Abstract
Phytochelatins, heavy-metal-binding polypeptides, are synthesized by phytochelatin synthase (PCS) (EC 2.3.2.15). Previous studies on plants overexpressing PCS genes yielded contrasting phenotypes, ranging from enhanced cadmium tolerance and accumulation to cadmium hypersensitivity. This paper compares the effects of overexpression of AtPCS1 and CePCS in tobacco (Nicotiana tabacum var. Xanthi), and demonstrates how the introduction of single homologous genes affects to a different extent cellular metabolic pathways leading to the opposite of the desired effect. In contrast to WT and CePCS transformants, plants overexpressing AtPCS1 were Cd-hypersensitive although there was no substantial difference in cadmium accumulation between studied lines. Plants exposed to cadmium (5 and 25 μM CdCl2) differed, however, in the concentration of non-protein thiols (NPT). In addition, PCS activity in AtPCS1 transformants was around 5-fold higher than in CePCS and WT plants. AtPCS1 expressing plants displayed a dramatic accumulation of γ-glutamylcysteine and concomitant strong depletion of glutathione. By contrast, in CePCS transformants, a smaller reduction of the level of glutathione was noticed, and a less pronounced change in γ-glutamylcysteine concentration. There was only a moderate and temporary increase in phytochelatin levels due to AtPCS1 and CePCS expression. Marked changes in NPT composition due to AtPCS1 expression led to moderately decreased Cd-detoxification capacity reflected by lower SH:Cd ratios, and to higher oxidative stress (assessed by DAB staining), which possibly explains the increase in Cd-sensitivity. The results indicate that contrasting responses to cadmium of plants overexpressing PCS genes might result from species-dependent differences in the activity of phytochelatin synthase produced by the transgenes.
Keywords: Cadmium, γ-glutamylcysteine, glutathione, phytochelatins, tobacco
Introduction
Phytochelatins (PCs) are small, heavy metal-binding, cysteine-rich polypeptides with the general structure of ((γ-Glu-Cys)nGly (n=2–11)), present in plants, fungi, and other organisms (Grill et al., 1985; Gekeler et al., 1988; Piechalak et al., 2002). They are synthesized from glutathione (GSH) in the presence of heavy metals by the enzyme phytochelatin synthase (PCS) (Grill et al., 1989; Tomaszewska et al., 1996; Vatamaniuk et al., 2000) and form complexes with some of those ions, subsequently transported from the cytosol into the vacuole (Salt and Rauser, 1995).
PCs are necessary for cadmium tolerance in plants and all the mutants reported to lack the ability to synthesize those peptides are Cd hypersensitive (Howden et al., 1995). Thus, it was reasonable to expect that overexpression of PCS in plants, with the aim of overproducing these metal-chelating peptides, may result in higher Cd tolerance and accumulation. It was considered that such transgenic plants could be used in the phytoremediation of cadmium-contaminated soils. The results on overexpression of PCS reported in the last few years, have been contradictory, however. For example, as expected, overexpression of the AtPCS1 gene in Escherichia coli (Sauge-Merle et al., 2003) and Saccharomyces cerevisiae (Vatamaniuk et al., 1999), enhanced cadmium tolerance and accumulation. Surprisingly, its overexpression in Arabidopsis thaliana led to Cd-hypersensitivity despite the enhanced phytochelatin production (Lee et al., 2003a, b; Li et al., 2004). By contrast, expression of the wheat phytochelatin synthase gene TaPCS1 in tobacco (Nicotiana glauca) resulted in increased Cd and Pb tolerance and accumulation (Gisbert et al., 2003; Martinez et al., 2006). However, as recently reported by Pomponi et al.(2006), Nicotiana tabacum expressing AtPCS1 displayed enhanced cadmium tolerance and accumulation, but only when plants were cultivated in culture medium supplied with GSH. Interestingly, expression of the same gene in Brassica juncea led to higher Cd and Zn tolerance, but significantly lower accumulation of those elements in both root and shoot tissues (Gasic and Korban, 2007). Despite the vast literature on the role of phytochelatins in heavy metal detoxification, it is still impossible to explain such differences among the reported phenotypes. Researchers used different PCS genes and plant species for transformation, which could have contributed to the observed disparities. Therefore, overexpression of PCS genes from different species in one model organism could yield important insights into their functions and answer some of the questions raised by the researchers.
This work addresses the mechanisms underlying the variation in response to cadmium reported for PCS overexpressing plants. It attempts to determine if the plant phenotype resulting from PCS overexpression could depend on the gene used for transformation. Two phytochelatin synthase genes: AtPCS1 from A. thaliana (Ha et al., 1999) and CePCS from Caenorhabditis elegans (Clemens et al., 2001; Vatamaniuk et al., 2001) were introduced into one model species, tobacco (N. tabacum var. Xanthi). This is the first study comparing the effects of the overexpression of two different PCS genes in the same organism.
Materials and methods
Plant expression constructs, transformation and selection
The AtPCS1 and CePCS gene constructs used for transformation derived from constructs used for functional expression in Schizosaccharomyces pombe (Cazalé and Clemens, 2001; Clemens et al., 2001, respectively). The expression cassettes were subcloned into pRT100 between the CaMV 35S promoter and the CaMV polyadenylation signal. The resulting cassettes were transferred as HindIII fragments into the binary plasmid vector pCB302 (Xiang et al., 1999). The constructs were introduced into Agrobacterium GV3101 strain. Tobacco (N. tabacum var. Xanthi) plants (seeds from stock of the Institute of Biochemistry and Biophysics PAS, Warszawa, Poland) were transformed by the standard leaf disc method (Horsh et al., 1985). F1 seeds were collected from generation F0 with confirmed gene presence (PCR) and expression (RT-PCR). The primer sequences used for the PCR reaction were: AtPCS1 forward 5′-GGTGGCTGAGATCCGAATTA-3′; reverse 5′-TGAGCTGCTTCATTTCATGC-3′; CePCS forward 5′-GCAAAATGTAATCGTCTAAAATCTACAG-3′; reverse 5′-TTCTAATGGATCACATAATAAGAATTGA-3′.
Expression analysis (RT-PCR)
Total RNA was extracted from around 100 mg of frozen tissue using an RNeasy Plant Mini-Kit (Qiagen). Following DNase digestion (Invitrogen), 1 μg of RNA from each sample was used for 20 μl RT-reaction (as recommended by the manufacturer, Fermentas). The RT-PCR product for the Tac9 actin gene was used as a control to confirm that equal amounts of RNA were used in each reaction. PCR was carried out with 2 μl of the RT reaction product using the specific primers for AtPCS1 and CePCS as described above and for Tac9 as: Tac9 forward 5′-CCTCCCACATGCTATTCTCC-3′; reverse 5′-AGAGCCTCCAATCCAGACAC-3′.
Experimental plant material and hydroponic conditions
Tobacco seeds were sterilized and germinated on agar plates, positioned vertically, on quarter-strength Knop's medium, 1% (w/v) agar, and 2% (w/v) sucrose. For the selection of transgenic plants and to determine the segregation ratio of Basta-resistant:Basta-sensitive seedlings, the herbicide Basta was added to the medium at a concentration of 10 μg ml−1. Seedlings were grown for 3 weeks in a climate chamber; temperature 24 °C, photoperiod 16/8 h day/night; quantum flux density (PAR) 250 μmol m−2 s−1 fluorescent Flora tubes, and subsequently transferred to 2.0 l pots containing quarter-strength Knop's liquid medium. In addition, to check the efficiency of the selection, after 2 d of growth in hydroponics the seedlings were sprayed with Basta (100 μg ml−1) for three consecutive days, and grown under control conditions for the next 8–10 d. The presence of the transgene was then checked by PCR.
All experiments were performed on Basta-resistant plants grown in hydroponics on quarter-strength Knop's medium under the conditions described above. The aerated nutrient solution was changed weekly. Initially, experiments were conducted on seven and 10 independent lines representing AtPCS1 and CePCS tobacco transformants, respectively. For further detailed studies the following lines (with 3:1 segregation ratio of Basta-resistant:Basta-sensitive T1 seedlings) were selected: four lines expressing AtPCS1 (PaII3, PaII4, PaII8, PaII12), five lines expressing CePCS (Pc30, PcII1, PcII3, PcII4, PcII5), and wild-type Xanthi.
Cadmium treatments
For each cadmium treatment, 5-week-old plants were placed in pots containing nutrient solution as described above, with five replicate pots per treatment. After 1 week of growth the nutrient solution was replaced by a fresh one of the same composition and cadmium chloride was added to achieve a final concentration of 5–35 μM. Untreated plants were grown in parallel under the same conditions. At the end of the incubation period (2–6 d) shoots and roots were used for further analysis.
Cadmium tolerance determination: hydroponic experiments
Appearance assessment and growth assay: After 6 d of growth at 0, 25 μM CdCl2 or 35 μM CdCl2, the condition of the plants was assessed (leaf colour, presence of necrosis). They were photographed, then shoots and roots were separated and dried for 4 d at 60 °C in an oven and their dry matter was determined. Based on dry weight, the tolerance index was calculated separately for shoots and roots of cadmium exposed plants as the percentage of dry weight of untreated (control) plants of the same line.
Pigment analysis: One of the first effects of cadmium toxicity on plants is chlorosis. Thus, the determination of photosynthetic pigment concentrations could be used to assess cadmium tolerance. After 2 d of growth at 0 or 25 μM CdCl2, the fourth leaf (counting from the top) was collected, frozen, and stored at –80 °C. Chlorophyll a and b and carotenoid contents were measured spectrophotometrically in acetone extracts according to Lichtenthaler and Wellburn (1983).
Cadmium tolerance determination: growth of plants on contaminated soil
To assess the tolerance of plants to the presence of Cd, Zn, and Pb in industrially contaminated soil, 4-week-old hydroponically grown tobacco seedlings were planted in pots containing 2.5 kg of contaminated industrial soil (Cd 18.6±1.2 mg kg−1 DW, Zn 2374±142 mg kg−1 DW, Pb 363±142 mg kg−1 DW, pH ∼7.0) and uncontaminated control soil (commercially obtained). They were grown for 6 weeks in a greenhouse, 24–27/18–20 °C day/night, photoperiod 16/8 h day/night, 65–75% humidity. Plant appearance was assessed and height and number of leaves were measured each week. Total dry weight of above-ground plant parts, expressed as a percentage of dry weight of plants grown on control soil, served as an indicator of plant tolerance to the industrial soil. This experiment was planned to demonstrate, in addition to tolerance level, the suitability of transformation with PCS genes for phytoremediation.
Determination of cadmium accumulation: plants grown in hydroponic culture
After 2–6 d of 5 or 25 μM CdCl2 treatment roots were washed briefly in distilled water, then for 15 min in ice-cold 5 mM CaCl2, then again twice in water. Roots and shoots were dried for 4 d at 60 °C in an oven and their dry matter was determined. Dried plant material was used for the determination of cadmium concentration by AAS.
Determination of cadmium accumulation: plants grown on contaminated soil
At the end of the growth period on contaminated soil (details described above) upper leaves, lower leaves, and a stem were collected, dried in 60 °C for 4 d and weighed. Cadmium contents were determined by AAS.
Determination of cadmium concentration
Dried plant samples were digested in 65% HNO3 and 39% H2O2 (9:1, v:v) in a closed system microwave mineralizer (Milestone Ethos). Cadmium was measured using a flame atomic absorption spectrophotometer (TJA Solution Solar M). Certified reference material (Virginia tobacco leaves CTA-VTL-2) was included in the analysis.
Non-protein thiol analysis
Several modifications of the method for non-protein thiol (NPT) analysis by Sneller et al. (2000) were tested. The method described below resulted from many trials and was used for NPT analysis of all tobacco samples.
At the end of the cadmium treatment, the fourth leaf (counting from the top) and roots were harvested for NPT analysis and processed immediately. NPT were extracted by homogenizing 200–300 mg of fresh material in a mortar, using a pestle and quartz sand in 1.78 ml of 6.3 mM ice-cold diethylenetriaminepenta-acetic acid (DTPA), 100 μl of 1 N NaOH, and 100 μl of 6 M NaBH4 (in 0.1 N NaOH). N-acetyl-L-cysteine was added during homogenization as an internal standard to a final concentration of 10 μM. The homogenates were centrifuged for 5 min at 10 000 g. 250 μl of plant extract were mixed with 10 μl of 20 mM monobromobimane and 450 μl of 200 mM HEPPS buffer, pH 8.2, containing 6.3 mM DTPA. Derivatization was carried out for 30 min in a water bath at 45 °C, in the dark. The reaction was stopped by the addition of 300 μl of 1 M methanesulphonic acid. The sample was filtered through a Costar Spin-X centrifuge tube with a nylon filter (0.22 μm). The samples were stored in the dark at 4 °C prior to the HPLC analysis. NPT were separated on a Nova-Pak C18 analytical column (60 Å, 4 μm, 3.9×300 mm, Waters) at 37 °C and were eluted with a slightly concave gradient of methanol and water, both with 0.1% (v/v) TFA, using fluorescence detection (Waters 464). The injection volume was 10 μl and the total analysis time was 70 min. The analytical data were integrated using Waters Millenium Software. Phytochelatin concentrations were corrected for derivatization efficiency according to Sneller et al. (2000). Calibration was based on GSH concentrations ranging from 5–20 μM.
Phytochelatin synthase activity assay
Phytochelatin synthase activity was determined in crude extracts according to a modified protocol by Finkemeier et al. (2003). Plant tissue (200 mg) was extracted in 2 ml of buffer containing 20 mM HEPES-NaOH, pH 7.5, 10 mM β-mercaptoethanol, 100 μM CdSO4, 20% (w/v) glycerol, and 100 mg ml−1 polyvinylpyrrolidone by homogenization in a mortar, using some quartz sand. Following centrifugation at 13 000 g for 10 min at 4 °C, the assay contained 400 μl extract and 100 μl reaction buffer (25 mM glutathione, 100 μM CdSO4, 10% (w/v) glycerol, and 250 mM HEPES-NaOH, pH 8.0) and protease inhibitor mix ‘Complete’ as recommended by the manufacturer (Sigma). The incubation was carried out at 35 °C for 90 min and terminated by addition of 125 μl 20% (w/v) trichloroacetic acid. SH-groups were derivatized using monobromobimane, and HPLC analysis was performed as described above.
H2O2 histochemical staining as an indication of oxidative stress level
Hydrogen peroxide accumulation was visualized with 3,3′-diaminobenzidine (DAB) according to Thordal-Christensen et al. (1997). Five-week-old tobacco plants grown in hydroponic culture were used for the experiment. Briefly, fourth leaves (counting from the top) excised from cadmium treated plants (2 d at 25 μM CdCl2) or from untreated plants were placed in Petri dishes containing DAB solution (1 mg ml−1). Plates were left in a climate chamber at 24 °C in darkness and DAB staining was assessed visually 8 h later.
Determination of ascorbate and dehydroascorbate content
Ascorbate and dehydroascorbate contents in fourth leaves and roots of 6-week-old cadmium treated (2 d and 3 d at 25 μM CdCl2) and untreated plants were determined according to the protocol for tobacco by Kampfenkel et al. (1994).
Determination of GSH/GSSG level
GSH and GSSG levels were determined according to the modified methods of Zhang and Kirkham (1996) and Somparn et al. (2007). The fourth leaves of 6-week-old tobacco plants were homogenized in 5 ml of ice-cold 5% meta-phosphoric acid. The homogenate was centrifuged for 20 min at 10 000 g at 4 °C. The supernatant was collected and neutralized with 5 M KOH. One ml of the neutralized supernatant was mixed with 100 μl of water and used for the enzymatic assay of total glutathione. In addition, another 1 ml of the neutralized extract was mixed with 100 μl of 33 mM 1-methyl-2-vinylpyridine (M2VP), a GSH scavenger, incubated for 2 min at room temperature and used for the assay of GSSG. For the enzymatic assay of glutathione, 330 μl of 0.3 mM DTNB in 150 mM NaPO4 (pH 7.2) containing 15 mM EDTA and 0.04% BSA were mixed with 50 μl of the sample and 290 μl of 150 mM NaPO4 (pH 7.2). The reaction was started by adding 330 μl of 150 mM NaPO4 (pH 7.2) containing 1 mM EDTA, 0.02% BSA, 0.6 mM NADPH, and glutathione reductase (1.2 U ml−1) and the absorbance was recorded at 412 nm for 3 min. Glutathione levels were determined against a standard curve. GSH content was calculated as the difference between total glutathione and GSSG.
Statistical analysis
Statistical significance was evaluated at the 0.05 probability levels using Student's t test. All experiments were repeated at least three times.
Results
Selection of transgenic lines overexpressing AtPCS1 and CePCS
After transformation and regeneration, the presence of the transgene was confirmed by PCR for 14 and 19 lines of tobacco transformed with AtPCS1 or CePCS construct, respectively. RT-PCR analysis of PCR-positive plants confirmed the expression of introduced genes in eight AtPCS1 and 11 CePCS harbouring lines (data not shown). The seeds of RT-PCR positive plants were collected and used for further tests.
All seedlings able to develop on agar-plates containing Basta and subsequently transferred to the hydroponic culture, also survived spraying three times with this agent, which indicated 100% efficiency of the selection. PCR examination showed that all Basta-resistant plants contained the transgene (data not shown). Transgenic lines (of both F0 and F1 generations) and the wild type grown under control conditions did not differ from each other in their development, appearance, and reproduction (data not shown).
Overexpression of AtPCS1, but not CePCS results in increased sensitivity to cadmium
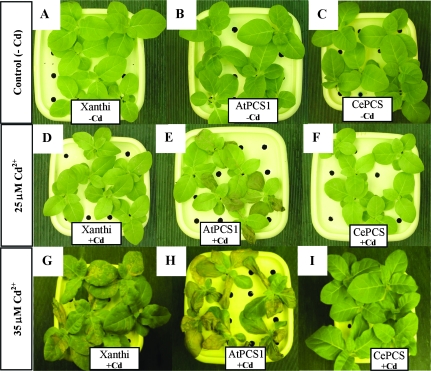
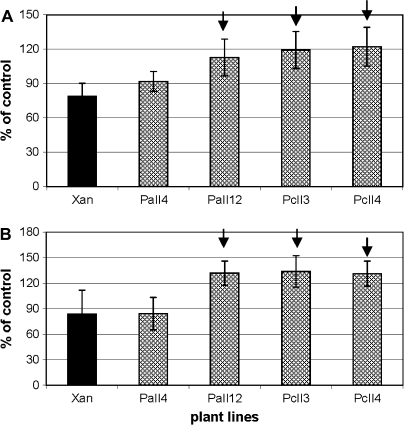
In the first experiment, 3-d exposure to 25 μM CdCl2 in hydroponic cultures resulted in a substantial difference in the plant appearance (Fig. 1). In contrast to the green, healthy-looking leaves of CePCS transformants and wild-type Xanthi plants (Fig. 1D, F), all tobacco lines expressing AtPCS1 had leaves with necrotic spots and wilted areas (Fig. 1E). The difference in the level of tolerance to cadmium between Xanthi and CePCS did not become evident until exposure to a higher concentration of this element, 35 μM Cd2+ (Fig. 1G, I). These results demonstrated that expression of AtPCS1, but not CePCS, led to enhanced cadmium sensitivity. It is important to note that both types of transformants did not show any phenotypic differences from WT when grown under control conditions without Cd2+ (Fig. 1A–C). To test whether the necrotic spots on leaves developing in AtPCS1-expressing plants were preceded by a loss of photosynthetic pigments, the concentrations of chlorophyll and carotenoids were determined. After 2 d of exposure to 25 μM CdCl2, however, both AtPCS1 and CePCS-expressing plants had slightly higher chlorophyll (Fig. 2A) and carotenoid (Fig. 2B) contents compared with WT.
Fig. 1.
Appearance of 6-week-old AtPCS1 (line PaII4; E, H) and CePCS (line PcII4; F, I) transformed, and wild type var. Xanthi (D, G) plants cultivated for 3 d in hydroponic culture containing 25 μM (D–F) or 35 μM (G–I) CdCl2. Untreated control (–Cd) plants: wild type Xanthi (A); AtPCS plants (B); CePCS plants (C).
Fig. 2.
Total chlorophyll (A) and carotenoid (B) content in the fourth leaf of 6-week-old tobacco plants after 2 d of 25 μM CdCl2 treatment shown as a percentage of untreated (control) plants of the same line. Values correspond to means ±SD (n=4); those significantly different from wild type var. Xanthi are indicated with the arrow (P <0.05). Concentration of chlorophyll (μg g–1 FW) in untreated (control) plants: Xanthi (609±147); PaII4 (572±57); PaII12 (586±77); PcII3 (590±61); PcII4 (791±77). Concentration of carotenoids (μg g–1 FW) in untreated (control) plants: Xanthi (94±23); PaII4 (89±9); PaII12 (80±10); PcII3 (88±6); PcII4 (113±11).
When tested for the response to cadmium in industrially contaminated soil (accompanied by elevated Pb and Zn levels), AtPCS1 transformants produced a slightly lower (decreased by ∼20%) shoot biomass relative to CePCS plants and the wild type (data not shown). The slower growth of AtPCS1-expressing plants might have partially resulted from their increased sensitivity to cadmium.
Overexpression of PCS did not lead to higher cadmium accumulation
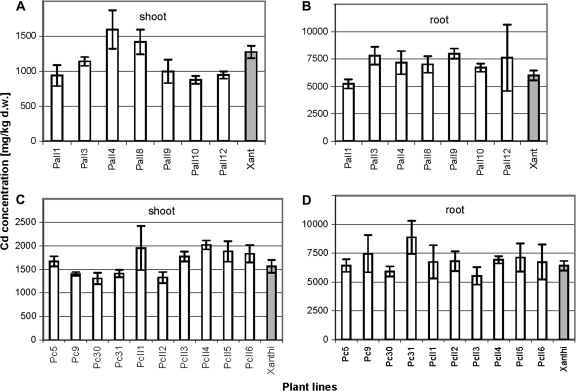
The determination of cadmium content in AtPCS1 and CePCS plants after 6 d of exposure to 25 μM CdCl2 in liquid medium demonstrated that the overexpression of both genes in tobacco did not cause dramatic differences in metal accumulation (Fig. 3A–D). The moderate decrease in the cadmium concentration in shoots (by 15–25%) was detected in some AtPCS1 transformed lines (Fig. 3A). Similar to the hydroponic experiments, no significant difference between the lines studied was found in cadmium concentrations in above-ground parts of plants grown for 6 weeks on cadmium-contaminated industrial soil (data not shown). Therefore, transformation with AtPCS1 and CePCS does not seem to improve the suitability of plants for phytoremediation of cadmium-contaminated soil. For that purpose, an increase in metal accumulation by at least 100–200% is required.
Fig. 3.
Cadmium accumulation in the shoot and the root of 6-week-old tobacco plants transformed with AtPCS1 (A, B) and CePCS (C, D) and wild type (var. Xanthi) cultivated for 6 d in hydroponic culture containing 25 μM CdCl2. Error bars represent SD at P ≤0.05) (n=3).
Expression analysis and selection of transgenic lines used for further experiments
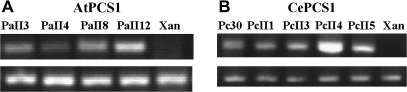
Semi-quantitative expression analysis was performed for selected T1 lines. The results shown in Fig. 4 demonstrate different levels of PCS expression. In order to correlate the level of phytochelatin synthase expression with the detected phenotype, two lines of both AtPCS1 (PaII4, PaII12) and CePCS (PcII3, PcII4) expressing plants, one with a high, the other with a low expression level, were chosen for further analysis.
Fig. 4.
The expression level of AtPCS1 (A) and CePCS (B) in independent plant lines tested by RT-PCR. Lower lines show the RT-PCR product for actin used as a control for equal amounts of RNA used in each reaction.
Overexpression of AtPCS1 and CePCS resulted in only a moderate increase of phytochelatin levels
To investigate the effects of PCS overexpression on PC accumulation, plants were exposed to 5 μM CdCl2 and to a more toxic concentration (25 μM), and harvested after 2 d and 3 d of exposure. The total PC levels are shown in Fig. 5, and the concentrations of individual PC species (PC2, PC3, and PC4) synthesized at 25 μM cadmium are given in Fig. 6. In general, the contents of phytochelatins were much higher in leaves than in roots and increased with Cd concentration and the duration of metal treatments. PCs were not detected in the absence of cadmium from the growth medium (data not shown).
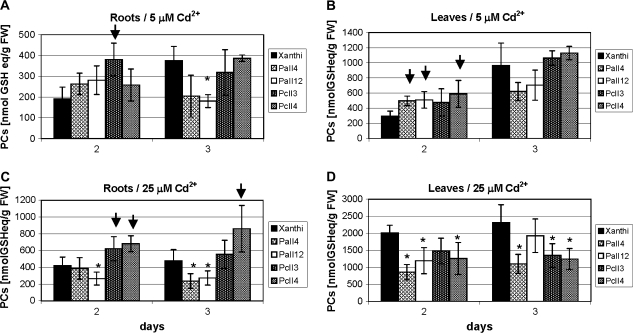
Fig. 5.
Total PC levels in roots (A, C) and leaves (B, D) of 6-week-old tobacco plants (wild type var. Xanthi, AtPCS1-expressing lines, PaII4, PaII12; CePCS-expressing lines, PcII3, PcII4) after 2 d or 3 d of 5 μM (A, B) or 25 μM (C, D) CdCl2 exposure. PCs were not detected in plants grown on medium without cadmium. Values correspond to means ±SD (n=5); those significantly higher from wild type var. Xanthi are indicated with the arrow, significantly lower are marked with the asterisk (P <0.05).
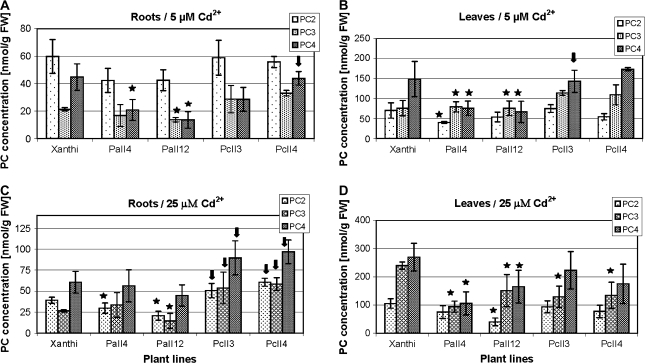
Fig. 6.
Concentration of major PC species in roots (A, C) and leaves (B, D) of 6-week-old tobacco plants (wild type var. Xanthi; AtPCS1-expressing lines, PaII4 and PaII12; CePCS-expressing lines, PcII3 and PcII4) after 3 d of 5 μM (A, B) and 25 μM (C, D) CdCl2 treatment. Values correspond to means ±SD (n=5); those significantly higher from wild type var. Xanthi are indicated with the arrow, significantly lower are marked with the asterisk (P <0.05).
There was a moderate difference in the total PC concentration between the WT, AtPCS1 and CePCS transformants. As shown in Fig. 5A and B, after 2 d of 5 μM CdCl2 exposure, an increase by ∼30–40% in PC production in leaves, and to a lesser extent in roots of both types of transformants was observed. However, on the third day of Cd treatment, the PC level in AtPCS1 expressing plants, in contrast to CePCS transformants, began to decrease both in roots and leaves (Fig. 5A, B). This tendency was more pronounced in roots of plants grown at 25 μM cadmium (Fig. 5C). However, in leaves the level of total PC was lower in all transgenic lines relative to WT (Fig. 5C, D).
Figure 6 shows levels of the major PC species synthesized in response to a 3 d exposure to cadmium. In general, at 5 μM Cd2+ the prevalent PC species in roots was PC2, and in leaves PC4 (Fig. 6A, B), whereas at 25 μM Cd2+ it was PC4 both in roots and leaves (Fig. 6C, D). The difference between both types of transformants and the WT is in the composition of synthesized PCs. Thus, at 5 μM Cd2+ the concentration of PC3 and PC4 in roots and leaves of AtPCS1 plants was lower compared to Xanthi, whereas in CePCS plants the concentration has not changed or has slightly increased (Fig. 6A, B). A similar tendency was detected at more toxic 25 μM Cd2+ in roots (Fig. 6C). In turn, in leaves the concentrations of almost all forms of PCs were reduced in AtPCS1 transformants (Fig. 6C), while in CePCS ones the reduction of only PC3 was observed (Fig. 6D).
The analysis of the content of cysteine, the substrate for GSH used subsequently for PC synthesis, showed that this amino acid was not the limiting factor for phytochelatin production. The cysteine level was similar in all plant lines tested and even slightly higher under cadmium exposure than in control conditions (data not shown).
Overexpression of AtPCS1 results in dramatic γ-glutamylcysteine accumulation and glutathione depletion in leaves
Overexpression of PCS did not influence GSH and γ-glutamylcysteine (γ-EC) concentrations in plants grown under control conditions without cadmium. There was no significant difference in their levels in either leaves or roots between the wild type and transgenic plants. The content of GSH was generally higher in leaves (200–320 nmol g−1 FW) compared with roots (140–180 nmol g−1 FW). In turn, in all plant lines studied γ-EC level in leaves was very low, ranging from 5–10 nmol g−1 FW, whereas in roots it was close to the detection limit, which stays in accordance with the data reported in the literature (Arisi et al., 1997; Creissen et al., 1999).
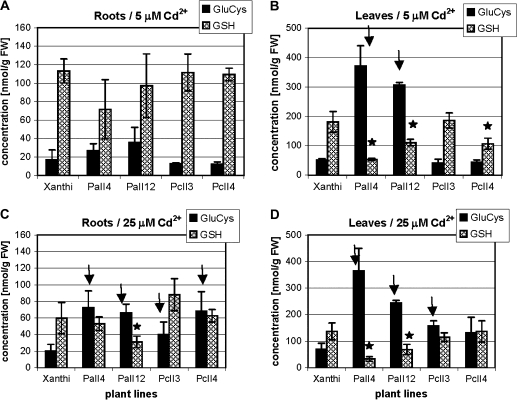
The concentration of γ-EC and GSH after 3 d of exposure to 5 μM and 25 μM cadmium is shown in Fig. 7. No significant difference was detected between the root GSH levels of two types of transformants and WT at the less toxic 5 μM cadmium (Fig. 7A); however, at 25 μM cadmium the level of γ-EC in roots of AtPCS1 and CePCS expressing plants increased markedly (Fig. 7C). In leaves, the overexpression of AtPCS1, in contrast to CePCS, resulted in a substantial decrease in glutathione content and in a dramatic increase in γ-EC after 3 d of exposure to both 5 μM and 25 μM cadmium (Fig. 7B, D). CePCS-expressing plants, in contrast to AtPCS1 ones, showed leaf GSH levels similar to WT on both cadmium treatments (Fig. 7B, D) whereas the γ-EC concentration was increased relative to WT only at 25 μM CdCl2 (Fig. 7D).
Fig. 7.
γ-Glutamylcysteine and glutathione levels in roots and leaves of 6-week-old tobacco plants (wild type var. Xanthi; AtPCS1-expressing lines, PaII4 and PaII12; CePCS-expressing lines, PcII3 and PcII4) after 3 d of 5 μM (A, B) or 25 μM CdCl2 (C, D) cadmium treatment. Values correspond to means ±SD (n=5); those significantly higher from wild type var. Xanthi are indicated with the arrow, significantly lower are marked with the asterisk (P <0.05).
Overexpression of AtPCS1 leads to a moderate decrease in cadmium detoxification capacity
In order to correlate the concentration of cadmium and NPTs in AtPCS1, CePCS and WT plants after heavy metal exposure of different duration, the cadmium content was determined in the fourth leaf and roots of plants also used for NPT analyses following 3 d of exposure to 5 μM CdCl2. There were no substantial differences between the lines (data not shown), thus the observed Cd hypersensitive phenotype of the AtPCS1 plants did not result from higher Cd accumulation in leaves.
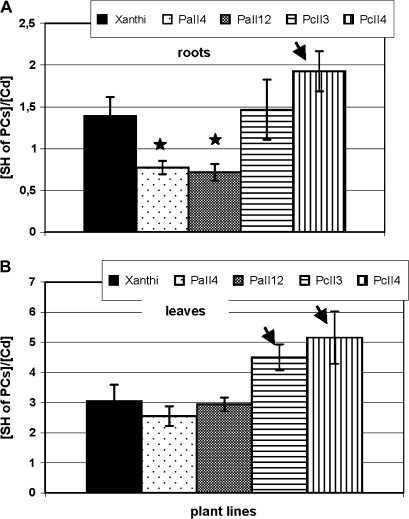
The stoichiometric relationship between the molar concentrations of sulphhydryl groups in PCs and the Cd concentration after 3 d at 5 μM CdCl2 is shown in Fig. 8. The PC-SH:Cd ratio was lower in AtPCS1 plants relative to CePCS and WT plants only in roots. In contrast, the PC-SH:Cd ratio in leaves of CePCS transformants was higher than in AtPCS1 ones and Xanthi. The lower ratio of PC sulphhydryl groups to cadmium indicated a lower Cd detoxification capacity of those plants and may be partially responsible for their reported hypersensitive phenotype. CePCS overexpressing plants seem to have an increased cadmium detoxification capacity compared with the AtPCS1 transformants and WT.
Fig. 8.
Stoichometry of SH of PCs to cadmium (nmol SH/nmol Cd) in roots (A), and leaves (B) of 6-week-old tobacco plants (wild type var. Xanthi; AtPCS1-expressing lines, PaII4, PaII12; CePCS-expressing lines, PcII3, PcII4) after 3 d at 5 μM CdCl2 Values correspond to means ±SD (n=5); those significantly higher from wild type var. Xanthi are indicated with the arrow, significantly lower are marked with the asterisk (P <0.05).
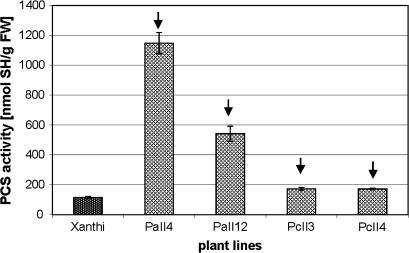
PCS activity is considerably higher in AtPCS1-expressing plants compared with WT and CePCS plants
To verify if the observed changes in thiol composition due to AtPCS1 and CePCS overexpression resulted from the difference in phytochelatin synthase activity, a PCS assay was performed using leaf protein extracts. It demonstrated that PCS activity in AtPCS1 plants from both studied lines was dramatically higher than in WT, whereas CePCS1 expression resulted in a much smaller, although significant, increase in PCS activity (Fig. 9).
Fig. 9.
Activity of phytochelatin synthase in extracts from tobacco plants (wild type var. Xanthi; AtPCS1-expressing lines, PaII4 and PaII12; CePCS-expressing lines, PcII3 and PcII4). Values correspond to means ±SD (n=3); those significantly higher from wild type var. Xanthi are indicated with the arrow (P <0.05).
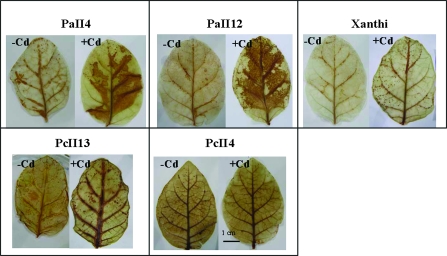
Overexpression of AtPCS1 leads to increased oxidative stress in the presence of cadmium
It is known that GSH plays a major role in the control of the cell redox state. Therefore, it was assumed that the Cd-hypersensitivity of AtPCS1 plants reported in this paper, manifested by necrosis on leaves, may result from the decrease in GSH levels (found in AtPCS1 plants; Fig. 7B, D), which probably contributes to the generation of oxidative stress. To investigate the level of oxidative stress in leaves in the presence of cadmium, hydrogen peroxide formed in leaf tissues was visualized using histochemical staining with DAB. After 2 d of 25 μM CdCl2 exposure, the staining intensity was higher in leaves of AtPCS1 transformants than in those of CePCS or WT plants (Fig. 10). Thus, the oxidative stress generated in the presence of cadmium was higher in Cd-hypersensitive AtPCS1 plants.
Fig. 10.
Hydrogen peroxide accumulation in leaves of 5-week-old hydroponically grown tobacco plants wild type var. Xanthi and transformed with AtPCS1 (lines PaII4 and PaII12) and CePCS (lines PcII3 and PcII4). Fourth leaves (counting from the top) excised from 5-week old plants after 2 d of 25 μM CdCl2 exposure (+Cd) or growth in the absence of cadmium (–Cd) were placed in Petri dishes containing DAB solution (1 mg ml−1). Plates were left in a climate chamber at 24 °C in darkness and DAB staining was assessed visually 8 h later.
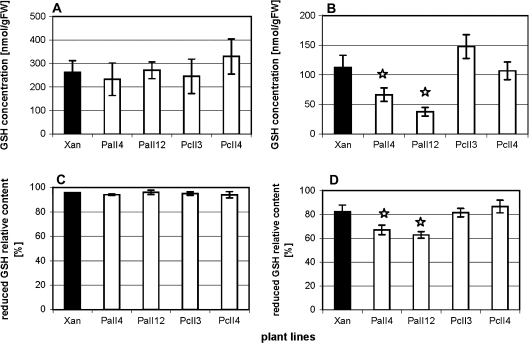
In the presence of cadmium, AtPCS1 overexpression affects GSH/GSSG level and ascorbate contents
It is well known that the ascorbate–glutathione cycle is crucial for the removal of H2O2 from the cell metabolism (Noctor and Foyer, 1998; Kanwischer et al., 2005; Semane et al., 2007), therefore both components of the cellular antioxidative system were examined in control (–Cd) plants and after exposure to 25 μM CdCl2.
The results of glutathione quantification are shown in Fig. 11. The increased sensitivity to cadmium of AtPCS1 expressing tobacco was accompanied not only by higher H2O2 level (Fig. 10) and reduced concentration of glutathione (Fig. 7) but also by a change in the GSH redox status. A decreased relative content of reduced GSH in leaves of AtPCS1 plants relative to Xanthi and CePCS ones (Fig. 11B) confirms an elevated level of oxidative stress generated by cadmium in these plants (as detected by DAB staining; Fig. 10). Total GSH concentration and the reduced GSH relative content were not statistically different between lines not exposed to cadmium (Fig. 11A, C).
Fig. 11.
Total GSH concentration (A, B) and relative content of its reduced form (C, D) in leaves of 6-week-old tobacco plants: wild type var. Xanthi and transformed with AtPCS1 (lines PaII4 and PaII12) and CePCS (lines PcII3 and PcII4). Plants were grown under control conditions without cadmium (A, C) or exposed for 3 d to 25 μM CdCl2 (B, D). The redox status of glutathione is indicated by the ratio of (total glutathione–2×GSSG) to total glutathione. Values correspond to means ±SD (n=4); those significantly lower from wild type var. Xanthi are indicated with the asterisk (P <0.05).
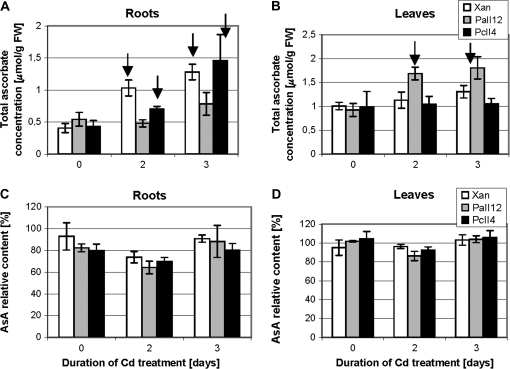
As demonstrated in Fig. 11A and B, no difference was found in total root and leaf ascorbate concentration, nor in the relative content of its reduced form (AsA) (Fig. 12C, D) between both types of transformants and WT plants when grown under control conditions (without cadmium). However, 2 d and 3 d treatment with 25 μM CdCl2 resulted in a significant increase in root ascorbate pool of WT and CePCS1 transformants, whereas in AtPCS1 its concentration remained unchanged and similar to that of untreated plants (Fig. 12A). On the other hand, AtPCS1 expressing plants showed a significant increase in leaf total ascorbate level after a 2 d cadmium treatment, whereas in CePCS1 and WT leaf AsA content did not change relative to plants grown on medium without the metal (Fig. 12B). An increased pool of ascorbate in leaves of AtPCS1 transformants could be related to higher levels of oxidative stress. Interestingly, cadmium treatment did not affect the relative content of the reduced ascorbate form in roots and leaves of all tested plant lines (Fig. 12C, D) which indicates that the cellular system maintaining the balance between the reduced and oxidized form of this compound has not been affected.
Fig. 12.
Total ascorbate concentration (A, B) and relative content of its reduced form AsA (C, D) in roots (A, C) and leaves (B, D) of 6-week-old tobacco plants: wild type var. Xanthi and transformed with AtPCS1 (lines PaII4 and PaII12) and CePCS (lines PcII3 and PcII4). Plants were grown under control conditions (–Cd) or exposed for 2 d and 3 d to 25 μM CdCl2. Values correspond to means ±SD (n=4). Significant differences between control (not exposed to cadmium) and Cd-treated plants of the same line are indicated with the arrow (P < 0.05).
Discussion
Phytochelatins play a major role in constitutive cadmium (Clemens et al., 1999) and arsenate (Bleeker et al., 2006) tolerance. Following the isolation of the first genes encoding phytochelatin synthase from different organisms by three independent groups (Clemens et al., 1999, 2001; Ha et al., 1999; Vatamaniuk et al., 1999, 2001), many researchers tried to overexpress PCS to enhance plant heavy metal tolerance and accumulation. The results on overexpression of different PCS genes in various plant species published in the last few years have, however, been contradictory thus far, with plant responses reported to range from increased cadmium tolerance and accumulation (Lee et al., 2003a; Gisbert et al., 2003; Martinez et al., 2006; Pomponi et al., 2006), through increased cadmium tolerance accompanied by its decreased accumulation (Gasic and Korban, 2007), to cadmium hypersensitivity without increased accumulation (Lee et al., 2003a, b; Li et al., 2004).
To date only papers describing overexpression of AtPCS1 (in A. thaliana, N. tabacum, and B. juncea), TaPCS1 (in N. glauca and cad1-3 Arabidopsis mutant), and SpPCS1 (in N. tabacum) are available, and each focused on one donor PCS gene and one target species. Cd-hypersensitivity was reported so far only as a result of AtPCS1 overexpression (Lee et al., 2003a,b; Li et al., 2004) but not in all the target species (Pomponi et al., 2006; Gasic and Korban, 2007) or lines tested (Lee et al., 2003a, b). A plausible explanation for the observed disparities in response to cadmium has not yet been found. It has been suggested that different PCS expression levels and genetic differences between target plant species (in particular with regard to the regulation of the glutathione synthetic pathway or the downstream processing of PC–Cd complexes) may contribute to the diverse phenotypes of PCS transformants. In this study, the experimental system was simplified by introducing two different phytochelatin synthase genes AtPCS1 and CePCS into one model organism—tobacco. This approach allowed the interspecific variability to be eliminated and to focus only on the possible functional differences between PCS enzymes as a reason for the distinct phenotypes observed.
This work demonstrated that the effects of overexpression of AtPCS1 and CePCS in the same species were different. Overexpression of AtPCS1 led to increased cadmium sensitivity (Fig. 1), similar to the majority of literature reports (Lee et al., 2003a, b; Li et al., 2004). On the other hand, tobacco plants transformed with CePCS were more tolerant to cadmium relative to WT (which became evident only after the exposure to 35 μM Cd2+). In addition, both types of transformants did not show substantially increased cadmium accumulation (Fig. 3), which is consistent with a number of papers describing AtPCS1 overexpression (Lee et al., 2003a, b; Li et al., 2004; Gasic and Korban, 2007).
This study showed that increased sensitivity to Cd of AtPCS1-expressing tobacco plants (relative to WT) was accompanied by dramatic γ-EC accumulation and glutathione depletion (Fig. 7B, D). Similar results, though not as pronounced, were reported by Li et al. (2004) for AtPCS1-overexpressing, Cd-hypersensitive A. thaliana plants. On the other hand, CePCS-overexpressing plants had GSH levels similar to WT, but increased γ-EC concentrations (Fig. 7D). The transformants differed also with respect to PC accumulation. There was only a moderate and temporary increase in their level due to AtPCS1 expression after only 2 d of exposure to low 5 μM cadmium followed by a decrease later on, whereas in CePCS plants the PC level was generally higher (Fig. 5A–C). As demonstrated by other researchers, overexpression of PCS in tobacco did not result in an increase of PC levels, unless GSH was added to the medium (Pomponi et al., 2006) or additional genes from the GSH biosynthetic pathways were introduced (Wawrzyński et al., 2006). The moderate increase in phytochelatin levels in AtPCS1 transformants reported in this study suggested that Cd-hypersensitivity of AtPCS1-overexpressing plants did not result from the accumulation of phytochelatins at the supra-optimal levels as initially proposed by Lee et al. (2003b). However, in the recent communication of Kim and Lee (2007) it was shown that Cd-hypersensitivity of A. thaliana overexpressing AtPCS1 did not result from the accumulation of NPT at supra-optimal levels. Instead, an unknown disruption in cellular metal homeostasis under Cd-stress due to the properties of the enzyme itself (e.g. binding metal ions by the enzyme) was proposed as the mechanism for the observed increase in Cd sensitivity of transgenic plants.
In this study, PCS genes of different origin were introduced into the same species, and therefore the difference in Cd-sensitivity and NPT concentrations in the lines studied might result from PCS protein specificity. Indeed, it was found that AtPCS1-expressing plants had ∼5-fold higher PCS enzymatic activity than CePCS-transformed and WT plants (Fig. 9). Knowing this, it is tempting to hypothesize that a higher rate of PCS activity in AtPCS1-expressing plants and a concomitantly higher rate of PC formation would result in faster depletion of GSH in those plants and a concomitant increase of γ-glutamylcysteine synthetase activity due to alleviation of the feedback inhibition of this enzyme by GSH. The high level of newly-synthesized γ-EC may not have been used for the glutathione and subsequent PC synthesis because, in the presence of Cd ions, the activity of glutathione synthetase, the second enzyme in the PC synthesis pathway, seems to be limiting (Rauser et al., 1991). As well as changes in cellular thiol concentrations, disturbances in the downstream processing of PC–Cd complexes due to PCS overexpression might appear. If the formation rate of PC–Cd complexes in AtPCS1 expressing plants, initially higher than in CePCS ones and WT plants, would exceed the rate of their ATP-dependent transport into the vacuole, PC–Cd would accumulate in the cytosol of those plants and probably be degraded (most likely to γ-EC, but at present the products of PC degradation are unknown). In addition, it cannot be excluded that the enzyme itself may take part in PC cleavage under certain conditions. It has been reported recently that AtPCS1 can catalyse the cleavage of GSH-bimane conjugates to γ-EC-bimane, particularly under cadmium exposure (Grzam et al., 2006; Blum et al., 2007). Moreover, as shown by Tsuji et al. (2005), this enzyme may cleave not only GSH but also PCs to supply the γ-EC unit for elongation of the PC chain. As a result of possible degradation, one can assume that both glutathione and PC concentrations would be decreased and the γ-glutamylcysteine level elevated. The decrease in PC levels in roots and leaves of AtPCS1 plants relative to WT after longer treatments with lower Cd concentration (5 μM), or in roots after higher 25 μM, could be taken to suggest degradation of PCs depending on the intracellular conditions. As a result, despite the higher PCS activity, the total PC level might not significantly increase in the AtPCS1 transformants. It seems possible that due to PC–Cd degradation in the cytosol, the amount of free Cd ions could be elevated, leading to more pronounced toxic effects, and in particular that Cd-detoxification capacity decreased, as reflected by the PC-SH:Cd ratio, (Fig. 8).
However, elevated γ-glutamylcysteine formation in the PCS activity assay, that could indicate a peptidase activity of PCS, was not observed. It seems possible that either peptidase or PC formation activity of the enzyme might be regulated in intact cells by factors not present in the extract. This could be dependent, for example, on the efficiency of the transport of PC–Cd complexes into the vacuole. Presently however, it can be only speculated about the disturbances in the PC pathway and on the regulation of PC transport and the products of their degradation. The vacuolar transporters of PC–Cd, probably members of MRP family (Rea, 1999), still remain unknown. Interestingly, the decrease in cadmium tolerance was not observed when AtPCS1 was targeted to A. thaliana chloroplasts (Picault et al., 2006), which is consistent with the above hypothesis about the role of vacuolar transport in the generation of the Cd hypersensitivity phenomenon due to AtPCS1 overexpression.
Cadmium induces oxidative stress, although it is not a Fenton reaction type metal (Jonak et al., 2004; Semane et al., 2007). Glutathione plays a major role in the control of the cell redox state (Noctor et al., 1998) and the inability to maintain sufficient GSH levels under cadmium exposure may lead to ROS production. Enhanced concentration of γ-EC, as shown by Creissen et al. (1999) might be an additional factor in oxidative stress generation. Thus Cd-hypersensitivity of AtPCS1-expressing tobacco could result, among others, from higher oxidative stress due to leaf GSH depletion together with γ-EC accumulation (Fig. 7D). As expected, H2O2 production in the presence of the metal was higher in leaves of AtPCS1 transformants relative to CePCS and WT (Fig. 10). In parallel, the decrease in GSH level (Fig. 7), a lower relative content of reduced GSH (Fig. 11) and an increase in leaf ascorbate level (Fig. 12B) were detected. The importance of AsA accumulation could probably be explained by its involvement in non-enzymatic antioxidative activity in AtPCS1 plants with a higher level of H2O2 (Fig. 10) (Noctor and Foyer, 1998; Noctor, 2006; Semane et al., 2007). It is known, that the total ascorbate pool is not stable and changes, among others, depending on the developmental stage as well as in response to the level of oxidative stress (Noctor, 2006). As shown by Franceschi and Tarlyn (2002), AsA is synthesized in photosynthetic organs and subsequently transported to non-photosynthetic tissues like roots, although the mechanism of the regulation of the transport has not been elucidated yet. Therefore, it cannot be excluded that its regulation is part of a plant's antioxidative defence system. An observed contrasting pattern of AsA accumulation in leaves and roots of AtPCS1 plants, compared to CePCS and Xanthi ones, could be the manifestation of the compensation mechanism under the diversified level of oxidative stress in tested plant lines. A similar mechanism was previously demonstrated by Kanwischer et al. (2005) for GSH-deficient cad2 mutants. Furthermore, the maintenance of the AsA relative content in all studied plants exposed to cadmium at the level of control plants grown without the metal in the medium (Fig. 12C, D), indicates that the efficiency of the AsA/DHA antioxidative system has not been affected in cadmium-treated plants. To summarize, cadmium hypersensitivity of AtPCS1 plants relative to CePCS ones could be linked to the generation of a higher level of oxidative stress. However, to elucidate the underlying mechanism it will be necessary to examine in detail the ascorbate–glutathione antioxidative network. Moreover, to supplement the short-term experiments, long-term ones will need to be conducted for further understanding of these processes. Recently, it has been clearly demonstrated, that acclimation to cadmium stress is also crucial for good performance in contaminated environments of hyperaccumulators like T. caerulescens (Küpper et al., 2007).
The monitoring of cysteine, the substrate for GSH biosynthesis, revealed that its concentration was similar in all plant lines tested and not decreased by cadmium treatments (data not shown). This result is in accordance with the literature suggesting that the level of cysteine is not a major factor deciding about the rate of glutathione biosynthesis under cadmium exposure (Noctor et al., 1998). According to Noctor et al. (1998), the other substrates from the GSH/PC biosynthesis pathway, glutamic acid and glycine, are not rate-limiting; therefore, their level was not checked in this study.
Lee et al. (2003a) characterizing the effects of AtPCS1 overexpression in A. thaliana reported that the decrease of Cd-tolerance was only noticed in lines with the highest AtPCS1 expression level. In the present study, two lines of AtPCS1 and CePCS transformants chosen for detailed investigation (PaII4, PaII12, and PcII3; PcII4, respectively) differed in the expression level (Fig. 4). However, no correlation was detected between the expression level (mRNA) and the monitored phenotype.
In conclusion, by expressing AtPCS1 and CePCS in the same species (thus eliminating the interspecific differences), the first evidence has been obtained that the diverse effects of overexpression may result from the functional differences between the enzymes from diverse organisms. The interrelationship between the PCS enzymatic activity/the rate of PCs biosynthesis, and their transport to vacuole/degradation, is probably different in AtPCS1 and CePCS plants and possibly one of the factors contributing to the observed distinct sensitivity to cadmium. The disturbances of thiol homeostasis, due to increased PCS activity over a certain threshold, and probably the lack of the synchronization between PC–Cd formation and their transport to the vacuole, may increase the oxidative stress level and decrease the cadmium detoxification capacity (as in the case of AtPCS1-expressing plants) leading to Cd-hypersensitivity. This study demonstrates how much the overexpression of a single gene can interfere in related metabolic processes occurring in the cell, leading to results that are opposite from those expected. Thus not all PCS genes would be suitable for the transformation of all plant species for the phytoremediation purposes.
Acknowledgments
The paper has been financially supported by the FP5 EU grant METALLOPHYTES ‘An integrated approach towards removal by plants of toxic metals from polluted soils’, QLRT-2000-00479; by STSM-COST-859-81, and by the State Committee for Scientific Research, KBN, through the Faculty of Biology, Warsaw University intramural grant, BW No. 1755-65.
Glossary
Abbreviations
- PC
phytochelatin
- PCS
phytochelatin synthase
- GSH
glutathione
- γ-EC
γ-glutamylcysteine
- NPT
non-protein thiols
- DAB
3,3′-diaminobenzidine
- WT
wild type
References
- Arisi A-CM, Noctor G, Foyer CH, Jouanin L. Modification of thiol contents in poplars (Populus tremula×P. alba) overexpressing enzymes involved in glutathione synthesis. Planta. 1997;203:362–372. doi: 10.1007/s004250050202. [DOI] [PubMed] [Google Scholar]
- Bleeker PM, Hakvoort HW, Bliek M, Souer E, Schat H. Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. The Plant Journal. 2006;45:917–929. doi: 10.1111/j.1365-313X.2005.02651.x. [DOI] [PubMed] [Google Scholar]
- Blum R, Beck A, Korte A, Stengel A, Letzel T, Lendzian K, Grill E. Function of phytochelatin synthase in catabolism of glutathione-conjugates. The Plant Journal. 2007;49:740–749. doi: 10.1111/j.1365-313X.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- Cazalé AC, Clemens S. Arabidopsis thaliana expresses a second functional phytochelatin synthase. FEBS Letters. 2001;507:215–219. doi: 10.1016/s0014-5793(01)02976-3. [DOI] [PubMed] [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO Journal. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Schroeder JI, Degenkolb T. Caenorhabditis elegans expresses a functional phytochelatin synthase. European Journal of Biochemistry. 2001;268:3640–3643. doi: 10.1046/j.1432-1327.2001.02293.x. [DOI] [PubMed] [Google Scholar]
- Creissen G, Firmin J, Fryer M, et al. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. The Plant Cell. 1999;11:1277–1292. doi: 10.1105/tpc.11.7.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkemeier I, Kluge C, Metwally A, Georgi M, Grotjohann N, Dietz KJ. Alterations in Cd-induced gene expression under nitrogen deficiency in Hordeum vulgare. Plant, Cell and Environment. 2003;26:821–833. doi: 10.1046/j.1365-3040.2003.01014.x. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Tarlyn NM. L-ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiology. 2002;130:649–656. doi: 10.1104/pp.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic K, Korban SS. Expression of Arabidopsis phytochelatin synthase inIndian mustard (Brassica juncea) plants enhances tolerance for Cd and Zn. Planta. 2007;225:1277–1285. doi: 10.1007/s00425-006-0421-y. [DOI] [PubMed] [Google Scholar]
- Gekeler W, Grill E, Winnacker EL, Zenk MH. Algae sequester heavy metals via synthesis of phytochelatin complexes. Archives of Microbiology. 1988;150:197–202. [Google Scholar]
- Gisbert C, Ros R, De Haro A, Walker DJ, Pilar BM, Serrano R, Navarro-Aviñó J. A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochemical and Biophysical Research Communications. 2003;303:440–445. doi: 10.1016/s0006-291x(03)00349-8. [DOI] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985;230:674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Grill E, Löffler S, Winnacker EL, Zenk MH. Phytochelatins, the heavy metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase) Proceedings of the National Academy of Sciences, USA. 1989;86:6838–6842. doi: 10.1073/pnas.86.18.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzam A, Tennstedt P, Clemens S, Hell R, Meyer AJ. Vacuolar sequestration of glutathione-S-conjugates outcompetes a possible degradation of the glutathione moiety by phytochelatin synthase. FEBS Letters. 2006;580:6384–6390. doi: 10.1016/j.febslet.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connel MJ, Goldsbrough PB, Cobbett CS. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. The Plant Cell. 1999;11:153–1164. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsh RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SC, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Andersen CR, Cobbett CS. Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiology. 1995;107:1059–1066. doi: 10.1104/pp.107.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C, Nakagami H, Hirt H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiology. 2004;136:3276–3283. doi: 10.1104/pp.104.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfenkel K, Van Montagu M, Inzé D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Analytical Biochemistry. 1994;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- Kanwischer M, Porfirova S, Bergmüller E, Dörmann P. Alterations in tocopherol cyclase axctivity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiology. 2005;137:712–723. doi: 10.1104/pp.104.054908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee S. Overexpression of Arabidopsis phytochelatin synthase (AtPCS1) does not change the maximum capacity for non-protein thiol production induced by cadmium. Journal of Plant Biology. 2007;502:220–223. [Google Scholar]
- Küpper H, Parameswaran A, Leitenmaier B, Trtilek M, Šetlik I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytologist. 2007;175:655–674. doi: 10.1111/j.1469-8137.2007.02139.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Petros D, Moon JS, Ko TS, Goldsbrough PB, Korban SS. Higher levels of ectopic expression of Arabidopsis phytochelatin synthase do not lead to increased cadmium tolerance and accumulation. Plant Physiology and Biochemistry. 2003a;41:903–910. [Google Scholar]
- Lee S, Moon JS, Ko TS, Petros D, Goldsbrough PB, Korban SS. Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiology. 2003b;131:656–663. doi: 10.1104/pp.014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dhankher OP, Carreira L, Lee D, Chen A, Schroeder JI, Balish RS, Meagher RB. Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant and Cell Physiology. 2004;45:1787–1797. doi: 10.1093/pcp/pch202. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions. 1983;603:591–592. [Google Scholar]
- Martínez M, Bernal P, Almela C, Vélez D, García-Augustín P, Serrano R, Navarro-Aviñó J. An engineered plant that accumulates higher levels of heavy metals than Thlaspi caerulescens, with yields of 100 times more biomass in mine soils. Chemosphere. 2006;64:478–485. doi: 10.1016/j.chemosphere.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Noctor G. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant, Cell and Environment. 2006;29:409–425. doi: 10.1111/j.1365-3040.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. Journal of Experimental Botany. 1998;49:623–647. [Google Scholar]
- Picault N, Cazalé AC, Beyly A, Cuiné S, Carrier P, Luu DT, Forestier C, Peltier G. Chloroplast targeting of phytochelatin synthase in Arabidopsis: effects on heavy metal tolerance and accumulation. Biochimie. 2006;88:1743–1750. doi: 10.1016/j.biochi.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Piechalak A, Tomaszewska B, Baralkiewicz D, Malecka A. Accumulation and detoxification of lead ion in legumes. Phytochemistry. 2002;60:153–162. doi: 10.1016/s0031-9422(02)00067-5. [DOI] [PubMed] [Google Scholar]
- Pomponi M, Censi V, Di Girolamo V, De Paolis A, di Toppi LS, Aromolo R, Costantino P, Cardarelli M. Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta. 2006;223:180–190. doi: 10.1007/s00425-005-0073-3. [DOI] [PubMed] [Google Scholar]
- Rauser WE, Schupp R, Rennenberg H. Cysteine, γ-glutamylcysteine and glutathione levels in maize seedlings. Plant Physiology. 1991;97:128–138. doi: 10.1104/pp.97.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea PA. MRP subfamily ABC transporters from plants and yeast. Journal of Experimental Botany. 1999;50:895–913. [Google Scholar]
- Salt DE, Rauser WE. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiology. 1995;107:1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauge-Merle S, Cuiné S, Carrier P, Lecomte-Pradines C, Luu DT, Peltier G. Enhanced toxic metal accumulation in engineered bacterial cells expressing Arabidopsis thaliana phytochelatin synthase. Applied and Environmental Microbiology. 2003;69:490–494. doi: 10.1128/AEM.69.1.490-494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semane B, Cuypers A, Smeets K, Van Belleghem F, Horemans N, Schat H, Vangronsveld J. Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiologia Plantarum. 2007;129:519–528. [Google Scholar]
- Sneller F, van Heerwaarden LM, Koevoets PL, Vooijs R, Schat H, Verkleij JA. Derivatization of phytochelatins from Silene vulgaris, induced upon exposure to arsenate and cadmium: comparison of derivatization with Ellman;s reagent and monobromobimane. Journal of Agriculture and Food Chemistry. 2000;48:4014–4019. doi: 10.1021/jf9903105. [DOI] [PubMed] [Google Scholar]
- Somparn N, Kukongviriyapan U, Tassaneeyakul W, Jetsrisuparb A, Kukongviriyapan V. Modification of CYP2E1 and CYP3A4 activities in haemoglobin E-beta thalassemia patients. European Journal of Clinical Pharmacology. 2007;63:43–50. doi: 10.1007/s00228-006-0224-x. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei YD, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew reaction. The Plant Journal. 1997;11:1187–1194. [Google Scholar]
- Tomaszewska B, Tukendorf A, Baralkiewicz D. The synthesis of phytochelatins in lupin roots treated with lead ions. The Science of Legumes. 1996;3:206–217. [Google Scholar]
- Tsuji N, Nishikori S, Iwabe O, Matsumoto S, Shiraki K, Miyasaka H, Takagi M, Miyamoto K, Hirata K. Comparative analysis of the two-step reaction catalyzed by prokaryotic and eukaryotic phytochelatin synthase by an ion pair liquid chromatography assay. Planta. 2005;222:181–191. doi: 10.1007/s00425-005-1513-9. [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OP, Mari S, Lu YP, Rea PA. AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proceedings of the National Academy of Sciences, USA. 1999;96:7110–7115. doi: 10.1073/pnas.96.12.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk OP, Bucher EA, Ward JT, Rea PA. A new pathway for heavy metal detoxification in animals. Phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. Journal of Biological Chemistry. 2001;276:20817–20820. doi: 10.1074/jbc.C100152200. [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OP, Mari S, Lu YP, Rea PA. Mechanism of heavy metal ion activation of phytochelatin (PC) synthase. Journal of Biological Chemistry. 2000;275:31451–31459. doi: 10.1074/jbc.M002997200. [DOI] [PubMed] [Google Scholar]
- Wawrzyński A, Kopera E, Wawrzyńska A, Kamińska J, Bal W, Sirko A. Effects of simultaneous expression of heterologous genes involved in phytochelatin biosynthesis on thiol content and cadmium accumulation in tobacco plants. Journal of Experimental Botany. 2006;57:2173–2182. doi: 10.1093/jxb/erj176. [DOI] [PubMed] [Google Scholar]
- Xiang C, Han P, Lutziger I, Wang K, Oliver DJ. A mini binary vector series for plant transformation. Plant Molecular Biology. 1999;40:711–717. doi: 10.1023/a:1006201910593. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kirkham MB. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytologist. 1996;132:361–373. doi: 10.1111/j.1469-8137.1996.tb01856.x. [DOI] [PubMed] [Google Scholar]