Abstract
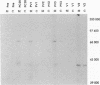
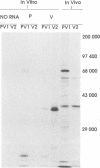
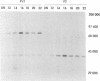
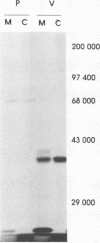
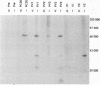
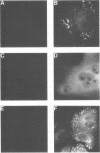
An edited mRNA transcribed from the phosphoprotein (P) gene of measles virus (MV) has been predicted to encode a cysteine-rich protein designated V. This mRNA contains a single additional nontemplated G residue which permits access to an additional protein-coding reading frame. Such an edited P gene-specific mRNA has been detected in MV-infected cells, but no corresponding protein has yet been identified in vivo. We report the use of antisera directed against synthetic peptides corresponding to five different regions of the predicted MV V protein amino acid sequence to analyse MV-specific proteins synthesized in vivo and in vitro. The MV V protein (40 kDa) was detected in MV-infected cells in a diffuse cytoplasmic distribution, a predominant subcellular localization distinct from that of virus nucleocapsids. The protein was found to be phosphorylated and to be maximally synthesized at 16 h postinfection, when MV-specific structural protein synthesis was also maximal. Antiserum directed against a peptide (PV2) corresponding to amino acids 65 to 87 of the V protein amino acid recognized the P protein but not the V protein, indicating that the P and V proteins may be folded differently at or near this region so that the PV2 sequence is in an exposed position at the surface of the P protein but not at the surface of the V protein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib G., Briedis D. J. The predicted primary structure of the measles virus hemagglutinin. Virology. 1986 Apr 30;150(2):479–490. doi: 10.1016/0042-6822(86)90312-0. [DOI] [PubMed] [Google Scholar]
- Alkhatib G., Massie B., Briedis D. J. Expression of bicistronic measles virus P/C mRNA by using hybrid adenoviruses: levels of C protein synthesized in vivo are unaffected by the presence or absence of the upstream P initiator codon. J Virol. 1988 Nov;62(11):4059–4069. doi: 10.1128/jvi.62.11.4059-4069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Billeter M., ter Meulen V. Purification and molecular weight determination of measles virus genomic RNA. J Gen Virol. 1983 Jun;64(Pt 6):1409–1413. doi: 10.1099/0022-1317-64-6-1409. [DOI] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Baczko K., Schmid A., Ter Meulen V. Cloning of DNA corresponding to four different measles virus genomic regions. Virology. 1984 Jan 15;132(1):147–159. doi: 10.1016/0042-6822(84)90099-0. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Kaelin K., Baczko K., Billeter M. A. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989 Mar 10;56(5):759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., KATZ S. L., HOLLOWAY A. Development of attenuated measles-virus vaccines. A summary of recentinvestigation. Am J Dis Child. 1962 Mar;103:335–340. doi: 10.1001/archpedi.1962.02080020347030. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F. Measles virus. Historical review, isolation, and behavior in various systems. Am J Dis Child. 1962 Mar;103:282–287. [PubMed] [Google Scholar]
- Gibbs P. E., Zouzias D. C., Freedberg I. M. Differential post-translational modification of human type I keratins synthesized in a rabbit reticulocyte cell-free system. Biochim Biophys Acta. 1985 Mar 20;824(3):247–255. doi: 10.1016/0167-4781(85)90055-7. [DOI] [PubMed] [Google Scholar]
- Graves M. C. Measles virus polypeptides in infected cells studied by immune precipitation and one-dimensional peptide mapping. J Virol. 1981 Apr;38(1):224–230. doi: 10.1128/jvi.38.1.224-230.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Bando H., Tsurudome M., Kawano M., Nishio M., Ito Y. Sequence analysis of the phosphoprotein (P) genes of human parainfluenza type 4A and 4B viruses and RNA editing at transcript of the P genes: the number of G residues added is imprecise. Virology. 1990 Sep;178(1):321–326. doi: 10.1016/0042-6822(90)90413-l. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund G. A., Tyrrell D. L., Bradley R. D., Scraba D. G. The molecular length of measles virus RNA and the structural organization of measles nucleocapsids. J Gen Virol. 1984 Sep;65(Pt 9):1535–1542. doi: 10.1099/0022-1317-65-9-1535. [DOI] [PubMed] [Google Scholar]
- Mumford R. A., Pickett C. B., Zimmerman M., Strauss A. W. Protease activities present in wheat germ and rabbit reticulocyte lysates. Biochem Biophys Res Commun. 1981 Nov 30;103(2):565–572. doi: 10.1016/0006-291x(81)90489-7. [DOI] [PubMed] [Google Scholar]
- Nakai T., Shand F. L., Howatson A. F. Development of measles virus in vitro. Virology. 1969 May;38(1):50–67. doi: 10.1016/0042-6822(69)90127-5. [DOI] [PubMed] [Google Scholar]
- Ohgimoto S., Bando H., Kawano M., Okamoto K., Kondo K., Tsurudome M., Nishio M., Ito Y. Sequence analysis of P gene of human parainfluenza type 2 virus: P and cysteine-rich proteins are translated by two mRNAs that differ by two nontemplated G residues. Virology. 1990 Jul;177(1):116–123. doi: 10.1016/0042-6822(90)90465-4. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A. RNA editing by G-nucleotide insertion in mumps virus P-gene mRNA transcripts. J Virol. 1990 Sep;64(9):4137–4145. doi: 10.1128/jvi.64.9.4137-4145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Berkovich A., Rozenblatt S., Bellini W. J. Use of antibodies directed against synthetic peptides for identifying cDNA clones, establishing reading frames, and deducing the gene order of measles virus. J Virol. 1985 Apr;54(1):186–193. doi: 10.1128/jvi.54.1.186-193.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rima B. K., Lappin S. A., Roberts M. W., Martin S. J. A study of phosphorylation of the measles membrane protein. J Gen Virol. 1981 Oct;56(Pt 2):447–450. doi: 10.1099/0022-1317-56-2-447. [DOI] [PubMed] [Google Scholar]
- Rubenstein P., Deuchler J. Acetylated and nonacetylated actins in Dictyostelium discoideum. J Biol Chem. 1979 Nov 10;254(21):11142–11147. [PubMed] [Google Scholar]
- Southern J. A., Precious B., Randall R. E. Two nontemplated nucleotide additions are required to generate the P mRNA of parainfluenza virus type 2 since the RNA genome encodes protein V. Virology. 1990 Jul;177(1):388–390. doi: 10.1016/0042-6822(90)90497-f. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Tanabayashi K., Hishiyama M., Yamada Y. K., Yamada A., Sugiura A. Detection and characterization of mumps virus V protein. Virology. 1990 Sep;178(1):247–253. doi: 10.1016/0042-6822(90)90400-l. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Lamb R. A., Paterson R. G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988 Sep 9;54(6):891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Curran J., Kolakofsky D. Editing of the Sendai virus P/C mRNA by G insertion occurs during mRNA synthesis via a virus-encoded activity. J Virol. 1990 Jan;64(1):239–246. doi: 10.1128/jvi.64.1.239-246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Curran J., Orvell C., Kolakofsky D. Mapping of monoclonal antibodies to the Sendai virus P protein and the location of its phosphates. J Virol. 1988 Jun;62(6):2200–2203. doi: 10.1128/jvi.62.6.2200-2203.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorburger K., Kitten G. T., Nigg E. A. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. EMBO J. 1989 Dec 20;8(13):4007–4013. doi: 10.1002/j.1460-2075.1989.tb08583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]