Abstract
Patients with dementia have been shown to have disturbed sleep/wake rhythms. There is evidence of impairment in endogenous generation of rhythms and deficient environmental cues in this population. This study sought to examine patterns of rest/activity rhythms as they relate to dementia severity. Three days of actigraphy were collected from 150 nursing-home patients with dementia and used to compute rhythm parameters. Dementia severity was estimated with the Mini-Mental State Examination (MMSE). The relationship between rhythm parameters and dementia severity was examined. Rhythm parameters were not associated with dementia in the sample as a whole, but relationships emerged when the sample was divided on the basis of overall rhythm robustness (F-statistic). Within the group with less robust rhythms, those with stronger rhythms had less severe dementia. In the group with more robust rhythms, milder dementia was associated with having an earlier acrophase (timing of the peak of the rhythm) and narrower peak of the rhythm (shorter duration of peak activity). These results suggested a three-stage model of rest/activity rhythm changes in dementia in which dementia patients have a rapid decline in rhythmicity followed by a slight return to stronger rhythms. In the later stages of dementia, rhythms decline even further.
Keywords: dementia, circadian rhythms, actigraphy
Introduction
Patients with dementia are characterized by impairments in memory and other cognitive domains. In addition, they often display fragmentation in their sleep/wake patterns, such that they frequently wake up during the night and frequently fall asleep during the day. In fact, it has been shown that these patients rarely spend a full hour awake during the day or a full hour asleep during the night (Jacobs et al 1989; Pat-Horenczyk et al 1998). Whereas in healthy older adults, sleep/wake patterns follow a regular 24-hour cycle, or circadian rhythm, patients with dementia often show little evidence of rhythmicity. It remains unclear, however, whether these disturbed sleep patterns are indicative of deterioration of the endogenous generation of circadian rhythms, impairment in the homeostatic drive for sleep that usually builds up during wakefulness, other neurological dysfunction, or a combination of factors. To better understand the underlying mechanisms of sleep fragmentation, a number of investigators have measured circadian rhythms in patients with dementia.
There is good reason to expect impairment in circadian rhythms in patients with dementia, particularly those with Alzheimer’s disease (AD), as circadian rhythms are generated in the suprachiasmatic nucleus (SCN) and there is often degeneration of the neurons of the SCN in this population (Swaab et al 1985). At the cellular level, there is a decrease in the activity of individual neurons within the SCN in patients with AD (Hoogendijk et al 1996). Edgar et al (1993) lesioned the SCN in squirrel monkeys and produced fragmented sleep/wake patterns similar to those seen in dementia. However, rhythms were relatively intact as long as 10% of the SCN neurons were undamaged. Given these results, it would be surprising not to find disturbed circadian rhythms in these patients, but one might expect problems to develop only at later stages of neurodegeneration. Nevertheless, conflicting results regarding the nature of circadian rhythms have been reported.
In studies of core body temperature rhythms, dementia patients were found to have worse (Campbell et al 1986; Harper et al 2001), similar (Prinz et al 1984; Satlin et al 1995), or even better circadian temperature rhythms (Touitou et al 1986) when compared with non-demented older adults. Studies that examined melatonin rhythms found greater rhythm disturbances in dementia patients (Uchida et al 1996; Mishima et al 1999) or no difference between groups (Ohashi et al 1999).
It can be difficult to gather data on core body temperature or hormonal (such as melatonin) circadian rhythms because of noncompliance with the often invasive data collection techniques. Partly because of these difficulties, several investigators have measured rest/activity rhythms. It would be expected that there might be differences in rest/activity rhythms in dementia patients compared with non-demented older adults, owing not only to the deterioration of the SCN as mentioned above, but also to weaker environmental cues that have been shown to be critical for rhythm entrainment (Aschoff 1960). Studies of rest/activity rhythms in dementia have been more consistent than those of other rhythms, although some discrepancies have arisen. Studies have found impaired rhythmicity (lower amplitude and shifted timing) (Witting et al 1990; Satlin et al 1991, 1995; van Someren et al 1996; Ancoli-Israel et al 1997; Pollak and Stokes 1997) or no difference (Campbell et al 1986; Aharon-Peretz et al 1991; Mishima et al 1997) when comparing demented subjects with non-demented older adults.
Several studies of dementia patients have documented low levels of exposure to bright light, the strongest environmental cue for synchronization of circadian rhythms. Campbell et al (1988) found that community-dwelling elderly with mild AD received, on average, less than 30 minutes of bright light exposure per day. Even lower levels of bright light exposure have been found in those who are institutionalized (Ancoli-Israel et al 1991). In addition, those with the most severe dementia received the lowest levels of light exposure (Ancoli-Israel et al 1997; Shochat et al 2000). Other environmental factors that have an effect on circadian rhythms, such as physical activity and regular social interaction, are often deficient in patients with dementia. Van Someren et al (1996) found that severity of rhythm disturbance was related to bright light exposure and activity level. It has been proposed that these environmental deficiencies may be at the heart of circadian rhythm disturbances in dementia, the so-called “environmental hypothesis” (Harper et al 2001).
The results of these studies of circadian rhythms in patients with dementia are difficult to synthesize because of the different, and sometimes conflicting, patterns of results. As reviewed by Ancoli-Israel et al (2002), there are several elements of study design and sample selection that may have contributed to these discrepancies. On average, the studies included small sample sizes (just under 20 patients with dementia) and patients at various stages of severity and with different forms of dementia, including AD, multi-infarct dementia, and frontotemporal dementia. Satlin et al (1995) argue that there may even be subgroups of AD patients who differ in terms of circadian rhythms.
The goal of the present study was to examine the relationship between dementia and patterns of rest/activity rhythms. It was hypothesized that, after controlling for the effects of other factors (light exposure, ambulatory status, sex, age, medical burden, and use of sedating medications), rest/activity rhythms would remain stable for individuals in the early stages of dementia but show increasing deterioration in those whose dementia was progressively more severe.
Methods
The data for these analyses were collected in two separate studies examining treatments for agitation, sleep, and circadian rhythm disruption in institutionalized older adults with dementia residing in San Diego, California between the years 1989 and 2000.
Sample
Participants from the two studies were 188 older adults residing in skilled-care nursing homes. Patients were excluded if they had had a prior severe stroke or a history of a primary psychiatric disorder preceding the suspected onset of their dementia. Participants in the second study were also given an ophthalmic examination and were excluded if they suffered from clinically significant visual impairment. Written informed consent was obtained from each subject’s legal guardian, and verbal assent was obtained from subjects with sufficient cognitive abilities to respond. Verbal consent was also obtained from each subject’s primary care physician. Thirty-eight subjects were dropped from the analyses because of incomplete or missing actigraphy data (n = 12) or incomplete assessments (n = 26). The remaining 150 subjects had a mean age of 84.1 years (SD = 7.8; range 60–100 years) and consisted of 105 women and 45 men. The mean Mini-Mental State Examination (MMSE) score for the sample was 8.5 (SD = 7.6; range 0–27). A histogram of MMSE scores for the sample is shown in Figure 1. The majority of subjects scored in the severe dementia range. In the first study, subjects needed only to have a diagnosis of dementia in their medical chart. The subjects in the second study were required to have a diagnosis of probable or possible AD based on the NINCDS-ARDA diagnostic criteria (McKhann et al 1984) as determined by a board-certified neurologist.
Figure 1.
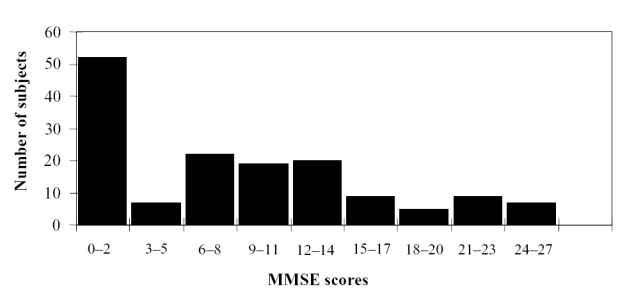
Distribution of Mini-Mental State Examination (MMSE) scores for all participants (n = 150).
Measures
The MMSE was administered to participants at the beginning of the study to assess severity of cognitive impairment (Folstein et al 1975). This instrument is used to assess various areas of memory and cognitive functioning, with scores ranging from 0 (severe dementia) to 30 (no dementia). The psychometric properties of the MMSE have been reviewed in detail (Tombaugh and McIntyre 1991). The MMSE is considered to be a valid assessment instrument because it correlates well with other tests of cognitive functioning, shows longitudinal changes that parallel cognitive decline in dementia, and has moderate to high sensitivity and specificity.
Apparatus
To assess rest/activity rhythms, information was collected with an Actillume monitor (Ambulatory Monitoring, Ardsley, NY, USA). The Actillume is an actigraph, a small device similar to a watch, worn on the dominant wrist. Actigraphy has been well documented to correlate with standard EEG recordings for distinguishing wake from sleep (Ancoli-Israel, Cole, et al 2003). The Actillume contains a piezoelectric linear accelerometer that is sensitive to movements of 0.003 g and above, making it sensitive to even small body movements, a photometric transducer (sensitive from < 0.01 to > 100 000 lux with measurements roughly log linear from a range below moonlight to the brightest summer day at noon), and a microprocessor and 32 K memory chip for processing and storing data. The Actillume was set to record the activity level every 10 seconds and to store the data as the maximum activity level recorded in one of the previous six 10-second blocks. Ambient light levels were sampled once per minute. At the end of the recording period, the data were downloaded into a desktop computer, edited for Actillume removals (eg, shower times) and data artifacts, and raw data were exported for analyses. Minutes of light exposure over 1000 lux while patients were out of bed was computed for each day and averaged across days of recording.
Procedure
The participants from both studies were recruited from nursing homes. Their legal guardian was contacted to obtain written informed consent and assent was obtained from participants whenever possible. Verbal consent was also obtained from the participant’s primary care physician. Medical information was abstracted from nursing-home records. This study was approved by the UCSD Committee on the Protection of Human Subjects.
As part of the larger treatment studies, participants wore the Actillume for 3 days of baseline monitoring, 10 days of treatment, and 5 days of follow-up. Only data from the 3-day baseline period were used for these analyses.
Data analyses
Rest/activity rhythms were computed from maximum minute-by-minute activity levels measured with the Actillume. Activity data were log transformed because of non-normal distributions. An extension of the traditional cosinor model was used to compute measures of circadian rhythmicity (Martin et al 2000; Ancoli-Israel, Gehrman, et al 2003). The extended model computes the standard cosinor parameters used to describe circadian rhythms: the mesor (mean), amplitude (peak), and acrophase (timing of the peak). Two additional parameters are computed: the width of the rhythm at its peak (α) and the steepness of the rise and fall of the curve (β; the curve approaches a square wave as β increases). The traditional cosinor model has been criticized for use with activity data because these data tend to follow more of a square-wave pattern rather than a true sinusoid (van Someren et al 1999). The addition of the α and β parameters allows the fitted curve to conform to a true sinusoidal pattern, a square wave, or patterns in between. To test the goodness-of-fit of the extended model, a pseudo-F-statistic was computed (Gallant 1987). A larger F-statistic suggested a better fit of the model, which was interpreted as overall more robust circadian rhythmicity. Once these analyses were performed, parameter estimates and F-statistics for each subject were retained for further analyses.
The relationships between MMSE score and the rhythm parameters could have been nonlinear, so quadratic and cubic polynomial terms were also included for MMSE. Multi-collinearity of polynomial terms was avoided by using the Gramm-Schmidt orthogonalization procedure (Seber 1977). This involved centering MMSE, regressing the polynomial terms on the lower order terms, and then retaining the residuals for use in the analyses. This produced linear, quadratic, and cubic terms that were orthogonal to each other.
To control for the influence of potential confounding variables, several covariates were included in all analyses. Covariates examined were age, sex, ambulatory status, medical burden, use of sedating medications, and bright light exposure. Ambulatory status was a trichotomous variable defined as wheelchair-bound (50.7%), walks with the use of a supportive device such as a walker (16.0%), or ambulatory without assistance (33.3%). Medical burden was computed as the total number of medical diagnoses recorded in each subject’s medical chart other than a diagnosis of dementia (mean [SD] = 2.4 [1.7] diagnoses). Sedating medications were defined as sedative-hypnotics, antihistamines, and tranquilizers. Subjects were assigned a value of 1 if they took any of these medications on a daily basis (52.3%) or 0 if they did not (47.7%). Light exposure was defined as the average minutes of exposure to light over 1000 lux per day (mean [SD] = 38.4 [82.7] minutes).
The multivariate models described above were repeated in two stages. First, only the effect of MMSE was included (MMSE centered as well as the quadratic and cubic orthogonal polynomials). The covariates were then added to the models. In this way, it could be determined if MMSE score was related to the circadian rhythm parameters both with and without controlling for the effects of the covariates. Normal score transforms (Blom’s method) of dependent variables were used throughout.
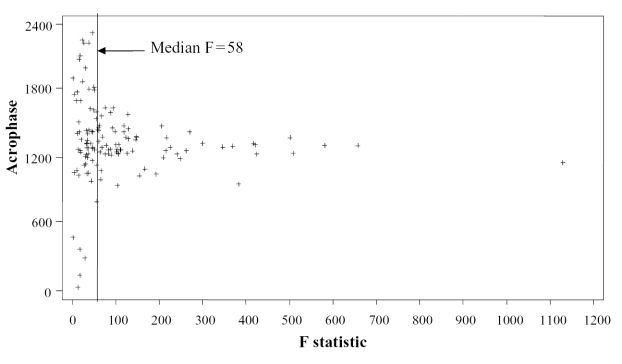
Rest/activity data were stored once per minute for 3 days, resulting in a possible total of 4320 data points. The degrees of freedom were extremely large; thus, even a very small degree of rhythmicity was likely to be statistically significant. Figure 2 shows a plot of acrophase versus the F-statistic based on maximum activity. Subjects with an F-statistic above the median F of 58 had their acrophases fall within a relatively narrow range around the mean (10 am–5 pm), whereas those with lower F-statistics had acrophases that appeared to be randomly distributed across the 24-hour day. Rhythm parameters based on low F-values may not be very meaningful, even when judged to be statistically significant. Therefore, all analyses were repeated separately for those with less robust (F < 58) and more robust (F ≥ 58) rhythms, with 75 subjects in each group. Better model fit, as indicated by the F-statistic, was utilized in this manner as an index of the robustness of circadian rhythmicity.
Figure 2.
Scatter plot of the acrophases (timing of the peak of the rhythm) expressed as clock time versus the value of the goodness-of-fit F-statistic. A vertical line indicates the median F-statistic of 58.
All analyses were repeated using only the subjects with a diagnosis of probable or possible AD (n = 111) to see if there were different results with a more homogeneous sample of patients with AD rather than with a mix of dementia types.
Results
Rhythm parameters
The extended cosine model was fitted to the baseline activity data of the subjects. Descriptive statistics of the parameters are given in Table 1. The extended cosinor model provided a statistically significant fit compared with the traditional cosinor model in all but three cases.
Table 1.
Parameters obtained from extended cosinor model (n = 150)
| Parameter | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Based on average activity | ||||
| Amplitude | 0.39 | 0.33 | 0.04 | 2.28 |
| Acrophase (phi) | 13:40 | 5:09 | 00:06 | 23:54 |
| Mesor | 0.82 | 0.24 | 0.34 | 1.70 |
| Alpha | −0.06 | 0.59 | −1.0 | 1.0 |
| Beta | 550.4 | 817.9 | 0.3 | 3760.4 |
| F-statistic for model fit | 75.7 | 124.4 | 2.2 | 831.8 |
| Based on maximum activity | ||||
| Amplitude | 0.49 | 0.38 | 0.02 | 2.38 |
| Acrophase (phi) | 14:07 | 3:40 | 0:46 | 23:54 |
| Mesor | 1.11 | 0.25 | 0.41 | 1.77 |
| Alpha | −0.20 | 0.51 | −1.0 | 1.0 |
| Beta | 413.7 | 570.5 | 0.4 | 3477.9 |
| F-statistic for model fit | 112.3 | 148.0 | 1.78 | 1129.6 |
Results for entire study sample
In models with only MMSE included, there were no statistically significant relationships with the rhythm parameters; ie, dementia level was not related to the rest/activity rhythm. The significant findings for the model, including covariates, are listed in Table 2. The rhythm parameters were related to several of the covariates, but again, there were no significant relationships with MMSE scores.
Table 2.
Statistically significant relationships between covariates and circadian rhythm parameters based on multivariate tests in the entire sample; all effects with p < 0.10 included
| Parameter | Effect | F | p | Effect | Effect size (Cohen’s d) |
|---|---|---|---|---|---|
| Amplitude | Sedating medications | F(2,139) = 3.27 | 0.041 | Higher for those on sedating medications | 0.30 |
| Medical burden | F(2,139) = 3.02 | 0.052 | Decreased as burden increased | 0.29 | |
| Beta | Age | F(2,139) = 2.95 | 0.056 | Increased as age increased | 0.29 |
| Acrophase | Sedating medications | F(2,139) = 6.09 | 0.003 | Later for those on sedating medications | 0.42 |
| Minutes >1000 lux | F(2,139) = 2.86 | 0.061 | Later as light exposure increased | 0.29 | |
| Alpha | Sex | F(2,139) = 4.98 | 0.008 | Higher for men | 0.38 |
| Age | F(2,139) = 4.59 | 0.012 | Increased as age increased | 0.36 |
Results for patients with less robust activity rhythms
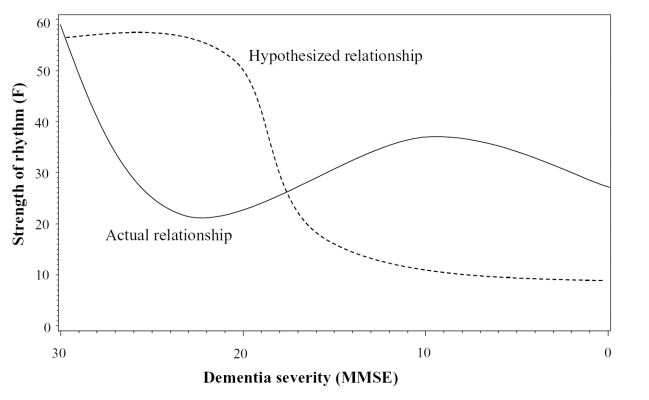
In subjects with less robust rhythms (F < 58), MMSE was related to the β parameter (F(2,68) = 3.37; p = 0.04) such that less demented subjects had higher values of β (ie, a steeper transition from activity to inactivity and vice versa). The cubic term (MMSE3) was significantly related to α (F(2,68) = 6.43; p = 0.003) and the F-statistic (F(2,68) = 5.10; p = 0.009); ie, subjects with an MMSE score of approximately 20 or above had a higher F (more robust rhythm) and lower α (wider peak suggesting more sustained periods of daytime activity). With more severe dementia, both parameters decreased. The α parameter increased again in severely demented subjects, whereas the F-statistic was higher for those in the 5–9 range of MMSE and then fell again for those below that level (Figure 3).
Figure 3.
Plot of the relationship between Mini-Mental State Examination (MMSE) score and the goodness-of-fit F-statistic. The dashed line indicates the hypothesized relationship, according to which rhythms would remain robust until the more severe stages of dementia. Once neuronal damage passes a critical threshold, there is a rapid decline in rhythmicity. In contrast, the solid line represents the actual relationship observed in those with less robust rhythms.
Results for patients with more robust activity rhythms
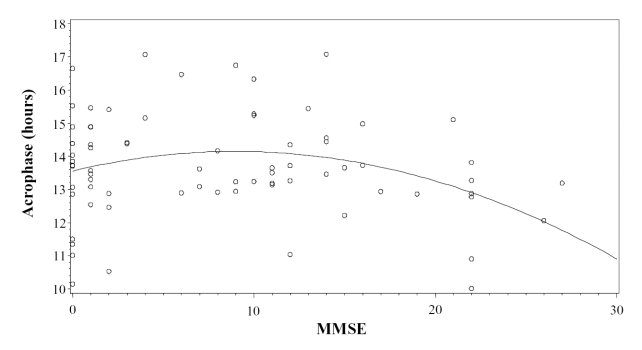
For subjects with robust rhythmicity (F ≥ 58), the quadratic term of MMSE was related to acrophase (F(2,68) = 3.53; p = 0.035) and the α parameter (F(2,68) = 4.21; p = 0.019). For both parameters, the highest values were for subjects with an MMSE score between approximately 7 and 13. Acrophase was earlier for those with higher MMSE scores (Figure 4).
Figure 4.
Scatter plot of the relative timing of rhythms (acrophases) versus the dementia severity (Mini-Mental State Examination; MMSE) in subjects whose F-statistic was above the median, indicating more robust rhythms.
When the models were repeated with the covariates included, all of the effects of MMSE remained statistically significant within this group, regardless of the robustness of the rhythm. However, most of the effects of the covariates seen in the whole sample were no longer statistically significant. Use of sedating medications was related to acrophase (F(2,63) = 7.84; p = 0.001) and amplitude (F(2,63) = 3.65; p = 0.032) in those with less robust rhythms. Regular use of sedating medications was associated with higher amplitude (0.68 vs 0.69) and earlier acrophase (13:42 vs 14:32).
When the analyses were restricted to those with a diagnosis of possible or probable AD, the results were essentially the same (ie, the same variables were statistically significant with similar p-values).
Discussion
Contrary to expectations, sleep/wake circadian rhythm parameters were not associated with MMSE score in the sample as a whole. Relationships emerged only when the sample was divided on the basis of the overall robustness of rest/activity rhythms. In subjects with less robust rhythms, milder dementia was associated with rhythms that had better model fit (higher F), were steeper (higher β), and had wider peaks (lower α). The functional implications of these results are that these patients had more rhythmic patterns of activity, transitioned between inactivity and activity quickly (ie, got up and going more easily), and were able to sustain high periods of activity for longer periods of time during the day. Therefore, although model fit in general was not associated with severity of dementia, among those with less robust rhythms, those with better model fit had less dementia. Among subjects with more robust rhythms, milder dementia was associated with having an earlier rhythm (acrophase) and narrower peak (lower α). This pattern of results raises three issues: (1) Why is there a difference between these groups? (2) What do these results mean for each group? (3) How do these results compare with those of other studies?
Why is there a difference between these groups?
There was a difference between subjects with less robust rhythms and those with more robust rhythms. As mentioned earlier, it is not clear how large the F-statistic for goodness-of-fit must be before concluding that there is a meaningful rhythm in the data, but statistical significance as the sole criterion may not be useful because of the large number of data points. It is possible that some subjects with low F-statistics do not have a meaningful degree of rhythmicity in their data in spite of statistical significance. Their rhythm parameters may have little meaning. This appears to be the case particularly for the acrophase, as plotted in Figure 2. By examining the group as a whole, these subjects may be adding random variability that would make it difficult to detect patterns in the data for the rest of the subjects. However, the fact that there were several systematic relationships in the less robust rhythm group suggests that the rhythm parameters are not merely random variation in all subjects with low F-statistics. These data lend support to the hypothesis of Satlin and colleagues (1995) that there may be distinct subtypes of dementia patients in terms of their circadian rhythms.
It is also important to consider that, once the data were dichotomized on the basis of rhythm robustness, most of the effects of the covariates were no longer statistically significant. It is likely that the grouping variable served as somewhat of a “proxy” indicator of the effects of the covariates, essentially replacing them in the analyses. The two groups of subjects (less vs more robust rhythms) were compared on all covariates using t-tests with no statistically significant differences between groups. It may be that the relationship between the grouping factor of rhythm robustness and the covariates is multidimensional, which would not be detected by simple group comparisons. Whether or not the grouping is related to covariates does not detract from the meaning of the results, but rather serves only as a possible explanation for observed differences between groups.
What do these results mean for each group?
Regardless of the source of these differences, it is important to consider their potential clinical meaning. MMSE score was related to various rhythm parameters for both those with less and those with more robust rhythms. As hypothesized, subjects with higher MMSE scores had more robust rhythms overall, although this relationship emerged only for the group with less robust rhythms. As shown in Figure 3, there was a decrease in the F-statistic during the earlier stages of dementia, which was followed by an increase in some subjects with severe dementia. At the bottom end of the scale of MMSE scores, rhythms once more declined. The initial decline supports the first hypothesis that activity rhythms would decline as dementia severity progressed, but the later resurgence of rhythmicity is surprising. One conjectural explanation for these results is that there are two sources of synchronization of rhythms: the endogenous output by the SCN and entrainment by the environment. Perhaps in the early stages of dementia, there is damage to the SCN that produces a decline in rhythmicity. Once the SCN deteriorates, environmental cues take on a larger role and lead to a resynchronization of circadian rhythms until the end stages of dementia, at which point even environmental cues lose their potency. This hypothesis is supported by the results of the comparisons of rhythm parameters between those with severe dementia and those with mild or moderate dementia. Patients in this study with mild to moderate dementia had an acrophase that was on average 2 hours earlier than that of patients with severe dementia.
The lack of a relationship between the F-statistic and MMSE score for the group with stronger rhythms may be due to different subgroups of dementia. These results show that poor rhythmicity is not a necessary by-product of dementia and that some individuals are able to maintain rest/activity rhythms even in the presence of severe dementia. The different results that have been reported in previous studies may reflect heterogeneity in the manifestation of dementia. The current study was the first to detect such subsamples, probably because of the large sample size compared with prior research. It would be interesting to examine whether these two groups show different patterns of neurodegeneration on post-mortem pathological examinations.
In subjects with more robust rhythms, MMSE score was related to acrophase in a quadratic manner. Subjects with higher MMSE scores had earlier acrophases, as shown in Figure 4. The relationship is quadratic rather than linear owing to a small return to earlier acrophases for those with very low MMSE scores. It should be noted that those with less severe dementia had rhythms that peaked earlier than the overall mean acrophase of 14.12 hours. At the earlier stages of dementia, subjects were able to maintain what may be a “healthier” acrophase until a particular stage of neurodegeneration. At that point, patients drifted towards a later acrophase, which may reflect an “environmentally driven” acrophase.
The results found with the α and β parameters are somewhat more difficult to interpret. Severity of dementia appears to be related to the steepness and peakedness of the rhythm. A rhythm that is steeper may reflect an individual who is able to transition from sleep to wakefulness, and vice versa, relatively quickly. They are able to “get going” faster than someone with a rhythm that is not as steep. The α parameter may reflect an individual’s ability to maintain longer periods of activity or wakefulness during the day. Both of these parameters could be related to overall medical health. The relationship between MMSE score and the α parameters was in opposite directions for those with less robust versus more robust rhythms, which provides further evidence for qualitative differences between these two groups.
How do these results compare with those of other studies?
The results of this study are mixed, which reflects the nature of the literature as a whole. Most previous investigations have documented a decline in rest/activity rhythms in dementia. Most of those studies found effects by comparing non-demented older adults to patients with dementia, whereas this study included only demented patients. Another difficulty with comparing these results with those of previous studies is that those studies provided very little descriptive information on the severity and/or type of dementia of their subjects, often referring to their patient population only as demented. The results could be interpreted very differently depending on the severity and type of the patients’ dementia. Perhaps this study, which included a broader range of dementia severity than previous studies, is a more accurate depiction of the true relationship between dementia and rest/activity rhythms. The results of the current study were the same when the analyses were restricted to subjects with a diagnosis of probable or possible AD as opposed to all patients with dementia, so it does not appear that heterogeneity of dementia subtype was a factor.
Another possible confounding factor in previous studies was the effect of covariates. In the entire sample, rhythm parameters were significantly associated with sedating medications (amplitude, acrophase), age (α and β parameters), sex (α parameter), medical burden (amplitude), and daily light exposure (acrophase). Most previous studies did not examine these variables, so it is possible that their results were confounded. In the only other study to examine the effects of covariates, van Someren and colleagues (1996) found that nonparametric measures of rest/activity rhythms were related to the total amount of activity during the day, age of onset of dementia, season of the year, and environmental light exposure. There may be other factors that were not considered in their study, such as other medical or environmental characteristics that might affect rest/activity rhythms and may explain the discrepancies among findings. Of note, the average light exposure of this sample was higher than that reported in other studies of similar samples. Inspection of the data revealed that there was a small group of subjects with high levels of light exposure that were having an inordinate effect on the mean for the group. Overall, most of the subjects had less than 30 minutes of bright light exposure.
In addition, conclusions cannot be drawn about the relative contributions of endogenous and exogenous factors to rest/activity rhythm deterioration. It is unclear if disturbed rhythms are the result of decreased SCN activity, environmental cues, or a combination of both. Rigorous experimental designs, such as the constant routine, should be employed in future studies, although this may prove difficult, if not impossible, with this population.
Lastly, the results obtained in this study may have been affected by the measure of dementia employed. As discussed above, the MMSE is considered to be a useful measure of dementia severity that is quick to administer. Other neuropsychological instruments take much longer to administer but provide more detailed information about specific domains of functioning and have a wider range of scores. In the sample we studied, the MMSE was not sensitive enough to detect subtle differences in cognitive functioning in patients with the most severe dementia (ie, patients with MMSE = 0). Perhaps the relationship between dementia and activity rhythms might have been clearer if a more sensitive measure of dementia had been used. As is always the case in research, practical constraints limit the ability of an investigator to use the most thorough assessments of every variable measured. On the other hand, the MMSE score was a significant predictor of survival: subjects with higher MMSE scores lived longer than those with lower scores, so lack of sensitivity was not an issue for these analyses. Future studies should try to replicate these findings with more extensive neuropsychological tests.
Overall, these results inspire a three-stage model of rest/activity rhythm changes in dementia. At the earlier stages of dementia, in which rest/activity rhythms are generally more robust, the endogenous rhythmic output is at its strongest and patients are able to maintain an acrophase that falls earlier in the day. As dementia progresses, a subset of patients will be able to maintain healthy rhythms. For everyone else the output of the SCN declines, producing a poorer circadian rhythm with a peak that becomes gradually later in the day. Subjects then enter a stage in their dementia in which weaker endogenous output competes with environmental cues, producing disturbed rhythms. As endogenous output declines even further, environmental cues take precedence and rhythms become somewhat more robust, although at a later acrophase, which falls in the mid afternoon.
In conclusion, not all patients with dementia have rest/activity disturbances; nevertheless, rest/activity rhythms do appear to play an important role in dementia. Rhythms do not change in the same manner for all patients as dementia progressively worsens, and having dementia does not necessarily mean that having a rhythm disturbance is inevitable. The potential outcomes of rest/activity rhythm disturbance remain to be examined. It may be possible to provide interventions that maintain patients with dementia at the first stage in this model in which their rhythms remain relatively strong. Future studies are needed to better understand the complex interplay of endogenous and environmental factors in the regulation and decline of rest/activity rhythms in dementia.
Acknowledgments
Sonia Ancoli-Israel was supported by NIA AG08415, NCI CA85264, NIA AG15301, the Department of Veterans Affairs VISN-22 Mental Illness Research, Education and Clinical Center (MIRECC), and the Research Service of the Veterans Affairs San Diego Healthcare System. The project could not have been completed without the cooperation of the administration, staff and patients at the nursing homes participating in this study and without the help of Drs Ruth Pat-Horenczyk, Jody Corey-Bloom, Donald Connor, Leah Levi, and the UCSD staff and student volunteers who spent countless hours with the patients.
References
- Aharon-Peretz J, Masiah A, Pillar T, et al. Sleep-wake cycles in multi-infarct dementia and dementia of the Alzheimer type. Neurology. 1991;41:1616–19. doi: 10.1212/wnl.41.10.1616. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi CA, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Gehrman P, Martin JL, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Jones DW, Hanger MA, et al. Sleep in the nursing home. In: Kuna ST, Suratt PM, Remmers JE, editors. Sleep and respiration in aging adults. New York: Elsevier; 1991. pp. 77–84. [Google Scholar]
- Ancoli-Israel S, Klauber MR, Jones DW, et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin JL, Kripke DF, et al. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. JAGS. 2002;50:282–9. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harbor Symp Quant Biol. 1960;25:11–26. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Gillin JC, Kripke DF. Ambulatory recording of rest/activity, body temperature and light exposure in normal elderly and Alzheimer’s disease. Sleep Res. 1986;15:264. [Google Scholar]
- Campbell S, Kripke DL, Gillin JC, et al. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiol Behav. 1988;42:141–4. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–79. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallant AR. Nonlinear statistical models. New York: Wiley; 1987. [Google Scholar]
- Harper DG, Stopa EG, McKee AC, et al. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Arch Gen Psychiatry. 2001;58:353–60. doi: 10.1001/archpsyc.58.4.353. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJG, van Someren EJW, Mirmiran M, et al. Circadian rhythm-related behavioral disturbances and structural hypothalamic changes in Alzheimer’s disease. Int Psychogeriatrics. 1996;8(Suppl 3):245–52. doi: 10.1017/s1041610297003426. [DOI] [PubMed] [Google Scholar]
- Jacobs D, Ancoli-Israel S, Parker L, et al. Twenty-four-hour sleep-wake patterns in a nursing home population. Psychol Aging. 1989;4:352–6. doi: 10.1037//0882-7974.4.3.352. [DOI] [PubMed] [Google Scholar]
- Martin J, Marler M, Shochat T, et al. Circadian rhythms of agitation in institutionalized patients with Alzheimer’s disease. Chronobiol Int. 2000;17:405–18. doi: 10.1081/cbi-100101054. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mishima K, Okawa M, Satoh K, et al. Different manifestations of circadian rhythms in senile dementia of Alzheimer’s type and multi-infarct dementia. Neurobiol Aging. 1997;18:105–9. doi: 10.1016/s0197-4580(96)00167-4. [DOI] [PubMed] [Google Scholar]
- Mishima K, Tozawa T, Satoh K, et al. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol Psychiatry. 1999;45:417–21. doi: 10.1016/s0006-3223(97)00510-6. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Okamoto N, Uchida K, et al. Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s type. Biol Psychiatry. 1999;45:1646–52. doi: 10.1016/s0006-3223(98)00255-8. [DOI] [PubMed] [Google Scholar]
- Pat-Horenczyk R, Klauber MR, Shochat T, et al. Hourly profiles of sleep and wakefulness in severely versus mild-moderately demented nursing home patients. Aging Clin Exp Res. 1998;10:308–15. doi: 10.1007/BF03339793. [DOI] [PubMed] [Google Scholar]
- Pollak CP, Stokes PE. Circadian rest-activity rhythms in demented and nondemented older community residents and their caregivers. JAGS. 1997;45:446–52. doi: 10.1111/j.1532-5415.1997.tb05169.x. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Christie C, Smallwood R, et al. Circadian temperature variation in healthy aged and in Alzheimer’s disease. J Gerontol. 1984;39:30–5. doi: 10.1093/geronj/39.1.30. [DOI] [PubMed] [Google Scholar]
- Satlin A, Teicher MH, Lieberman HR, et al. Circadian locomotor activity rhythms in Alzheimer’s disease. Neuropsychopharmacology. 1991;5:115–26. [PubMed] [Google Scholar]
- Satlin A, Volicer L, Stopa EG, et al. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol Aging. 1995;16:765–71. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- Seber GAF. Linear regression analysis. New York: Wiley; 1977. [Google Scholar]
- Shochat T, Martin J, Marler M, et al. Illumination levels in nursing home patients: effects on sleep and activity rhythms. J Sleep Res. 2000;9:373–80. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. JAGS. 1991;40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Touitou Y, Reinberg A, Bogdan A, et al. Age-related changes in both circadian and seasonal rhythms of rectal temperature with special reference to senile dementia of Alzheimer type. Gerontology. 1986;32:110–18. doi: 10.1159/000212774. [DOI] [PubMed] [Google Scholar]
- Uchida K, Okamoto N, Ohara K, et al. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996;717:154–9. doi: 10.1016/0006-8993(96)00086-8. [DOI] [PubMed] [Google Scholar]
- van Someren EJW, Hagebeuk EEO, Lijzenga C, et al. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol Psychiatry. 1996;40:259–70. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- van Someren EJW, Swaab DF, Colenda CC, et al. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16:505–18. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, et al. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:563–72. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]