Abstract
The arbuscular mycorrhizal (AM) symbiosis enhances plant tolerance to water deficit through the alteration of plant physiology and the expression of plant genes. These changes have been postulated to be caused (among others) by different contents of abscisic acid (ABA) between AM and non-AM plants. However, there are no studies dealing with the effects of exogenous ABA on the expression of stress-related genes and on the physiology of AM plants. The aim of the present study was to evaluate the influence of AM symbiosis and exogenous ABA application on plant development, physiology, and expression of several stress-related genes after both drought and a recovery period. Results show that the application of exogenous ABA had contrasting effects on AM and non-AM plants. Only AM plants fed with exogenous ABA maintained shoot biomass production unaltered by drought stress. The addition of exogenous ABA enhanced considerably the ABA content in shoots of non-AM plants, concomitantly with the expression of the stress marker genes Lsp5cs and Lslea and the gene Lsnced. By contrast, the addition of exogenous ABA decreased the content of ABA in shoots of AM plants and did not produce any further enhancement of the expression of these three genes. AM plants always exhibited higher values of root hydraulic conductivity and reduced transpiration rate under drought stress. From plants subjected to drought, only the AM plants recovered their root hydraulic conductivity completely after the 3 d recovery period. As a whole, the results indicate that AM plants regulate their ABA levels better and faster than non-AM plants, allowing a more adequate balance between leaf transpiration and root water movement during drought and recovery.
Keywords: ABA, arbuscular mycorrhiza, drought, recovery, stress-related gene
Introduction
Plants in nature are continuously exposed to several biotic and abiotic stresses, water deprivation being one of the commonest. Soils too dry for crop production have been estimated to cover 28% of the Earth's land surface (Bray, 2004). Nevertheless, plants have developed several physiological, biochemical, and molecular mechanisms in order to cope with drought stress.
Many of the plant responses to soil drying occur via chemical signals such as the phytohormone abscisic acid (ABA) (Wilkinson and Davies, 2002) and it is well known that the endogenous levels of ABA in vegetative plant tissues rise in response to stresses that cause a plant water deficit (Taylor et al., 2000; Bray, 2002; Zhang et al., 2006). Moreover, a clear relationship between plant ABA content and plant tolerance to water deficit has been described (Kulkarni et al., 2000; Liu et al., 2005). The protective effect of ABA is based on the fact that ABA primarily promotes stomatal closure to minimize transpirational water loss and then it mitigates stress damage through the activation of many stress-responsive genes, which collectively increase the plant stress tolerance (Bray, 1997, 2002; Zhang et al., 2006). Drought stress-inducible genes can be divided into two groups: functional and regulatory. Functional genes are those that have a specific function in acquiring drought tolerance such as water channels and other transporters, detoxification enzymes, protection molecules such as late embryogenesis abundant (LEA) proteins, key enzymes for osmolyte biosynthesis, or different proteases. Regulatory genes are those that regulate the expression of the functional genes and include, among others, transcription factors, protein kinases and phosphatases, and those involved in abscisic acid biosynthesis. For a recent review see Shinozaki and Yamaguchi-Shinozaki (2007).
Besides the natural responses of plants against drought, it must be considered that most terrestrial plants can establish a symbiotic association with the arbuscular mycorrhizal (AM) fungi. When the AM symbiosis is established the fungus receives carbon molecules from the plant, and the plant receives nutrients (especially phosphorus) and water from the fungus (Harrison, 2005; Gosling et al., 2006). In this way, AM plants are usually more tolerant to several stresses, including drought, than non-AM plants (Augé, 2001, 2004; Ruiz-Lozano, 2003, Ruiz-Lozano et al., 2006).
The beneficial effect of AM symbiosis under drought-stress conditions has been studied largely at the physiological level including regulation of transpiration rate or increasing root water absorption (Augé, 2001, 2004). More recently, it has also been noted that, under drought-stress conditions, AM and non-AM plants regulate differently the expression of several stress-related genes in root tissues (Ruiz-Lozano et al., 2006). These stress-related genes include a P5CS gene (Porcel et al., 2004), encoding for Δ1-pyrroline-5-carboxylate synthetase, the enzyme that catalyses the rate-limiting step in the biosynthesis of proline, which is a robust osmotic and antioxidant agent (Aral and Kamoun, 1997). Genes regulated by AM symbiosis under drought conditions also included the late embryogenesis abundant (LEA) proteins (Porcel et al., 2005). The specific function of LEA proteins during osmotic stress is not well understood, but could include protein stabilization, ion binding, antioxidant function, or membrane stabilization and folding (for a recent review see Tunnacliffe and Wise, 2007). Other genes regulated differently by AM symbiosis during drought are those encoding for plasma membrane aquaporins (PIP) proteins (Porcel et al., 2006; Aroca et al., 2007). Aquaporins are membrane intrinsic proteins that facilitate water and small neutral solutes flow, always following an osmotic gradient (Maurel, 2007). Finally, a differential expression in AM plants of a nced gene has also been described (Jahromi et al., 2008). The nced genes encode for 9-cis-epoxycarotenoid dioxygenase (NCED), the key enzyme for the biosynthesis of ABA (Schwartz et al., 2003).
The changes described above for the expression of stress-related genes under drought conditions by the AM symbiosis have been postulated to be caused (among others) by different contents of ABA between AM and non-AM plants (Ruiz-Lozano et al., 2006). Indeed, all the above-described genes are regulated by ABA (Strizhov et al., 1997; Kamisugi and Cuming, 2005; Aroca et al., 2006). Moreover, it has been described that AM symbiosis regulates ABA contents of the host plant under drought conditions (Goicoechea et al., 1997; Estrada-Luna and Davies, 2003). However, there are no studies dealing with the effects of exogenous ABA on the expression of stress-related genes and on the physiology of AM plants. In addition, the studies mentioned above which deal with the different regulation of stress-related genes in AM and non-AM plants have been carried out only at the root level, where the physical interaction between the plant and the fungus takes place. It is, however, known that the AM symbiosis also alters the physiology and metabolism of the aerial part of the plant (Toussaint, 2007). On the other hand, only a few studies concerning drought tolerance enhancement by AM symbiosis have paid attention to the behaviour of the host plant during recovery from the stress (Dell'Amico et al., 2002; Caravaca et al., 2005). However, it has been proposed that the importance of the capacity to recover from the stress is similar to the capacity of tolerating the stress itself (Grzesiak et al., 2006; Oukarroum et al., 2007). A comprehensive evaluation is therefore needed of the physiological and molecular changes experienced by AM plants, as affected by the plant ABA level, during drought and after recovery.
The aim of the present research was to evaluate the influence of AM symbiosis and exogenous ABA application on plant development, physiology, and expression of several stress-related genes after both drought and a recovery period. The starting hypothesis are that (i) the physiological and molecular responses of AM and non-AM plants to drought will differ; (ii) the application of exogenous ABA to plants will change the response of the plant to drought; and (iii) the recovery from the stress will be different in AM and non-AM plants and it will be affected by the exogenous ABA. To achieve our objective, lettuce plants were inoculated or not with the AM fungus Glomus intraradices and subjected to 10 d of water deficit and/or recovered for three additional days. Plants were also treated or not exogenously with an ABA solution just before and during the water-deficit treatment.
Materials and methods
Experimental design and statistical analysis
The experiment consisted of a randomized complete block design with two inoculation treatments: (i) non-inoculated control plants (NI), (ii) plants inoculated with the AM fungus Glomus intraradices (Schenck and Smith) BEG 121 (Gi). There were forty replicates of each microbial treatment, totalling 80 pots (one plant per pot), so that half of the plants were cultivated under well-watered conditions throughout the entire experiment and the other half were subjected to drought stress for 10 d before harvest. In addition, half of the plants from each inoculation treatment were supplied with exogenous ABA while the other half remained ABA-free. Finally, a group of plants were maintained for three additional days after the drought stress period in order to allow recovery from the stress.
Data were subjected to analysis of variance (ANOVA) with inoculation treatment, ABA treatment, and water regime as sources of variation, and followed by Duncan's multiple range test (Duncan, 1955). Percentage values were arcsin transformed before statistical analysis.
Soil and biological materials
Loamy soil was collected from the Zaidin Experimental Station (Granada, Spain), sieved (2 mm), diluted with quartz-sand (<1 mm) (1:1, soil:sand, v/v) and sterilized by steaming (100 °C for 1 h on three consecutive days). The soil had a pH of 8.1 (water); 1.81% organic matter, nutrient concentrations (mg kg−1): N, 2.5; P, 6.2 (NaHCO3-extractable P); K, 132.0. The soil texture was made up of 35.8% sand, 43.6% silt, and 20.5% clay.
Three seeds of lettuce (Lactuca sativa L. cv. Romana) were sown in pots containing 750 g of the same soil/sand mixture as described above and thinned to one seedling per pot after emergence.
Mycorrhizal inoculum was bulked in an open-pot culture of Zea mays L. and consisted of soil, spores, mycelia, and infected root fragments. The AM species was Glomus intraradices (Schenck and Smith) isolate BEG 121. Ten grams of inoculum with about 60 infective propagules per gram (according to the most probable number test), were added to appropriate pots at sowing time.
Uninoculated control plants received the same amount of autoclaved mycorrhizal inoculum together with a 2 ml aliquot of a filtrate (<20 μm) of the AM inoculum in order to provide a general microbial population free of AM propagules.
Growth conditions
Plants were grown in a controlled environmental chamber with 65–75% RH, day/night temperatures of 25/15 °C, and a photoperiod of 16 h at a photosynthetic photon flux density (PPFD) of 350 μmol m−2 s−1 measured with a light meter (Li-Cor, Lincoln, NE, USA, model LI-188B).
In the middle of the experiment, non-AM plants received an application (10 ml per pot) of Hewitt's nutrient solution (Hewitt, 1952) in order to enhance plant growth and obtain AM and non-AM plants of comparable size before starting the drought and recovery treatments. One day before starting the drought stress treatment, 4 d after starting the drought stress, and 9 d after starting the drought stress (just 1 d before harvesting), half of plants from each microbial treatment received 10 ml pot−1 of an aqueous ABA solution (100 μM). The group of plants allowed to recover from drought stress for three additional days under well-watered conditions received an additional application of ABA (10 ml per pot) the day before their harvest. The concentration of ABA and the systemic application were selected as the most convenient in preliminary experiments which were tested from 10 μM to 1 mM ABA.
Soil moisture was measured with a ML2 ThetaProbe (AT Delta-T Devices Ltd., Cambridge, UK) as previously described (Porcel and Ruiz-Lozano, 2004). Water was supplied daily to maintain soil at field capacity during the first 7 weeks after planting. Then, half of the plants were allowed to dry until soil water content reached 75% field capacity, while the other half were maintained at field capacity. The soil water content was measured daily with the ThetaProbe ML2 before rewatering (at the end of the afternoon), reaching a minimum soil water content ranging from 65–70% field capacity. The amount of water lost was added to each pot in order to keep the soil water content at the desired level of 75% field capacity (Porcel and Ruiz-Lozano, 2004). Plants were maintained under such conditions for an additional 10 d before harvesting or before being rewatered to field capacity for three additional days.
Parameters measured
Biomass production:
At harvest (60 d after planting), the shoot and root system were separated and the shoot dry weight (DW) measured after drying in a forced hot-air oven at 70 °C for 2 d.
Symbiotic development:
The percentage of mycorrhizal root infection in lettuce plants was estimated by visual observation of fungal colonization after clearing washed roots in 10% KOH and staining with 0.05% trypan blue in lactic acid (v/v), according to Phillips and Hayman (1970). The extent of mycorrhizal colonization was calculated according to the gridline intersect method (Giovannetti and Mosse, 1980).
Leaf transpiration rate:
Leaf transpiration rate was determined by a gravimetric method (Aroca et al., 2007). Surfaces of the pots were covered with aluminium foil. The pot-plant system was weighed and referred as W0. The pot-plant system was weighed again after 2 h and referred as Wf. Leaf transpiration rate was calculated as: (W0–Wf)/t×A, where t is the time in seconds, and A is the leaf area in m2. Leaf area was calculated as follows: leaves of a whole plant were detached and scanned (hp scanjet 5550c, Hewlett Packard, Palo Alto, CA). The corresponding images were analysed with Adobe Photoshop CS (Adobe Systems Incorporated, San Jose, CA).
Root hydraulic conductivity (Lo):
In the present work, Lo was measured on detached roots exuding under atmospheric pressure (Aroca et al., 2007). Under these conditions, water is only moving because of the osmotic gradient between the soil solution and the root tissue. Therefore, according to Steudle (2000), the water would be moving mainly by the cell-to-cell path. This path predominates under conditions where the transpiration stream is restricted, contrary to the apoplastic path (Steudle, 2000).
Pots were immersed in aerated nutrient solution. Plants were cut below the cotyledons and a pipette with a silicone tube was attached to the stem. The liquid exuded in the first 15 min was discarded to avoid phloem contamination. The exudate of the following 2 h was collected with a syringe and weighed. The osmolarities of the exuded sap and the nutrient solution were determined using a cryoscopic osmometer (Osmomat 030, Gonotec Gmbh, Berlin, Germany). Osmotic root hydraulic conductance (Lo) was calculated as Lo=Jv/ΔΨ, where Jv is the exuded sap flow rate and ΔΨ the osmotic potential difference between the exuded sap and nutrient solution.
ABA content:
ABA was measured on 250 mg of frozen root or the youngest leaves of the tissues that were immersed in 2 ml distilled water and incubated for 24 h at 4 °C in the dark (Pardossi et al., 1992; Aroca et al., 2003). Quantitative analysis was performed on crude aqueous extracts using an ELISA assay based on a monoclonal antibody designed specifically against (S)-cis,trans-ABA (BT-GB-252A, Babraham Biosciences Technologies, Cambridge, UK). The procedure of Walker-Simmons (1987) was followed. Briefly, microtitration plates were coated overnight at 4 °C with BSA-ABA (Blintsov and Gussakovskaya, 2004), and then rinsed three times with 50 mM TRIS–HCl (pH = 7.8), 1 mM MgCl2, 150 mM NaCl, 0.1% (p/v) BSA, and 0.05% (v/v) Tween 20. After that, 200 μl of the samples and standards incubated overnight with the antibody were added and plates incubated at room temperature for 150 min. The plates were rinsed again as described above and incubated for 2 h at room temperature with Anti-rat IgG (1:1000). Finally, the plates were rinsed again and p-nitrophenyl phosphate added. Plates were incubated until the absorbance at 405 nm of the non-ABA sample was 1. Absorbance was inversely proportional to the amount of ABA. Three independent samples were assayed for each treatment. All sample results were the average of three serial dilutions within the linear range of the ABA standard curve.
Northern blot analysis
Total RNA was isolated from lettuce roots and leaves by phenol/chloroform extraction according to the method described by Kay et al. (1987). Northern blot with Lsnced (accession no. AB120109), LsPIP2 (accession no. AJ937963), Lsp5cs (accession no. AJ715852), and Lslea (accession no. AJ704826) genes were carried out as previously described (Porcel et al., 2006). Hybridizations were conducted overnight at 65 °C under standard conditions (Sambrook et al., 1989). After washing twice for 5 min at room temperature in 2× SSC and 0.1% SDS, and twice for 15 min at 65 °C with 0.5× SSC and 0.1% SDS, membranes were exposed to the phosphorimager. The amount of rRNA in the membranes was quantified using Quantity One software (Bio-Rad, Hemel Hempstead, UK) in ethidium bromide-stained membranes. After the northern blot, the hybridization signals were analysed with a phosphorimager and quantified using the same software. Transcript accumulation levels for each gene probe (in arbitrary units) were divided by the corresponding amount of rRNA in the membrane (also in arbitrary units). Each quantification of signals on screens and of rRNA in the membranes was repeated three times and the average value for each was used for normalization. Northern blot analyses were repeated twice with different set of plants.
Results
Mycorrhizal colonization
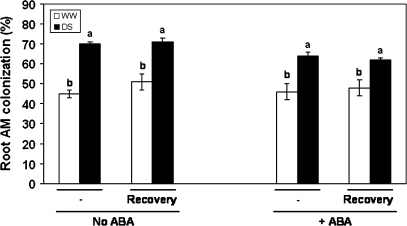
Uninoculated control plants did not show mycorrhizal colonization. Plants inoculated with G. intraradices showed a percentage of root colonization ranging from 45% to 70% of root length (Fig. 1). The application of ABA did not affect the colonization of root by G. intraradices. The only differences in root colonization were due to the water regime. In fact, plants cultivated under well-watered conditions showed 45% to 50% of mycorrhizal root length. In contrast, plants subjected to drought showed 62% to 70% of root colonization.
Fig. 1.
Percentage of mycorrhizal root length in lettuce plants inoculated with G. intraradices. See legend for Fig. 2.
Plant growth
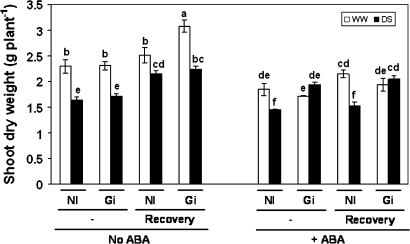
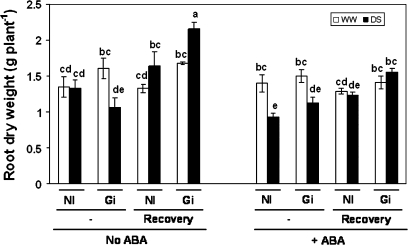
In order to obtain AM and non-AM plants of similar size before starting the drought and recovery treatments, non-AM plants received an application of nutrient solution. Therefore, under well-watered conditions AM and non-AM plants had similar shoot and root dry weights either with ABA or without ABA (Figs 2, 3). Under drought-stress conditions and in the absence of exogenous ABA, AM and non-AM plants also exhibited similar shoot dry weight, while roots of AM plants grew more after the recovery period (Fig. 3). By contrast, in the presence of exogenous ABA and drought stress, AM plants had 34% more shoot biomass than non-AM plants, but the difference in root dry weight was not significant.
Fig. 2.
Shoot dry weight (g plant−1) in lettuce plants. Plants were either inoculated with G. intraradices (Gi) or remained non-inoculated (NI). White bars represent plants cultivated under well-watered conditions and black bars represent plants subjected to drought stress. Half of the plants were allowed to grow for an additional 3 d period in order to recover from drought (recovery) and the other half was harvested just after the drought stress period (–). Finally, plants were supplied with exogenous ABA just before and during the drought stress or recovery periods (+ABA) or did not receive ABA (No ABA). Bars represent means plus the standard error (n=5). Means followed by the same letter are not significantly different (P < 0.05) as determined by Duncan's multiple range test.
Fig. 3.
Root dry weight (g plant−1) in lettuce plants. See legend for Fig. 2.
Shoot dry weight decreased in all treatments as a consequence of drought, except in plants inoculated with G. intraradices and supplied with exogenous ABA that showed similar shoot dry weight to those under well-watered conditions.
The application of exogenous ABA decreased shoot dry weight in all treatments, except in AM plants subjected to drought. Exogenous ABA also decreased the root dry weight of non-AM plants subjected to drought and of AM plants subjected to drought after the recovery period. By contrast, exogenous ABA did not affect the root dry weight of plants maintained under well-watered conditions.
In the absence of ABA the 3 d recovery period allowed further increase of shoot dry weight of the AM plants kept under well-watered conditions (Fig. 2). Drought-stressed plants also increased plant growth during the recovery period, mainly the root dry weight of AM plants (Fig. 3). In the presence of ABA, the recovery period did not significantly increase the shoot dry weight, either under well-watered conditions or under drought-stress conditions. By contrast, the root dry weight of AM plants increased significantly after the recovery period.
Root hydraulic conductivity (Lo)
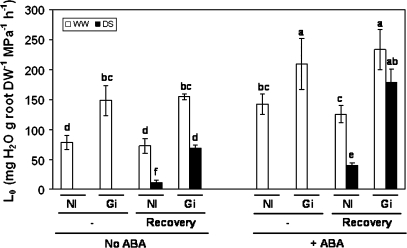
Plants subjected to drought stress were unable to exude after detachment of the shoots. Hence, Lo could not be measured in any of the treatments subjected to drought. Lo could only be measured in well-watered plants and in plants recovered from drought (Fig. 4).
Fig. 4.
Root hydraulic conductivity (Lo; mg H2O g−1 root DW MPa−1 h−1) in lettuce plants. See legend for Fig. 2.
Well-watered AM plants had higher Lo than non-AM plants, both with and without ABA application (increasing by 47% and 89%, respectively). This effect was also maintained after the recovery period. AM plants subjected to drought stress and recovered for 3 d also showed higher Lo than non-AM plants, both with and without ABA application (increasing by 346% and 520%, respectively).
The application of ABA to plants cultivated under well-watered conditions enhanced Lo by 81% in non-AM plants and by 41% in AM ones. The same trend was observed after the extra 3 d recovery period for AM and non-AM plants maintained under well-watered conditions.
Plants that had been subjected to drought stress and recovered for 3 d also showed an important increase of Lo by ABA application (260% increase for non-AM plants and 158% increase for AM plants), AM plants always exhibited higher Lo than non-AM plants. However, even after recovery from the stress, the plants that had been subjected to drought stress always showed lower Lo than those maintained under well-watered conditions. Only the AM plants that had exogenous ABA added recovered their Lo to values similar to those of well-watered plants.
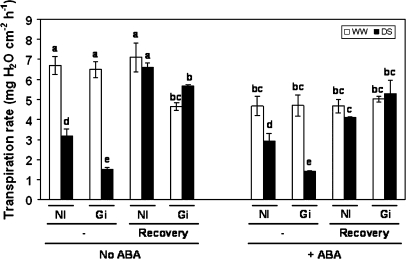
Transpiration rate
Under well-watered conditions the transpiration rate was similar in AM and non-AM plants, regardless of ABA application (Fig. 5). By contrast, under drought-stress conditions, AM plants showed a lower transpiration rate than non-AM plants, both with and without ABA application.
Fig. 5.
Transpiration rate (mg H2O cm−2 h−1) in lettuce plants. See legend for Fig. 2.
Drought stress decreased the transpiration rate both in AM and in non-AM plants, this decrease being more pronounced in AM plants. After the recovery from stress for 3 d, all the treatments recovered their transpiration rate to levels similar to those of well-watered plants. This effect was unrelated to ABA application.
The application of ABA decreased the transpiration rate both in AM and in non-AM plants, but only under well-watered conditions. In plant subjected to drought, the application of ABA did not reduce the transpiration rate further.
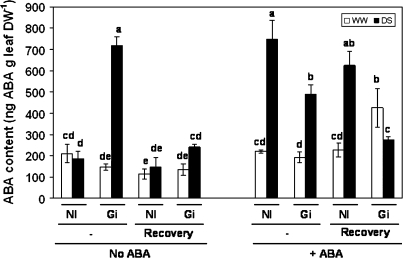
ABA content
ABA content was measured in leaves and roots. In leaves, the drought stress considerably enhanced (by 388%) the ABA content in plants colonized by G. intraradices, while it had no effect on non-AM plants (Fig. 6). However, after the recovery period, AM plants subjected to drought considerably decreased their ABA content reaching levels similar to those of non-inoculated plants maintained under well-watered conditions. By contrast, when exogenous ABA was added to the plant, the non-AM plants also increased their ABA content considerably as a consequence of drought. Under such conditions, the increase of ABA content was even higher for non-AM plants than for AM ones. In the presence of exogenous ABA, the level of ABA in leaves of non-AM lettuce plants that had been previously subjected to drought did not significantly decrease after recovery, while the decrease after recovery in AM plants was significant (by 44%).
Fig. 6.
ABA content (ng ABA g−1 leaf DW) in leaves of lettuce plants. See legend for Fig. 2.
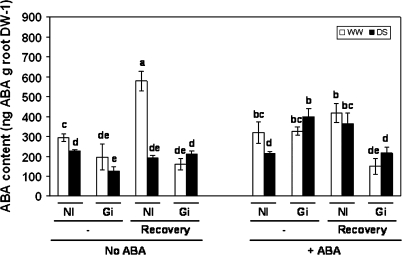
In roots, the picture is considerably different (Fig. 7). In fact, drought stress did not increase the ABA content in roots. Surprisingly, roots of non-AM plants cultivated under well-watered conditions enhanced their ABA content after the 3 d recovery period. The application of exogenous ABA to the plants enhanced the ABA content of plants inoculated with G. intraradices, mainly when subjected to drought stress (increasing by 216% compared with the absence of exogenous ABA). In the presence of exogenous ABA, the recovery from drought only decreased the content of ABA in the roots of AM plants, while non-AM plants even increased their ABA content.
Fig. 7.
ABA content (ng ABA g−1 root DW) in roots of lettuce plants. See legend for Fig. 2.
Gene expression
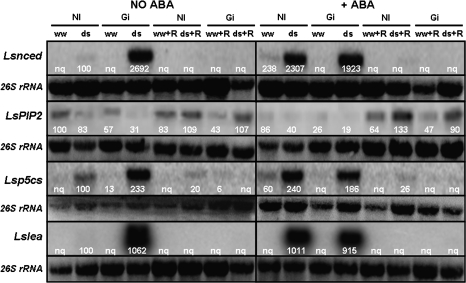
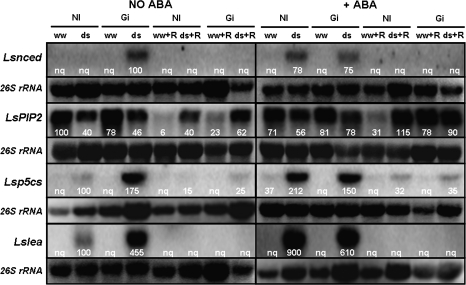
The expression of four stress-related plant genes was analysed by northern blot both in shoot and root tissues of lettuce plants under the various growing conditions tested in this work. Except for the LsPIP2 gene, the pattern of gene expression was quite similar in shoots and roots (Figs 8, 9).
Fig. 8.
Northern blot of total RNA (15 μg) from lettuce shoots using Lsnced (accession no. AB120109), LsPIP2 (accession no. AJ937963), Lsp5cs (accession no. AJ715852), and Lslea (accession no. AJ704826) gene probes. Treatments are designed as NI, non-inoculated controls or Gi, plants inoculated with G. intraradices. Plants were cultivated under well-watered conditions (ww) or subjected to drought stress (ds) with (+ABA) or without addition of exogenous ABA (No ABA). Half of the plants were allowed to grow for an additional 3 d period in order to recover from drought (ww+R or ds+R) and the other half was harvested just after the drought stress period. The panel under each northern blot shows the amount of 26S rRNA loaded for each treatment. Numbers close to each northern blot represent the relative gene expression after normalization to rRNA.
Fig. 9.
Northern blot of total RNA (15 μg) from lettuce roots. See legend for Fig. 8.
Gene Lsnced
In the absence of exogenous ABA, the expression of the Lsnced gene was only remarkable in shoots and roots of plants colonized by G. intraradices and subjected to drought, although the expression of this gene could also be detected in shoots of non-AM plants subjected to drought (Figs 8, 9). The application of exogenous ABA considerably enhanced the expression of this gene in shoots and roots of non-AM plants subjected to drought, while in AM plants it did not significantly change the expression pattern in roots (Fig. 9) or decreased it slightly in shoots (Fig. 8). Well-watered plants and plants recovered from drought did not express the Lsnced gene.
Gene LsPIP2
In the absence of exogenous ABA, the expression of the LsPIP2 gene was inhibited by drought stress both in the shoots and roots, mainly in AM plants, that showed the lowest rate of gene expression (Figs 8, 9). In shoots, the AM plants cultivated under well-watered conditions always exhibited about 45% reduction of gene expression compared with non-AM plants (Fig. 8). In any case, in shoots, AM and non-AM plants that had been subjected to drought, recovered the expression rate of the LsPIP2 gene after the recovery period. By contrast, in roots, the expression rate of the LsPIP2 gene in the plants that had been previously subjected to drought only partially recovered after the recovery period (Fig. 9). The expression rate of this gene in roots of AM and non-AM plants that were maintained under well-watered conditions was considerably lower than before the recovery period.
The application of exogenous ABA considerably inhibited the gene expression in shoots of AM and non-AM plants, regardless of water treatment (Fig. 8). By contrast, in roots, the application of ABA to these plants did not significantly change the rate of gene expression (Fig. 9). The plants with added exogenous ABA having recovered from a previous drought also recovered the expression rate of the LsPIP2 gene in shoots and in roots. By contrast, roots of non-AM plants that had been maintained under well-watered conditions showed reduced gene expression (Fig. 9).
Gene Lsp5cs
In the absence of exogenous ABA, this gene was only expressed in plants subjected to drought, mainly in AM plants (both shoots and roots) (Figs 8, 9). A residual expression was observed after the recovery period in plants that had been previously drought-stressed. In any case, the application of exogenous ABA considerably induced the expression of this gene in shoots and roots of non-AM plants only (even under well-watered conditions) so, under drought-stress conditions, the expression of that gene was higher than in AM plants. Again, a residual expression of the Lsp5cs gene was observed after the recovery period in plants that had previously been drought-stressed.
Gene Lslea
In the absence of exogenous ABA, the Lslea gene was only expressed in plants subjected to drought, mainly in AM plants (both shoots and roots) (Figs 8, 9). No expression was detected in well-watered plants or after the recovery period. The application of exogenous ABA considerably induced the expression of this gene in shoots and roots, mainly in the case of non-AM plants. In fact, after the addition of exogenous ABA, the expression of that gene under drought-stress conditions was higher in non-AM plants than in AM ones. Again, no expression was detected in well-watered plants or after the recovery period.
Discussion
The plant hormone ABA plays a major role in plant responses to several stresses, mainly those having a dehydration component (Zhang et al., 2006). ABA regulates plant water status through guard cells and growth as well as by the induction of genes that encode enzymes and other proteins involved in cellular dehydration tolerance (Bray, 2002; Xiong and Zhu, 2003). It has been shown that ABA regulates important plant processes such as Lo (Quintero et al., 1999; Hose et al., 2000; Wan et al., 2004; Schraut et al., 2005; Aroca, 2006), transpiration rate (Netting, 2000; Holbrook et al., 2002; Wilkinson and Davies, 2002; Zhang et al., 2006), and expression and activity of certain aquaporins (Suga et al., 2002; Jang et al., 2004; Zhu et al., 2005; Aroca et al., 2006), among many other drought-induced genes (Bray, 1997, 2002; Zhang et al., 2006). Thus, ABA is considered the most important stress signal transduction pathway among all the plant responses to stresses (Zhang et al., 2006). At the same time, the AM symbiosis has been shown to modulate the same physiological processes and to improve plant tolerance to water deficit (for reviews see Augé, 2001, 2004; Ruiz-Lozano, 2003). Indeed, improved Lo and transpiration rate in AM plants during drought stress have been observed (Augé, 1989; Ruiz-Lozano et al., 1995; Green et al., 1998; Sánchez-Blanco et al., 2004; Aroca et al., 2007). Modulation of aquaporin genes by the AM symbiosis during salt, cold, and drought stresses has also been described (Ouziad et al., 2005; Porcel et al., 2006; Aroca et al., 2007; Jahromi et al., 2008). This is why, in this study, the combined effects of the AM symbiosis and exogenous ABA application on the physiological and molecular responses of lettuce plants to drought stress and after recovery have been analysed.
Plant growth
Although it has been suggested that a high level of exogenous ABA can inhibit plant growth under non-stressful conditions, an increased ABA content is beneficial for plants under environmental stress as a result of ABA-induced changes at the cellular and whole-plant levels (Xiong and Zhu, 2003). However, it is not clear at the moment, whether ABA reduces, enhances or maintains plant growth during drought (Quarrie, 1991; Mäkelä et al., 2003). Results obtained in this study indicate that exogenous ABA decreased shoot dry weight in all plants and also the root dry weight of non-AM plants subjected to drought. An exception was found in AM plants subjected to drought that maintained their shoot and root growth as a consequence of exogenous ABA (Figs 2, 3). A reduction in plant growth by the application of exogenous ABA has previously been described in several plants (Finkelstein et al., 2002; Yang et al., 2007).
The AM symbiosis generally increases host plant growth due to improved plant nutrition (Smith and Read, 1997). In studies on the water relations of plants confined to containers, it is often difficult to compare treatments if plants are not of comparable size, since unequal size causes different degrees of soil water depletion, plant transpiration, and, consequently, unequal stress (Goicoechea et al., 1997). In this study, non-AM plants received a single application of nutrient solution in the middle of the experiment in order to obtain AM and non-AM plants of similar size before starting the drought and recovery treatments. Therefore, the effects of the different treatments on transpiration and Lo can be seen as direct effects not mediated by plant size.
Plant stress-related genes
Plant responses to water deficit involve changes in gene expression resulting in modifications of plant physiology that lead to enhanced drought tolerance. The expression of four plant stress-related genes has been analysed in root and shoot tissues after the different treatments. The genes selected for this study were Lsnced, encoding for NCED, a PIP2 aquaporin-encoding gene, the gene Lsp5cs, encoding P5CS, and the Lslea gene encoding for a dehydrin (LEA proteins). The Lsnced, Lsp5cs, and Lslea genes showed a very similar pattern of expression and this was also similar in root and shoot tissues. They were all induced by drought stress in AM plants. In non-AM plants, the induction by drought stress was remarkable when ABA was applied exogenously to the plants. Under such conditions, the expression was higher than in AM plants. The induction of nced genes by drought stress has been observed previously in a variety of plants (Iuchi et al., 2000; Tan et al., 2003; Rodrigo et al., 2006; Wan and Li, 2006). In addition, Cheng et al. (2002) demonstrated in Arabidopsis that a minimum level of ABA is required in plant tissues for the full induction of a nced gene, which could explain its induction by exogenous ABA in non-AM plants. The Lsp5cs and Lslea genes were mainly expressed in drought-stress treatments, demonstrating that they are important for the plant response against water deficit, as it has previously been shown (Kishor et al., 1995; Cellier et al., 1998; Giordani et al., 1999) and confirming their usefulness as stress markers (Porcel et al., 2004, 2005). The facts that genes encoding dehydrins and P5CS are regulated by both ABA-dependent and ABA-independent pathways and that both pathways may have cumulative effects, have been proved (Chandler and Robertson, 1994; Giordani et al., 1999; Ábrahám et al., 2003). This can explain the important induction of the expression of these genes in non-AM plants fed with exogenous ABA. In addition, in this study, the expression of Lsnced, Lsp5cs, and Lslea genes and the accumulation of ABA correlate very well in shoots. By contrast, no clear correlation between ABA levels with the expression of these three genes can be found in roots, even after exogenous application of ABA. However, bearing in mind that oxidative degradation of ABA to phaseic acid and dihydrophaseic acid can be extremely rapid (Zeevaart, 1999), it is nearly impossible to predict the percentage of externally applied ABA that reaches the site of action and acts on it. For instance, Zhang et al. (1995) found that after 24 h of feeding maize leaves with ABA, only 8% remain unmodified. To explain the different correlation between ABA content with gene expression in roots and shoots it must also be considered that, as the soil is drying, ABA serves as a long-distance stress signal synthesized in the roots and released by root tissues to the xylem for its translocation to the shoot (Hartung et al., 2005). Differences in the rate of ABA metabolism between root and shoot may also account for this discrepancy. Factors such as recirculation and exudation to the rhizosphere may decrease the internal root ABA concentration as well (Peuke et al., 2002; Hartung et al., 2005). In any case, ABA could also act during drought treatment in the roots, as shown by changes in gene expression in the roots, simply by changing its internal localization, moving from symplastic to apoplastic zones (Cohen et al., 1999; Wilkinson and Davies, 2002).
Regarding LsPIP2, the effects of ABA on PIP aquaporin gene expression are not fully understood, the effect being positive or negative depending on the gene analysed and the plant genotype (Lian et al., 2006). Here, ABA down-regulated LsPIP2 gene expression in shoots, but had no effect in roots. A down-regulation by ABA of some PIP aquaporin genes in shoot tissues has been described before by Lian et al. (2006) in rice plants. This behaviour can be postulated as a mechanism of cell water conservation (Smart et al., 2001).
On the other hand, the modulation by the AM symbiosis of the expression of genes Lsnced, LsPIP2, Lsp5cs, and Lslea has previously been analysed in roots of lettuce plants in relation to the tolerance towards osmotic stresses (Porcel et al., 2004, 2005, 2006; Ruiz-Lozano et al., 2006; Jahromi et al., 2008). By contrast, the expression pattern of these genes has never been analysed in the shoots of mycorrhizal lettuce plants or after exogenous ABA application or recovery from the stress. A discrepancy was found here in the pattern of Lsp5cs and Lslea genes in roots as compared with the previous studies. In fact, it has previously been shown that roots of AM plants subjected to drought had reduced expression of Lsp5cs and Lslea genes compared with roots of non-AM plants (Porcel et al., 2004, 2005). This is exactly the same result that was found here in plants that were fed with exogenous ABA, but it is contrary to the result of plants that did not receive exogenous ABA. To explain these contradictory results it must be considered that the plants used for the previous studies were cultivated under greenhouse conditions and subjected to a more severe stress (before daily watering the soil water content decreased to 55–60% of the field capacity) (Porcel et al., 2004, 2005). By contrast, the experiment described here was conducted in a controlled-environment chamber and the stress imposed was less severe (before daily watering the soil water content only decreased to 65–70% of field capacity). Thus, it may be possible that, in this work, the level of ABA in the plants (at least in the non-AM plants) was lower than in the previous works (not measured). It is known that a minimum level of ABA is required to up-regulate the expression of ABA-induced genes (Cohen et al., 1999). Thus, only after the addition of exogenous ABA, was the expression of the Lsp5cs and Lslea genes in the non-AM plants up-regulated remarkably, as can be seen in Figs 8 and 9. The ABA content of AM plants was higher than in non-AM plants in shoots and increased considerably in non-AM plants after the addition of exogenous ABA (Fig. 6). In roots, this was not so, perhaps because of differences in the rate of ABA metabolism, recirculation, and exudation to the rhizosphere or changes in its internal localization (Hartung et al., 2005; Wilkinson and Davies, 2002), as discussed above.
Plant water relations
Plant water status is determined by the balance between the water lost in transpiration and water uptake from the soil (Aroca et al., 2007; Galmés et al., 2007). In this study, AM and non-AM plants showed similar transpiration rates under well-watered conditions. The application of exogenous ABA decreased the transpiration rate of well-watered plants and did not affect the transpiration of drought-stressed plants. By contrast, under drought-stress conditions, AM plants showed a lower transpiration rate than non-AM plants, while Lo was always higher in AM plants than in non-AM plants. Enhanced Lo in AM plants has been previously shown in tomato and rosemary plants (Dell'Amico et al., 2002; Sánchez-Blanco et al., 2004). The application of exogenous ABA also enhanced Lo in all treatments, in agreement with previous findings (Quintero et al., 1999; Hose et al., 2000; Wan et al., 2004; Schraut et al., 2005; Aroca, 2006). The intimate mechanisms involved in the ABA promotion of root water transport remain largely unexplored (Aroca, 2006). However, the regulation of Lo has been related to changes in aquaporin activity and/or abundance (Javot and Maurel, 2002; Luu and Maurel, 2005; Beaudette et al., 2007). The role played by aquaporins in water transport may become crucial under water stress, when conditions of reduced transpiration do not allow high driving forces derived from significant water potential gradients. Under these conditions, water transport through the cell-to-cell pathway predominates in the plant and it is expected that aquaporins regulate water movement (Javot et al., 2003; Luu and Maurel, 2005). On the other hand, it is known that ABA modulates the expression of some PIP genes in roots and leaves (Suga et al., 2002; Jang et al., 2004; Zhu et al., 2005; Aroca et al., 2006). Thus, the regulation of Lo by ABA may be linked to the modulation of aquaporins. However, whether or not ABA increases Lo through a direct or an indirect interaction with aquaporins remains unclear.
The effects of drought on Lo depend on stress intensity (Siemens and Zwiazek, 2004). Under moderate to severe water deficit, Lo generally decreases and it generally rises again after rewatering (Siemens and Zwiazek, 2004). In this study Lo was not measurable under drought-stress conditions. In the plants that had been previously drought-stressed, Lo could only be measured after the 3 d recovery period. Nevertheless, even after rewatering, plants did not completely recover Lo values (except AM plants fed with ABA), while the transpiration rate recovered completely. Such behaviour indicates that water should be following an apoplastic path as reported before for other plant species subjected to drought (Ionenko et al., 2003; Siemens and Zwiazek, 2004) and that, in AM plants, ABA could be promoting symplastic water movement.
The process of recovery after water stress has been studied in several plants at the physiological level, but it has seldom been linked with changes in aquaporin expression and/or activation. In this study the expression of the LsPIP2 gene was inhibited by drought in roots and also in shoots of lettuce plants, coinciding with a decrease in Lo (until the values were not measurable). The expression of this gene in shoots rose again in drought-stressed plants after the 3 d recovery period, while in roots it was almost constant. Lo increased after the recovery period. The inoculation with G. intraradices decreased the expression of LsPIP2 in shoots, regardless of water status and ABA application. By contrast, mycorrhization did not significantly alter the pattern of gene expression in roots, but Lo was always enhanced by mycorrhization. Finally, the application of exogenous ABA decreased the expression of this gene in shoots of plants not subjected to the recovery period and did not significantly change the expression in roots. On the contrary, Lo was increased by exogenous ABA in all treatments. A similar discrepancy between the effect of exogenous ABA on Lo and aquaporin gene expression has also been found by Beaudette et al. (2007). Thus, it can be concluded that there is no correlation between changes in Lo due to mycorrhizal colonization or ABA application with the expression of LsPIP2 gene. By contrast, there is correlation between the reduction of Lo by drought stress and the decrease of the LsPIP2 gene expression in roots and shoots. The lack of complete correlation between the expression of aquaporin genes and Lo has also been described by Galmés et al. (2007). It may be due to the fact that the regulation of aquaporin activity is not only restricted to the transcriptional level. Post-transcriptional regulation via phosphorylation, methylation, re-localization, or changes in cytosolic pH has been described (Maurel, 2007). Besides, aquaporins are not the unique way to control Lo, symplastic movement of water via plasmodesmata may contribute significantly to Lo depending on the exact environmental circumstances (Galmés et al., 2007). Finally, it must be considered that aquaporins constitute a multiple gene family in plants (Maurel, 2007) and only one aquaporin gene was analysed in this study. Thus, the differential effect of other aquaporins not analysed here can account for the changes observed in Lo.
Conclusion
In conclusion, in this work it is shown that the addition of exogenous ABA considerably enhanced the ABA content in shoots of non-AM plants, concomitantly with the expression of the stress marker genes Lsp5cs and Lslea and the gene Lsnced. By contrast, the addition of exogenous ABA decreased the content of ABA in shoots of AM plants and did not produce any further enhancement of the expression of these three genes. These contrasting effects of exogenous ABA in AM and non-AM plants can be related to the fact that AM plants always exhibited higher values of Lo and reduced transpiration rate under drought stress, suggesting better water regulation in AM plants under drought stress. The application of exogenous ABA allowed AM plants only to maintain shoot biomass production unaltered by drought stress. Exogenous ABA enhanced Lo in all treatments and, again, from the plants that had been subjected to stress, only the AM ones recovered completely their Lo after the 3 d recovery period. As a whole, the results indicate that AM plants regulate better and faster their ABA levels than non-AM plants, allowing in this way a more adequate balance between leaf transpiration and root water movement during drought and recovery.
Acknowledgments
This work was financed by CICYT-FEDER (Project AGL2005-01237). R Aroca was financed by the Spanish Ministry of Education and Science throughout the Juan de la Cierva program. We thank Jorge García-Tello for correcting the English text.
References
- Ábrahám E, Rigó G, Székely G, Nagy R, Koncz C, Szabados L. Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Molecular Biology. 2003;51:363–372. doi: 10.1023/a:1022043000516. [DOI] [PubMed] [Google Scholar]
- Aral B, Kamoun P. The proline biosynthesis in living organisms. Amino Acids. 1997;13:189–217. [Google Scholar]
- Aroca R. Exogenous catalase and ascorbate modify the effects of abscisic acid (ABA) on root hydraulic properties in Phaseolus vulgaris L. plants. Journal of Plant Growth Regulation. 2006;25:10–17. [Google Scholar]
- Aroca R, Ferrante A, Vernieri P, Chrispeels MJ. Drought, abscisc acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Annals of Botany. 2006;98:1301–1310. doi: 10.1093/aob/mcl219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R, Porcel R, Ruiz-Lozano JM. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytologist. 2007;173:808–816. doi: 10.1111/j.1469-8137.2006.01961.x. [DOI] [PubMed] [Google Scholar]
- Aroca R, Vernieri P, Irigoyen JJ, Sánchez-Díaz M, Tognoni F, Pardossi A. Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Science. 2003;165:671–679. [Google Scholar]
- Augé RM. Do VA mycorrhizae enhance transpiration by affecting host phosphorus content? Journal of Plant Nutrition. 1989;12:743–753. [Google Scholar]
- Augé RM. Water relations, drought and vesicular–arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11:3–42. [Google Scholar]
- Augé RM. Arbuscular mycorrhizae and soil/plant water relations. Canadian Journal of Soil Science. 2004;84:373–381. [Google Scholar]
- Beaudette PC, Chlup M, Yee J, Emery RJN. Relationships of root conductivity and aquaporin gene expression in Pisum sativum: diurnal patterns and the response to HgCl2 and ABA. Journal of Experimental Botany. 2007;58:1291–1300. doi: 10.1093/jxb/erl289. [DOI] [PubMed] [Google Scholar]
- Blintsov AN, Gussakovskaya MA. Immunochemical approach to the problem of differential determination of natural forms of abscisic acid. Biochemistry (Moscow) 2004;69:1099–1108. doi: 10.1023/b:biry.0000046883.07615.e5. [DOI] [PubMed] [Google Scholar]
- Bray EA. Plant responses to water deficit. Trends in Plant Science. 1997;2:48–54. [Google Scholar]
- Bray EA. Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant, Cell and Environment. 2002;25:153–161. doi: 10.1046/j.1365-3040.2002.00746.x. [DOI] [PubMed] [Google Scholar]
- Bray EA. Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. Journal of Experimental Botany. 2004;55:2331–2341. doi: 10.1093/jxb/erh270. [DOI] [PubMed] [Google Scholar]
- Caravaca F, Alguacil MM, Hernández JA, Roldán A. Involvement of antioxidant enzyme and nitrate reductase activities during water stress and recovery of mycorrhizal Myrtus communis and Phillyrea angustifolia plants. Plant Science. 2005;169:191–197. [Google Scholar]
- Cellier F, Conéjéro G, Breitler JC, Casse F. Molecular and physiological responses to water deficit in drought-tolerant and drought-sensitive lines of sunflower. Plant Physiology. 1998;116:319–328. doi: 10.1104/pp.116.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler PM, Robertson M. Gene expression regulated by abscisic acid and its relation to stress tolerance. Annual Review of Plant Physiology and Plant Molecular Biology. 1994;47:113–141. [Google Scholar]
- Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leun P, Nambara E, Asami T, Seo M. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signalling and abscisic acid biosynthesis and functions. The Plant Cell. 2002;14:2723–2743. doi: 10.1105/tpc.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Moses MS, Plants AL, Bray EA. Multiple mechanisms control the expression of abscisic acid (ABA)-requiring genes in tomato plants exposed to soil water deficit. Plant, Cell and Environment. 1999;22:989–998. [Google Scholar]
- Dell'Amico J, Torrecillas A, Rodríguez P, Morte A, Sánchez-Blanco MJ. Responses of tomato plants associated with the arbuscular mycorrhizal fungus Glomus clarum during drought and recovery. Journal of Agricultural Science. 2002;138:387–393. [Google Scholar]
- Duncan DB. Multiple range and multiple F-tests. Biometrics. 1955;11:1–42. [Google Scholar]
- Estrada-Luna AA, Davies FT. Arbuscular mycorrhizal fungi influence water relations, gas exchange, abscisic acid and growth of micropropagated chile ancho pepper (Capsicum annuum) plantlets during acclimatization and post-acclimatization. Journal of Plant Physiology. 2003;160:1073–1083. doi: 10.1078/0176-1617-00989. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signalling in seeds and seedlings. The Plant Cell. 2002;14:S15–S45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Pou A, Alsina MM, Tomás M, Medrano H, Flexas J. Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): relationship with ecophysiological status. Planta. 2007;226:671–681. doi: 10.1007/s00425-007-0515-1. [DOI] [PubMed] [Google Scholar]
- Giordani T, Natali L, D'Ercole A, Pugliesi C, Fambrini M, Vernieri P, Vitagliano C, Cavallini A. Expression of a dehydrin gene during embryo development and drought stress in ABA-deficient mutants of sunflower (Helianthus annuus L.) Plant Molecular Biology. 1999;39:739–748. doi: 10.1023/a:1006194720022. [DOI] [PubMed] [Google Scholar]
- Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular-arbuscular infection in roots. New Phytologist. 1980;84:489–500. [Google Scholar]
- Goicoechea N, Antolin MC, Sánchez-Díaz M. Gas exchange is related to the hormone balance in mycorrhizal or nitrogen-fixing alfalfa subjected to drought. Physiologia Plantarum. 1997;100:989–997. [Google Scholar]
- Gosling P, Hodge A, Goodlass G, Bending GD. Arbuscular mycorrhizal fungi and organic farming. Agriculture, Ecosystems and Environment. 2006;113:17–35. [Google Scholar]
- Green CD, Stodola A, Augé RM. Transpiration of detached leaves from mycorrhizal and non-mycorrhizal cowpea and rose plants given varying abscisic acid, pH, calcium and phosphorus. Mycorrhiza. 1998;8:93–99. [Google Scholar]
- Grzesiak MT, Grzesiak S, Skoczowski A. Changes of leaf water potential and gas exchange during and after drought in triticale and maize genotypes differing in drought tolerance. Photosynthetica. 2006;44:561–568. [Google Scholar]
- Harrison MJ. Signaling in the arbuscular mycorrhizal symbiosis. Annual Review of Microbiology. 2005;59:19–42. doi: 10.1146/annurev.micro.58.030603.123749. [DOI] [PubMed] [Google Scholar]
- Hartung W, Schraut D, Jiang F. Physiology of abscisic acid (ABA) in roots under stress: a review of the relationship between root ABA and radial water and ABA flows. Australian Journal of Agricultural Research. 2005;56:1253–1259. [Google Scholar]
- Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. Technical Communication 22, Farnham Royal, Commonwealth Agricultural Bureaux, Bucks; 1952. [Google Scholar]
- Holbrook NM, Shashidnar VR, James RA, Munss R. Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. Journal of Experimental Botany. 2002;53:1503–1514. [PubMed] [Google Scholar]
- Hose E, Steudle E, Hartung W. Abscisic acid and hydraulic conductivity of maize roots: a study using cell- and root-pressure probes. Planta. 2000;211:874–882. doi: 10.1007/s004250000412. [DOI] [PubMed] [Google Scholar]
- Ionenko IF, Anisimov AV, Romanov AV. Effect of water stress and mercuric chloride on the translational diffusion of water in maize seedlings roots. Russian Journal of Plant Physiology. 2003;50:79–83. [Google Scholar]
- Iuchi S, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K. A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiology. 2000;123:553–562. doi: 10.1104/pp.123.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi F, Aroca R, Porcel R, Ruiz-Lozano JM. Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microbial Ecology. 2008;55:45–53. doi: 10.1007/s00248-007-9249-7. [DOI] [PubMed] [Google Scholar]
- Jang JY, Kim DG, Kim YO, Kim JS, Kang H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Molecular Biology. 2004;54:713–725. doi: 10.1023/B:PLAN.0000040900.61345.a6. [DOI] [PubMed] [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, et al. Role of a single aquaporin isoform in root water uptake. The Plant Cell. 2003;15:509–522. doi: 10.1105/tpc.008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javot H, Maurel C. The role of aquaporins in root water uptake. Annals of Botany. 2002;90:301–313. doi: 10.1093/aob/mcf199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisugi Y, Cuming AC. The evolution of the abscisic acid-response in land plants: comparative analysis of group 1 LEA gene expression in moss and cereals. Plant Molecular Biology. 2005;59:723–737. doi: 10.1007/s11103-005-0909-z. [DOI] [PubMed] [Google Scholar]
- Kay R, Chau A, Daly M. Duplication of CaMV 35S promoter sequences creates a strong enhancer for plants genes. Science. 1987;236:1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- Kishor PB, Hong Z, Miao GH, Hu CA, Verma DPS. Overexpression of Δ1-pyrroline-5-carboxilate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiology. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni MJ, Prasad TG, Sashidhar VR. Genotypic variation in ‘early warning signals’ from roots in drying soil: intrinsic differences in ABA synthesising capacity rather than root density determines total ABA ‘message’ in cowpea (Vigna unguiculata L.) Annals of Applied Biology. 2000;136:267–272. [Google Scholar]
- Lian HL, Yu X, Lane D, Sun WN, Tang ZC, Su WA. Upland rice and lowland rice exhibit different PIP expression under water deficit and ABA treatment. Cell Research. 2006;16:651–660. doi: 10.1038/sj.cr.7310068. [DOI] [PubMed] [Google Scholar]
- Liu F, Jensen CR, Andersen MN. A review of drought adaptation in crop plants: changes in vegetative and reproductive physiology induced by ABA-based chemical signals. Australian Journal of Agricultural Research. 2005;56:1245–1252. [Google Scholar]
- Luu D-T, Maurel C. Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant. Cell and Environment. 2005;28:85–96. [Google Scholar]
- Mäkelä P, Munns R, Colmer TD, Peltonen-Sainio P. Growth of tomato and an ABA-deficient mutant (sitiens) under saline conditions. Physiologia Plantarum. 2003;117:58–63. [Google Scholar]
- Maurel C. Plant aquaporins: novel functions and regulation properties. FEBS Letters. 2007;581:2227–2236. doi: 10.1016/j.febslet.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Netting AG. pH, abscisic acid and the integration of metabolism in plants under stressed and non-stressed conditions: cellular responses to stress and their implication for plant water relations. Journal of Experimental Botany. 2000;51:147–158. doi: 10.1093/jexbot/51.343.147. [DOI] [PubMed] [Google Scholar]
- Oukarroum A, El Madidi S, Schansker G, Strasser RJ. Probing the response of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environmental and Experimental Botany. 2007;60:438–446. [Google Scholar]
- Ouziad F, Wilde P, Schmelzer E, Hildebrandt U, Bothe H. Analysis of expression of aquaporins and Na+/H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environmental and Experimental Botany. 2006;57:177–186. [Google Scholar]
- Pardossi A, Vernieri P, Tognoni F. Involvement of abscisic-acid in regulating water status in Phaseolus vulgaris during chilling. Plant Physiology. 1992;100:1243–1250. doi: 10.1104/pp.100.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuke AD, Jeschke WD, Hartung W. Flows of elements, ions and abscisic acid in Ricinus cummunis under potassium limitation. Journal of Experimental Botany. 2002;53:241–250. doi: 10.1093/jexbot/53.367.241. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Hayman DS. Improved procedure of clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society. 1970;55:159–161. [Google Scholar]
- Porcel R, Aroca R, Azcón R, Ruiz-Lozano JM. PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Molecular Biology. 2006;60:389–404. doi: 10.1007/s11103-005-4210-y. [DOI] [PubMed] [Google Scholar]
- Porcel R, Azcón R, Ruiz-Lozano JM. Evaluation of the role of genes encoding for Δ1-pyrroline-5-carboxylate synthetase (P5CS) during drought stress in arbuscular mycorrhizal Glycine max and Lactuca sativa plants. Physiological and Molecular Plant Pathology. 2004;65:211–221. [Google Scholar]
- Porcel R, Azcón R, Ruiz-Lozano JM. Evaluation of the role of genes encoding for dehydrin proteins (LEA D-11) during drought stress in arbuscular mycorrhizal Glycine max and Lactuca sativa plants. Journal of Experimental Botany. 2005;56:1933–1942. doi: 10.1093/jxb/eri188. [DOI] [PubMed] [Google Scholar]
- Porcel R, Ruiz-Lozano JM. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany. 2004;55:1743–1750. doi: 10.1093/jxb/erh188. [DOI] [PubMed] [Google Scholar]
- Quarrie SA. Implications of genetic differences in ABA accumulation for crop production. In: Davies WJ, Jones HG, editors. Abscisic acid: physiology and biochemistry. Oxford: BIOS Scientific Publishers; 1991. pp. 227–243. [Google Scholar]
- Quintero JM, Fournier JM, Benlloch M. Water transport in sunflower root sytems: effects of ABA, Ca2+ status and HgCl2. Journal of Experimental Botany. 1999;50:1607–1612. [Google Scholar]
- Rodrigo MJ, Alquezar B, Zacarías L. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck) Journal of Experimental Botany. 2006;57:633–643. doi: 10.1093/jxb/erj048. [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano JM. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza. 2003;13:309–317. doi: 10.1007/s00572-003-0237-6. [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano JM, Azcón R, Gómez M. Effects of arbuscular mycorrhizal Glomus species on drought tolerance: physiological and nutritional plant responses. Applied and Environmental Microbiology. 1995;61:456–460. doi: 10.1128/aem.61.2.456-460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Lozano JM, Porcel R, Aroca R. Does the enhanced tolerance of arbuscular mycorrhizal plants to water deficit involve modulation of drought-induced plant genes? New Phytologist. 2006;171:693–698. doi: 10.1111/j.1469-8137.2006.01841.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis TA. Molecular cloning: a laboratory manual. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sánchez-Blanco MJ, Ferrández T, Morales MA, Morte A, Alarcón JJ. Variations in water status, gas exchange, and growth in Rosmarinus officinalis plants infected with Glomus deserticola under drought conditions. Journal of Plant Physiology. 2004;161:675–682. doi: 10.1078/0176-1617-01191. [DOI] [PubMed] [Google Scholar]
- Schraut D, Heilmeier H, Hartung W. Radial transport of water and abscisic acid (ABA) in roots of Zea mays under conditions of nutrient deficiency. Journal of Experimental Botany. 2005;56:879–886. doi: 10.1093/jxb/eri080. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Zeevaart JAD. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiology. 2003;131:1591–1601. doi: 10.1104/pp.102.017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- Siemens JA, Zwiazek JJ. Changes in water flow properties of solutions culture-grown trembling aspen (Populus tremuloides) seedlings under different intensities of water-deficit stress. Physiologia Plantarum. 2004;121:44–49. doi: 10.1111/j.0031-9317.2004.00291.x. [DOI] [PubMed] [Google Scholar]
- Smart LB, Moskal WA, Cameron KD, Bennett AB. MIP genes are down-regulated under drought stress in Nicotiana glauca. Plant and Cell Physiology. 2001;42:686–693. doi: 10.1093/pcp/pce085. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. San Diego: Academic Press; 1997. [Google Scholar]
- Steudle E. Water uptake by plants roots: and integration of views. Plant and Soil. 2000;226:45–56. [Google Scholar]
- Strizhov N, Abraham E, Okresz L, Blickling S, Zilberstein A, Schell J, Koncz C, Szabados L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. The Plant Journal. 1997;12:557–569. doi: 10.1046/j.1365-313x.1997.00557.x. [DOI] [PubMed] [Google Scholar]
- Suga S, Komatsu S, Maeshima M. Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant Cell Physiology. 2002;43:1229–1237. doi: 10.1093/pcp/pcf148. [DOI] [PubMed] [Google Scholar]
- Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR. Molecular characterization of the Arabidopsis 9-cis-epoxycarotenoid dioxygenase gene family. The Plant Journal. 2003;35:44–56. doi: 10.1046/j.1365-313x.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- Taylor IB, Burbidge A, Thompson AJ. Control of abscisic acid synthesis. Journal of Experimental Botany. 2000;51:1563–1574. doi: 10.1093/jexbot/51.350.1563. [DOI] [PubMed] [Google Scholar]
- Toussaint JP. Investigating physiological changes in the aerial parts of AM plants: what do we know and where should we be heading? Mycorrhiza. 2007;17:349–353. doi: 10.1007/s00572-007-0133-6. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812. doi: 10.1007/s00114-007-0254-y. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M. ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiology. 1987;84:61–66. doi: 10.1104/pp.84.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Li L. Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cis-epoxycarotenoid dioxygenase gene. Biochemical and Biophysical Research Communications. 2006;347:1030–1038. doi: 10.1016/j.bbrc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Wan X, Steudle E, Hartung W. Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and HgCl2. Journal of Experimental Botany. 2004;55:411–422. doi: 10.1093/jxb/erh051. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant, Cell and Environment. 2002;25:195–210. doi: 10.1046/j.0016-8025.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu JK. Regulation of abscisic acid biosynthesis. Plant Physiology. 2003;133:29–36. doi: 10.1104/pp.103.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yu CL, Shi F, Wei YQ, Wang CC, Hu HT, Cheng CG. Effects of abscisic acid on growth and dehydration tolerance of Cynanchum komarovii seedlings. Plant Growth Regulation. 2007;51:177–184. [Google Scholar]
- Zeevaart JAD. Abscisic acid metabolism and its regulation. In: Hooykaas MA, Hall MA, Libbenga KR, editors. Biochemistry and molecular biology of plant hormones. New York: Elsevier Science; 1999. pp. 189–207. [Google Scholar]
- Zhang J, Jia W, Yang J, Ismail AM. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Research. 2006;97:111–119. [Google Scholar]
- Zhang J, Zhang X, Liang J. Exudation rate and hydraulic conductivity of maize roots are enhanced by soil drying and abscisic acid treatment. New Phytologist. 1995;131:329–336. [Google Scholar]
- Zhu C, Schraut D, Hartung W, Schäffner AR. Differential responses of maize MIP genes to salt stress and ABA. Journal of Experimental Botany. 2005;56:2971–2981. doi: 10.1093/jxb/eri294. [DOI] [PubMed] [Google Scholar]