Abstract
Programmed cell death (PCD) in petals provides a model system to study the molecular aspects of organ senescence. In this study, the very early triggering signal for PCD during the senescence process from young green buds to 14-d-old petals of Tulipa gesneriana was determined. The opening and closing movement of petals of intact plants increased for the first 3 d and then gradually decreased. DNA degradation and cytochrome c (Cyt c) release were clearly observed in 6-d-old flowers. Oxidative stress or ethylene production can be excluded as the early signal for petal PCD. In contrast, ATP was dramatically depleted after the first day of flower opening. Sucrose supplementation to cut flowers maintained their ATP levels and the movement ability for a longer time than in those kept in water. The onset of DNA degradation, Cyt c release, and petal senescence was also delayed by sucrose supplementation to cut flowers. These results suggest that intracellular energy depletion, rather than oxidative stress or ethylene production, may be the very early signal to trigger PCD in tulip petals.
Keywords: ATP, DNA degradation, intracellular energy depletion, programmed cell death, senescence, tulip petal
Introduction
Programmed cell death (PCD) is a genetically controlled process for the removal of redundant, misplaced, or damaged cells, and is associated with common morphological and biochemical changes during the development of multicellular organisms (Hoeberichts and Woltering, 2002; Gunawardena et al., 2004; Swidzinski et al., 2004). PCD is also an integral part of the life cycle of multicellular organisms, including animals and plants (Green, 1998). This process can be induced by various stimuli, such as developmental signals and environmental cues (Xu and Hanson, 2000). PCD occurs in plants during developmental processes, such as embryogenesis, development of vascular tissues, senescence, and sex determination in unisexual plants, as well as during interactions with pathogens (Lam et al., 2001).
Senescence, or ageing, is the final stage of development and precedes cell and organ death. Petal senescence, tightly regulated developmentally, is considered to be a genetically programmed event that culminates in PCD (Rubinstein, 2000; Rogers, 2006). The processes of senescence and senescence-induced PCD are regulated by a co-ordinated signalling pathway, which is consistent with the view that senescence involves PCD (reviewed by Coupe et al., 2004).
Apoptosis, the most widely studied form of PCD, has been investigated in animal cells. The crucial biochemical effectors of apoptotic cell death are the caspases, a family of cysteine proteases. These enzymes are activated during apoptosis and are responsible for the observed morphological changes and lead to cell death. The release of cytochrome c (Cyt c) from mitochondria to the cytosol is considered to be a prerequisite for apoptosis. Following its release, the cell will die due to the collapse of electron transport, the generation of reactive oxygen species (ROS), and a reduction in ATP generation (Yang et al., 1997; Green, 1998). While these events have been observed in plant PCD (Balk et al., 1999; Kim et al., 2003; Vacca et al., 2004), they are not associated with every type of PCD in plants (Pennell and Lamb, 1997). The release of Cyt c into the cytosol has been reported in Zea mays cell suspension following D-mannose treatment (Stein and Hansen, 1999) and in Arabidopsis cell cultures after oxidative stress (Tiwari et al., 2002). In addition, DNA fragmentation is a characteristic marker of PCD in many plants (Green and Kroemer, 2004; Rogers, 2005) and has been observed in the flowers of Actinidia deliciosa (Coimbra et al., 2004), the petal and ovary of Pisum sativum (Orzaez and Granell, 1997a, b), and petals of Alstroemeria peruviensis (Wagstaff et al., 2003) and Petunia inflata (Xu and Hanson, 2000). Although DNA fragmentation is observed during PCD in many plants, some plant cells show DNA degradation rather than classical DNA laddering (Yamada et al., 2006).
Petal senescence, an active process, is a useful model system for studying the molecular mechanisms of organ senescence and requires de novo gene expression at both the transcriptional and translational levels (Lawton et al., 1990; Noodén et al., 1997). Several genes encoding 1-aminocyclopropane-1-carboxylate (ACC) synthase, ACC oxidase, and cysteine proteinase are up-regulated during petal wilting in senescing Dianthus caryophyllus (carnation) flowers (Sugawara et al., 2002). A type of cysteine endoproteinase that may mediate petal PCD has been identified in senescent Hemerocallis (daylily) petals (Valpuesta et al., 1995). The biochemical changes and increases in hydrolytic enzymes and respiratory activity, associated with petal senescence that consists of the breakdown of macromolecules, can be used to predict PCD (Rubinstein, 2000).
Accelerated generation and/or accumulation of ROS have emerged as important signals in the activation of plant PCD, including petal cells in some species (Rubinstein, 2000; Hoeberichts and Woltering, 2002; De Pinto et al., 2006). In contrast, antioxidant enzymes, such as catalase (CAT) and ascorbate peroxidase (APX), both of which are well-known reductants of hydrogen peroxide (H2O2), are considered to play a role as a defence line against ROS-induced PCD in petals and other organs of the plant (Bartoli et al., 1995; Rubinstein, 2000; De Pinto et al., 2006). In addition to oxidative stress (Robson and Vanlerberghe, 2002), ethylene generation/production that is closely related to abscission (Nukui et al., 2004; Rogers, 2006) and pollination (Xu and Hanson, 2000) have been proposed as signals for PCD during petal senescence. However, even in species-specific ethylene-regulated petal senescence, the primary signal initiating the ethylene cascade remains elusive (Rogers, 2006). Petals are not usually green, and an early step in their development is a conversion of chloroplasts to chromoplasts. This conversion has been compared with the chromoplasts/gerontoplast transition (Thomas et al., 2003), with the suggestion that petals are most similar to senescent leaves. This agrees with the very early cell death seen in flowers (Wagstaff et al., 2003) presumably associated with nutrient mobilization. Although progress in the understanding of PCD in floral organs has advanced, the very early signals responsible for either the initiation or the regulation of PCD during natural petal senescence remain unknown. During apoptosis in animal cells, glucose deprivation, leading to ATP depletion and a fall in energy charge potentials, has been reported as a signal to trigger DNA fragmentation and mitochondrial dysfunction (Comelli et al., 2003; Liu et al., 2003). Despite some similarities between animal and plant PCD at a cellular level, few studies have attempted in plants to investigate the relationship between the depletion of intracellular energy and PCD events during the natural senescence process.
The large petals of tulip (Tulipa gesneriana) have a lifespan of 2 weeks, running from petal emergence to flower death, and have a repetitive diurnal petal opening and closing movement similar to other spring-blossoming flowering species, such as Ficaria, Galanthus, and Crocus (van Doorn and van Meeteren, 2003). Previously, we showed that reversible phosphorylation of aquaporin, the water channel protein in the plasma membrane, and water transpiration are involved in temperature-dependent opening and closing of young tulip petals (Azad et al., 2004a, b, 2007). Although this movement is physiologically interesting during tulip petal development, it is significantly lost after the third day of petal opening. Accordingly, it was hypothesized that the onset of PCD of petals caused the loss of the ability for petal movement, and an attempt was made to identify the very early signal for initiating PCD. In the study reported herein, tulip petal PCD was characterized during its entire floral life by evaluating a number of parameters, and the possibility is discussed that depletion of intracellular energy sources, rather than oxidative stress or ethylene production, triggers PCD in the early stage of tulip petals, as has been described in apoptosis in animal cells (Comelli et al., 2003; Liu et al., 2003; Tome et al., 2004).
Materials and methods
Plant materials and growth conditions
Ten bulbs of tulip (T. gesneriana L. cv. Ile de France) were sown per plastic box (length = 65 cm, width = 22 cm, height = 19 cm) containing a mixture of soil used for flower plantation. In the mixture of soil, the ratio of potting compost (Juntendo Co. Ltd, Japan) to organic phosphoric and compound fertilizers (Juntendo Co. Ltd, Japan) was 3:1. The plastic boxes planted with tulip bulbs were kept in an open place near the greenhouse where the outdoor temperature was –2 °C to 8 °C from November to February. Boxes were watered once every 2 d if there was no rain or snow in the interim. Following the first day of bud emergence, tulip plants were date-tagged and then grown in the greenhouse. Each day, the temperature was set at 20 °C for 8 h during daylight to initiate petal opening and to maintain the open state, and at 5 °C for 16 h from the late evening to early morning to cause petal closing and to maintain closure. Plants were watered once every 2 d. To investigate the time-course of morphological and molecular changes, the lifespan of the corolla was divided into several stages: bud 1 (B1), bud 2 (B2), and bud 3 (B3) before petal opening, and 0-, 3-, 6-, 9-, 12-, and 14-d-old flowers after petal opening (Fig. 1a), with the first day of petal opening being designated as day zero. Based on the similar size of buds, at least three sets (in each set, n=5) of plants were selected for observation or sample collection. To record the observations for morphological changes, the whole bud or flower was imaged by a Nikon digital camera (model Coolpix2000; Nikon Imaging Products Division, Tokyo, Japan). Samples of buds or petals of each stage were frozen with liquid N2 and stored at −80 °C until required.
Fig. 1.
Morphological changes during the lifespan of petals in intact plants. (a) Different stages during the lifespan of corolla in intact plants (a typical observation, n=5); (b) Chlorophyll (filled diamonds) and anthocyanin (filled triangles) contents in buds and petals (mean ±SEM, n=5); (c) petal movement capability (filled diamonds) and fresh weight (FW) (filled triangles) of petals (mean ±SEM, n=5).
Chemicals
All chemicals were obtained from Wako Pure Chemical Industries, Osaka, Japan, unless noted otherwise. Primary antibody for Cyt c and avidin–peroxidase conjugate (secondary antibody) were obtained from Sigma-Aldrich Co., USA.
Experimental design and statistical analysis of the data
To investigate the morphological and biochemical events during the time-course of tulip petal senescence, intact and cut flowers were used. All of the study parameters reported herein were carried out with the intact flowers. For some specific study parameters, cut flowers were used in addition. In the case of investigations with cut flowers, at day 0 of petal opening, flowers with petals of similar sizes whose stem length without any leaves was 10 cm were cut from intact plants. The cut flower experiments involved two treatment groups: a control group kept in distilled water (dH2O) and a group maintained in dH2O supplemented with 10 mM sucrose. The control and sucrose-supplemented groups were incubated in the greenhouse. The daily temperature cycle in the greenhouse was identical to that described previously for the cultivation of the intact plants. The growing solutions were replaced every 12 h. Both groups of flowers were observed each day for analysis of morphological study parameters, such as petal movement capability, wilting, and the floral lifespan. All petals from 0-, 3-, 6-, 9-, and 12-d-old flowers (n=5) of each group were collected, frozen in liquid N2, and stored at –80 °C until required for analysis of biochemical study parameters, such as determination of adenosine nucleotide (ANT) contents for adenylate energy charge (AEC) analyses, DNA fragmentation, DNase activity, and Cyt c release from mitochondria to the cytosol.
For statistical comparison, Student's t-test was used. P-values <0.05 were considered to be statistically significant. Data are given as the mean ±SEM or as noted in the figure legends.
Petal movement and FW measurement
The method for analysing petal movement has been described previously (Azad et al., 2004a). In brief, the apertures of opposite petals in the open state at 20 °C and the closed state at 5 °C were measured. The opposite petal apertures in the closed state on the previous night were subtracted from those in the open state on the following day to determine the opening of petals on that day. The petal movement capability on a specified day was normalized to that of day 0. For fresh weight (FW), five corollas (whole bud or flower) without any stem harvested at the B1, B2, B3, 0-, 3-, 6-, 9-, 12-, and 14-d-old stages were weighed immediately, frozen in liquid N2, and kept at –80 °C until required.
Determination of chlorophyll and anthocyanin content
Frozen buds or petals were ground to a fine powder in liquid N2 and extracted with ice-cold 80% acetone in 2.5 mM sodium phosphate buffer, pH 7.8. The extracts were centrifuged at 15 000 g at 4 °C for 15 min and the supernatant was used for spectrophotometric determination of chlorophyll and anthocyanin contents. For anthocyanin, the absorbance (A) was set at 565 nm (ε565=14.2 mM−1 cm−1). Chlorophyll was determined according to the following formula: chlorophyll a + b [μg ml−1] = A645×20.3 + A663×8.02 (Porra et al., 1989).
ANT analysis using high-performance liquid chromatography (HPLC)
ANTs were extracted from buds or petals with 5% (w/v) trichloroacetic acid (TCA), using a method described previously (Shibata et al., 1987). Following centrifugation, the supernatant was treated five times with an equal volume of diethyl ether to remove the acid before filtering it through a 0.45 μm Millipore filter. HPLC analysis of the filtered supernatant was performed with a Hitachi HPLC system, D-2000 Elite (Hitachi, Japan) equipped with a UV monitor and a Chromato Processor (Hitachi, Japan). An anion exchange column (Hitachi Packed column #3013-N) was used at a flow rate of 0.5 ml min−1 at 60 °C under a pressure of <10 MPa. The AEC level was determined using the formula AEC=(ATP+0.5ADP)/(ATP+ADP+AMP) (Yang et al., 2004).
Protein extraction and quantification
For protein extraction, frozen tissues were homogenized at 4 °C in ice-cold homogenization buffer [25 mM TRIS-HCl, pH 7.5, 0.2 M sucrose, 1 mM dithiothreitol (DTT), 2 mM EDTA, 2 mM EGTA, 15 mM β-mercaptoethanol, and 0.1% (w/v) bovine serum albumin (BSA)] using a chilled mortar and pestle. A 2 ml aliquot of homogenization buffer was used per gram of tissues. The homogenate was then filtered through four layers of cheesecloth and the pH of the filtrate was adjusted to 7.5 with concentrated TRIS-HCl buffer. After successive centrifugation of the filtrate at 3000 g for 5 min and at 13 000 g for 15 min, the resulting supernatant was used as total protein extract. Protein concentration was determined by the Bradford assay (1976) (Bio-Rad Laboratories, Hercules, CA, USA) using BSA as the standard.
DNA fragmentation analysis
DNA was extracted from petals of every stage by using the DNeasy Plant Minikit (QIAGEN, Valencia, CA, USA) according to the manufacturer's instructions. An aliquot (5 μg) of extracted DNA was electrophoresed onto a 1.4% agarose gel, stained with ethidium bromide, and observed under UV illumination (UVP BioImaging System, USA) equipped with an Olympus digital camera (model C-3040ZOOM, Olympus Optical Co. Ltd, Tokyo, Japan).
In-gel DNase activity assay
An equal amount of total protein (20 μg) from petals of every stage was used for electrophoresis on 12.5% SDS–polyacrylamide gels. The resolving gel contained 15 μg ml−1 salmon DNA as the substrate. Following electrophoresis, DNase activity in-gel was detected according to the method described previously (Xu and Hanson, 2000).
Cellular fractionation and immunoblotting
Cellular fractionation was carried out using a method described previously (Xu and Hansen, 2000) with the following modifications. A 2 g aliquot of buds or petals was ground in grinding buffer [150 mM Tricine-KOH, containing 5% (w/v) sucrose, 5 mM MgCl2, 5 mM KCl, and 5 mM EDTA; pH 7.5] for 1 min at 4 °C. Extracts were filtered through four layers of cheesecloth, and the filtrate was centrifuged at 1600 g for 5 min at 4 °C. The resultant supernatant was then centrifuged at 16 000 g for 15 min at 4 °C. Following the second centrifugation, the supernatant thus obtained was considered to represent the cytosolic fraction. Total protein (10 μg) in the cytosolic fraction was separated on a 12.5% SDS–polyacrylamide gel, and immunoblotting was performed according to the method described by Azad et al. (2004a) using monoclonal anti-Cyt c as the primary antibody and avidin–peroxidase conjugate as the secondary antibody.
APX and CAT activities
Buds or petals (200 mg) were ground with 3 vols (w/v) of grinding buffer [50 mM potassium phosphate, 1 mM EDTA, 1 mM L(+)-ascorbic acid; pH 7.0] for 1 min at 4 °C, and centrifuged at 12 000 g for 10 min at 4 °C. The activities of APX and CAT in the supernatant were determined using the methods described by Madhusudhan et al. (2003) and Chiou and Tzeng (2000), respectively.
Measurement of H2O2 content
H2O2 content was quantified by chemiluminescence (Guan et al., 2000). Tissues of buds or petals (0.5 g) were ground in liquid nitrogen and extracted in 3 ml of ice-cold 5% (w/v) TCA. The crude extracts were centrifuged for 15 min at 8000 g, and 0.5 ml of supernatant was passed through a column, previously equilibrated with 5% TCA, containing 1.0 g of Dowex resin (1-X2, 200–400 mesh, Cl form, Dow Chemical Company, MI, USA). The column was washed with 3.5 ml of 5% TCA, and all eluates were pooled. The H2O2 content was measured by adding 0.5 ml of eluate to a solution containing 0.2 ml of 1 M 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo[1,2-a]pyrazin-3-1 hydrochloride and 0.01 ml of 10 mM Fe2SO4, and the volume was made up to 2.0 ml with 0.2 M NH4OH, pH 10.0.
Determination of protease activity
Protease activity was measured using azo-casein as the substrate based on the method described previously (Serafini-Fracassini et al., 2002). A 250 mg aliquot of frozen tissues of buds or petals from every stage was homogenized at 4 °C in 3 vols of ice-cold buffer (100 mM TRIS-HCl, pH 7.5, and 15 mM β-mercaptoethanol). After successive centrifugation of the homogenate at 3000 g for 5 min and at 13 000 g for 15 min, the resulting supernatant was used for protease assay. To study the effects of protease inhibitors, the sample was pre-treated at room temperature for 1 h with 1 mM phenylmethylsulphonyl fluoride (PMSF) or 0.1 mM leupeptin, and the residual proteolytic activity was measured.
Measurement of ethylene production
The rate of ethylene production by the whole flower kept in water was measured by enclosing the flowers in 960 ml airtight polypropylene containers (one flower per container) for 24 h at 20 °C. A 1 ml gas sample was taken into a hypodermic syringe from the container through a rubber septum in the lid of the container, and analysed with a gas chromatograph (Shimadzu, GC-14APS, Japan) equipped with a flame ionization detector and an activated alumina column.
Results
Morphological changes during the time-course of petal senescence in intact plants
The developmental stages of the tulip from the bud to the flower and the changes in morphological characteristics of the petals are shown in Fig. 1a. Almost 100% of the B1 buds and ∼75% and 25% of the B2 and B3 buds were green from the bottom. In the 0-d-old petals, the proximal portion, near the peduncle, was green while the remainder was only pigmented. This green colour disappeared completely in the 3-d-old petals. The analysis of chlorophyll and anthocyanin contents during petal development confirmed this observation (Fig. 1b). The growth of petals from bud to flower coincided with an increase in FW and preceded petal development (Fig. 1c). By day 6, the average FW of petals had increased ∼2-fold when compared with the FW in 0-d-old petals. The FW began to decrease from day 9 onwards, suggesting the start of wilting that may be associated with the loss of turgor. However, apparent wilting was observed morphologically on day 12 (Fig. 1a). Following the start of wilting, the petals began to lose pigment, and on day 14, petals became detached from their receptacles. Tulip petals exhibit a diurnal rhythm, opening during the day and closing at night. However, this movement was only significant until day 6. Figure 1c describes the time-dependent changes in petal movement capability (the ability to repeat the temperature-dependent cycle of petal opening and closing during the day and night, respectively). The movement capability of petals increased 3-fold on day 3, which indicates full ability to open and close. After day 3, older flowers began to respond less to the temperature, because the ability of the petals to move was noticeably lower than that of younger flowers.
Observation of DNA degradation and divalent cation-dependent DNase activity during petal senescence in intact plants
Figure 2a shows that nuclear DNA remains unfragmented until day 3. The integrity of nuclear DNA remained intact on days 4 and 5 (data not shown). However, DNA degradation was observed on day 6 and was present in all the subsequent stages. The assay for DNase activity revealed a single band at approximately the 30 kDa position (Fig. 2b). DNase activity was first detected in 3-d-old petals, gradually increased with petal development, and reached a plateau after day 6.
Fig. 2.
DNA fragmentation and DNase activity during the time-course of petal senescence in intact plants. DNA fragmentation (a) and DNase activity (b) were determined as described in the Materials and methods. The typical results of repeated experiments (n=5) are shown.
Since Ca2+ and Mg2+ have been shown to enhance DNase activity during petal senescence (Xu and Hanson, 2000), the effects of divalent cations on its activity in the extract from 14-d-old petals were investigated. Enzyme activity was detected in buffer containing 20 μM CaCl2 and 10 μM MgCl2, and was not enhanced when the concentrations of either calcium or magnesium were increased to as high as 1 mM (data not shown), indicating that the concentration of bivalent ions in the basic buffer was at a saturation level. The addition of 1 mM EDTA to the buffer inhibited DNase activity completely, and the activity could not be restored by adding either CaCl2 or MgCl2.
Cyt c is released and ATP depletion occurs at the early stage of petal senescence in intact plants
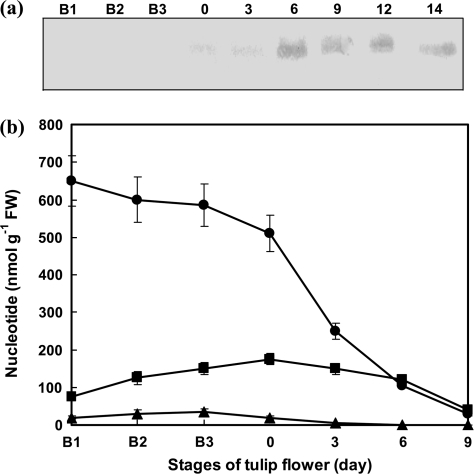
The presence of Cyt c in the cytosol was not detected in the B1, B2, and B3 stages (Fig. 3a). Cyt c could be detected in the cytosolic fraction prepared from 0-d-old petals, but became prominent in 6-d-old petals and subsequent stages (Fig. 3a).
Fig. 3.
Appearance of cytochrome c in the cytosol (a) and ANT contents (b) during the lifespan of tulip petals in the intact plant. (a) The typical results of repeated experiments (n=5) are shown. (b) ATP (filled circles), ADP (filled squares), and AMP (filled triangles) contents are shown in the graph (mean ±SEM, n=5).
The generation of the ANT family (ATP, ADP, and AMP), especially ATP, reflects mitochondrial functional activity. High levels of ATP could be measured in B1, B2, and B3 buds, as well as in 0-d-old petals (Fig. 3b). In 3-d-old petals, the measured ATP level was only about half of that measured in 0-d-old petals. Only trace amounts of ATP were found in the petals in the later stages (Fig. 3b). Since the intracellular high energy phosphate content is reflected by AEC (Atkinson, 1968; Hardie and Hawley, 2001), the AEC was calculated using the levels of ATP, ADP, and AMP. The AEC levels in B1, B2, B3, 0-, 3-, 6-, and 9-d-old petals were 0.923±0.009, 0.877±0.007, 0.857±0.006, 0.848±0.004, 0.802±0.003, 0.730±0.006, and 0.704±0.004, respectively. The values indicated that the buds had a higher energy supply. However, the AEC was maintained at >0.8 in up to 3-d-old petals.
Oxidative stresses, ethylene generation, and protease activity are evident at the final stages during petal senescence in intact plants
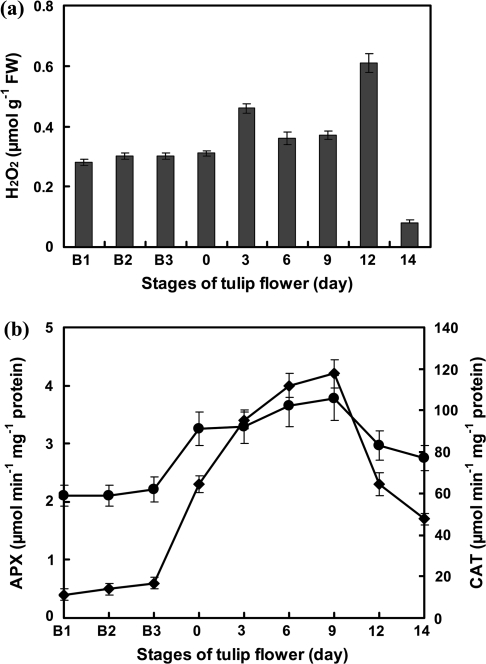
H2O2 content was almost constant from B1 buds to 0-d-old flowers (Fig. 4a). In 3-d-old petals, the levels suddenly increased transiently and returned to the previous levels until day 9. On day 12, the levels rose again. On day 14, they fell abruptly to almost 13% of that measured on day 12. Because H2O2 levels rose suddenly at day 3, its levels were also investigated at days 2 and 4. The levels were close to those measured on days 0 and 6. Because there is a close connection between the endogenous H2O2 levels and the activities of antioxidant enzymes (De Pinto et al., 2006), the activities of APX and CAT were measured. Their activities did not change considerably up to the B3 stage (Fig. 4b). Figure 4b showed that the activities of these two antioxidant enzymes increased after the B3 stage and reached their highest levels on day 9. After day 9, their activities gradually decreased.
Fig. 4.
H2O2 level and antioxidant enzyme activities in the lifespan of tulip petals in intact plants. (a) H2O2 contents were the mean ±SEM of four experiments. (b) Ascorbate peroxidase (APX, filled squares) and catalase (CAT, filled circles) activities are the mean ±SEM of five independent experiments.
Although a small amount of ethylene production was evident in 12-d-old petals, a considerable level of ethylene was measured only in the late stage associated with petal abscission (Fig. 5a). There were no significant differences in protease activity from bud to day 9 of petal opening (Fig. 5b). Protease activity increased conspicuously with the petal wilting on day 12. Protease activity at all stages up to day 9 could not be inhibited by 0.1 mM leupeptin. However, ∼40% of protease activity in 14-d-old petals was inhibited by 0.1 mM leupeptin (Fig. 5b) and the inhibition was not further increased by higher concentrations of leupeptin. The fact that 1 mM PMSF caused insignificant inhibition suggests that cysteine-type proteases might be involved in the late stage of tulip petal senescence.
Fig. 5.
Detection of ethylene production (a) and protease activity (b) during the time-course of petal senescence in intact plants. Samples extracted from 14-d-old petals were used to investigate the effects of protease inhibitors (inset in b). To calculate the inhibition rate compared with the control (without inhibitor), the residual proteolytic activity was measured. Data in both panels are the mean ±SEM (n=4).
Sucrose supplementation to cut flowers maintains ATP levels and petal movement capability
ATP levels fell dramatically in petals after day 0 (Fig. 3b), and movement capability (see Materials and methods) also decreased after day 3 (Fig. 1c). It was shown that petal opening and closing movement in cut flowers can be reproduced by changing the temperature in the dark; petals open at 20 °C and opened petals close at 5 °C (Azad et al., 2004a). The effect of sucrose on petal movement capability and ATP levels, as well as AEC, was investigated over the floral lifespan in cut flowers. Three-day-old flowers supplemented with sucrose (10 mM) had a 1.3-fold increased movement capability compared with those treated without sucrose (Fig. 6). Sucrose supplementation increased petal movement ability progressively up to day 9 (3-fold on day 6 and 6.3-fold on day 9), in contrast to movement measured in flowers incubated in sucrose-free dH2O. Figure 6 further shows that the supply of sucrose significantly affects the time-dependent pattern of the petal movement curve by changing the peak from day 3 (in sucrose-untreated flowers) to day 4. This result indicates that a supply of energy is necessary to maintain petal movement. Moreover, sucrose supplementation lengthened the floral lifespan, because morphologically evident wilting was observed on day 13 and petal detachment occurred on day 16. Morphologically evident wilting in cut flowers that were not supplemented with sucrose was observed on day 11 and petal detachment occurred on day 13. Table 1 shows that the flowers supplemented with sucrose maintained a higher level of ATP compared with the control flowers, and the level of AEC was maintained at >0.8 at all stages until day 9. In contrast, the AEC level dropped below 0.75 after 3 d in the control flowers.
Fig. 6.
Petal movement capability in cut flowers grown in distilled water (filled diamonds) or distilled water containing 10 mM sucrose (filled triangles). Data are the mean ±SEM of five independent experiments.
Table 1.
The effect of sucrose supplementation on the levels of ANT and AEC in petals of cut flowers
| Stages of petals | –Sucrose | + Sucrose (10 mM) | ||||||
| AMP | ADP | ATP | AEC | AMP | ADP | ATP | AEC | |
| 0 | 12.1±3.8 | 190.1±18.6 | 514.7±51.1 | 0.851±0.003 | 11.9±3.6 | 191.8±20.3 | 517.7±47.8 | 0.846±0.001 |
| 3 | 9.8±3.2 | 63.2±5.9 | 117.3±12.3 | 0.783±0.006 | 7.2±2.8 | 76.4±7.1 | 202.3±21.6 | 0.842±0.005 |
| 6 | 7.1±2.3 | 54.6±4.7 | 59.4±6.8 | 0.717±0.006 | 2.2±1.2 | 56.2±6.3 | 107.4±11.1 | 0.818±0.006 |
| 9 | 4.7±2.1 | 44.7±4.3 | 42.5±4.1 | 0.707±0.009 | 1.5±1.1 | 39.1±4.2 | 69.8±7.4 | 0.810±0.007 |
| 12 | 2.9±1.2 | 31.5±3.7 | 26.8±3.4 | 0.696±0.009 | 1.3±1.0 | 25.4±3.1 | 32.6±3.3 | 0.749±0.029 |
Data are the mean ±SEM of three independent experiments.
Sucrose supplementation to cut flowers delays DNA degradation and Cyt c release
The effect of sucrose supplementation on the appearance and/or intensities of the DNA degradation, DNase activity, and extent of release of Cyt c was investigated in petals of cut flowers. Although DNA degradation in cut flowers without sucrose began on day 3 and became more evident in the subsequent stages, DNA remained almost intact even after day 9 in petals of sucrose-supplemented flowers (Fig. 7a, b). The appearance of bivalent cation-dependent DNase activity was also delayed by sucrose supplementation and detected minimally on day 6 (Fig. 7d). In contrast, the petals collected from flowers kept in sucrose-free water showed readily detectable DNase activity from day 3 (Fig. 7c). Figure 7f shows that the amount of Cyt c released into the cytosol did not increase significantly up to day 6 in petals from sucrose-supplemented flowers. The figure also shows that the amount of cytosolic Cyt c in 6-d-old petals of sucrose-treated cut flowers seemed to be only about a quarter of the amount of cytosolic Cyt c in petals of sucrose-untreated flowers (Fig. 7e).
Fig. 7.
Analysis of DNA fragmentation, DNase activity, and cytosolic Cyt c in petals of cut flowers. DNA fragmentation was analysed at each stage of petals when flowers were grown without sucrose (a) and with 10 mM sucrose in distilled water (b), and the extent of DNase activity was determined when grown without sucrose (c) and with 10 mM sucrose (d). Cytosolic Cyt c was probed with anti-Cyt c in petals of each stage when flowers were grown without sucrose (e) and with sucrose (f). (a–f) Representative results of repeated experiments (n=4) are shown.
Discussion
Morphological changes concur with biochemical features in PCD
Comparing the time-courses of different parameters over the senescence period provided a better understanding of natural senescence in tulip petals. This study reports that the biochemical features of PCD coincided with the morphological changes during the floral lifespan of tulip.
DNA fragmentation is a hallmark trait for PCD in many plant cells, including pollination-induced petal senescence in Petunia inflata (Xu and Hanson, 2000), natural petal senescence in Pisum sativum (Orzaez and Granell, 1997b) and Sandersonia aurantiaca (Eason and Bucknell, 2001), and harvest-induced senescence in Brassica oleracea (Coupe et al., 2004) and Asparagus officinalis (Eason et al., 2002). However, the time at which DNA fragmentation begins may vary depending on the type of plant and plant organ, and becomes apparent earlier in the induced senescence than in natural senescence (Coupe et al., 2004). In natural petal senescence of P. inflata (Xu and Hansan, 2000), DNA fragmentation was evident at day 6 after flower opening, and, prior to visible wilting, DNA degradation was reported in petals of Antirrhinum majus, Argyranthemum frutescens, and Petunia hybrida (Yamada et al., 2006). It was possible to detect the onset of DNA degradation at day 6 when petal movement capability in the intact plant was very low. These results indicate that DNA fragmentation or degradation may be a common feature associated with petal senescence. DNA fragmentation, in both animals and plants, is catalysed by a number of endonucleases, and even a single DNase is sufficient to degrade single- or double-stranded DNA (Krishnamurthy et al., 2000; Xu and Hanson, 2000). The bivalent cation-dependent DNase activity (Fig. 2b) is a feature in the floral senescence of many plants (Rubinstein, 2000; Xu and Hanson, 2000). However, the timing of DNase expression in natural petal senescence may be species specific (Langston et al., 2005). In this study, the DNase activity observed from day 3 may be related to the onset of DNA degradation that was detected at day 6.
In apoptosis, release of Cyt c from the mitochondria into the cytosol precedes any morphological changes (Yang et al., 1997). This study shows that the translocation of Cyt c into the cytosol started at day 0 of petal opening following the disappearance of chlorophyll (Figs 1a, b, 3a). The translocation of Cyt c into the cytosol was detected during stress-induced PCD of other plants (Balk et al., 1999; Stein and Hansen, 1999; Tiwari et al., 2002). In PET1 cytoplasmic male sterility of sunflower, there is Cyt c release into the cytosol followed by changes in cell morphology, loss of outer mitochondrial membrane integrity, and fall in the respiratory control ratio (Balk and Leaver, 2001).
Results in this study indicate that a low level of H2O2 may be generated in the early stages of petal senescence (Fig. 4a). The threshold H2O2 may be required for biosynthesis and development-related modifications of the structural components of cell walls (Ros Barcelo et al., 2004), as well as in cell signalling as a second messenger (McInnis et al., 2006). The transient increase of H2O2 levels in 3-d-old petals may be under the control of antioxidant enzymes or ROS scavengers to minimize cytotoxicity (Vacca et al., 2004) since the APX and CAT activities increased after the B3 stage and are maintained up to day 9 (Fig. 4b). The ROS/H2O2-dependent PCD may depend on the location, timing, and level of ROS/H2O2 production, and cannot be effective as long as the antioxidant enzymes are maintained in cells at sufficient levels (Vacca et al., 2004; De Pinto et al., 2006; McInnis et al., 2006). Because the activities of APX and CAT decreased after day 9 and the H2O2 level again increased, this relationship supports the notion that the ROS-dependent PCD may not be active in the early stages of tulip petal senescence.
Ethylene is a clear regulator of petal senescence in carnation, Petunia, tobacco, and orchids following contact between pollen and the stigmatal surface; however, it appears to play little or no part in senescence of other species including lilies such as Hemerocallis and Alstroemeria (Wagstaff et al., 2005; Rogers, 2006). The abscission-associated ethylene production by tulip petals reported here coincided with the production of ethylene in carnations, resulting in wilting of the flower (Nukui et al., 2004; van Doorn, 2004; Rogers, 2006). The cysteine-type protease activity following the wilting of tulip petals is consistent with cysteine proteinase expression during the onset of petal wilting in carnation flowers undergoing natural senescence (Sugawara et al., 2002). However, the timing of expression of this cysteine protease is much later than in Alstroemeria (Wagstaff et al., 2002) and Hemerocallis (Valpuesta et al., 1995).
Exogenous energy source supplementation delays petal senescence and the onset of PCD-associated biochemical markers
Supplementation of exogenous sugars to cut flowers generally delays visible senescence in petals of many flowers such as Sandersonia sp., Iris sp., and carnations (reviewed by van Doorn, 2004). However, the extent of delay in visible senescence by sugar feeding differs considerably depending on plant species. For example, exogenous sugar in cut flowers delayed it by ∼7, 2, and 0.5 d in carnation, Sandersonia sp., and Iris sp. flowers, respectively (controls have a lifespan of ∼7, 7, and 4 d, respectively). In addition to visible senescence, sugar supplementation delays the rise in ethylene production in carnation petals and expression of the protease gene associated with PCD in Sandersonia sp. petals (reviewed by van Doorn, 2004). In this study, sucrose supplementation to cut flowers restored and maintained the temperature-dependent movement ability of petals for as long as day 9 (Fig. 6), and delayed visible petal senescence by at least 2 d and 4 d compared with petal senescence in intact plants and cut flowers without sucrose, respectively. These results suggest that transport activity in stems remains almost intact. The levels of AEC were maintained for 9 d following sucrose feeding. The onset of DNA degradation, DNase activity, and the release of Cyt c from mitochondria to the cytosol in petals was delayed by incubating cut flowers with sucrose. The longer floral lifespan in sucrose-treated cut flowers might be due to the delay in appearance of these PCD events. Results in this study therefore show that energy source supplementation to cut flowers delays the appearance of important biochemical events reported in different types of plant PCD and apoptosis in animal cells (Xu and Hansen, 2000; Nakamura and Wada, 2000; Liu et al., 2003; Coimbra et al., 2004). Indeed, a threshold level of AEC of ∼0.8 may be necessary to maintain the capability of petals of intact plants to open and close (Figs 1c, 3b). Although cut flowers incubated without sucrose supplementation exhibited maximum petal movement capability on day 3, this capability was lower than observed in the flowers of intact plant (Figs 1c, 6). Morphologically evident petal wilting in sucrose-untreated cut flowers occurred earlier than in flowers of intact plants. Moreover, ATP and AEC levels in the petals of these flowers were lower than those in petals of intact plants (Fig. 3b and Table 1). These differences may be due to the presence of leaves in the intact plant. In addition, perhaps the parameters of PCD (DNase activity, DNA degradation, and Cyt c release) appeared earlier or became more extensive in sucrose-free cut flowers than in petals of intact plants because of the absence of leaves and lower levels of ATP and AEC. Little information is available, however, to address whether cell death in petals on intact plants would be triggered by energy source depletion. Petal senescence in uncut flowers has rarely been studied, and seldom has a comparison been made between senescent petals on cut flowers and senescent petals that remain attached to the plant. Our present study demonstrates a comparison of some study parameters (discussed above) between petal senescence in intact plants and in cut flowers. This comparison supports the hypothesis that energy depletion triggers PCD events through a similar pathway in the early stage of petal senescence in cut and uncut flowers.
Intracellular energy depletion is a primary early signal for PCD during tulip petal senescence
The results reported here seem to suggest that PCD in the very early stage of tulip petal senescence might be triggered by signals other than ethylene, H2O2, and proteinase activity. The loss of chlorophyll and RuBisCO (data not shown) during bud development indicates chloroplast transition to chromoplasts (Thomas et al., 2003). The release of Cyt c from mitochondria to the cytosol is an indicator of mitochondrial disintegration. The decrease in activity of Cyt c oxidase, an indicator for the activity of respiration, was observed in petals on day 3 (data not shown), which suggests the inactivation of photosynthetic as well as respiration activities after flower opening. ATP is necessary to prevent DNA fragmentation (Nakamura and Wada, 2000), and to maintain mitochondrial transmembrane potential (Liu et al., 2003) and cell volume regulation by an ATP-regulated ion channel (Okada et al., 2001). However, all these events are evident only in animal cells. Although the natural and induced senescence process in leaves has been characterized by a decline in photosynthetic capacity, chlorophyll destruction, visible leaf yellowing, and down-regulation of photosynthetic genes (Mishina et al., 2007), there have been few reports on energy or ATP levels correlating with plant PCD and/or senescence. Data collected in this study showed that maintaining a supply of energy using sucrose to cut tulip flowers could (i) generate ATP; (ii) maintain the level of AEC; (iii) delay the onset of DNA degradation and increased DNase activity; and (iv) lower the extent of mitochondrial Cyt c release, which will result in a longer floral lifespan. These data shown herein are consistent with the hypothesis that intracellular energy depletion or ATP deficiency plays the primary role as a signal to induce PCD in the early stage of tulip petal senescence.
Acknowledgments
AKA was supported by a Japan Society for the Promotion of Science (JSPS) fellowship (P05199). The work was supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture, Japan (Nos 17.05199 and 19580106-00).
Glossary
Abbreviations
- ACC
1-aminocyclopropane-1-carboxylate
- AEC
adenylate energy charge
- ANT
adenosine nucleotide
- APX
ascorbate peroxidase
- CAT
catalase
- Cyt c
cytochrome c
- dH2O
distilled water
- FW
fresh weight
- H2O2
hydrogen peroxide
- PCD
programmed cell death
- PMSF
phenylmethylsulphonyl fluoride
- ROS
reactive oxygen species
- TCA
trichloroacetic acid
References
- Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968;7:4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Azad AK, Sawa Y, Ishikawa T, Shibata H. Temperature-dependent stomatal movement in tulip petals controls water transpiration during flower opening and closing. Annals of Applied Biology. 2007;150:81–87. [Google Scholar]
- Azad AK, Sawa Y, Ishikawa T, Shibata H. Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals. Plant and Cell Physiology. 2004a;45:608–617. doi: 10.1093/pcp/pch069. [DOI] [PubMed] [Google Scholar]
- Azad AK, Sawa Y, Ishikawa T, Shibata H. Characterization of protein phosphatase 2A acting on phosphorylated plasma membrane aquaporin of tulip petals. Bioscience, Biotechnology and Biochemistry. 2004b;68:1170–1174. doi: 10.1271/bbb.68.1170. [DOI] [PubMed] [Google Scholar]
- Balk J, Leaver CJ. The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. The Plant Cell. 2001;13:1803–1818. doi: 10.1105/TPC.010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Leaver CJ, McCabe PF. Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Letters. 1999;463:151–154. doi: 10.1016/s0014-5793(99)01611-7. [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Simontachhi M, Guiamet J, Montaldi E, Puntarlo S. Antioxidant enzymes and lipid peroxidation during aging of Chrysanthemum morifolium RAM petals. Plant Science. 1995;104:161–168. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle for protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Tzeng WF. The roles of glutathione and antioxidant enzymes in menadion-induced oxidative stress. Toxicology. 2000;154:75–84. doi: 10.1016/s0300-483x(00)00321-8. [DOI] [PubMed] [Google Scholar]
- Coimbra S, Torrão L, Abreu I. Programmed cell death induces male sterility in Actinidia deliciosa female flowers. Plant Physiology and Biochemistry. 2004;42:537–541. doi: 10.1016/j.plaphy.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Comelli M, Pancrazio FD, Mavelli I. Apoptosis is induced by decline of mitochondrial ATP synthesis in erythroleukemia cells. Free Radical and Biological Medicine. 2003;34:1190–1199. doi: 10.1016/s0891-5849(03)00107-2. [DOI] [PubMed] [Google Scholar]
- Coupe SA, Watson LM, Ryan DJ, Pinkney TT, Eason JR. Molecular analysis of programmed cell death during senescence in Arabidopsis thaliana and Brassica oleracea: cloning broccoli LSD1, Bax inhibitor and serine palmitoyltransferase homologues. Journal of Experimental Botany. 2004;55:59–68. doi: 10.1093/jxb/erh018. [DOI] [PubMed] [Google Scholar]
- De Pinto MC, Paradisco A, Leonetti P, De Gara L. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. The Plant Journal. 2006;48:784–795. doi: 10.1111/j.1365-313X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- Eason JR, Bucknell TT. DNA processing during tepal senescence of Sandersonia aurantiaca. Acta Horticulturae (ISHS) 2001;543:143–146. [Google Scholar]
- Eason JR, Pinkney TT, Johnston JW. DNA fragmentation and nuclear degradation during harvest-induced senescence of asparagus spears. Postharvest Biology and Technology. 2002;26:231–235. [Google Scholar]
- Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Guan LM, Shao J, Scandalios JG. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. The Plant Journal. 2000;22:87–95. doi: 10.1046/j.1365-313x.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Gunawardena AH, Greenwood JS, Dengler NG. Programmed cell death remodels lace plant leaf shape during development. The Plant Cell. 2004;16:60–73. doi: 10.1105/tpc.016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- Hoeberichts FA, Woltering EJ. Multiple mediators of plant programmed cell death: interplay of conserved cell death mechanisms and plant-specific regulators. BioEssays. 2002;25:47–57. doi: 10.1002/bies.10175. [DOI] [PubMed] [Google Scholar]
- Kim M, Ahn JW, Jin UH, Choi D, Paek KH, Pai HS. Activation of the programmed cell death pathway by inhibition of proteasome function in plants. Journal of Biological Chemistry. 2003;278:19406–19415. doi: 10.1074/jbc.M210539200. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy KV, Krishnaraj R, Chozhavendan R, Christopher FS. The programme of cell death in plants and animals—a comparison. Current Science. 2000;79:1169–1181. [Google Scholar]
- Lam E, Kato N, Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- Langston BJ, Bai S, Jones ML. Increases in DNA fragmentation and induction of a senescence-specific nuclease are delayed during corolla senescence in ethylene-insensitive (etr1-1) transgenic petunias. Journal of Experimental Botany. 2005;56:15–23. doi: 10.1093/jxb/eri002. [DOI] [PubMed] [Google Scholar]
- Lawton KA, Raghothama KG, Goldsbrough PB, Woodson WR. Regulation of senescence-related gene expression in carnation flower petals by ethylene. Plant Physiology. 1990;93:1370–1375. doi: 10.1104/pp.93.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Song XD, Liu W, Zhang TY, Zuo J. Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line. Journal of Cell and Molecular Medicine. 2003;7:49–56. doi: 10.1111/j.1582-4934.2003.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudhan R, Ishikawa T, Sawa Y, Shigeoka S, Shibata H. Characterization of ascorbate peroxidase in plastids of tobacco BY-2 cells. Physiologia Plantarum. 2003;17:550–557. doi: 10.1034/j.1399-3054.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- McInnis SM, Desikan R, Hancock JT, Hiscock SJ. Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk? New Phytologist. 2006;172:221–228. doi: 10.1111/j.1469-8137.2006.01875.x. [DOI] [PubMed] [Google Scholar]
- Mishina TE, Lamb C, Zeier J. Expression of nitric oxide degrading enzyme induces a senescence programme in Arabidopsis. Plant, Cell and Environment. 2007;30:39–52. doi: 10.1111/j.1365-3040.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Wada Y. Properties of DNA fragmentation activity generated by ATP depletion. Cell Death and Differentiation. 2000;7:477–484. doi: 10.1038/sj.cdd.4400677. [DOI] [PubMed] [Google Scholar]
- Noodén LD, Guiamet JJ, John I. Senescence mechanisms. Physiologia Plantarum. 1997;101:746–753. [Google Scholar]
- Nukui H, Kudo S, Yamashita A, Satoh S. Repressed ethylene production in the gynoecium of long-lasting flowers of the carnation ‘White Candle’: role of the gynoecium in carnation flower senescence. Journal of Experimental Botany. 2004;55:641–650. doi: 10.1093/jxb/erh081. [DOI] [PubMed] [Google Scholar]
- Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) Journal of Physiology. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzaez D, Granell A. DNA fragmentation is regulated by ethylene during petal senescence in Pisum sativum. The Plant Journal. 1997a;11:137–144. [Google Scholar]
- Orzaez D, Granell A. The plant homologue of the defender against apoptotic death gene is down-regulated during senescence of flower petals. FEBS Letters. 1997b;404:275–278. doi: 10.1016/s0014-5793(97)00133-6. [DOI] [PubMed] [Google Scholar]
- Pennell RI, Lamb C. Programmed cell death in plants. The Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thomson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta. 1989;975:384–394. [Google Scholar]
- Robson CA, Vanlerberghe GC. Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and -independent pathways of programmed cell death. Plant Physiology. 2002;129:1908–1920. doi: 10.1104/pp.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HJ. Cell death and organ development in plants. Current Topics in Developmental Biology. 2005;71:225–261. doi: 10.1016/S0070-2153(05)71007-3. [DOI] [PubMed] [Google Scholar]
- Rogers HJ. Programmed cell death in floral organs: how and why do flowers die? Annals of Botany. 2006;97:309–315. doi: 10.1093/aob/mcj051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros Barcelo AR, Gomes-Ros LV, Gabaldon C, Lopez-Serrano M, Pomar F, Carrion JS, Pedreno MA. Basic peroxidase: the gateway for lignin evolution? Phytochemistry Review. 2004;3:61–78. [Google Scholar]
- Rubinstein B. Regulation of cell death in flower petals. Plant Molecular Biology. 2000;44:303–318. doi: 10.1023/a:1026540524990. [DOI] [PubMed] [Google Scholar]
- Serafini-Fracassini D, Duca SD, Monti F, Poli F, Sacchetti G, Bregoli AM, Bionti S, Mea MD. Transglutatminase activity during senescence and programmed cell death in the corolla of tobacco (Nicotina tabacum) flowers. Cell Death and Differentiation. 2002;9:309–321. doi: 10.1038/sj.cdd.4400954. [DOI] [PubMed] [Google Scholar]
- Shibata H, Sawa Y, Ochiai H, Kawashima T, Yamane K. A possible regulation of carbamoylphosphate synthetase and aspartate carbamoyltransferase in chloroplasts. Plant Science. 1987;51:129–133. [Google Scholar]
- Stein JC, Hansen G. Mannose induces an endonuclease responsible for DNA laddering in plant cells. Plant Physiology. 1999;121:71–79. doi: 10.1104/pp.121.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H, Shibuya K, Yoshioka T, Hashiba T, Satoh S. Is a cysteine proteinase involved in the regulation of petal wilting in senescing carnation (Dianthus caryophyllus) flowers? Journal of Experimental Botany. 2002;53:407–413. doi: 10.1093/jexbot/53.368.407. [DOI] [PubMed] [Google Scholar]
- Swidzinski JA, Leaver CJ, Sweetlove LJ. A proteomic analysis of plant programmed cell death. Phytochemistry. 2004;65:1829–1838. doi: 10.1016/j.phytochem.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Thomas H, Ougham HJ, Wagstaff C, Stead AD. Defining senescence and death. Journal of Experimental Botany. 2003;54:1127–1132. doi: 10.1093/jxb/erg133. [DOI] [PubMed] [Google Scholar]
- Tiwari BS, Belenghi B, Levine A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiology. 2002;128:1271–1280. doi: 10.1104/pp.010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome ME, Lutz NW, Briehl MM. Overexpression of catalase or Bcl-2 alters glucose and energy metabolism concomitant with dexamethasone resistance. Biochimica et Biophysica Acta. 2004;1693:57–72. doi: 10.1016/j.bbamcr.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Vacca RA, de Pinto MC, Valenti D, Passerella S, Marra E, De Gara L. Reactive oxygen species production, impairment of glucose oxidation and cytosolic ascorbate peroxidase are early events in heat-shock induced programmed cell death in tobacco BY-2 cells. Plant Physiology. 2004;134:1100–1112. doi: 10.1104/pp.103.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valpuesta V, Lange NE, Guerrero C, Reid MS. Up-regulation of a cysteine protease accompanies the ethylene-insensitive senescence of daylily (Hemerocallis) flowers. Plant Molecular Biology. 1995;28:575–582. doi: 10.1007/BF00020403. [DOI] [PubMed] [Google Scholar]
- Van Doorn WG. Is petal senescence due to sugar starvation? Plant Physiology. 2004;134:35–42. doi: 10.1104/pp.103.033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, van Meeteren U. Flower opening and closure: a review. Journal of Experimental Botany. 2003;54:1801–1812. doi: 10.1093/jxb/erg213. [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Chanasut U, Harren FJM, Laarhoven LJ, Thomas B, Rogers HJ, Stead AD. Ethylene and flower longevity in Alstroemeria: relationship between tepal senescence, abscission and ethylene biosynthesis. Journal of Experimental Botany. 2005;56:1007–1016. doi: 10.1093/jxb/eri094. [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Leverentz M, Griffiths G, Thomas B, Chanasut U, Stead A, Rogers HJ. Cysteine protease gene expression and proteolytic activity during senescence of Alstroemeria petals. Journal of Experimental Botany. 2002;53:233–240. doi: 10.1093/jexbot/53.367.233. [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Malcolm P, Rafiq A, Leverentz M, Griffiths G, Thomas B, Stead A, Rogers H. Programmed cell death (PCD) processes begin extremely early in Alstroemeria petal senescence. New Phytologist. 2003;160:49–59. doi: 10.1046/j.1469-8137.2003.00853.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hanson MR. Programmed cell death during pollination-induced petal senescence in petunia. Plant Physiology. 2000;122:1323–1333. doi: 10.1104/pp.122.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Ichmura K, van Doorn WG. DNA degradation and nuclear degeneration during programmed cell death in petals of Antirrhinum, Argyranthemum, and Petunia. Journal of Experimental Botany. 2006;57:3543–3552. doi: 10.1093/jxb/erl100. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yang MS, Yu LC, Gupta RC. Analysis and changes in energy and redox states in HepG2 hepatoma and C6 glioma cells upon exposure to cadmium. Toxicology. 2004;201:105–113. doi: 10.1016/j.tox.2004.04.007. [DOI] [PubMed] [Google Scholar]