Abstract
Freshly harvested sunflower (Helianthus annuus L.) seeds are considered to be dormant because they fail to germinate at relatively low temperatures (10 °C). This dormancy results mainly from an embryo dormancy and disappears during dry storage. Although endogenous ethylene is known to be involved in sunflower seed alleviation of dormancy, little attention had been paid to the possible role of cyanide, which is produced by the conversion of 1-aminocyclopropane 1-carboxylic acid to ethylene, in this process. The aims of this work were to investigate whether exogenous cyanide could improve the germination of dormant sunflower seeds and to elucidate its putative mechanisms of action. Naked dormant seeds became able to germinate at 10 °C when they were incubated in the presence of 1 mM gaseous cyanide. Other respiratory inhibitors showed that this effect did not result from an activation of the pentose phosphate pathway or the cyanide-insensitive pathway. Cyanide stimulated germination of dormant seeds in the presence of inhibitors of ethylene biosynthesis, but its improving effect required functional ethylene receptors. It did not significantly affect ethylene production and the expression of genes involved in ethylene biosynthesis or in the first steps of ethylene signalling pathway. However, the expression of the transcription factor Ethylene Response Factor 1 (ERF1) was markedly stimulated in the presence of gaseous cyanide. It is proposed that the mode of action of cyanide in sunflower seed dormancy alleviation does not involve ethylene production and that ERF1 is a common component of the ethylene and cyanide signalling pathways.
Keywords: Cyanide, dormancy, ethylene, germination, Helianthus annuus L. (sunflower)
Introduction
Cyanide is a compound known to stimulate germination and to release dormancy of seeds of many species (Taylorson and Hendricks, 1973; Roberts and Smith, 1977; Bogatek and Lewak, 1988; Côme et al., 1988; Bethke et al., 2006). Seed dormancy is defined as the property of a seed that prevents its germination in apparently favourable conditions (Finch-Savage and Leubner-Metzger, 2006). Despite the well-described effects of cyanide on germination and dormancy, the cellular bases of its mechanism are poorly understood, and seem moreover to vary from one species to another. Different hypotheses have been proposed to explain the stimulatory effect of cyanide on germination and dormancy (Côme and Corbineau, 1989). According to Taylorson and Hendricks (1973), the cyanhydric gas could react with L-cysteine to give the β-L-cyanoalanine necessary for the synthesis of arginine and aspartic acids which could be limiting factors for germination. Hagesawa et al. (1994) suggested that this increase in the amino acid pool might also promote germination by decreasing the water potential in embryonic axis. However, other respiratory inhibitors which are not metabolized, such as NaN3 or Na2S, have the same effect as KCN in various species (Roberts and Smith, 1977; Côme and Corbineau, 1989). Some studies proposed that the beneficial effect of cyanide on germination might involve the cyanide-insensitive pathway (Esashi et al., 1979, 1981b; Upadhyaya et al., 1983), the pentose phosphate pathway (Roberts and Smith, 1977; Côme and Corbineau, 1989), the glycolysis (Bogatek, 1995) or the hydrolysis of oligosaccharides and their catabolism (Bogatek and Lewak, 1991; Bogatek et al., 1999). Cyanide is also known to interact with reactive oxygen species (ROS) metabolism; it is an inhibitor of Cu/Zn superoxide dismutase (SOD) (Bowler et al., 1992) and catalase (CAT) (Tejera García et al., 2007) and it has been demonstrated to induce oxidative stress and lipid peroxidation in animals (Johnson et al., 1987; Gunasekar et al., 1998). Oracz et al. (2007) recently demonstrated that cyanide could trigger protein oxidation during sunflower seed dormancy alleviation. At last, cyanide might also interplay with the ethylene signalling pathway. Indeed, hydrogen cyanide is a co-product of ACC oxidase, which converts ACC to ethylene (Peiser et al., 1984), and it has been proposed to stimulate ethylene biosynthesis via a feedback effect (Pirrung and Brauman, 1987). Thus Smith and Arteca (2000) demonstrated that the ACC synthase gene ACS6 was activated by cyanide in Arabidopsis. However, it is actually not known whether ethylene and cyanide share some molecular components of their downstream transduction pathways.
The putative relationship between cyanide and ethylene signalling pathways might be particularly relevant for sunflower seeds, whose dormancy is broken by ethylene (Corbineau et al., 1990). The inability of freshly harvested sunflower seeds to germinate at temperatures below c. 15 °C results from an embryo dormancy which is gradually eliminated during dry storage (Corbineau et al., 1990; Corbineau and Côme, 2003). Embryo dormancy is characterized by the inhibition of radicle extension thus preventing excised embryos to grow (Finch-Savage and Leubner-Metzger, 2006). Little attention has been paid to the possible role of cyanide in sunflower seed dormancy, despite the close relationship between ethylene and cyanide pathways. Therefore, sunflower seed is a very good model for studying the mechanisms of action of cyanide in seed germination, as related to ethylene. In particular, it could help to get a better understanding of the molecular events occurring downstream of the cyanide signal, and to determine whether it might share some homology with the ethylene transduction pathway. In Arabidopsis, ethylene is perceived by a family of five membrane-associated receptors: ETR1, ERS1, ETR2, ERS2, and EIN4 falling into two subfamilies (Wang et al., 2002). ETR1 and ERS1 belong to subfamily 1, and subfamily 2 is composed by ETR2, ERS2, and EIN4 (Moussatche and Klee, 2004). The ethylene receptors modulate the activity of the Raf-like kinase CTR1 which is a negative regulator of the pathway (Gao et al., 2003; Huang et al., 2003). Further downstream elements include positive regulators (EIN2, EIN5, EIN6) and transcription factors (EIN3 and EIL1), the former regulating gene expression of other transcription factors such as ERF1 (Guo and Ecker, 2004), which belongs to a large family of 65 ERF genes (Riechmann et al., 2000). ERF proteins bind to the GCC motif found in the promoter region of ethylene-regulated genes (Gu et al., 2002). ERF genes can also be activated by regulatory compounds such as ABA, jasmonate (Lorenzo et al., 2003; Zhang et al., 2004) or salicylic acid (Gu et al., 2002) which suggests that they might play a pivotal role in various cellular transduction pathways. Leubner-Metzger et al. (1998) demonstrated that ethylene-responsive element binding protein (EREBP) expression was involved in the regulation of glucanase during tobacco seed germination. Interestingly, recent studies also proposed that ERF genes would play a role in the germination of seeds of other species (Song et al., 2005; Pirrello et al., 2006).
The aims of the present study were to investigate whether exogenous cyanide might improve the germination of dormant sunflower seeds and to determine if this effect could result from an interaction with the ethylene biosynthesis or transduction pathway. It is demonstrated that cyanide activates some part of the ethylene transduction pathway but without stimulating ethylene production.
Materials and methods
Plant material
Sunflower (Helianthus annuus L., cv. LG5665) seeds were harvested in 2005 and 2006 near Montélimar (Drôme, France) and purchased from Limagrain. At harvest, dormant seeds were stored at –30 °C until use in order to maintain their dormancy or stored dry at 20 °C and 75% relative humidity for at least 3–4 months to break their dormancy. All the results presented in this study represent a mean of the data obtained from seeds harvested in both 2005 and 2006.
Germination tests
Germination assays were performed with naked seeds (i.e. seeds without pericarp) in darkness in 9 cm Petri dishes (25 seeds per dish, eight replicates) on a layer of cotton wool moistened with deionized water or with various solutions (ACC, AOA, CoCl2, and AIB). Petri dishes were placed at 10 °C, a suboptimal temperature for dormant sunflower seed germination (Corbineau et al., 1990). A seed was considered as germinated when the radicle had elongated to 2–3 mm. Germination counts were made daily for 14 d and the results presented are the means of the germination percentages obtained in eight replicates ±SD as a function of time. Germination speed is also expressed as T50 which corresponds to the time necessary to obtain 50% germination.
Treatments at various moisture contents
In order to equilibrate seeds at various moisture contents, naked seeds were incubated in the presence of appropriate amounts of deionized water in tightly closed glass containers placed on a roller overnight at 10 °C.
Seed moisture content was determined by oven-drying the seeds at 105 °C for 48 h.
Cyanide treatment
Sunflower seeds were treated by gaseous 1 mM HCN as described by Bogatek and Lewak (1988). Naked dry seeds were placed in a tightly closed container (500 ml) on a layer of cotton wool moistened with deionized water (50 seeds per container). A glass tube containing 5 ml of 0.1 M KCN solution placed in the container was used as a source of gaseous HCN, which was produced by acidifying the KCN solution with 5 ml of lactic acid (10%, v/v). After 1–48 h of treatment in darkness at 10 °C, containers were opened and gaseous cyanide released. Seeds were then rinsed carefully three times with deionized water before germination tests or biochemical analyses.
2,5-Norbornadiene treatment
Treatment or germination of seeds in the presence of 2,5-norbornadiene (NBD) was performed in tightly closed container (500 ml) in which 3.5 ml of NBD was injected. In each container, 50 sunflower embryos were placed on a layer of cotton wool moistened with deionized water at 10 °C.
NBD treatment was also carried out simultaneously with the cyanide treatment in 500 ml containers. After 3 h of treatment in the presence of gaseous HCN and NBD, embryos were rinsed three times with deionized water and placed on a layer of cotton wool moistened with deionized water in the presence of NBD at the same concentration in tightly closed 500 ml containers for germination assay.
Results presented correspond to the means of three replicates ±SD.
Determination of cyanide content
Measurement of HCN content was carried out according to Nagashima (1977) with some modifications. Naked seeds (0.8 g FW) were ground in a mortar in the presence of 8 ml of 0.02 M NaOH and 2.5 ml of the resulting extract were transferred to the main compartment of a 20 ml Warburg flask. The small chamber in the centre of the flask contained 0.4 ml of 0.4 M NaOH, in order to absorb liberated HCN, and 1 ml of 0.4 M KHSO4 was placed in the side arm. The flask was tightly closed and the content of the main compartment was acidified with the KHSO4 solution. After 2 h of incubation under agitation at room temperature, the NaOH solution, which absorbed HCN, was used for cyanide determination.
Cyanide content was determined spectrophotometrically, the reaction mixture containing 0.4 ml of 0.4 M NaOH (containing absorbed HCN), 1 ml phosphate buffer solution (0.1 M, pH 5.2), 50 ml of 1% (w/v) chloramine T and 0.6 ml of γ-picoline-barbituric acid reagent. Change in absorbance was monitored at 605 nm after 5 min at 25 °C, and compared with the absorbance obtained with known amounts of KCN.
Results are expressed as μmol g−1 DW and correspond to the means of the values ±SD obtained with three replicates from three independent experiments.
Measurement of ethylene production
The ethylene production was measured with 20 embryonic axes placed in 10 ml flasks containing 200 μl deionized water or ACC solution (1 mM). After 20 min of incubation, flasks were tightly closed with serum caps and placed at 20 °C. After 3 h, a 2.5 ml gas sample was taken from each flask and injected into a gas chromatograph (Hewlett Packard 5890 series II) equipped with a flame ionization detector and an activated alumina column for ethylene determination.
Results are the means of five measurements ±SD and are expressed as nl ethylene h−1g−1 DW.
Extraction of total RNA
Axes isolated from embryos using a sharp scalpel blade were frozen in liquid nitrogen, and then stored at –80 °C until use. For each extract, 25 axes were ground to a fine powder in liquid nitrogen, and total RNA was extracted by a hot phenol procedure according to Verwoerd et al. (1989). RNA concentration was determined spectrophotometrically at 260 nm.
Design of primers
The oligonucleotide primer sets used for real-time qPCR and semi-quantitative RT-PCR analysis were designed on the basis of sunflower gene or EST sequences. ACO primers were chosen in a conserved region (using multiple alignment, ClustalW) between sunflower ACO1, ACO2, and ACO3 genes in order to have the whole expression of the three isoforms (GenBank accession numbers: U62555, U62554, L29405). Candidate ACS, ETR2, ERF1, CTR1, and ERS1 sequences were found in the CGP EST database of sunflower (http://cgpdb.ucdavis.edu/) using the BLAST algorithm. Names of used EST, homology percentage with other plant sequences, amplified probe length and primer sets sequences are listed in Table 1.
Table 1.
Main characteristics of genes and primer sequences used in the present work
| Name of H. annnus L. gene | GenBank or CGP EST accession number | Amplification product size | Primer sequences | Homology percentage of EST sequences with other plants (plant, accession number) |
| HaACO | L 29405 | 124 | Left: GAAGTGTATGGAGCAGAGGTTT | |
| Right: GTTGGAGGTAGGGCGATG | ||||
| HaACS | QHG4g06.yg.ab1 | 126 | Left: CGGTTATTAGAGGGGGTAGTG | 80 (Arabidopsis thaliana, NM_116016) |
| Right: TATTGTGTCGGGAGGAGGA | ||||
| HaETR2 | QHF11C05.yg.ab1 | 131 | Left: AGTCGGAAGGCTCTGGTG | 77 (Cucumis sativus, AB026500) |
| Right: TCCTGTGGGATACGGAACT | ||||
| HaCTR1 | QHG13O10.yg.ab1 | 166 | Left: CCGTCCACTCTCTTGTAGGT | 84 (Arabidopsis thaliana, At5g03730.1) |
| Right: TCGTCGTCTGGCTCTTCT | ||||
| HaERF1 | QH_CA_Contig5791 | 176 | Left: TCTTGACTCAATCCAACACC | 86 (Arabidopsis thaliana, AF076278) |
| Right: ACTCTTGGTTTTCCACCACT | ||||
| HaERS1 | CHA_M2_Contig3981 | 346 | Left: GCCTATTTCTCCATTCCGTTG | 86 id bp (Chrysanthemum×morifolium, |
| Right: CGTGTCTTCCAGTTTCTTCTTG | AB128828) | |||
| HaETR1 | none | 208 | Left: CCDGTRATTAAYCAAGTKTT | 91 (Arabidopsis thaliana, NP 176808) |
| Right: GAAGGAAGCATCAAWACCAT | ||||
| HaEF1 | QH_CA_Contig2764 | 159 | Left: TCTCCACTCCTCCAACAC | 98 id aa (Lycopersicon esculentum, Swissprot |
| Right: CTCAATCACTCGCTACACC | accession P17786) | |||
| Haβ-tubulin | QH_CA_Contig4019 | 132 | Left: GGCGTCTACCTTCATTGGT | 88 (Arabidopsis thaliana AAK96884) |
| Right: TCCATCTCATCCATTCCTTC |
In addition to a high homology score with the Arabidopsis thaliana sequence, ACS-like sunflower EST (QHG4g06.yg.ab1) contains an aminotransferase class I and II conserved domain that is ACS specific. ACS primers were designed in the conserved region of three Arabidopsis thaliana ACS (GenBank accession numbers: NM116016, NM 122719, NM 100030) and sunflower ACS-like EST sequences to have the total ACS expression. ERF1-like sunflower EST displays 86% amino acid homology compared to the Arabidopsis thaliana ERF1 sequence (AF076278) and contains an AP2 domain which is a DNA-binding domain found in plant transcription regulators such as EREBP (ethylene responsive element binding protein).
Degenerate primers were designed based on the consensus sequence of A. thaliana, Lycopersicon esculentum, Nicotiana tabacum, Cucumis melo, and Brassica oleracea ETR1 (GenBank accession numbers: NM 105305, U47279, AF022727, AB052228, and AF0447476, respectively) to amplify ETR1-like probe from sunflower. Because of the high sequence similarity between ETR1 and ERS1, the primers designed target an ETR1 sequence out of common sequence with the ERS1 gene.
The gene-specific primers used were designed with Primer3 Input software. The length of all PCR products ranged from 120 bp to 346 bp. The PCR products were cloned into pCR2.1 vectors (Invitrogen) and sequence analysis confirmed the correct amplicons produced from each pair of primers. Sequences presenting some differences with the corresponding EST, such as ACS, ERF, and ERS and the sunflower ETR1-homologue, were referenced in the GenBank database with the accession numbers: EY255087, EY255088, EY255089, and EY255090, respectively.
Real-time quantitative and semi-quantitative RT-PCR
Total RNA (4 μg) was treated with DNase I (Sigma) and then was reverse transcribed with Revertaid H minus M-MuLV RT (Fermentas) for 2 h at 42 °C. After enzyme inactivation (10 min at 95 °C), the first strand cDNA obtained was checked by 1% agarose gel electrophoresis. The amplifications were performed with real-time PCR (iCycler iQ, Bio-Rad) using 5 μl of 50 times diluted cDNA solution for HaACO, HaACS, HaETR2, HaERF1, and 10 times diluted cDNA solution for HaCTR1. As an internal standard, a fragment of sunflower β-tubulin or EF1α gene was used. Real-time PCR reactions were performed with the Absolute qPCR Syber Green Fluorescein mix (Abgene, Epsom, UK) and 0.25 μM of each primer in a 25 μl reaction volume. They were initiated at 94 °C for 15 min followed by 40 cycles at 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s. Calculation of Critical threshold (Ct) and relative expressions of genes were performed using the iCycler iQ software (Bio-Rad).
Semi-quantitative RT-PCR studies were performed for HaETR1 and HaERS1 genes. Five μl of 10 times diluted cDNA reaction mix was used in a 25 μl PCR reaction containing 1.5 mM MgCl2, 10 pM dNTP mix, 50 pM of each primer, and 1 unit of Taq polymerase (Invitrogen). The thermocycling conditions were as follows: 5 min at 95 °C, 25 cycles of 30 s at 94 °C, 30 s at 54°C, and 1 min at 72 °C, and a final extension of 5 min at 72 °C.
Results
Effects of cyanide on germination of dormant seeds
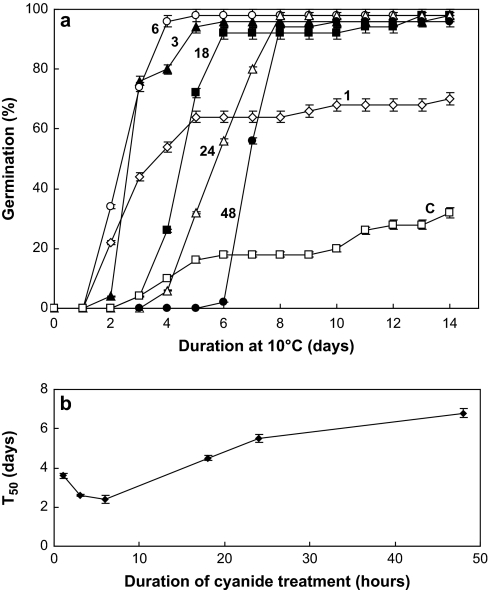
Freshly harvested naked sunflower seeds germinated poorly at 10 °C (Fig. 1, curve C), only 30% of seeds being able to germinate after 14 d. Cyanide treatments (1 mM) for 1–48 h strongly stimulated the subsequent germination of dormant embryos at 10 °C. The total population was able to germinate at 10 °C when the duration of the CN treatment exceeded 1 h. The improving effect of cyanide depended on the duration of the treatment. It was optimal after a 3–6 h treatment (Fig. 1a), the time to obtain 50% germination being then around 2.4–2.6 d (Fig. 1b). Seeds did not germinate in the prolonged presence of HCN, from 18–48 h (Fig. 1a), and therefore the T50 values increased regularly to reach c. 6.5 d after 48 h of treatment (Fig. 1b).
Fig. 1.
Effects of 1, 3, 6, 18, 24, and 48 h of HCN treatment (1 mM) of dormant sunflower embryos on the time-course of germination at 10 °C (a) and on the time to obtain 50% germination (T50) (b) (C, non-treated control embryos placed directly on water). Time 0 corresponds to the beginning of the treatment by HCN of seeds imbibed at 10 °C, for various durations, prior to HCN removal. Means of eight replicates ±SD.
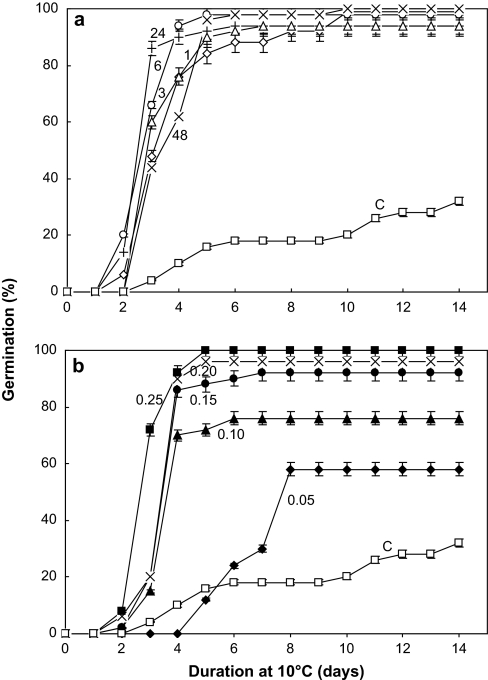
Three hours of treatment with cyanide (1 mM) allowed the germination of dormant embryos at 10 °C whatever cyanide was applied after 1 h to 48 h of seed imbibition on water (Fig. 2a). This beneficial effect did not require full seed imbibition since it stimulated the germination of dormant embryos treated in the dry state, i.e. at 0.05 g H2O g−1 DW (Fig. 2b). However, embryos were able to germinate fully after treatment when their moisture content was above 0.15 g H2O g−1 DW (Fig. 2b), the higher the moisture content, the faster the germination.
Fig. 2.
Effects of a 3 h treatment with 1 mM HCN applied after various durations of pre-imbibition on water at 10 °C indicated on the graph (1, 3, 6, 24, and 48 h) (a) or applied to embryos equilibrated at different moisture contents (0.05, 0.10, 0.15, 0.20, and 0.25 g H2O g−1 DW) (b) prior to germination at 10 °C (C, non-treated control embryos placed directly on water). Means of eight replicates ±SD.
Cyanide content in embryonic axis
Cyanide was not detected in axes isolated from dry dormant and non-dormant embryos (Table 2). Its content reached very low values, close to 6–7 μmol g−1 DW, when embryos were imbibed on water for 3 h or 24 h (Table 2). Treatment of embryos for 3 h by gaseous cyanide resulted in a dramatic increase in cyanide content, which was much higher in non-dormant embryos than in dormant ones. It subsequently decreased markedly after 24 h of seed imbibition and reached c. 94 and 207 μmol g−1 DW in dormant and non-dormant embryos, respectively (Table 2).
Table 2.
Changes, during imbibition at 10 °C, in cyanide content in axes isolated from dormant and non-dormant sunflower embryos, treated or not by gaseous cyanide (1 mM) during the first 3 h of their imbibition
| Seed | Duration of imbibition (h) | Cyanide content (μmol g −1 DW) of axis isolated from | |
| Control embryos | CN-treated embryos | ||
| Dormant | 0 | nd | – |
| 3 | 6.9±0.9 | 755.8±15.9 | |
| 24 | 6.7±0.9 | 94.0±11.5 | |
| Non dormant | 0 | nd | – |
| 3 | 6.9±0.9 | 1903.5±50.9 | |
| 24 | 7.9±0.8 | 207.4±12.5 | |
Mean ±SD of three measurements; nd, non detectable.
Effects of respiratory inhibitors
In order to determine whether the cyanide improving effect on germination was related to its action on the respiratory pathway, germination assays were performed in the presence of NaN3, an inhibitor of the cyanide-sensitive cytochrome oxidase, and SHAM, an inhibitor of the cyanide alternative oxidase (Day et al., 1980). NaN3 (1 mM) stimulated the germination of dormant embryos, but only 56% germinated after 14 d, and the stimulatory effect of cyanide on germination was maintained even in the presence of NaN3 (Table 3). SHAM did not strongly alter the stimulatory effect of HCN. After 3 h treatment in the presence of HCN and SHAM, 88% of embryos germinated after 14 d at 10 °C (Table 3). SHAM applied alone had no marked effect on germination.
Table 3.
Germination of naked dormant seeds incubated at 10 °C on water after 3 h of treatment by various compounds
| Treatment | Germination (%) after | ||
| 3 d | 7 d | 14 d | |
| Water (control) | 4±3 | 18±3 | 32±3 |
| HCN (1 mM) | 76±3 | 96±3 | 98±1 |
| NaN3 (1 mM) | 8±3 | 40±3 | 56±3 |
| NaN3 (1 mM) + HCN (1 mM) | 38±4 | 98±2 | 98±2 |
| SHAM (1 mM) | 2±3 | 20±3 | 46±4 |
| HCN (1 mM) + SHAM (1 mM) | 64±3 | 78±2 | 88±2 |
Mean of 8 replicates ±SD.
Relationship between ethylene synthesis and signalling pathway and cyanide
The effect of HCN on the germination of dormant embryos was studied in the presence of inhibitors of ethylene synthesis and perception. Cyanide treatment (3 h) and subsequent germination of dormant embryos at 10 °C were therefore carried out in the presence of amino-oxyacetic acid (AOA), an inhibitor of ACC synthase activity, α-aminoisobutyric acid (AIB) and cobalt chloride (CoCl2), inhibitors of ACC oxidase, and 2,5-norbornadiene (NBD), an inhibitor of ethylene action (Lau and Yang, 1976; Peiser, 1989; Sisler and Serek, 1999). When applied alone, i.e. without cyanide treatment, AOA and AIB had no significant effect on germination at 10 °C; when CoCl2 allowed 54% germination after 14 d (Table 4). NBD slightly inhibited the germination of dormant embryos at 10 °C. All the inhibitors of ethylene production used did not markedly modify the stimulatory effect of cyanide on the germination of dormant sunflower seeds (Table 4). On the contrary, the beneficial effect of cyanide treatment was suppressed in the presence of NBD, only 22% of the seed population germinating at 10 °C (Table 4).
Table 4.
Germination of dormant embryos, treated or not by cyanide, at 10 °C in the presence of α-aminoisobutyric acid (AIB), amino-oxyacetic acid (AOA), cobalt chloride (CoCl2), or 2,5-norbornadiene (NBD)
| Treatment | Germination (%) after | ||
| Inhibitors of ethylene synthesis or perception | Gaseous HCN | 7 d | 14 d |
| None (water) | – | 18±3 | 32±3 |
| + | 96±2 | 96±2 | |
| AOA (1 mM) | – | 14±2 | 38±2 |
| + | 78±4 | 98±2 | |
| CoCl2 (1 mM) | – | 22±2 | 54±2 |
| + | 58±4 | 78±3 | |
| AIB (1 mM) | – | 24±2 | 40±1 |
| + | 94±3 | 98±1 | |
| NBD (7 ml l−1) | – | 18±1 | 18±1 |
| + | 22±1 | 22±1 | |
Cyanide (1 mM) was applied during the first 3 h of imbibition. Mean of four replicates ±SD.
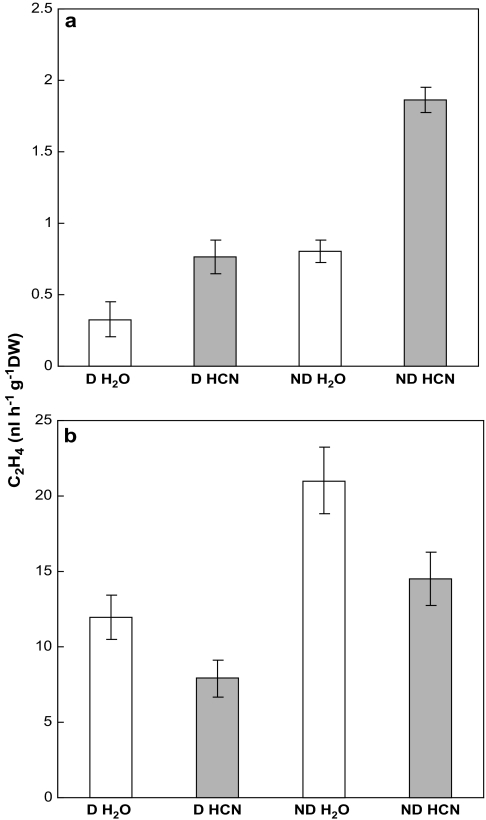
Cyanide treatment stimulated ethylene production in dormant and non-dormant axes (Fig. 3a). Although ethylene production was higher in non-dormant axes, this was always very low. Incubation of axes in the presence of ACC resulted in a strong increase in ethylene production (Fig. 3b), but, conversely to what was observed without ACC (Fig. 3a), cyanide appeared to have a slight inhibitory effect on ACC conversion to ethylene, either in dormant and non-dormant axes.
Fig. 3.
Ethylene emission by dormant (D) and non-dormant (ND) axes isolated from control or cyanide-treated sunflower embryos imbibed for 24 h at 10 °C on water. CN (1 mM) was applied during the first 3 h of imbibition. Ethylene measurements were performed after 5 h incubation at 20 °C of axes excised from embryos in the absence (a) or presence (b) of ACC 1 mM. Means of five replicates ±SD.
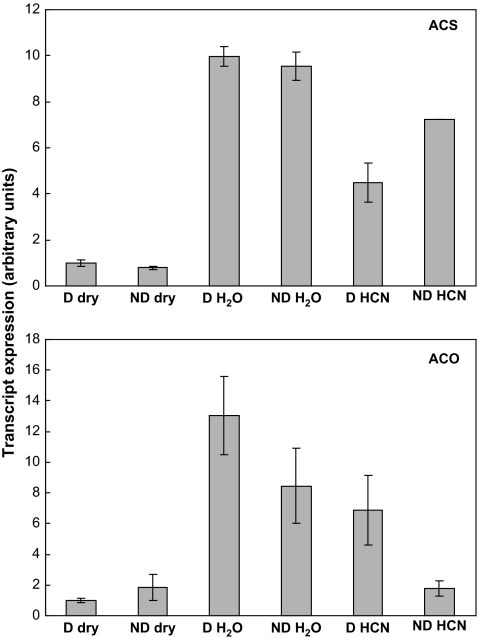
Expression of genes involved in ethylene production (HaACS and HaACO) and signal transduction (HaETR1, HaETR2, HaERS1, HaCTR1, and HaERF1) was studied by RT-PCR. HaACS and HaACO expression did not change significantly during after-ripening, but 24 h imbibition of naked seeds on water resulted in a marked increase in the level of their transcripts in the embryonic axes, irrespective of their level of dormancy (Fig. 4). However, cyanide treatment for 3 h resulted in a lower stimulation of HaACS and HaACO expression than in non-treated naked seeds.
Fig. 4.
ACS and ACO transcript expression in axes isolated from dormant (D) and non-dormant (ND) sunflower embryos before imbibition (dry) and from control (H2O) and CN-treated embryos (HCN) imbibed for 24 h at 10 °C on water. CN (1 mM) was applied during the first 3 h of imbibition. Data from real-time RT-PCR (mean of four biological replicates ±SD) are expressed in arbitrary units.
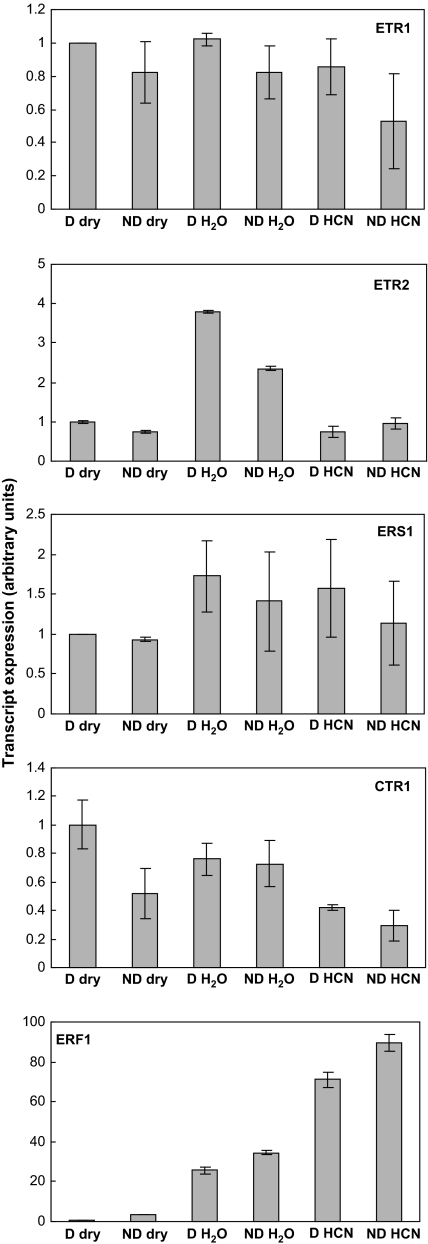
ETR1, ETR2, and ERS1 belong to the family of the ethylene receptors. Expression of HaETR1 and HaERS1 did not vary markedly among the various samples used in this study (Fig. 5). HaETR2 appeared to be up-regulated during imbibition of seeds on water, but not in the presence of cyanide. HaCTR1, a negative regulator of ethylene signalling, was always poorly expressed during seed imbibition (Fig. 5). The transcription factor HaERF1 was almost not expressed in dry dormant axes, but its amount increased more than 3-fold during after-ripening (Fig. 5). Seed imbibition was associated with a dramatic up-regulation of HaERF1 in dormant and non-dormant embryonic axes, and this effect was magnified by cyanide (Fig. 5). In dormant imbibed axes, cyanide increased by nearly three times the level of HaERF1 transcripts.
Fig. 5.
ETR1, ERS1, ETR2, CTR1, and ERF1 transcript expression in axes isolated from dry dormant (D) and non-dormant (ND) sunflower embryos before imbibition (dry) and from control (H2O) and CN-treated embryos (HCN) imbibed for 24 h at 10 °C on water. CN (1 mM) was applied during the first 3 h of imbibition. Data from real-time RT-PCR (ETR2, CTR1, and ERF1) and semi-quantitative RT-PCR (ETR1 and ERS1) (mean of four biological replicates ±SD) are expressed in arbitrary units.
Discussion
The data presented here show that sunflower embryo dormancy can be broken by gaseous cyanide treatment for as short a time as 1 h, thus allowing subsequent germination at 10 °C (Fig. 1a), as demonstrated by Oracz et al. (2007) and they allow a first set of putative mechanisms associated with the effects of this compound on dormancy alleviation to be proposed. Measurements of cyanide contents demonstrated that a 3 h treatment of seeds with gaseous cyanide led to a strong accumulation of this compound in the axis, thus suggesting that cyanide per se might be involved in dormancy alleviation. Nevertheless it decreased dramatically after 24 h of imbibition (Table 2), probably due to the action of detoxifying mechanisms. Surprisingly, cyanide content was more than two times higher in axes isolated from non-dormant seeds than in those isolated from dormant seeds, reaching a very high level that was potentially toxic for the cells (Table 2). Cyanide treatments longer than c. 6 h were associated with a decrease in germination rate (Fig. 1b), which is in agreement with the data obtained with other species showing that, at high concentrations, cyanide has to be removed from the germination medium in order to promote germination (Côme et al., 1988). Cyanide treatment carried out after various durations of imbibition ranging from 1 h to 48 h resulted in the same stimulatory effect on germination (Fig. 2a), which demonstrates that dormant embryos do not present a time window of cyanide sensivity during imbibition. On the contrary, the responsiveness of dormant seeds to a short treatment (1–3 h) with 50 ppm ethylene depends on the time at which it is applied during imbibition, being optimal after about 2 d of pre-imbibition on water (Corbineau and Côme, 2003). In addition, the beneficial effect of cyanide did not require full seed imbibition, since it was effective in promoting germination at 10 °C when embryo moisture content was as low as 0.05 to 0.10 g H2O g−1 DW (Fig. 2b). This improving effect probably results from gaseous cyanide trapped in the dry tissues during the treatment, which would become effective during subsequent seed imbibition at 10 °C. The respiratory inhibitors NaN3, an inhibitor of cytochrome oxidase, and SHAM, an inhibitor of cyanide-resistant alternative oxidase, were unable to mimic or to inhibit the beneficial effect of HCN on the germination of the dormant embryos (Table 3). These results rule out a direct effect of cyanide on respiration, through the control of oxidative electron transport, for example, inhibition of cytochrome oxidase or/and induction of an alternative, CN-insensitive pathway (Esashi et al., 1981b), or on the regulation of metabolic equilibrium, for example, glycolysis versus the pentose phosphate pathway (Esashi et al., 1979), as a mode of action for alleviating dormancy, as previously postulated for seeds of cocklebur (Esashi et al., 1981a; Upadhyaya et al., 1983), cereals (Roberts and Smith, 1977; Côme et al., 1988; Côme and Corbineau, 1989), or apple (Bogatek, 1995)
Among the possible mechanisms of cyanide action in plants, its interplay with ethylene metabolism has often been cited, mainly because cyanide is a co-product of ethylene biosynthesis (Peiser et al., 1984). Oxidation of ACC leads to ethylene and cyanoformic acid, which is subsequently converted to CO2 and hydrogen cyanide. Moreover, hydrogen cyanide by itself has been demonstrated to be involved in the ethylene biosynthesis pathway, through the stimulation of the ACS gene, for example (Smith and Arteca, 2000). None of the compounds inhibiting ethylene production through their action on ACS (i.e. AOA) or ACO (i.e. CoCl2 and AIB) was able to suppress the beneficial effect of cyanide on the germination of dormant embryos (Table 4). Germination occurred slowly in the presence of AOA and AIB, but was complete after 14 d at 10 °C (Table 4), and was only slightly reduced in the presence of CoCl2 (Table 4). This suggests that cyanide probably does not act on the germination of sunflower seeds through the stimulation of ethylene biosynthesis. Measurements of ethylene production and determination of the expression pattern of genes coding for the two main enzymes involved in ethylene biosynthesis, i.e. ACS and ACO, confirmed this hypothesis (Figs 3, 4). Cyanide did not markedly promote ethylene production in dormant axes, or at least not enough for this compound to allow their germination (Fig. 3). Indeed, Corbineau et al. (1990) demonstrated that ethylene stimulates the germination of dormant sunflower seeds at concentrations higher than 1 ppm when applied for a sufficient length of time, i.e. around 24 h. These conditions are therefore far from the one used in this study, since cyanide treatment, and therefore associated ethylene production, lasted for only 3 h before its removal. In addition, when isolated axes were incubated in the presence of ACC solution, cyanide slightly inhibited ACC-dependent ethylene production (Fig. 3b) suggesting that the improving effect of CN did not result from a stimulatory action of this compound on ACO activity. These data also demonstrate that cyanide even has an inhibitory effect on the expression of HaACS and HaACO (Fig. 4), conversely to what was shown by Smith and Arteca (2000) in Arabidopsis plants. This inhibitory effect of CN on HaACS and HaACO expression emphasizes the fact that CN does not act through the stimulation of the ethylene biosynthesis pathway. Various studies have indicated that ethylene could regulate its own synthesis through the stimulation of ACO activity, as for example in leaves of citrus (Riov and Yang, 1982) or in epicotyls of pea seedlings (Schierle et al., 1989). However, in sunflower seeds, Corbineau and Côme (1995), using CO2, an inhibitor of autocatalytic ethylene biosynthesis, suggested that this pathway did not exist and one can therefore exclude any feedback effect of accumulated ethylene on ACS and ACO activity or expression.
Since the stimulatory effect of CN was suppressed in the presence of NBD, an inhibitor of ethylene action (Table 3), the expression of different genes involved in ethylene signal transduction (ETR1, ETR2, ERS1, CTR1, and ERF1) was investigated in the axes of embryos treated or not by cyanide for 3 h. Expression of HaETR1 and HaERS1, which belong to the subfamily 1 of ethylene membrane-associated receptors (Wang et al., 2002), was roughly similar and almost unchanged whatever the treatment, whereas expression of HaETR2, a member of subfamily 2, was stimulated during imbibition on water, but not in the presence of cyanide (Fig. 5). Similarly, expression of HaCTR1, the negative regulator of ethylene signalling acting downstream of the receptors, was neither induced nor repressed by the cyanide treatment. These results show that the stimulatory effect of cyanide on germination is not associated with either induction or repression of ethylene receptors genes or of the next downstream component (i.e. HaCTR1). It is nevertheless difficult to compare the changes in expression induced by HCN to those induced by ethylene, which appear to be somewhat unpredictable. For example, in Arabidopsis, ethylene induces the expression of ERS1 and ETR2 (Hua et al., 1998), but represses that of ETR1 (Raz and Ecker, 1999). However, Qu et al. (2007) recently proposed a differential role to the genes belonging to subfamilies 1 and 2 in ethylene perception and transduction. Genes belonging to family 1 seem to have a predominant role in ethylene perception since loss of both ETR1 and ERS1 results in a strong constitutive ethylene-response phenotype. This could explain in part why the different ethylene receptors do not share a common expression pattern in the presence of ethylene, or as shown here, during seed germination. Although our data clearly show that cyanide does not act at the transcriptional level on ethylene receptors, its beneficial effect on germination nevertheless requires functional ethylene receptors, since cyanide is not active in the presence of NBD (Table 3). This therefore suggests that at least the first steps of the cyanide signal transduction pathway would be common with those of ethylene, but without requiring an active transcription of the receptor genes.
Expression of HaERF1 (Fig. 5), a transcription factor belonging to the ethylene response factor family, also suggests an overlap of the ethylene and cyanide pathways. Imbibition of either dormant or non-dormant seeds dramatically enhances HaERF1 expression, but it must be noted that the HaERF1 transcript was initially present at a very low level in dry seeds. This increase in HaERF1 expression was magnified in the presence of gaseous cyanide (Fig. 5), thus suggesting that the CN effect on dormant seed germination might involve activation of the transcription factor HaERF1. ERF1 and the other related genes from this family are known to be key actors of plant responses to ethylene (Chen et al., 2005). For example, overexpression of ERF1 confers constitutive ethylene responses in Arabidopsis (Solano et al., 1998). It is therefore postulated that ERF1 is a common component of the ethylene and cyanide pathways. This finding is in agreement with other data which demonstrate that ERF was also activated by jasmonate (Lorenzo et al., 2003; Zhang et al., 2007), ABA (Zhang et al., 2004; Xu et al., 2007) or salicylic acid (Xu et al., 2007; Zhang et al., 2007), thus confirming that different hormone signalling pathways come together to ERF protein. Besides their central role in the cross-talk of hormonal pathways, ERFs are involved in plant tolerance to various biotic or abiotic stresses (Park et al., 2001; Tang et al., 2006) but also in some developmental processes, such as seed germination (Song et al., 2005; Pirrello et al., 2006). Leubner-Metzger et al. (1998) hypothesized that EREBP-3 and EREBP-4, two members of the ERF family in tobacco, were playing a pivotal role in the response to ethylene during tobacco seed germination. The role of ERF in germination is confirmed in this study since the amount of ERF1 transcripts is higher in germinating sunflower seeds, i.e. non-dormant seeds, than in the dormant seeds (Fig. 5). Finally, it must also be emphasized that dry afterripening was associated with a marked increase in ERF transcription. Even if the levels of expression were rather low in the dry seeds, non-dormant axes contained five times more ERF transcripts than dormant ones (Fig. 5), thus underlining again that changes in gene expression may occur in anhydrobiosis, as already suggested by El-Maarouf-Bouteau et al. (2007) with seeds of the same species. This increase in expression of HaERF1, which governs the transduction of many signals, is particularly interesting.
In conclusion, this work allows a new mechanism of action of cyanide in seed dormancy alleviation to be proposed, which does not involve ethylene production but the ethylene transduction pathway, in particular through the regulation of HaERF1 transcription. Our results also underline the possible pivotal role of ERF1 in cross-talk between hormones and the regulation of developmental mechanisms. Future work aims to investigate whether cyanide signalling also interferes with ROS signalling, as recently suggested by Oracz et al. (2007).
Glossary
Abbreviations
- ACC
1-aminocyclopropane 1-carboxylic acid
- EST
expressed sequence tag
- qPCR
quantitative PCR
References
- Bethke PC, Libourel IGL, Reinöhl V, Jones RL. Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta. 2006;223:805–812. doi: 10.1007/s00425-005-0116-9. [DOI] [PubMed] [Google Scholar]
- Bogatek R. The possible role of fructose 2,6-bisphosphate in the cyanide-mediated removal of embryonic dormancy in apple. Physiologia Plantarum. 1995;94:460–464. [Google Scholar]
- Bogatek R, Lewak S. Effect of cyanide and cold treatment on sugar catabolism in apple seeds during dormancy removal. Physiologia Plantarum. 1988;73:406–411. [Google Scholar]
- Bogatek R, Lewak S. Cyanide controls enzymes involved in lipid and sugar catabolism in dormant apple embryos during culture. Physiologia Plantarum. 1991;83:422–426. [Google Scholar]
- Bogatek R, Côme D, Corbineau F, Picard MA, Żarska-Maciejewska B, Lewak S. Sugar metabolism as related to the cyanide-mediated elimination of dormancy in apple embryos. Plant Physiology and Biochemistry. 1999;37:577–585. [Google Scholar]
- Bowler C, Van Montagu M, Inzé D. Superoxide dismutase and stress tolerance. Annual Review of Plant Physiology and Plant Molecular Biology. 1992;43:83–116. [Google Scholar]
- Chen YF, Etheridge N, Schaller GE. Ethylene signal transduction. Annals of Botany. 2005;95:901–915. doi: 10.1093/aob/mci100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côme D, Corbineau F, Lecat S. Some aspects of metabolic regulation of cereal seed germination and dormancy. Seed Science and Technology. 1988;16:175–186. [Google Scholar]
- Côme D, Corbineau F. Some aspects of metabolic regulation of seed germination and dormancy. In: Taylorson RB, editor. Recent advances in the development and germination of seeds. New York: Plenum Press; 1989. pp. 165–179. [Google Scholar]
- Corbineau F, Côme D. Control of seed germination and dormancy by the gaseous environment. In: Kigel J, Galili G, editors. Seed development and germination. New York: Marcel Dekker; 1995. pp. 397–427. [Google Scholar]
- Corbineau F, Côme D. Germination of sunflower seeds as related to ethylene synthesis and sensitivity: an overview. In: Vendrell M, Klee H, Pech JC, Romojaro F, editors. Biology and biotechnology of the plant hormone ethylene III. Amsterdam: IOS Press, NATO Sciences Series; 2003. pp. 216–221. [Google Scholar]
- Corbineau F, Bagniol S, Côme D. Sunflower (Helianthus annuus) seed dormancy and its regulation by ethylene. Israel Journal of Botany. 1990;39:313–325. [Google Scholar]
- Day DA, Arron GP, Laties GG. Nature and control of respiratory pathways in plants: the interaction of cyanide-resistant respiration with the cyanide-sensitive pathways. In: Davies DD, editor. The biochemistry of plants. A comprehensive treatise, Vol. 2. Metabolism and respiration. New York: Academic Press; 1980. pp. 197–241. [Google Scholar]
- El-Maarouf Bouteau H, Job C, Job D, Corbineau F, Bailly C. ROS signaling in seed dormancy alleviation. Plant Signaling and Behavior. 2007;2:362–364. doi: 10.4161/psb.2.5.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esashi Y, Kusuyama K, Tazaki S, Ishihara N. Necessity of a balance between CN-sensitive and CN-resistant respirations for germination of cocklebur seeds. Plant and Cell Physiology. 1981a;22:65–71. [Google Scholar]
- Esashi Y, Ohhara Y, Okazaki M, Hishinuma K. Control of cocklebur seed germination by nitrogenous compounds: nitrite, nitrate, hydroxylamine, thiourea, azide, and cyanide. Plant and Cell Physiology. 1979;20:349–361. [Google Scholar]
- Esashi Y, Sakai Y, Ushizawa R. Cyanide-sensitive and cyanide-resistant respiration in the germination of cocklebur seeds. Plant Physiology. 1981b;67:503–508. doi: 10.1104/pp.67.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Gao Z, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. Journal of Biological Chemistry. 2003;278:34725–34732. doi: 10.1074/jbc.M305548200. [DOI] [PubMed] [Google Scholar]
- Gu YQ, Wildermuth MC, Chakravarthy S, Loh YT, Yang C, He X, Han Y, Martin GB. Tomato transcription factors Pti4, Pti5, and Pti6 activate defense responses when expressed in Arabidopsis. The Plant Cell. 2002;14:817–831. doi: 10.1105/tpc.000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekar PG, Borowitz JL, Isom GE. Cyanide-induced generation of oxidative species: involvement of nitric oxide synthase and cyclooxygenase-2. Journal of Pharmacology and Experimental Therapeutics. 1998;285:236–241. [PubMed] [Google Scholar]
- Guo H, Ecker JR. The ethylene signaling pathway: new insights. Current Opinion in Plant Biology. 2004;7:40–49. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. The Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ. Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. The Plant Journal. 2003;33:221–233. doi: 10.1046/j.1365-313x.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Conroy WG, Burris KD, Isom GE. Peroxidation of brain lipids following cyanide intoxication in mice. Toxicology. 1987;46:21–28. doi: 10.1016/0300-483x(87)90134-x. [DOI] [PubMed] [Google Scholar]
- Lau OL, Yang SF. Inhibition of ethylene production by cobaltous ion. Plant Physiology. 1976;58:114–117. doi: 10.1104/pp.58.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leubner-Metzger G, Petruzzelli L, Waldvogel R, Vögeli-Lange R, Meins F., Jr Ethylene-responsive element binding protein (EREBP) expression and the transcriptional regulation of class I β-1,3-glucanase during tobacco seed germination. Plant Molecular Biology. 1998;38:785–795. doi: 10.1023/a:1006040425383. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. The Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussatche P, Klee HJ. Autophosphorylation activity of the Arabidopsis ethylene receptor multigene family. Journal of Biological Chemistry. 2004;279:48734–48741. doi: 10.1074/jbc.M403100200. [DOI] [PubMed] [Google Scholar]
- Nagashima S. Spectrophotometric determination of cyanide with γ-picoline and barbituric acid. Analytica Chimica Acta. 1977;91:303–306. [Google Scholar]
- Oracz K, El-Maarouf Bouteau H, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. The Plant Journal. 2007;50:452–465. doi: 10.1111/j.1365-313X.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH. Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. The Plant Cell. 2001;13:1035–1046. doi: 10.1105/tpc.13.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser G. Effect of 2,5-norbornadiene upon ethylene biosynthesis in midclimacteric carnation flowers. Plant Physiology. 1989;90:21–24. doi: 10.1104/pp.90.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser G, Wang TT, Hoffman NE, Yang SF, Liu HW, Walsh CT. Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carboxylic acid during its conversion to ethylene. Proceedings of the National Academy of Sciences, USA. 1984;81:3059–3063. doi: 10.1073/pnas.81.10.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrello J, Jaimes-Miranda F, Sanchez-Ballesta MT, Tournier B, Khalil-Ahmad Q, Regad F, Latché A, Pech JC, Bouzayen M. Sl-ERF2, a tomato ethylene response factor involved in ethylene response and seed germination. Plant and Cell Physiology. 2006;47:1195–1205. doi: 10.1093/pcp/pcj084. [DOI] [PubMed] [Google Scholar]
- Pirrung MC, Brauman JI. Involvement of cyanide in the regulation of ethylene biosynthesis. Plant Physiology and Biochemistry. 1987;25:55–61. [Google Scholar]
- Qu X, Hall BP, Gao Z, Schaller GE. A strong constitutive ethylene-response phenotype conferred on Arabidopsis plants containing null mutations in the ethylene receptors ETR1 and ERS1. BioMed Central Plant Biology. 2007 doi: 10.1186/1471-2229-7-3. doi:10.1186/1471–2229–7–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz V, Ecker JR. Regulation of differential growth in the apical hook of Arabidopsis. Development. 1999;126:3661–3668. doi: 10.1242/dev.126.16.3661. [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Heard J, Martin G, et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290:2105–2010. doi: 10.1126/science.290.5499.2105. [DOI] [PubMed] [Google Scholar]
- Riov J, Yang SF. Effects of exogenous ethylene on ethylene production in citrus leaf tissue. Plant Physiology. 1982;70:136–141. doi: 10.1104/pp.70.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EH, Smith RD. Dormancy and the pentose phosphate pathway. In: Khan AA, editor. The physiology and biochemistry of seed dormancy and germination. Amsterdam: Elsevier; 1977. pp. 385–411. [Google Scholar]
- Schierle J, Rohwer F, Bopp M. Distribution of ethylene synthesis along the etiolated pea shoot and its regulation by ethylene. Journal of Plant Physiology. 1989;134:331–337. [Google Scholar]
- Sisler EC, Serek M. Compounds controlling the ethylene receptor. Botanical Bulletin of Academia Sinica. 1999;40:1–7. [Google Scholar]
- Smith JM, Arteca RN. Molecular control of ethylene production by cyanide in Arabidopsis thaliana. Physiologia Plantarum. 2000;109:180–187. [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signalling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes and Development. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CP, Agarwal M, Ohta M, Guo Y, Halfter U, Wang P, Zhu JK. Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drough stress responses. The Plant Cell. 2005;17:2384–2396. doi: 10.1105/tpc.105.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Newton RJ, Lin J, Charles TM. Expression of a transcription factor from Capsicum annuum in pine calli counteracts the inhibitory effects of salt stress on adventitious shoot formation. Molecular Genetics and Genomics. 2006;276:242–253. doi: 10.1007/s00438-006-0137-5. [DOI] [PubMed] [Google Scholar]
- Taylorson RB, Hendricks SB. Promotion of seed germination by cyanide. Plant Physiology. 1973;52:23–27. doi: 10.1104/pp.52.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejera García NA, Iribarne C, Palma F, Lluch C. Inhibition of the catalase activity from Phaseolus vulgaris and Medicago sativa by sodium chloride. Plant Physiology and Biochemistry. 2007;45:535–541. doi: 10.1016/j.plaphy.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Upadhyaya MK, Naylor JM, Simpson GM. The physiological basis of seed dormancy in Avena fatua. II. On the involvement of alternative respiration in the stimulation of germination by sodium azide. Physiologia Platarum. 1983;58:119–123. [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Research. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KLC, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. The Plant Cell. 2002;14:S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZS, Xia LQ, Chen M, et al. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Molecular Biology. 2007;65:719–732. doi: 10.1007/s11103-007-9237-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yao W, Dong N, Liang HX, Liu HX, Huang R. A novel ERF transcription activator in wheat and its induction kinetics after pathogen and hormone treatments. Journal of Experimental Botany. 2007;58:2993–3003. doi: 10.1093/jxb/erm151. [DOI] [PubMed] [Google Scholar]
- Zhang H, Huang Z, Xie B, Chen Q, Tian X, Zhang X, Zhang H, Lu X, Huang D, Huang R. The ethylene-, jasmonate-, abscisic acid-, and NaCl-responsive tomato transcription factor JERF1 modulates expression of GCC box-containing genes and salt tolerance in tobacco. Planta. 2004;220:262–270. doi: 10.1007/s00425-004-1347-x. [DOI] [PubMed] [Google Scholar]