Abstract
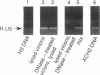
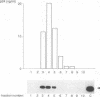
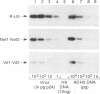
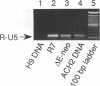
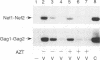
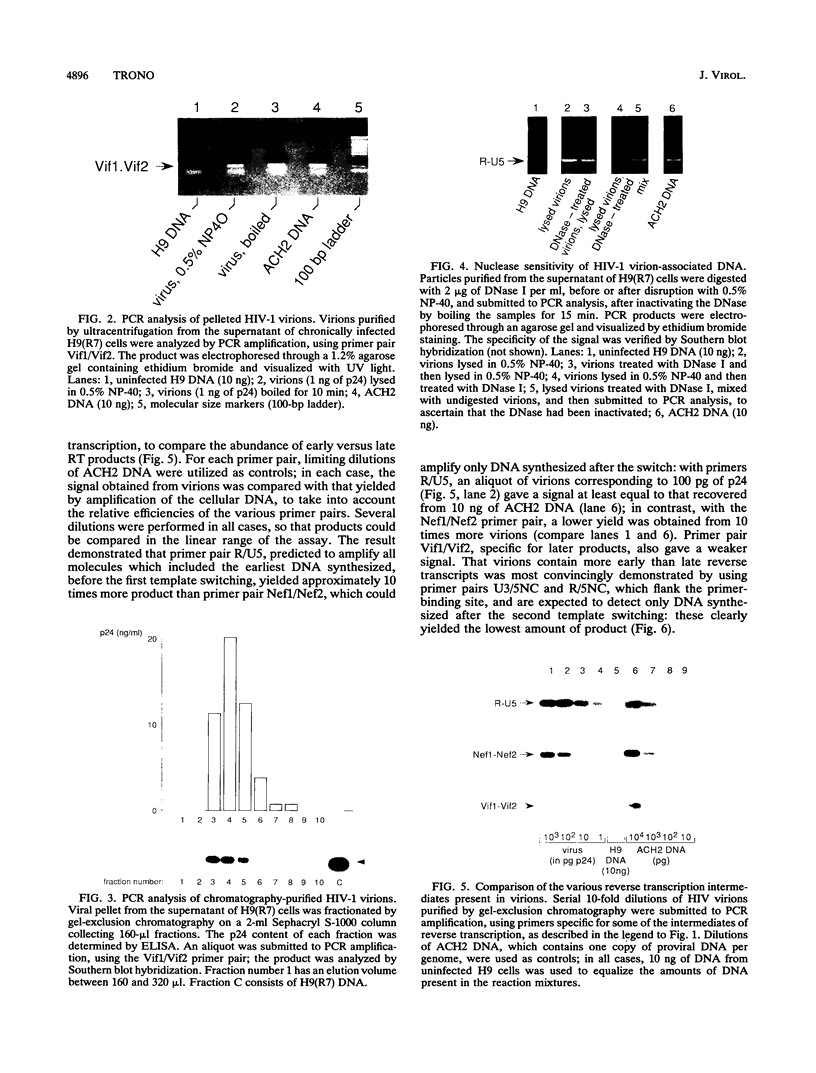
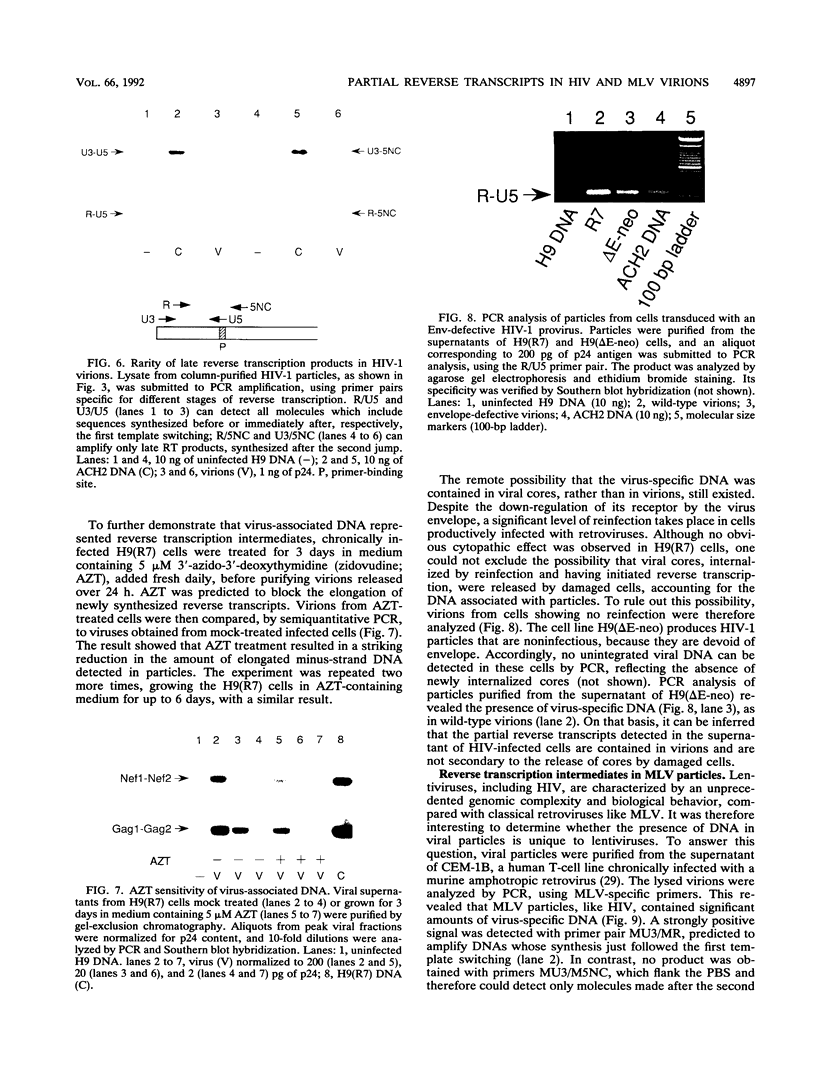
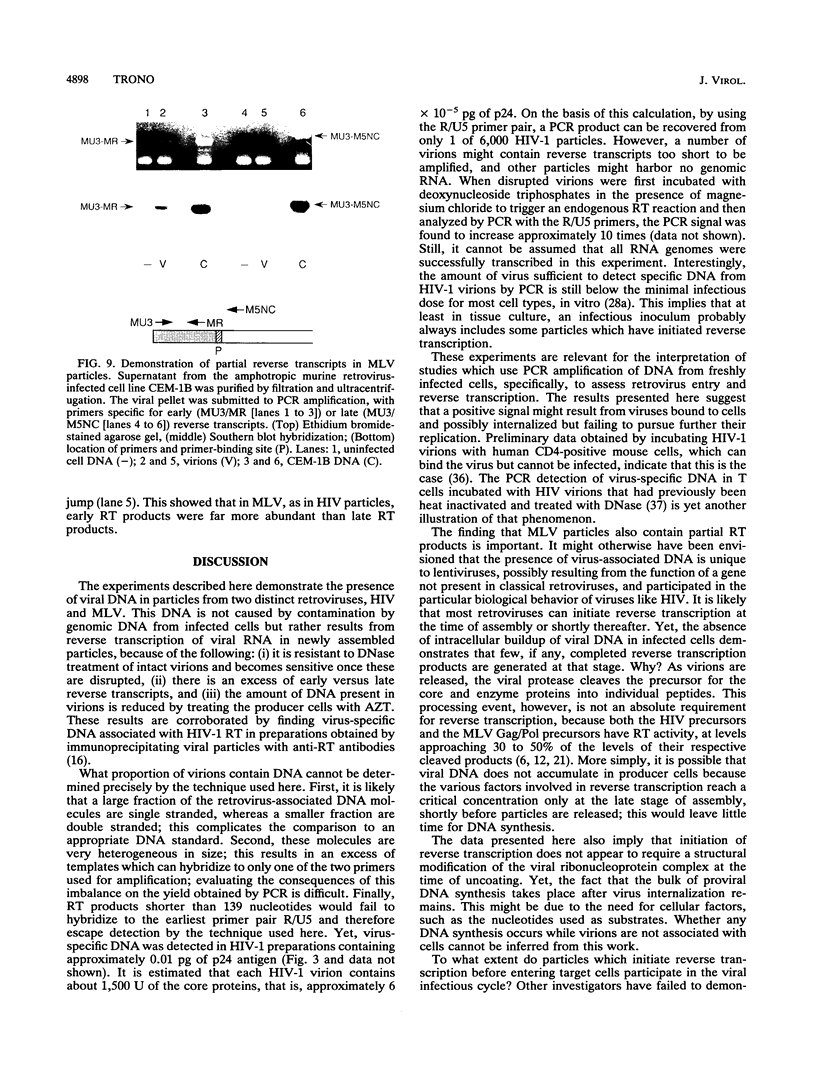
Reverse transcription of the retroviral genome is thought to start after virions enter target cells. Purified preparations of human immunodeficiency virus were found to contain virus-specific DNA, detectable by polymerase chain reaction amplification. This DNA resulted from reverse transcription in newly assembled virus particles and not from contamination by cellular DNA, because virions contained a striking excess of early versus late transcripts and because the accumulation of these products was sensitive to 3'-azido-3'-deoxythymidine (zidovudine) treatment of producer cells. A similar observation was made with murine amphotropic retrovirus particles. It is therefore likely that all retroviruses contain partial reverse transcripts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Besansky N. J., Butera S. T., Sinha S., Folks T. M. Unintegrated human immunodeficiency virus type 1 DNA in chronically infected cell lines is not correlated with surface CD4 expression. J Virol. 1991 May;65(5):2695–2698. doi: 10.1128/jvi.65.5.2695-2698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., McCain B., Maccain B., Benyeshh-Melnick M. The DNA of murine sarcoma-leukemia virus. Virology. 1971 Sep;45(3):697–706. doi: 10.1016/0042-6822(71)90183-8. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Evidence for circularization of the avian oncornavirus RNA genome during proviral DNA synthesis from studies of reverse transcription in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1329–1332. doi: 10.1073/pnas.73.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. A deletion mutation in the 5' part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J Virol. 1985 Mar;53(3):899–907. doi: 10.1128/jvi.53.3.899-907.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Baltimore D., Frankel A. D. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4045–4049. doi: 10.1073/pnas.88.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappes J. C., Conway J. A., Lee S. W., Shaw G. M., Hahn B. H. Human immunodeficiency virus type 2 vpx protein augments viral infectivity. Virology. 1991 Sep;184(1):197–209. doi: 10.1016/0042-6822(91)90836-z. [DOI] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Rein A., Shibuya M., Odaka T., Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from "immature" to "mature" core form and for virus infectivity. Virology. 1985 Sep;145(2):280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Byrn R., Groopman J., Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989 Sep;63(9):3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W. E., Varmus H. E., Garapin A. C., Bishop J. M. DNA of Rous sarcoma virus: its nature and significance. Science. 1972 Jan 7;175(4017):76–78. doi: 10.1126/science.175.4017.76. [DOI] [PubMed] [Google Scholar]
- Levinson W., Bishop J. M., Quintrell N., Jackson J. Presence of DNA in Rous sarcoma virus. Nature. 1970 Sep 5;227(5262):1023–1025. doi: 10.1038/2271023a0. [DOI] [PubMed] [Google Scholar]
- Marcon L., Michaels F., Hattori N., Fargnoli K., Gallo R. C., Franchini G. Dispensable role of the human immunodeficiency virus type 2 Vpx protein in viral replication. J Virol. 1991 Jul;65(7):3938–3942. doi: 10.1128/jvi.65.7.3938-3942.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Taylor J. M., Hull R. Retroid virus genome replication. Adv Virus Res. 1987;32:35–96. doi: 10.1016/s0065-3527(08)60474-1. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Norton W. A., Sattentau Q. J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991 Mar;65(3):1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Peng C., Ho B. K., Chang T. W., Chang N. T. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J Virol. 1989 Jun;63(6):2550–2556. doi: 10.1128/jvi.63.6.2550-2556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokutanda M., Rokutanda H., Green M., Fujinaga K., Ray R. K., Gurgo C. Formation of viral RNA-DNA hybrid molecules by the DNA polymerase of sarcoma-leukaemia viruses. Nature. 1970 Sep 5;227(5262):1026–1028. doi: 10.1038/2271026a0. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Synthesis of long, representative DNA copies of the murine RNA tumor virus genome. J Virol. 1975 Jan;17(1):168–174. doi: 10.1128/jvi.17.1.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Smotkin D., Baltimore D., Weinberg R. A. In vitro synthesis of infectious DNA of murine leukaemia virus. Nature. 1977 Sep 8;269(5624):122–126. doi: 10.1038/269122a0. [DOI] [PubMed] [Google Scholar]
- Ríman J., Beaudreau G. S. Viral DNA-dependent DNA polymerase and the properties of thymidine labelled material in virions of an oncogenic RNA virus. Nature. 1970 Oct 31;228(5270):427–430. doi: 10.1038/228427a0. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Haggerty S., Lamonica C. A., Meier C. M., Welch S. K., Wasiak A. J. Integration is not necessary for expression of human immunodeficiency virus type 1 protein products. J Virol. 1990 May;64(5):2421–2425. doi: 10.1128/jvi.64.5.2421-2425.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T. L., Dempsey M. P., Lamonica C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990 May;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Trono D., Feinberg M. B., Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989 Oct 6;59(1):113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]