Abstract
Regular scale patterning, restricted to the caudalmost tail and organized into two opposing rows on each side of the tail, is observed in few chondrichthyans. These evenly spaced scales, in dorsal and ventral rows, develop in an iterative sequence from the caudal tip, either side of the notochord. They are subsequently lost as a scattered pattern of placoid scales develops on the body and fins. An identical organized pattern is observed in tail scales of Scyliorhinus canicula (catshark), where the expression of sonic hedgehog signal is restricted to the epithelium of developing scales and remains localized to the scale pocket. Regulation of iterative scale position by sonic hedgehog is deeply conserved in vertebrate phylogeny.
These scales also reveal an archaic histological structure of a dentine type found in the oldest known shark scales from the Ordovician and Silurian. This combination of regulated pattern and ancient dentine occurs only in the tail, representing the primary scalation. Scattered body scales in elasmobranchs such as S. canicula originate secondarily from differently regulated development, one with typical orthodentine around a central pulp cavity. These observations emphasize the modular nature of chondrichthyan scale development and illustrate previously undetected variation as an atavism in extant chondrichthyan dentine.
Keywords: Scyliorhinus, chondrichthyan evolution, scale development, dentine structure
1. Introduction
Recent work has highlighted the presence of scale patterning early in the development of certain chondrichthyan taxa, including both major chondrichthyan groups, the Holocephali and Elasmobranchii (e.g. Miyake et al. 1999; Didier 2004; Freitas & Cohn 2004; Eames et al. 2007). This patterning is observed in bilateral scale rows along the body and rows associated with the lateral sensory canals; patterned scale rows have also been described in association with the tail fin (axial lobe). These latter scales occur in four rows, two on each side of the tail, one dorsal and one ventral. Within each row, scales are equidistant from one another (Scyliorhinus canicula: Ballard et al. 1993; Cephaloscyllium ventriosum: Eames et al. 2007; Heterodontus portusjacksoni, Heterodontus galeatus: Johanson et al. 2007). All these scale rows are lost during ontogeny and replaced by more randomly developing scattered body scales. The body scales show nearest neighbour irregular spacing related to proximity of nearby scales providing initiation factors (M. Smith & R. Fraser 2007, personal observation) and are not organized into rows of iterative, evenly spaced scales (also Reif 1985).
Johanson et al. (2007) suggested that linearly patterned rows of scales were plesiomorphic for chondrichthyans, as scale rows and a more strictly regulated scale development also characterized actinopterygians and sarcopterygians (Osteichthyes; Donoghue 2002; Sire & Akimenko 2004). The scattered chondrichthyan body scales were considered to be a derived feature within the clade. Differences in scale patterning between early, ordered rows and later developing, more scattered scales suggest that chondrichthyan scalation may be separated into discrete developmental modules (one for ordered rows and one for scattered scales). These separate developmental modules have different regulation and potentially different evolutionary histories.
Alongside this regular scale patterning, we observed that dentine in S. canicula (small-spotted catshark) tail fin scales is a type that is otherwise only known from the oldest shark scales, including those from the Ordovician Harding Sandstone (ca 450 Myr ago; Sansom et al. 1996) and those assigned to Elegestolepis from the Upper Silurian (ca 430 Myr ago; Karatajute-Talimaa 1973). This structural similarity suggests that patterned tail scales have an extremely deep phylogenetic history, retained for 450 Myr but now restricted to a brief period of ontogeny in living sharks.
2. Material and methods
Scyliorhinus canicula eggs from Tenby Aquarium (Pembrokeshire), Millport Field station Station (Glasgow University) and the London Aquarium were held under standard aquarium conditions. Staged embryos (Ballard et al. 1993) were removed from egg cases, anaesthetized (MS222) and tails anterior to the caudal fin were removed. These were processed in 4% PFA for in situ hybridization with a probe for Scshh (S. canicula sonic hedgehog gene), SEM or histological examination. Comparative material of fossil chondrichthyan scales was obtained from Harding Sandstone deposits, Colorado, USA (see also electronic supplementary material).
3. Results
(a) Macroscopic appearance
Scanning electron micrographs, photomacrographs and photomicrographs show two dorsal and ventral, laterally opposed rows of scales spaced symmetrically and iteratively from the extreme tip of the tail of a S. canicula embryo (stage 33). The older scales are more caudal, with a more irregular shape (figures 1c,f,g (versus figure 1b,e) and 2a,b), but all show a flat smooth surface (figure 1). Scales develop within a distinct epithelial pocket (figure 2a) with individual positions dorsoventrally staggered by half a scale pocket (figure 2a,b, arrows in figure 2a,b pass between two dorsal scales but through one ventral scale). Consequently, the dorsal and ventral rows are non-aligned with one more scale in the dorsal row than the ventral (9/8 in stage 31, with 10/9 in stage 33), indicating a regulated sequential timing of individual scale row development, synchronized between the rows (figure 2a,b).
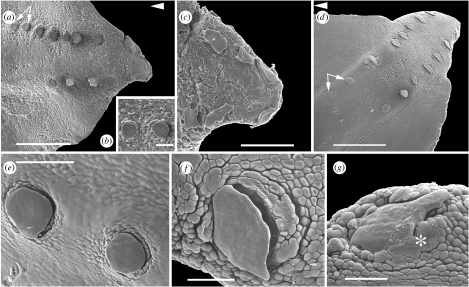
Figure 1.
SEM images showing S. canicula tail tip, with arrangement of two offset rows of scales. (a–c) Stage 31: (a) left lateral view, (b) newest round-topped scales (arrows), (c) caudalmost tail tip, terminal scale absent. (d–g) Stage 33: (d) left lateral view, one more scale on dorsal row (arrows indicate scales in (e)). (f,g) Older, lozenge-shaped scales, scale pocket epithelium (asterisk). Scale bars, (a) 0.50 mm, (b) 86 μm, (c) 176 μm, (d,f,g) 0.60 mm and (e) 120 μm. (a,d) Arrowheads indicate rostral.
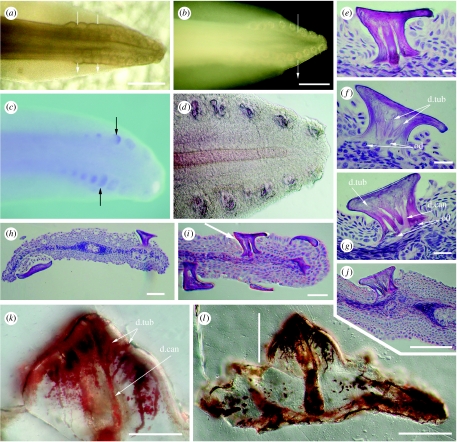
Figure 2.
Scale rows at S. canicula tail tip showing arrangement and histology compared to Ordovician scales from the Harding Sandstone. (a–d) Tail tip in left lateral view. (a) Transmitted light showing iterative scale pockets at stage 29 with an rostrocaudal stagger 9/10 dorsal and 8/9 ventral. (b) Incident light reflected from erupted scale surfaces showing tail shape (stage 33). (c) Photomacrograph (stage 32) and (d) Nomarski optics (DIC; stage 33) of whole mount in situ hybridization for Scshh showing expression patterns as iterative focal loci restricted to scale positions. (e–j) Scale histology in H&E stained vertical sections through the four scale rows (stage 33, DIC). (h) Scales either side of neural tube and notochord with large flat crowns and slender necks, (i) similar but ventral tail end (arrow, scale in (e)). (e) Mature scale showing two dentine canals and odontoblast cells below extended neck. (f) Young scale with shorter neck and odontoblast cell bodies in the dentine below the tubules. (g) More rostral, younger scale in (j) from dorsal row next to neural tube, with open base, several wide canals and odontoblast cell bodies below these. (k,l) Mineralized ground sections (DIC) of Ordovician shark microremains from Colorado (see also Sansom et al. 1996, fig. 1). (k) Higher magnification of crown dentine (line in (l)) of whole scale, mineral deposits in the spaces show tubular dentine with multiple branching from a wide central canal extending down through the extensive scale base. Abbreviations, d.can, dentine canal; d.tub, dentine tubule; od, odontoblasts. Scale bar, (a,c) 20 mm, (b) 500 μm, (d) 100 μm, (e–h) 10 μm and (i–l) 50 μm.
The wide, regular spacing within the row and the staggered temporal development are associated with a timing difference in focal expression of Scshh in the epithelium, each expression site localized to the scale pocket. This appearance with different levels of expression (figure 2c,d) confirms that the staggered timing of regulation is iterative in a caudal to rostral direction and also dorsal in advance of ventral (figure 2c,d). One new rostral scale, relative to older caudal scales (figure 2c), is characterized by a small rounded area of Scshh expression. This is a scale pocket, where all epithelium expresses Scshh compared with the older scales, where this expression becomes shifted to the posterior (caudal) margin of the scale (figure 2c, arrow).
(b) Microstructure
In section view, the overlap of the crown on the neck of the scales is more extensive ventrally (figure 2e–j) and vascular canals have not been observed. Dentine secreting cell bodies (odontoblasts, od) do not form a layer in a central pulp cavity as they do for orthodentine of placoid body scales but are restricted to the base of the dentine in the neck of Scyliorhinus tail scales. Each cell body occupies the wide, branching canals representing spaces for broad cell processes (dentine canals, d.can) leading into finer branches in the dentine of the scale crown (dentine tubules, d.tub). Younger scales with a less developed neck and broad base show odontoblast cells at the forming dentine surface here within the dentine canals (figure 2f). This is a non-vascular dentine with neither a central pulp cavity nor vascular neck or central canals in the scale base. Identification of this tail scale dentine as archaic is by comparison with dentine in the crown of Ordovician scales from the Colorado Harding Sandstone microremains (figure 2k,l). The Ordovician dentine tissue also has many wide branching canals for odontoblast cell processes leading into finer tubules in the crown. These cell process spaces are filled with iron oxide deposits, allowing identification as a branching, tubular dentine (Sansom et al. 1996). However, as a composite scale of many units to the crown (polyodontodes), this also has a central canal, assumed to be vascular, through the base.
4. Discussion
Body scales (including those on the head) of S. canicula develop in an irregular, dispersed, scattered pattern, with the position of new scales probably controlled by their nearest neighbours (Reif 1985, pl. 26; M. Smith & R. Fraser 2007, personal observation). This non-regular, non-iterative arrangement is characteristic of chondrichthyan scale development (Reif 1985). Scyliorhinus caniculus lacks a single ‘initiator’ scale at the posterior extremity of the tail, differing from Heterodontus in this feature (Johanson et al. 2007), but otherwise the regular iterative pattern is maintained. An iterative series implies regulated development of scale rows and data on Scshh expression illustrates one of the patterning genes used in this process, comparable to Shh patterning observed in zebrafish scales (Danio rerio; Sire & Akimenko 2004) and for sequential tooth pattern in the dentition of the trout (Onchorhyncus mykiss; Fraser et al. 2004). This regularity is plesiomorphic for chondrichthyans (phylogeny in the electronic supplementary information) and is retained only during early ontogenetic stages of living taxa (Johanson et al. 2007).
Dentine of the S. canicula tail scales is not orthodentine (regular cell process tubules formed from a layer of cells lining a central pulp cavity), but a branching pattern where wide canals branch into smaller and finer tubules. This is comparable to an ancient form of dentine lacking a central pulp cavity present in Ordovician shark scales (figure 2k,l; Sansom et al. 1996) and in those assigned to the Silurian Elegestolepis grossi (Karatajute-Talimaa 1973). Growth stages of the S. canicula tail scales show dentine development similar to the growth series described by Karatajute-Talimaa (1973, refigured in Smith & Hall 1990, fig. 1). These ancient scales have multiple dentine canals, housing putative odontoblast processes, but all placoid scales, as those of S. canicula body scales, have typical orthodentine (Reif 1980). Thus, S. canicula tail scale dentine is similar to that of the oldest fossil chondrichthyans rather than a modern type as in typical placoid scales.
Elegestolepis and the Harding Sandstone taxa have not yet been resolved within a chondrichthyan phylogeny (Sansom et al. 1996), but they are generally believed to be phylogenetically basal forms. The tail scales of S. canicula appear only during egg capsule development and not only retain a plesiomorphic organization or patterning (relative to osteichthyans), but also an early, phylogenetically basal form of dentine that can be traced back over more than 400 Myr. More scattered body scales develop later in chondrichthyan ontogeny, composed of typical orthodentine. These observations indicate that chondrichthyan scales on different regions of the body can be considered as independent developmental modules. We suggest that the ‘patterned tail scale’ module represents the more ancient, relative to the scattered body scales, which may be related to suggestions that the tail is itself an independent developmental unit relative to the rest of the body (reviewed in Handrigan 2003). Data on pattern regulation and histology of tail scales in other taxa, and the histology of bilaterally axially patterned scales on the body during early ontogeny, for example, in holocephalans (Didier 2004) and other elasmobranchs (Miyake et al. 1999; Freitas & Cohn 2004) would test these hypotheses.
Supplementary Material
Technical information; phylogenetic relationships of the Gnathostomata
References
- Ballard W.W, Mellinger J, Lechenault H. A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish. J. Exp. Zool. 1993;267:318–336. doi:10.1002/jez.1402670309 [Google Scholar]
- Didier D.A. Phylogeny and classification of extant Holocephali. In: Carrier J.C, Musik J.A, Heithaus M.R, editors. Biology of sharks and their relatives. CRC Press; London, UK: 2004. pp. 115–135. [Google Scholar]
- Donoghue P.C.J. Evolution of development of the vertebrate dermal and oral skeletons: unravelling concepts, regulatory theories, and homologies. Paleobiology. 2002;28:474–507. doi:10.1666/0094-8373(2002)028<0474:EODOTV>2.0.CO;2 [Google Scholar]
- Eames B.F, Allen N, Young J, Kaplan A, Helms J.A, Schneider R.A. Skeletogenesis in the swell shark Cephaloscyllium ventriosum. J. Anat. 2007;210:542–554. doi: 10.1111/j.1469-7580.2007.00723.x. doi:10.1111/j.1469-7580.2007.00723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser G.J, Graham A, Smith M.M. Conserved deployment of genes during odontogenesis across osteichthyans. Proc. R. Soc. B. 2004;271:2311–2317. doi: 10.1098/rspb.2004.2878. doi:10.1098/rspb.2004.2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas R, Cohn M.J. Analysis of EphA4 in the lesser spotted catshark identifies a primitive gnathostome expression pattern and reveals co-option during evolution of shark-specific morphology. Dev. Genes Evol. 2004;214:466–472. doi: 10.1007/s00427-004-0426-0. doi:10.1007/s00427-004-0426-0 [DOI] [PubMed] [Google Scholar]
- Handrigan G.R. Concordia discors: duality in the origin of the vertebrate tail. J. Anat. 2003;202:255–267. doi: 10.1046/j.1469-7580.2003.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson Z, Smith M.M, Joss J. Early scale development in Heterodontus (Heterodontiformes; Chondrichthyes): a novel chondrichthyan scale pattern. Acta Zool. 2007;88:249–256. doi:10.1111/j.1463-6395.2007.00276.x [Google Scholar]
- Karatajute-Talimaa V. Elegestolepis grossi gen. et sp. nov., ein neuer Typ der Placoidschuppe aus dem Oberen Silur der Tuwa. Palaeontographica A. 1973;143:35–50. [Google Scholar]
- Miyake T, Vaglia J.L, Taylor L.H, Hall B.K. Development of dermal denticles in skates (Chondrichthyes, Batoidea): patterning and cellular differentiation. J. Morphol. 1999;241:61–81. doi: 10.1002/(SICI)1097-4687(199907)241:1<61::AID-JMOR4>3.0.CO;2-S. doi:10.1002/(SICI)1097-4687(199907)241:1<61::AID-JMOR4>3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- Reif W.-E. Development of dentition and dermal skeleton in embryonic Scyliorhinus canicula. J. Morphol. 1980;166:275–288. doi: 10.1002/jmor.1051660303. doi:10.1002/jmor.1051660303 [DOI] [PubMed] [Google Scholar]
- Reif W.-E. Squamation and ecology of sharks. Cour. Forsch. Inst. Sencken. 1985;78:1–255. [Google Scholar]
- Sansom I.J, Smith M.M, Smith M.P. Scales of thelodont and shark-like fishes from the Ordovician of Colorado. Nature. 1996;379:628–630. doi:10.1038/379628a0 [Google Scholar]
- Sire J.-Y, Akimenko M.E. Scale development in fish: a review, with description of sonic hedgehog (shh) expression in the zebrafish (Danio rerio) Int. J. Dev. Biol. 2004;48:233–247. doi:10.1387/ijdb.15272389 [PubMed] [Google Scholar]
- Smith M.M, Hall B.K. Development and evolutionary origins of vertebrate skeletal and odontogenic tissues. Biol. Rev. 1990;65:277–373. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical information; phylogenetic relationships of the Gnathostomata