Abstract
Lipid modifying enzymes play a key role in the development of cold stress tolerance in cold-resistant plants such as cereals. However, little is known about the role of the endogenous enzymes in cold-sensitive species such as cotton. Delta 12 fatty acid desaturases (FAD2), known to participate in adaptation to low temperatures through acyl chain modifications were used in gene expression studies in order to identify parameters of plant response to low temperatures. The induction of microsomal delta 12 fatty acid desaturases at an mRNA level under cold stress in plants is shown here for first time. Quantitative PCR showed that though both delta 12 omega 6 fatty acid desaturase genes FAD2-3 and FAD2-4 identified in cotton are induced under cold stress, FAD2-4 induction is significantly higher than FAD2-3. The induction of both isoforms was light regulated, in contrast a third isoform FAD2-2 was not affected by cold or light. Stress tolerance and light regulatory elements were identified in the predicted promoters of both FAD2-3 and FAD2-4 genes. Di-unsaturated fatty acid species rapidly increased in the microsomal fraction isolated from cotton leaves, following cold stress. Expression analysis patterns were correlated with the observed increase in both total and microsomal fatty acid unsaturation levels suggesting the direct role of the FAD2 genes in membrane adaptation to cold stress.
Keywords: Cold stress, cotton, desaturase, FAD2, lipid modification
Introduction
Plant response to cold stress is a complex process involving several membrane, cytoskeletal, and cytosolic elements responsible for cold perception, signal transmission, and, finally, development of cold stress resistance through the up-regulation of cold-related genes (Jonak et al., 1996; Murata and Los, 1997; Jaglo-Ottosen et al., 1998; Johnson and Cornell, 1999; Knight, 2000; Abdrakhamanova et al., 2003). As with any adaptation process, resistance to cold stress results from an interplay of several pathways dependent on hormonal regulation as well as on other environmental and physiological factors (Fowler and Limin, 2004; Li et al., 2004; Ludwig et al., 2005; Vandenbussche et al., 2003).
Cold stress can be differentially defined depending on the plant species. Chilling, non-freezing temperatures may lead to the acclimation of cold-tolerant species, including the development of resistance under freezing temperatures. Such temperatures, however, may result in damage of cold-sensitive plants affecting growth and yield. In addition, anti-freezing proteins induced in cold-resistant plants under cold temperatures may exist in cold-sensitive plants with the same catalytic activity. However, their induction in the latter occurs in response to pathogens instead of cold (Kirsch et al., 1997; Gobel et al., 2001).
The study of low temperature adaptation mechanisms of the cold-tolerant plant Arabidopsis thaliana are of great interest for other cold-tolerant species such as cereals. However, little is known about the mechanisms of cold perception by cold-sensitive plants and the possibility of existence of cold-acclimation processes in such species.
Cotton is a subtropical crop of high economical interest and its cultivation has been expanded from tropical and subtropical to colder regions. Temperatures under 15 °C adversely affect plant development, resulting in poor germination and plant exposure to attack by fungi and other disease-causing organisms affecting plant development and final yield. Although the cultivation of the plant has been extended to cold regions, the mechanisms of adaptation of such varieties under such conditions remain unknown. Furthermore, there is no information at a molecular level related to the response of cotton to low temperatures.
A group of enzymes responsible for membrane lipid modification and, consequently, membrane re-ordering are the fatty acid desaturases. Their role in the production of unsaturated fatty acid species has been demonstrated using prokaryotic and yeast mutants as well as gene expression analysis. In the Synechocystis sp. PCC 6803 the levels of the mRNAs transcribed from the genes that encode the delta 6, delta 12, and omega 3 desaturases increased about 10-fold, but at different rates, upon a decrease in temperature from 34 °C to 22 °C, whereas the level of the mRNA for the delta 9 desaturase remained constant (Los et al., 1997). FAD2 desaturases present particular interest since their modifying ability under cold-stress responses has been attributed solely to post-transcriptional/post-translational modifications rather than an increase in their mRNA levels (Tang et al., 2005). With the exception of FAD8 and FAD7 omega 3 desaturases (Berberich et al., 1998; Matsuda et al., 2005), which are induced under low temperatures, the induction of other desaturases under stress responses has not been demonstrated in higher plants (Falcone et al., 2004). A microarray analysis has also suggested induction in delta 9 desaturase under cold stress in A. thaliana (Kreps et al., 2002).
Microsomal omega 6 desaturases (FAD2) are the only desaturases isolated and characterized in G. hirsutum. They insert a double bond between carbons 12 and 13 of mono-unsaturated oleic acid to generate di-unsaturated linoleic acid (Panpoom et al., 1998). The FAD2-1 (Liu et al., 2001) is a seed-specific desaturase responsible for the polyunsaturated fatty acids in the seed oil of cultivated cotton. FAD2-2 has a low level constitutive expression and is expressed at a low level throughout seed development (Pirtle et al., 2001). A third member of the family, a FAD2-3 gene, was cloned (Pirtle et al., 2001). The study suggested a possible light regulation of this member and showed the existence of a big intron in the 5′-UTR that was suggested to enhance gene expression. Both the genomic and cDNA sequence of a fourth member of the FAD2 family (FAD2-4) have been reported (IL Pirtle et al., unpublished data).
In this study, the response and adaptation of cotton to low temperatures was studied through expression analysis of the FAD2 genes, and was aimed at the identification of response stages as well as of other environmental factors that may affect this response.
Materials and methods
Plant material
Plant material (cotelydons, new leaves, and roots) was obtained from G. hirsutum, Acala SJ2 from Delta and Pine land. Plants were grown in a Percival Scientific, Inc. Series 982 Microprocessor.
Material was collected from 7-d-old plants grown under a photoperiod of 16:8 h (light:dark) and a light intensity of 45 μmol m−2 s−1 diffuse light. Plants were grown at 32 °C for the first 4 d in order to speed up germination and at 22 °C for the last 3 d. Cold-stress treatments were performed under continuous light of 45 μmol m−2 s−1 (l) and 15 μmol m−2 s−1 (dl, dim light). Plant material was collected, snap-frozen in liquid nitrogen, and stored at –80 °C. Microsomes were prepared by homogenizing fresh material and, following filtration, spinning it at 2000 g for 15 min to collect nuclei and entire chloroplasts, and then at 10 000 g for 30 min to collect the remaining fragmented chloroplasts and mitochondria, and finally at 100 000 g for 1 h where the microsome-enriched fraction was collected.
Gene isolation cloning and expression analysis
Cloning and quantitative PCR:
RNA for RT-PCR reactions was isolated with the RNA isolation kit (Qiagen). A 1200 bp fragment from the coding region of the delta 12 fatty acid desaturase (FAD2-3) (AF331163) was isolated using the following primers: Forward: 5′-ATGGGTGCAGGTGGCAGAATGTCGG-3′, Reverse: 5′-GGTGAGCAGAGCAGCAAAGGTGTA-3′. Cloning of the PCR products was performed using TOPO TA cloning in a pCR II TOPO vector (Invitrogen, UK).
For the purpose of real-time quantitative-PCR, 2 μg of total RNA extracted from cotyledons and roots was used. RNA was transcribed using Superscript II Reverse Transcriptase (Invitrogen, UK) and random hexamers. qPCR was performed in a 20 μl total volume of Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, UK) following addition of the cDNA and the gene-specific primers. Reactions were performed in an Opticon2 DNA engine (MJ Research). Triplicates were used in each experiment and two experiments were performed. Primers designed for ubiquitin 4 (Forward: 5′-GAAGGCATTCCACCTGACCAAC-3′ and Reverse: 5′-CAAGCACAAGAAGAAGAAGGTCAAG-3′) were used in the following thermocycler conditions: 94 °C for 2 min, 36 cycles of 94 °C for 30 s, 60 °C for 20 s, 72 °C for 20 s, plate read at 81 °C for 0.2 s followed by 72 °C for 2 min. For FAD2-1 the following primers were used: Forward: 5′-ATTTCGGGGTGTTGAACAAAGTGTT-3′ and Reverse-5′-CCCTCCACATTGCCTTGTAAATC-3′. Thermocycler conditions were: 94 °C for 2 min, 36 cycles of 94 °C for 30 s, 62 °C for 18 s, 72 °C for 18 s, plate read at 80 °C for 0.2 s followed by 72 °C for 2 min. An 189 bp fragment was amplified using primers designed according to the FAD2-2 sequence: Forward: 5′-GATGAGAGGAGCTTTATCAACTGTG-3′ and Reverse: 5′-TAGACAGGCATCCCATCGAACTG-3′. Thermocycler conditions were: 94 °C for 2 min, 36 cycles of 94 °C for 30 s, 64 °C for 18 s, 72 °C for 18 s, plate read at 80 °C for 0.2 s followed by 72 °C for 2 min. The following specific primers designed for FAD2-3 were used: Forward: 5′-TACGACTCATCCGAATGGGACT-3′ and Reverse: 5′-TCTCCCAATATTGGTTTTATTGCCTTA-3′. Thermocycler conditions were: 94 °C for 2 min, 36 cycles of 94 °C for 30 s, 62 °C for 18 s, 72 °C for 18 s, plate read at 80 °C for 0.2 s followed by 72 °C for 2 min. For FAD2-4 the following specific primers were used: Forward: 5′-CACTACGACTCATCCGAATGGGATT-3′ and Reverse: 5′-TCGCCCAATATTGGCTTTATTGCCTTT-3′. Thermocycler conditions were: 94 °C for 2 min, 36 cycles of 94 °C for 30 s, 65 °C for 18 s, 72 °C for 18 s, plate read at 80 °C for 0.2 s followed by 72 °C for 2 min. FAD2-3 primers amplified an 182 bp fragment and FAD2-4 primers an 185 bp fragment.
For AtFAD2, AtFAD8, AtFAD6, AtRD29, and Atubiquitin7 the following primers were used: AtFAD2F: 5′-CACTACGATTCATCAGAGTGGGACT-3′, AtFAD2R: 5′-TCTCCCAGAATTGGCTTTATCGCCTTT-3′, AtFAD8F: 5′-ATGGCGAGCTCGGTTTTATCAGAATG-3′, AtFAD8R: 5′-GGCCAAGGACAAAGAGAGCCCAGAAC-3′, AtFAD6F: 5′-CTTCCCAAAGAGGTGTTTGAGATTGATGA-3′, AtFAD6R: 5′-CATGACACACAGTCATTATCGTCTTCATC-3′, AtRD29F: 5′-CTGGAGGAGTACCGGAGATTGCTGA-3′, AtRD29R: 5′-CTGCACGGGAACAACAGTGGAGCCA-3′, AtUBA7F: 5′-GAGGGCATACCACCTGATCAAC-3′, AtUBA7R: 5′-GTCGGTTGGTTCGATATCAATCTCG-3′. Thermocycler conditions were: 94 °C for 2 min, 36 cycles of 94 °C for 30 s, 61 °C for 18 s, 72 °C for 90 s, plate read at 80 °C for 0.2 s followed by 72 °C for 2 min.
Quantitation of gene expression was performed as described by Livak and Schmittgen (2001). Ubiquitin was used as an internal control and the lowest expression of the 22 °C treatment as a calibrator (Livak and Schmittgen, 2001; Athanasiadou et al., 2005).
Northern and southern blotting
RNA used in gene expression analysis by northern blotting was isolated using a solution containing 8 M gunadinium chloride and 20 mM MES, pH 7.0. Gene expression analysis in cotton is hampered by difficulties in RNA extraction. Reducing temperature to 22 °C a few days before tissue collection in combination with a very fast extraction method improved RNA yield from cotyledons and reduced RNA degradation (results not shown). This is possibly due to reduction in phenolic compounds which may accumulate at higher levels under high temperatures (Rivero et al., 2001).
Northern blotting was used for gene expression analysis at high stringency conditions. An equal amount of RNA was loaded (10 μg) on a 1.2 (w/v) agarose gel containing formaldehyde and transferred to an N+ nylon membrane in 20× SSC (3 M NaCl, 0.3 M citric acid, pH 7.0). Following UV crosslinking, hybridization was performed in Dig Easy Hyb solution (Dig Northern Starter kit, Roche) for 16 h at 50 °C using 25 ng ml−1 probe. Membranes were washed twice for 15 min each with 2× SSC and 0.1% SDS at room temperature, twice for 15 min with 0.5× SSC and 0.1% SDS at 68 °C. Antibody hybridization and detection were performed according to the manufacturer's instructions.
Lipid extraction and identification
Lipid extraction from frozen material was performed according to Bligh and Dyer (1959). Preparation of methyl ester derivatives was performed according to Sen et al. (1981). Briefly the lipids were methylated by heating the samples at 70 °C for 30 min in boron trifluoride/methanol 14% and the FA methyl esters were extracted twice with diethyl ether which was dried with sodium sulphate The samples were then dried under N2, resuspended in hexane and analysed by GC.
GC analyses were performed by a Hewlett-Packard GC (flame-ionization detector) on a BPX70-coated fused-silica capillary column (25.0 m×0.32 mm i.d., 0.2 μm film thickness) (SGE, Ringwood, Australia) under programmed conditions: 90 °C for 1 min then rising at 7 °C min−1 to 120 °C, then at 3 °C min−1 to 160 °C, and finally at 4 °C min−1 to 200 °C for 6 min, followed by a post run at 240 °C for 2 min. Carrier gas was He at a flow rate of 1 ml min−1. Commercially obtained FA methyl esters were used as standards (Fig. 6a, e).
Fig. 6.
(a) GC profiles of fatty acid methyl esters of A, control; B, 10 °C/24 h, light; C, 10 °C/48 h, light; D, 10 °C/48 h, dark. The samples were extracted and analysed as described in the Materials and methods. Fatty acids were identified by comparison of retention times with those of the standard mixture shown in E (GC profiles of standard fatty acid methyl esters, obtained from 1.0 μl injections). Profiles presented are indicative of the one of the two experiments performed. (b) Ratios of unsaturated and saturated fatty acids under different temperature and light conditions. control: 22 °C; 24 h and 48 h at 10 °C, l, light; d, dark; y axis: ratio values. Cotyledons of 8–10 plants were used for each experiment and values are average of three independent experiments. SD (P <0.05).
Promoter prediction
Promoter prediction and search for regulatory elements was performed using the database available at: http://softberry.com. TSSs were determined using the TSSP program available at the site. NSITE-PL was used for recognition of plant regulatory motifs.
Results
Promoter prediction and identification of regulatory elements in the FAD2 promoter of A. thaliana and FAD2-3 and FAD2-4 promoters of G. hirsutum
Previous studies (Pirtle et al., 2001) suggested that the promoter region of the FAD2-3 contains several light-responsive elements. For our study, the TSSP program was used first for plant promoter prediction (Shahmuradov et al., 2005). The program predicted one promoter for FAD2-3 and one promoter for FAD2-4, considering that 2967 bps upstream the ATG are an intron (Pirtle et al., 2001). FAD2-3 and FAD2-4 TSS were predicted at position 188 of the submitted sequences, 3080 bps upstream the ATG. One enhancer and two promoters were predicted for AtFAD2. TSSs for the promoters were predicted 1372 and 1807 bps upstream the ATG and for the enhancer 1255 bps upstream the ATG (see Supplementary data at JXB online).
Light regulatory elements were identified in all promoters as well as stress-tolerance-related elements (ABRE and DREB) in the predicted promoters of FAD2-3 and FAD2-4 (Table 1; see Supplementary data at JXB online). However, ABRE1/2 and ABRE3 promoter elements of the RD29B were identified in the promoters of FAD2-3 and FAD2-4 of G. hirsutum, but not in the promoters of FAD2 from A. thaliana (see Supplementary data at JXB online).
Table 1.
Regulatory elements identified in common in the predicted promoters of FAD2-3 and FAD2-4 from G. hirsutum and FAD2 from A. thaliana (also see Supplementary data at JXB online)
| Promoter element | Description | GhFAD2-AtFAD2 |
| CHS/RE: BOXII | Part of a light responsive element/Petroselinum hortense | CCACGTGcCg |
| CHS/RE: ACE | ACE: cis-acting element involved in light responsiveness/Petroselinum hortense | CCACGTGcCg |
| AtEm6/RE: ABRE | ABRE: cis-acting element involved in the abscisic acid responsiveness/Arabidopsis thaliana | GgCACGTGGa |
| Adh/:-214 G-box | cis-acting regulatory element involved in light responsiveness | GgCACGTGGA |
| Chs/RE:G-box | cis-acting regulatory element involved in light responsiveness | tcCACGTGCC |
| Patatin/RE: G-box | cis-acting regulatory element involved in light responsiveness | TCCACGTGCC |
| vspB/RE: G-box | cis-acting regulatory element involved in light responsiveness | TcCACGTGCc |
| PI-II/RE:G-box | cis-acting regulatory element involved in light responsiveness | TcCACGTGCc |
| Em/RE: Em1b | G-box element required for transcription | tcCACGTGCC |
Expression analysis of FAD2 under low temperatures
Tissue-specific expression analysis of FAD2 using a full-length probe designed according to nucleotide information of the FAD2-3 and FAD2-4 mRNA (see Materials and methods), suggested that the gene is ubiquitously expressed in all tissues with a very low expression level in cotyledons and new leaves (Fig. 1a). For the northern study the gene is referred to as FAD2 since the full-length probe designed using two external primers cannot differentiate between FAD2-3 and FAD2-4 genes.
Fig. 1.
(a) Expression analysis of FAD2 in leaves (L), cotelydons (C), shoots (S), and roots (R). RNA was extracted from young leaves of 20-d-old plants as well as from cotyledons, shoots, and roots of 7-d-old plants. (b) Expression analysis of FAD2 in plants grown at different growth temperatures and transferred to 10 °C. Plants were grown for 4 d at 32 °C. Some plants were treated at 20 °C for 24 h. Then all plants were transferred to 10 °C and time points were collected as indicated. (c) Expression analysis of FAD2 under dim light and dark conditions in plants exposed to 10 °C. (d) Expression analysis of FAD2 in 7-d-old plants grown at 22 °C and exposed to 10 °C for 0, 24, 48, 72, and 96 h. Plants were kept in light (l), dim light (dl), or dark (d). The FAD2 expression is independent of light intensity. (e) Expression analysis of FAD2 in unwounded (0) and wounded plants.
FAD2-2 has an 85% identity at an amino acid level with FAD2-3 and FAD2-4 isoforms. FAD2-3 and FAD2-4 have a 98.5% identity at a nucleotide level and six amino acids difference. FAD2-3 and FAD2-4 were incorporated in the G. hirsutum genome following fusion of the two genomes of G. arboreum and G. raimondi. Primers designed according to the FAD2-4 sequence (Fig. 5b) detect the expected fragment in G. hirsutum but not in G. arboreum. Primers designed according to the FAD2-3 sequence detect a band in both G. hirsutum and G. arboreum (results not shown). In order to assess their role in the cold stress response, their expression patterns were followed using both northern analysis and quantitative PCR with gene-specific primers so that possible differences in the expression patterns of the three genes could be identified.
Fig. 5.
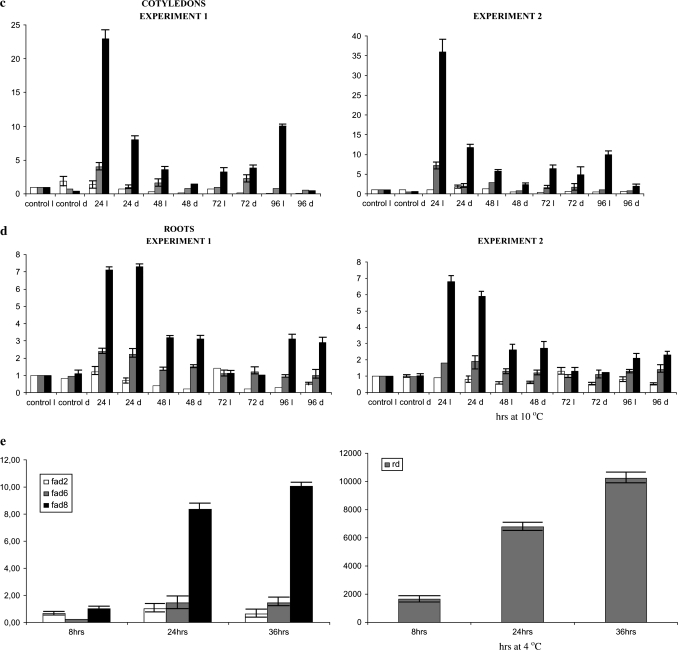
(a) Semi-quantitative PCR of FAD2 under cold stress (+RT. –RT, and no-RT control, C: 22 °C). (b) Alignment of FAD2-2, FAD2-3, and FAD2-4 sequences from G. hirsutum and FAD2 sequence from A. thaliana. Arrows indicate primers used in the QPCR. (c, d) Quantitative PCR of FAD2-2, FAD2-3, and FAD2-4 following treatment of cotton plants under growth temperature (22 °C, control) and 10 °C and two different light conditions [light (l), and dark (d.)] in cotyledons (c) and roots (d). The values of the y axis are the mean fold increase relative to the control light assigned value 1 (see Materials and methods). White, FAD2-2; grey, FAD2-3; black, FAD2-4. (e) Quantitative PCR of FAD2, FAD6, FAD8, and RD29 following treatment of Arabidopsis plants under 22 °C (control) and 4 °C (for 8, 24. and 36 h cold). The values of the y axis are relative to the calibrator (control assigned value 1).
Seven-day-old seedlings were collected at various time points following low temperature incubation. Although FAD2 is ubiquitously expressed, for the purposes of this experiment only cotyledons were used where the expression of the genes is very low under growth temperatures. It is known that cotton germination is adversely affected at termperatures under 15 °C. A study by Rikin et al. (1993) suggested that a temperature of 20 °C induces acclimation in cotton. Since their results were correlated with an increase in the di-unsaturated lipid content, FAD2 expression at 32 °C and 20 °C was checked. No difference in FAD2 expression was found in plants grown between 32 °C and 20 °C (in both cases FAD2 levels in cotelydons were very low) (Fig. 1b). It was assumed therefore that a low temperature study should be performed at temperatures below 15 °C. It was also assumed that temperatures under which FAD2 induction occurs could be acclimation temperatures, whereas no induction should be observed when plants are transferred from growth temperatures directly to cold temperatures (<4 °C).
Taking into account the promoter prediction analysis suggesting light regulation of the FAD2-3 and FAD2-4 genes, the experiments were performed under both light and dark conditions in order to test any possible differences in gene response. A 10 °C temperature was initially applied considering it to be a low temperature but not one that would cause direct tissue damage. Two different light intensities were applied in order to test the possible effects of light intensity on gene expression (45 μmol m−2 s−1 (l) and 15 μmol m−2 s−1 (dl, dim light) and in order to establish that the dim light used in the cold-stress experiments for the avoidance of the photoinhibition had no effect on gene expression. The results showed that induction of FAD2 following a 24 h incubation at 10 °C is light dependent (Fig. 1c). This mRNA accumulation showed a periodic behaviour, decreasing to the 22 °C levels following a 48 h incubation and re-establishing its 24 h induction after 96 h (Fig. 1d). The induction of FAD2 was inhibited by incubation at 10 °C under dark conditions but appeared unaffected by light intensity (Fig. 1d). The possibility that the gene may also respond to other factors such as wounding was also examined (Fig. 1e). FAD2 was shown to be wound-inducible, a fact that should be taken into account in the mRNA expression and lipid content analysis. The study by Pirtle et al. (2001) suggested that the members of the FAD2 gene family do not cross-hybridize and phylogenetic analysis with other desaturases shows that the FAD2 are distantly related to other FADs.
Incubation of plants under lower temperatures shifted gene expression from the 24 h mRNA accumulation to earlier time points (Fig. 2). The observed re-induction was also shifted from 96 h to 72 h when plants were stressed at 6 °C instead of at 10 °C (Fig. 2). RNA levels were adversely affected when temperature was reduced to 4 °C, resulting in a very poor RNA yield.
Fig. 2.
Expression analysis of FAD2 in cotyledons of 7-d-old plants exposed to different low temperatures. FAD2 is differentially expressed following exposure to different low temperatures.
The effect of gene induction under low, non-freezing temperatures on plant survival was tested by assessing plant survival following plant exposure to cold (4 °C) after plants had been exposed to 10 °C. It was found that prolonged exposure to low temperatures (either 10 °C alone or 10 °C followed by 4 °C had an adverse effect on plant survival (results not shown) (see Appendix 1 in the Supplementary data at JXB online). An important finding was, however, the fact that plants had attained a much higher survival rate when exposed to cold in the dark, confirming the dominant effect of photoinhibition that plants suffer under cold and light (Moon et al., 1995).
FAD2 expression in light and dark and its tissue-specific expression patterns
Since the induction of FAD2 observed is expected to play a decisive role in membrane modification and plant adaptation to low temperatures, an attempt was made to establish a time point after which dark conditions would affect gene expression. In Fig. 3a it appears that the FAD2 expression is reduced following a 24 h incubation in the dark. Under normal growth temperatures (22 °C), where no gene induction is observed, FAD2 mRNA levels appear reduced following at least a 4 h incubation in dark conditions (Fig. 3a).
Fig. 3.
(a) Expression of FAD2 in cotyledons of 7-d-old plants grown at 22 °C; dl, dim light; d: dark. FAD2 down-regulation starts following at least a 4 h treatment under dark conditions. (b) Accumulated mRNA of FAD2 under cold stress in the presence of light is still present following dark treatment. Plants were incubated for 16 h at 10 °C in dim light followed by an 8 h or 2 h treatment in the dark. 0 h at 10 °C represents plants grown at 22 °C in dim light. 24 h dark (d) represents plants grown at 22 °C followed by 10 °C treatment in the dark for 24 h.
The question of the physiological importance of light regulation on FAD2 under cold conditions was addressed. Considering a photoperiod of a 16 h day and an 8 h night, the effect of the absence of light on FAD2 in plants incubated for 16 h under light conditions followed by 8, 2, and 0 h dark (Fig. 3b) was tested. Incubation of plants at 10 °C under dark conditions resulted in FAD2 mRNA accumulation, comparable to the accumulation under light and the growth temperature. FAD2 mRNA accumulated for 16 h at 10 °C was not abolished, even following an 8 h incubation at 10 °C in the dark (Fig. 3b).
Since light appeared an important factor in FAD2 expression, the role of the enzyme in tissues where light is difficult to penetrate, such as roots (it is known that only 5% of light passes to the roots) was questioned. Roots were collected from light-grown (dl), dark-grown (d) plants and from plants where the roots were in absolute darkness (l/d).
Plants were treated at 22 °C for 24 h (0: dl, d, and l/d) as well as at 10 °C for 8, 24, 48, and 72 h. Although dark treatment abolished gene expression in cotyledons, the dark treatment did not affect gene expression in roots. However, when roots were under absolute darkness, but light was available for the cotyledons, a shift in gene expression was observed as early as 8 h instead of 24 h (Fig. 4).
Fig. 4.
Expression analysis of FAD2 in cotton under 10 °C and different light treatments. One day before cold treatment, some plants were transferred to darkness (d) or continuous light (dl). In some plants placed in light it was ensured that roots were completely covered so that no light penetrated (l/d). The experiment was repeated at 10 °C for 8 h and 24 h. Cotyledons and roots were collected as indicated.
Differentiation of expression patterns of the FAD2 genes identified in cotton (FAD2-2, FAD2-3, and FAD2-4) using real-time quantitative PCR
In order to confirm the results obtained from northern expression analysis, a real-time quantitative PCR approach was followed. A semi-quantitative PCR performed initially using two external primers showed that FAD2-3 (and FAD2-4) are detected following cold stress (Fig. 5a). For the qPCR, primers were designed so that they would specifically recognize the FAD2 genes identified in cotton, FAD2-1 (used as a negative control since no expression is expected in the photosynthetic tissue), FAD2-2, FAD2-3, and FAD2-4 (Fig. 5b). Since FAD2-3 and FAD2-4 genes exhibit a high nucleotide identity, primers were selected in a region such that the use of the suitable primer annealing temperature would not allow the detection of one of the two genes. i.e. FAD2-3 primers had a four nucleotide difference to FAD2-4 primers. Forward and reverse primers were selected so that they would have same melting temperature, and the maximum annealing temperature for signal detection was used. Primer specificity was confirmed by performing a PCR reaction on the genomic sequence of G. arboreum. Primers designed for the FAD2-3 QPCR detected a band in G. arboreum, however, primers designed for FAD2-4 did not detect a signal in G. arboreum confirming that the primers could only detect the FAD2-4 gene and do not cross-react with FAD2-3 (results not shown). The qPCR approach confirmed the northern experiment results, showing that both FAD2-3 and FAD2-4 isoforms are induced under cold stress in cotyledons and both are light regulated (Fig. 5c). It also confirmed the fact that, in root tissue, FAD2 induction is not light regulated.(Fig. 5c, d). However, it is clear that, in both cotyledons and roots, FAD2-4 is the gene responsible for supplying the majority of transcripts (Fig. 5c, d). Sequencing of the PCR products confirmed that FAD2-4 is the predominant gene induced under cold stress.
In order to confirm the difference in the induction of FAD2 from G. hirsutum from that of FAD2 genes from other species under cold stress, FAD2 from A. thaliana was quantitated using primers designed at the same region as that for FAD2-3 and FAD2-4 (Fig. 5e). Arabidopsis seedlings were treated at 4 °C under dim light prior to RNA extraction. RD29 was used as a positive control. FAD6 and FAD8 desaturases were used in order to compare their induction to that of FAD2. These results clearly show that both FAD2 and FAD6 are not induced under cold stress in A. thaliana and only in FAD8 was a 5-fold induction detected (Fig. 5e).
Lipid saturation under different temperature and light conditions
Analysis of acyl chain saturation by gas chromatography indicated an increase in di-unsaturated acyl chain species at low temperatures (10 °C) (Fig. 6a). As shown in Fig. 6a, the percentage of di-unsaturated lipids increased from 22.7% in plants grown at growth temperature to 28% in plants treated at 10 °C in light. An increase in di-unsaturation was observed in species containing 18 carbons in their acyl chains. The percentage of di-unsaturated species was light-dependent, the light effect was, however, prominent at low temperature (10 °C) (Fig. 6b). Total di-unsaturated lipid species increased, both in relation to saturated lipids as well as in relation to 18:1 and 18:3 lipids.
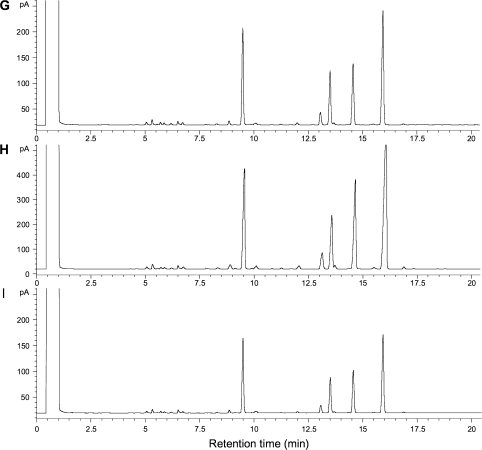
FAD2 are ER-localized microsomal desaturases containing a putative ER retrieval signal in their C-terminus (Dyer and Mullen, 2001; McCartney et al., 2004). In order to establish a relationship between FAD2-3 and FAD2-4 induction under cold stress and membrane adaptation to low-temperature conditions through the insertion of a second double bond to the mono-unsaturated lipids, GC analysis was performed with the microsomal fraction in plants treated for up to 96 h at 10 °C under both light and dark conditions. As shown in Fig. 7 and Table 2, there is a light-dependent increase in 18:2 lipid levels, showing the maximum increase 24 h following the application of low temperature. The increase in lipid unsaturation under cold stress is clearly light-dependent as far as it concerns the insertion of a second double bond in the mono-unsaturated lipid species a fact that is not apparent in the case of the mono and tri-unsaturated lipids (Table 2).
Fig. 7.
GC profiles of fatty acid methyl esters of A, control; B, 10 °C/24 h, light; C, 10 °C/24 h, dark; D, 10 °C/48 h, light; E, 10 °C/48 h, dark; F, 10 °C/72 h, light; G, 10 °C/72 h, dark; H, 10 °C/96 h, light; I, 10 °C/96 h, dark. The samples were extracted from the microsomal fractions and analysed as described in the Materials and methods. Profiles presented are indicative of the one of the two experiments performed.
Table 2.
Percentage of unsaturated and saturated fatty acids in lipid extracts of microsomal fractions from plants treated in different temperature and light conditions (l, light; d, dark), (24, 48, 72, and 96: h treatment)
| Treatment (% fatty acids) | |||||||||
| 22 °C l | 10 °C 24 l | 10 °C 24 d | 10 °C 48 l | 10 °C 48 d | 10 °C 72 l | 10 °C 72 d | 10 °C 96 l | 10 °C 96 d | |
| 16:0 | 25.0±0.6 | 23.8±0.4 | 25.1±1.6 | 23.4±2.5 | 21.6±0.6 | 25.9±0.6 | 24.1±2.8 | 22.3±1.6 | 27.8±0.8 |
| 18:0 | 3.7±0.6 | 4.5±0.4 | 3.8±0.1 | 4.0±0.2 | 3.7±0.0 | 3.4±0.1 | 3.7±0.0 | 4.0±0.0 | 3.3±0.1 |
| 18:1 | 12.7±0.1 | 16.0±0.2 | 16.2±0.4 | 15.3±0.8 | 13.6±0.1 | 15.1±0.4 | 16.4±0.5 | 11.7±0.1 | 15.1±0.0 |
| 18:2 | 22.5±0.2 | 26.7±0.3 | 24.8±0.4 | 24.0±0.6 | 20.3±0.2 | 23.3±0.3 | 18.5±0.5 | 20.2±0.1 | 17.8±0.0 |
| 18:3 | 36.3±0.1 | 29.2±0.5 | 30.1±0.7 | 33.6±0.9 | 40.0±0.2 | 32.5±0.3 | 37.4±1.8 | 41.8±1.3 | 36.1±0.9 |
Cotyledons of 8–10 plants were used for each experiment and values are average of two independent experiments. SD (P <0.05).
Discussion and conclusions
In order to study the G. hirsutum response to low temperatures and the possibility of plant acclimation under cold, the expression of a delta 12 fatty acid desaturase was followed.
The study of FAD2 isoforms appeared of particular interest since, in other plant species, the content in di- and tri-unsaturated fatty acids increases under cold stress. However, the increase in di-unsaturated fatty acids was not attributed to a rise in mRNA levels of the enzyme. A recent study on FAD2-3 from soybean (Li et al., 2006) was in accordance with the general acceptance indicating that the increase in di-unsaturated fatty acid composition under cold stress is due to post-transcriptional/post-translational modifications on the enzyme rather than to increases in its mRNA levels (Heppard et al., 1996; Falcone et al., 2004; Tang et al., 2005).
These results show for the first time an increase in mRNA levels of a membrane-modifying enzyme in cotton under cold stress. FAD2-3 and FAD2-4 mRNA levels increased as temperatures were lowered far below the lowest germination permissive temperature.
Promoter analysis of the delta 12 fatty acid desaturases led to the conclusion that the response of the gene under low temperatures and its effect on plant survival ability could be the result of the interplay of various factors generating signalling pathways, the cross-talk of which would determine gene response and plant adaptation to the new situation. Activation of the acclimation genes induced by ABA takes place as a result of interaction and eventual convergence of ABA-dependent and ABA-independent pathways. Although the ABA-signalling pathway is not so much directly related to the cold-stress response as to drought and salinity, abiotic factors that induce significant rises in its endogenous levels (Shinozaki and Yamaguchi-Shinozaki, 2000), its endogenous rise during winter seems to be due to dehydration. Its action converges with the dehydration effect on the Rd29A gene which contains in its promoter an ABRE and DRE/CRT (Liu et al., 1998). Analysis of the promoter elements of the FAD2 orthologues from G. hirsutum and A. thaliana indicated that ABRE1/2 and ABRE3 promoter elements of the RD29B were present in the promoters of FAD2-3 and FAD2-4 of G. hirsutum but absent from the predicted promoters of FAD2 from A. thaliana (see Supplementary data at JXB online).
Taking into account the thousand-fold induction of RD29 under cold stress (Fig. 5e), the significance of these elements in the promoter of a gene for its low-temperature induction could be speculated.
Signalling pathways may co-operate or antagonize the regulation of plant responses to stimuli. Differential regulation of gene response by transcription factors may determine the selected pathway under different environmental situations that share the same signal, for example, differential regulation of pathogen attack and wound response by MYC2 and ERF1 (Lorenzo and Solano, 2005). In A. thaliana a combination of low temperature and light is required for freezing tolerance enhancement (Wanner and Junttila, 1999). However, the light requirement for the enhancement of freezing tolerance was related to the accumulation of soluble sugars and proline rather than to the expression of cold-related genes.
The time of induction of both genes was dependent on the applied temperature. mRNA levels were higher at 10 °C whereas, when lower temperatures were applied, mRNA levels decreased, with a simultaneous shift from the 24 h induction to as early as 8 h at 6 °C and it was prolonged to 24 h. At 5 °C an induction at 8 h was observed. At 4 °C a low induction was observed as early as 8 h. This shift to earlier time points could be explained as an imminent cold perception as well as a mis-perception of cold stress, i.e. a strong cold stress could be perceived as mechanical damage. These results suggest that FAD2 may also respond to mechanical wounding (Fig. 1e).
Expression of both FAD2-3 and FAD2-4 under cold stress was light-dependent (Fig. 6). This could be due to an indirect hormonal effect or to a direct effect of light regulatory elements on the FAD2-3 ad FAD2-4 promoters. The fact that the light effect is likely to be the result of hormonal interplay was demonstrated by the tissue-specific expression patterns. Expression of FAD2 in roots was unaffected by light. This could be due to a differential hormonal regulation of the gene in roots and cotyledons. It is known that light inhibits ethylene synthesis, which in turn affects auxin distribution. The influence of light on gene expression under cold stress could therefore be regulated by the ethylene/auxin gradient which, in turn, is itself regulated by light (Vandenbussche et al., 2003). This could account for the earlier induction in FAD2 in cotyledons when no light is permitted to the roots. In addition, gene expression may be controlled by promoter domains differentially regulating induction or suppression in different tissues. This was demonstrated for another desaturase member, FAD7, the wound induction of which is mediated by distinct promoter domains (Nishiuchi et al., 1999). Whether light exerts a direct effect on cold-induced FAD2 requires a thorough analysis of their predicted promoter elements.
It is assumed that temperatures that induce an increase in the mRNA levels of FAD2-3 and FAD2-4 could be correlated to acclimation temperatures. As previously described (Chang et al., 2001), a 10 °C exposure of the chilling sensitive Vigna radiata (mungbean) protected it from injuries caused when plants were exposed to 4 °C. However, this was not the case for G. hirsutum. Acclimation experiments performed at a temperature of 10 °C, together with the lipid analysis data, suggested that, although the increase in mRNA levels may result in an increase in lipid unsaturation, no acclimation was achieved. A possible explanation could be that the 10 °C temperature causes damaging effects, the results of which cannot be overcome by the increase in lipid unsaturation. It is also possible that the increase in di-unsaturated fatty acid species at 10 °C could occur in order to protect the species from the direct 10 °C effect. The results indicated that the longer the exposure to low temperatures the higher the plant death rate was (see Supplementary data at JXB online). It was clear, however, that light played an important role in plant survival ability since plants achieved much higher survival rates when exposed to cold temperatures under dark conditions (Rikin et al., 1993). By contrast, although no increase in FAD2 occurs at 20 °C, increasing lipid unsaturation as observed by Rikin et al. (1993) may be achieved by post-transcriptional/translational modifications of FAD2 or other desaturase isoforms leading to the observed increase in the acclimation ability of the plant.
Supplementary data
Supplementary data can be found at JXB online and include the GhFAD2-3NSITEP results; the GhFAD2-4NSITEP results; the AtFAD2NSITEP results; and Appendix 1.
Supplementary Material
Acknowledgments
We would like to thank Dr N Argiriou for critical reading of the paper and Dr A Polidoros for useful suggestions for the QPCR analysis. We would also like to acknowledge the technical assistance of C Pasentsis as well as the assistance of Dr G Leondaritis for the presentation of the GC data. Part of the work was supported by the GSRT (Programme for the Support of Research Potential).
References
- Abdrakhamanova A, Wang QY, Khokhlova L, Nick P. Is microtubule disassembly a trigger for cold acclimation? Plant and Cell Physiology. 2003;44:676–686. doi: 10.1093/pcp/pcg097. [DOI] [PubMed] [Google Scholar]
- Athanasiadou R, Polidoros AN, Mermigka G, Nianiou-Obeidat I, Tsaftaris AS. Differential expression of CmPP16 homologues in pumpkin (Curcurbita maxima), winter squash (C. moschata) and their interspecific hybrid. Journal of Horticultural Science and Biotechnology. 2005;80:643–649. [Google Scholar]
- Berberich T, Harada M, Sugawara K, Kodama H, Iba K, Kusano T. Two maize genes encoding omega-3 fatty acid desaturase and their differential expression to temperature. Plant Molecular Biology. 1998;36:297–306. doi: 10.1023/a:1005993408270. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chang MY, Chen SL, Lee CF, Chen YM. Cold-acclimation and root temperature protection from chilling injury in chilling-sensitive mungbean (Vigna radiata L.) seedlings. Botanical Bulletin Academia Sinica. 2001;42:53–60. [Google Scholar]
- Dyer JM, Mullen RT. Immunocytological localization of two plant fatty acid desaturases in the endoplasmic reticulum. FEBS Letters. 2001;494:44–47. doi: 10.1016/s0014-5793(01)02315-8. [DOI] [PubMed] [Google Scholar]
- Falcone DL, Ogas JP, Somerville CR. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biology. 2004;4:17. doi: 10.1186/1471-2229-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DB, Limin AE. Interactions among factors regulating phenological development and acclimation rate determine low-temperature tolerance in wheat. Annals of Botany. 2004;94:717–724. doi: 10.1093/aob/mch196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel C, Feussner I, Schmidt A, Scheel D, Sanchez-Serrano J, Hamberg M, Rosahl S. Oxylipin profiling reveals the preferential stimulation of the 9-lipoxygenase pathway in elicitor-treated potato cells. Journal of Biological Chemistry. 2001;276:6267–6273. doi: 10.1074/jbc.M008606200. [DOI] [PubMed] [Google Scholar]
- Heppard EP, Kinney AJ, Stecca KL, Miao GH. Developmental and growth temperature regulation of two different microsomal omega-6 desaturase genes in soybeans. Plant Physiology. 1996;110:311–319. doi: 10.1104/pp.110.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Cornell RB. Amphitropic proteins: regulation by reversible membrane interactions (review) Molecular Membrane Biology. 1999;16:217–235. doi: 10.1080/096876899294544. [DOI] [PubMed] [Google Scholar]
- Jonak C, Kiegerl S, Ligterink W, Barker PJ, Huskisson NS, Hirt H. Stress signaling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proceedings of the National Academy of Sciences, USA. 1996;93:11274–11279. doi: 10.1073/pnas.93.20.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch C, Takamiya-Wik M, Reinold S, Hahlbrock K, Somssich IE. Rapid, transient, and highly localized induction of plastidial omega-3 fatty acid desaturase mRNA at fungal infection sites in Petroselinum crispum. Proceedings of the National Academy of Sciences, USA. 1997;94:2079–2084. doi: 10.1073/pnas.94.5.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H. Calcium signaling during abiotic stress in plants. International Review of Cytology. 2000;195:269–324. doi: 10.1016/s0074-7696(08)62707-2. [DOI] [PubMed] [Google Scholar]
- Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiology. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Developmental Cell. 2004;7:193–204. doi: 10.1016/j.devcel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Li L, Wang X, Gai J, Yu D. Molecular cloning and characterization of a novel microsomal oleate desaturase gene from soybean. Journal of Plant Physiology. 2006;164:1516–1526. doi: 10.1016/j.jplph.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Liu Q, Brubaker CL, Green AG, Marshall DR, Sharp PJ, Singh SP. Evolution of the FAD2-1 fatty acid desaturase 5′ UTR intron and the molecular systematics of Gossypium (Malvaceae) American Journal of Botany. 2001;88:92–102. [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. The Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Solano R. Molecular players regulating the jasmonate signalling network. Current Opinion in Plant Biology. 2005;8:532–540. doi: 10.1016/j.pbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Los DA, Ray MK, Murata N. Differences in the control of the temperature-dependent expression of four genes for desaturases in Synechocystis sp. PCC 6803. Molecular Microbiology. 1997;25:1167–1175. doi: 10.1046/j.1365-2958.1997.5641912.x. [DOI] [PubMed] [Google Scholar]
- Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O, Wasternack C, Boller T, Jones JD, Romeis T. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proceedings of the National Academy of Sciences, USA. 2005;102:10736–10741. doi: 10.1073/pnas.0502954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda O, Sakamoto H, Hashimoto T, Iba K. A temperature-sensitive mechanism that regulates post-translational stability of a plastidial omega-3 fatty acid desaturase (FAD8) in Arabidopsis leaf tissues. Journal of Biological Chemistry. 2005;280:3597–3604. doi: 10.1074/jbc.M407226200. [DOI] [PubMed] [Google Scholar]
- McCartney AW, Dyer JM, Dhanoa PK, Kim PK, Andrews DW, McNew JA, Mullen RT. Membrane-bound fatty acid desaturases are inserted co-translationally into the ER and contain different ER retrieval motifs at their carboxy termini. The Plant Journal. 2004;37:156–173. doi: 10.1111/j.1365-313x.2004.01949.x. [DOI] [PubMed] [Google Scholar]
- Moon BY, Higashi S, Gombos Z, Murata N. Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proceedings of the National Academy of Sciences, USA. 1995;92:6219–6223. doi: 10.1073/pnas.92.14.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Los DA. Membrane fluidity and temperature perception. Plant Physiology. 1997;115:875–879. doi: 10.1104/pp.115.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiuchi T, Kodama H, Yanagisawa S, Iba K. Wound-induced expression of the FAD7 gene is mediated by different regulatory domains of its promoter in leaves/stems and roots. Plant Physiology. 1999;121:1239–1246. doi: 10.1104/pp.121.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panpoom S, Los DA, Murata N. Biochemical characterization of a Δ12 acyl-lipid desaturase after overexpression of the enzyme in Escherichia coli. Biochimica et Biophysica Acta. 1998;1390:323–332. doi: 10.1016/s0005-2760(97)00190-2. [DOI] [PubMed] [Google Scholar]
- Pirtle IL, Kongcharoensuntorn W, Nampaisansuk M, Knesek JE, Chapman KD, Pirtle RM. Molecular cloning and functional expression of the gene for a cotton delta-12 fatty acid desaturase (FAD2) Biochimica et Biophysica Acta. 2001;1522:122–129. doi: 10.1016/s0167-4781(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Rikin A, Dillwith JW, Bergman DK. Correlation between the circadian rhythm of resistance to extreme temperatures and changes in fatty acid composition in cotton seedlings. Plant Physiology. 1993;101:31–36. doi: 10.1104/pp.101.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Ruiz JM, Garcia PC, Lopez-Lefebre LR, Sanchez E, Romero L. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Science. 2001;160:315–321. doi: 10.1016/s0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- Sen A, Williams WP, Quinn PJ. The structure and thermotropic properties of pure 1,2-diacylgalactosylglycerols in aqueous systems. Biochimica et Biophysica Acta. 1981;663:380–389. doi: 10.1016/0005-2760(81)90167-3. [DOI] [PubMed] [Google Scholar]
- Shahmuradov IA, Solovyev VV, Gammerman AJ. Plant promoter prediction with confidence estimation. Nucleic Acids Research. 2005;33:1069–1076. doi: 10.1093/nar/gki247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Current Opinion in Plant Biology. 2000;3:217–223. [PubMed] [Google Scholar]
- Tang GQ, Novitzky WP, Carol Griffin H, Huber SC, Dewey RE. Oleate desaturase enzymes of soybean: evidence of regulation through differential stability and phosphorylation. The Plant Journal. 2005;44:433–446. doi: 10.1111/j.1365-313X.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Vriezen WH, Smalle J, Laarhoven LJ, Harren FJ, Van Der Straeten D. Ethylene and auxin control the Arabidopsis response to decreased light intensity. Plant Physiology. 2003;133:517–527. doi: 10.1104/pp.103.022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner LA, Junttila O. Cold-induced freezing tolerance in Arabidopsis. Plant Physiology. 1999;120:391–400. doi: 10.1104/pp.120.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.