 1996;11:309–19. [Google Scholar]
- Harvey BH, McEwen BS, Stein DJ. Neurobiology of antidepressant withdrawal: implications for the longitudinal outcome of depression. Biol Psychiatry. 2003;54:1105–17. doi: 10.1016/s0006-3223(03)00528-6. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Naciti C, Brand L, et al. Endocrine, cognitive and hippocampal 5-HT1A/2A receptor changes evoked by a time-dependant sensitization (TDS) stress model in rats. Brain Res. 2003;983:97–107. doi: 10.1016/s0006-8993(03)03033-6. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Naciti C, Brand L, et al. Serotonin and stress: protective or malevolent actions in the biobehavioural response to repeated trauma. Ann N Y Acad Sci. 2005:1032. doi: 10.1196/annals.1314.035. In press. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Oosthuizen F, Brand L, et al. Stress-restress evokes sustained iNOS activity and altered GABA levels and NMDA receptors in rat hippocampus. Psychopharmacology. 2004;175:494–502. doi: 10.1007/s00213-004-1836-4. [DOI] [PubMed] [Google Scholar]
- Heiberg IL, Wegener G, Rosenberg R. Reduction of cGMP and nitric oxide has antidepressant-like effects in the forced swimming test in rats. Behav Brain Res. 2002;134:479–84. doi: 10.1016/s0166-4328(02)00084-0. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U. N-methyl-D-aspartate (NMDA) receptor based therapeutic approaches in schizophrenia. Int J Neuropsychopharmacol. 1999;2:211–17. doi: 10.1017/S1461145700001978. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC. The role of n-methyl-d-aspartate (NMDA) receptor-mediated neurotransmission in the pathophysiology and therapeutics of psychiatric syndromes. Eur Neuropsychopharmacol. 1998;8:141–52. doi: 10.1016/s0924-977x(97)00050-3. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Kremer I, Javitt DC, et al. Pilot-controlled trial of D-cycloserine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol. 2002;5:301–7. doi: 10.1017/S1461145702003061. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Khavandgar S, Dehpour AR. The involvement of endogenous opioids and nitroxidergic pathway in the anticonvulsant effects of foot-shock stress in mice. Epilepsy Res. 2002;49:131–42. doi: 10.1016/s0920-1211(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–9. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iga Y, Yoshioka M, Togashi H, et al. Inhibitory action of N omega-nitro-L-arginine methyl ester on in vivo long-term potentiation in the rat dendrate gyrus. Eur J Pharmacol. 1993;238:395–8. doi: 10.1016/0014-2999(93)90873-g. [DOI] [PubMed] [Google Scholar]
- Joseph MH, Kennett GA. Stress-induced release of 5-HT in the hippocampus and its dependence on increased tryptophan availability: an in vivo electrochemical study. Brain Res. 1983;270:251–7. doi: 10.1016/0006-8993(83)90598-x. [DOI] [PubMed] [Google Scholar]
- Kahn S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology. 2004;172:225–9. doi: 10.1007/s00213-003-1634-4. [DOI] [PubMed] [Google Scholar]
- Karten YJ, Nair SM, van Essen L, et al. Long-term exposure to high corticosterone levels attenuates serotonin responses in rat hippocampal CA1 neurons. Proc Natl Acad Sci USA. 1999;96:13456–61. doi: 10.1073/pnas.96.23.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298(Pt 2):249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. In: Manic-depressive insanity and paranoia. Barclay RM, translator; Robertson GM, editor. Edinburgh: ES: Livingstone Pr; 1921. [Google Scholar]
- Krukoff TL, Khalili P. Stress-induced activation of nitric oxide-producing neurons in the rat brain. J Comp Neurol. 1997;377:509–19. doi: 10.1002/(sici)1096-9861(19970127)377:4<509::aid-cne3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kurt M, Bilge SS, Aksoz E, et al. Effect of sildenafil on anxiety in the plus-maze test in mice. Pol J Pharmacol. 2004;56:353–7. [PubMed] [Google Scholar]
- Le Doux J. Fear and the brain: where are we and where are we going? Biol Psychiatry. 1998;44:1229–38. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, Flagel SB, et al. Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology. 1999;21:40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–53. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Lopez F, Flagel SB. Differential regulation of hippocampal glucocorticoid receptor mRNA abd fast feedback: relevance to posttraumatic stress disorder. Neuroendocrinology. 1999;11:11–17. doi: 10.1046/j.1365-2826.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Penalva RG, Flachskamm C, et al. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur J Neurosci. 2002;16:2441–52. doi: 10.1046/j.1460-9568.2002.02400.x. [DOI] [PubMed] [Google Scholar]
- Louzada PR, Lima ACP, Mendonca-Silva DL, et al. Taurine prevents the neurotoxicity of β-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J. 2004;18:511–18. doi: 10.1096/fj.03-0739com. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Moro MA, Lizasoain I, et al. Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology. 2003;28:1579–88. doi: 10.1038/sj.npp.1300187. [DOI] [PubMed] [Google Scholar]
- Madrigal JLM, Lizasoain I, Lorenzo P, et al. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor κβ-mediated mechanisms. J Neurochem. 2001;76:532–8. doi: 10.1046/j.1471-4159.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Delmeire L, et al. Elevated serum interleukin-6 and interleukin-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45:833–9. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Maes M, Mylle J, Delmeire L, et al. Pre-and post-disaster negative life events in relation to the incidence and severity of post-traumatic stress disorder. Psychiatry Res. 2001;105:1–12. doi: 10.1016/s0165-1781(01)00325-0. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Marcoli M, Maura G, Tortarolo M, et al. Serotonin inhibition of the NMDA receptor/nitric oxide/cyclic GMP pathway in rat cerebellum: involvement of 5-hydroxytryptamine 2C receptors. J Neurochem. 1997;69:427–30. doi: 10.1046/j.1471-4159.1997.69010427.x. [DOI] [PubMed] [Google Scholar]
- Marletta MA. Nitric oxide synthase structure and mechanisms. J Biol Chem. 1993;268:12231–4. [PubMed] [Google Scholar]
- Maura G, Marcoli M, Pepicelli O, et al. Serotonin inhibition of the NMDA receptor/nitric oxide/cyclic GMP pathway in human neocortex slices: involvement of 5-HT(2C) and 5-HT(1A) receptors. Br J Pharmacol. 2000;130:1853–8. doi: 10.1038/sj.bjp.0703510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaslin PP, Oh S. Nitric oxide and glutamate receptors. In: Stone TW, editor. CNS neurotransmitters and neuromodulators: glutamate. New York: CRC Pr; 1995. pp. 159–79. [Google Scholar]
- McEwen BS. Possible mechanisms for atrophy of the human hippocampus. Mol Psychiatry. 1997;2:255–62. doi: 10.1038/sj.mp.4000254. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McLeod TM, Lopez-Figueroa AL, Lopez-Figueroa MO. Nitric oxide, stress, and depression. Psychopharmacol Bull. 2001;35:24–41. [PubMed] [Google Scholar]
- McNally RJ. Experimental approaches to cognitive abnormality in posttraumatic stress disorder. Clin Psychol Rev. 1998;18:971–82. doi: 10.1016/s0272-7358(98)00036-1. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamada K, Noda Y, et al. Lower sensitivity to stress and altered monoaminergic neuronal function in mice lacking the NMDA receptor epsilon 4 subunit. J Neurosci. 2002;22:2335–42. doi: 10.1523/JNEUROSCI.22-06-02335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller M, Jones NM, Beart PM. Complex involvement of nitric oxide and cGMP at N-methyl-D-aspartic acid receptors regulating gamma-[3H]aminobutyric acid release from striatal slices. Neurosci Lett. 1995;190:195–8. doi: 10.1016/0304-3940(95)11538-8. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A, Furchgott R. International Union of Pharmacology Nomenclature in Nitric Oxide Research. Pharmacol Rev. 1997;49:137–42. [PubMed] [Google Scholar]
- Montgomery AJ, Bench CJ, Young AH, et al. PET Measurement of the influence of corticosteroids on serotonin-1A receptor number. Biol Psychiatry. 2001;50:668–76. doi: 10.1016/s0006-3223(01)01205-7. [DOI] [PubMed] [Google Scholar]
- Naskar R, Dreyer EB. New horizons in neuroprotection. Survey Opthalmol. 2001;45:S250–5. doi: 10.1016/s0039-6257(01)00198-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- O’Brien M, Nutt D. Loss of consciousness in posttraumatic stress disorder: a clue to etiology and treatment. Br J Psychiatry. 1998;173:102–4. doi: 10.1192/bjp.173.2.102. [DOI] [PubMed] [Google Scholar]
- O’Dell TJ, Huang PL, Dawson TM, et al. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science. 1994;265:542–6. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- Okada S, Yokotani K, Yokotani K. Inducible nitric oxide synthase is involved in corticotrophin-releasing hormone-mediated central sympatho-adrenal outflow in rats. Eur J Pharmacol. 2003;477:95–100. doi: 10.1016/j.ejphar.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Oosthuizen F. PhD thesis. Potchefstroom, South Africa: North-West University; 2003. The involvement of nitric oxide in a rodent model of post-traumatic stress disorder. [Google Scholar]
- Padovan CM, Del Bel EA, Guimaraes FS. Behavioral effects in the elevated plus maze of an NMDA antagonist injected into the dorsal hippocampus: influence of restraint stress. Pharmacol Biochem Behav. 2000;67:325–30. doi: 10.1016/s0091-3057(00)00361-0. [DOI] [PubMed] [Google Scholar]
- Post RM, Weiss SRB. Sensitization and kindling phenomena in mood, anxiety, and obsessive-compulsive disorders. The role of serotonergic mechanisms in illness progression. Biol Psychiatry. 1998;44:193–206. doi: 10.1016/s0006-3223(98)00144-9. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Robb SJ, Connor JR. Nitric oxide protects astrocytes from oxidative stress. Ann N Y Acad Sci. 2002;962:93–102. doi: 10.1111/j.1749-6632.2002.tb04059.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000a;48:755–65. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch General Psychiatry. 2000b;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, et al. Corticotropin-releasing factor in post-traumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54:1382–8. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Shiah I-Shin, Yatham LN. GABA function in mood disorders: an update and critical evidence. Life Sci. 1998;63:1289–303. doi: 10.1016/s0024-3205(98)00241-0. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs female rats are NMDA receptor-dependent. Eur J Neurosci. 2004;19:145–50. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Levine S, Thompson RF. Effect of adrenalectomy and demedullation on the stress-induced impairment of long-term potentiation. Neuroendocrinology. 1990;51:70–5. doi: 10.1159/000125318. [DOI] [PubMed] [Google Scholar]
- Skolnick P. Antidepressants for the new millenium. Eur J Pharmacol. 1999;375:31–40. doi: 10.1016/s0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:174–9. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, et al. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32:756–65. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Strohle A. The neuroendocrinology of stress and the pathophysiology and therapy of depression and anxiety. Nervenarzt. 2003;74:279–91. doi: 10.1007/s00115-002-1444-7. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Yagi G, Nakaki T, et al. Elevated plasma nitrate levels in depressive states. J Affect Disord. 2001;63:221–4. doi: 10.1016/s0165-0327(00)00164-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kimoto T, Tanabe N, et al. Corticosterone acutely prolonged N-methyl-D-aspartate receptor-mediated Ca2+ elevation in cultured rat hippocampal neurons. J Neurochem. 2002;83:1441–51. doi: 10.1046/j.1471-4159.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Endogenous nitric oxide inhibits NMDA-and-kainate resposes by a negative feedback system in rat hippocampal neurons. Brain Res. 1993;631:72–6. doi: 10.1016/0006-8993(93)91188-x. [DOI] [PubMed] [Google Scholar]
- Uys JDK, Stein DJ, Daniels WMU, et al. Animal models of anxiety disorders. Curr Psychiatry Rep. 2003;5:274–81. doi: 10.1007/s11920-003-0056-7. [DOI] [PubMed] [Google Scholar]
- Vahabzadeh A, Fillenz M. Comparison of stress-induced changes in noradrenergic and serotonergic neurons in the rat hippocampus using microdialysis. Eur J Neurosci. 1994;6:1205–12. doi: 10.1111/j.1460-9568.1994.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Rewilliger RMZ, Duman RS. Role of 5HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 1999;262:1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Vaiva G, Thomas P, Ducrocq F, et al. Low posttrauma GABA plasma levels as a predictive factor in the development of acute posttraumatic stress disorder. Biol Psychiatry. 2004;55:250–4. doi: 10.1016/j.biopsych.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Vallance P, Collier J. Fortnightly review biology and clinical revelance of nitric oxide. BMJ. 1994;309:453–7. doi: 10.1136/bmj.309.6952.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk BA. The body keeps the score [online] 1994 Accessed Mar 2000. URL: http://www.trauma-pages.com/vanderk4.htm.
- Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–84. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Vincent VA, De Groot CJ, Lucassen PJ, et al. Nitric oxide synthase and apoptotic cell death in brains of AIDS and AIDS dementia patients. AIDS. 1999;13:3127–326. doi: 10.1097/00002030-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Volke V, Wegener G, Bourin M, et al. Antidepressant- and anxiolytic-like effects of selective neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole (TRIM) in mice. Behav Brain Res. 2003;140:141–7. doi: 10.1016/s0166-4328(02)00312-1. [DOI] [PubMed] [Google Scholar]
- Volke V, Wegener G, Vasar E. Augmentation of the NO-cGMP cascade induces anxiogenic-like effect in mice. J Physiol Pharmacol. 2003;54:653–60. [PubMed] [Google Scholar]
- Wegener G, Volke V, Harvey BH, et al. Local but not systemic administration of serotonergic antidepressants decreases hippocampal nitric oxide synthase activity. Brain Res. 2003;959:128–34. doi: 10.1016/s0006-8993(02)03738-1. [DOI] [PubMed] [Google Scholar]
- Wink DA, Vodovotz Y, Grisham MB, et al. Antioxidant effects of nitric oxide. Methods Enzymol. 1999;301:413–24. doi: 10.1016/s0076-6879(99)01105-2. [DOI] [PubMed] [Google Scholar]
- Wolff DJ, Datto GA, Samatovicz The dual mode of inhibition of calmodulin-dependent nitric-oxide synthase by antifungal imidazole agents. J Biol Chem. 1993;268:9430–6. [PubMed] [Google Scholar]
- Yamada J, Saitow F, Satake S, et al. GABA(B) receptor-mediated presynaptic inhibition of glutamatergic and GABAergic transmission in the basolateral amygdala. Neuropharmacology. 1999;38:1743–53. doi: 10.1016/s0028-3908(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Yanik M, Vural H, Tutkun H, et al. The role of the arginine-nitric oxide pathway in the pathogenesis of bipolar affective disorder. Eur Arch Psychiatry Clin Neurosci. 2004;254:43–7. doi: 10.1007/s00406-004-0453-x. [DOI] [PubMed] [Google Scholar]
- Yeh CB, Leckman JF, Wan FJ. Characteristics of acute stress symptoms and nitric oxide concentration in young rescue workers in Taiwan. Psychiatry Res. 2002;112:59–68. doi: 10.1016/s0165-1781(02)00179-8. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Sensitization of the hypothalamic-pituitary-adrenal axis in posttraumatic stress disorder. Ann N Y Acad Sci. 1997;821:57–75. doi: 10.1111/j.1749-6632.1997.tb48269.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Neuroendocrinology of trauma and posttraumatic stress disorder. In: Yehuda R, editor. Psychological trauma. Washington: American Psychiatric Pr; 1998. pp. 97–125. [Google Scholar]
- Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry. 1993;33:479–86. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Schmeidler J, et al. Low cortisol and risk for PTSD in adult offspring of holocaust survivors. Am J Psychiatry. 2000;157:1252–9. doi: 10.1176/appi.ajp.157.8.1252. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Harvey H. Relevance of neuroendocrine alterations in PTSD to cognitive impairments of trauma survivors. In: Read D, Lindsay S, editors. Recollections of trauma: scientific research and clinical practice. New York: Plenum Pr; 1997. pp. 221–52. [Google Scholar]
- Yehuda R, Southwick SM, Hussbaum G, et al. Low urinary cortisol excretion in patients with PTSD. J Nerv Ment Dis. 1990;178:366–9. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yun HY, Dawson VL, Dawson TM. Nitric oxide in health and disease of the nervous system. Mol Psychiatry. 1997;2:300–10. doi: 10.1038/sj.mp.4000272. [DOI] [PubMed] [Google Scholar]


