Abstract
In plants, salicylic acid (SA) is a signalling molecule regulating disease resistance responses such as systemic acquired resistance (SAR) and the hypersensitive response (HR), and has been implicated in both basal and acquired thermotolerance. It has been shown that SA enhances heat-induced Hsp/Hsc70 accumulation in plants. To investigate the mechanism of how SA influences the heat shock response (HSR) in plants, tomato seedlings were treated with SA alone, heat shock, or a combination of both before analyses of hsp70 mRNA, heat shock factor (Hsf)–DNA binding, and gene expression of hsp70, hsfA1, hsfA2, and hsfB1. SA alone led to activation of Hsf–DNA binding, but not induction or transcription of hsp70 mRNA. SA had no significant effect on hsfA2 and hsfB1 gene expression, but potentiated the basal levels of hsfA1. In heat-shocked plants, Hsf–DNA binding was established, and increased hsfA1, hsfA2, and hsfB1 expression was followed by accumulation of Hsp70. SA plus heat shock showed enhanced Hsf–DNA binding, enhanced induction of hsp70 mRNA transcription, and gene expression of hsfA1, hsfA2, and hsfB1, resulting in potentiated levels of Hsp/Hsc70. Since increased hsp70 and hsf gene expression coincide with increased levels of Hsp70 accumulation, it is concluded that SA-mediated potentiation of Hsp70 is due to modulation of these Hsfs by SA. In our efforts to understand the role of Hsp70 in heat-related disease susceptibility, the degree of the complexity of the cross-talk between the pathways in which SA is involved, inter alia, the plant defence response, the HSR and thermotolerance, was further underscored.
Keywords: Heat shock, heat shock factors, HsfA1, HsfA2, HsfB1, Hsf–DNA binding, Hsp70, salicylic acid, tomato
Introduction
Plants, by virtue of their sessile nature, have evolved a remarkable repertoire of survival mechanisms, including defence against a range of pathogens as well as the ability to deal with fluctuations in temperature by, inter alia, developing a tolerance to heat or activating the heat shock response (HSR).
The plant defence mechanism is enabled by the establishment of a systemic acquired resistance (SAR) response and the hypersensitive response (HR). Salicylic acid (SA), a derivative of aspirin and a well-documented and widely used non-steroidal anti-inflammatory drug in mammalians, is essential for the establishment of SAR in plants (Klessig and Malamy, 1994; Dempsey et al., 1999).
Heat tolerance can be induced in a plant by prior exposure to moderately high temperatures which enables the plant to cope with subsequent, potentially lethal, heat exposure (Howarth and Ougham, 1993). This acclimatization is termed thermotolerance. SA has been found to be involved in both basal and acquired thermotolerance in plants (Dat et al., 1998a, b, 2000; Lopez-Delgado et al., 1998; Larkindale and Knight, 2002). In fact, it was recently shown that a PIP2-specific-phospholipase was induced by heat acclimation and was mediated by increased levels of free SA (Liu et al., 2006a, b).
SA also has the ability to enhance the HSR by potentiating the heat-induced levels of the heat shock protein 70 (Hsp70) in both mammalian cells (Jurivich et al., 1992) and plants (Cronjé and Bornman, 1999; Cronjé et al., 2004). In mammalian cells, SA-induced activation of heat shock factor (Hsf) was shown to be the result of phosphorylation of the serine residues, an event that does not lead to transcriptional activation of heat shock genes, whereas in heat-induced stress, phosphorylation of threonine residues occurs (Jurivich et al., 1995). Furthermore, SA treatment at normal temperatures is able to enhance Hsf–DNA binding, but does not cause induction of Hsp70 (Jurivich et al., 1992).
The HSR in plants and mammals is regulated by a set of highly conserved proteins known as heat shock proteins (Hsps), and expression of Hsps is governed by Hsfs. Interestingly, the number of Hsfs in plants far exceeds those found in mammalian cells, most probably to equip plants with the ability to deal with various forms of external stressors at any time during their lifespan. There are 15 known Hsfs in Arabidopsis thaliana (Nover et al., 2001) and >21 in Solanum lycopersicum (formerly Lycopersicon esculentum), and all are thought to play key roles in stress responses (Scharf et al., 1998b). In tomato, only two of these Hsfs, HsfA2 and HsfB1, are heat inducible (Scharf et al., 1990), but their expression is controlled by HsfA1, referred to as the master regulator of the HSR (Mishra et al., 2002). In fact, the interaction of HsfA2 with HsfA1 is imperative for the co-localization of HsfA2 into the nucleus (Scharf et al., 1998a). HsfA2 is seen as the ‘work horse’ of the stress response and becomes the most dominant Hsf during a heat stress (Mishra et al., 2002). When cells are exposed to various stress conditions, i.e. heat stress, the Hsfs that reside in the cytosol dissociate from the Hsp (e.g. Hsp70), are activated, and undergo trimerization. These Hsf trimers are phosphorylated and translocated to the nucleus where they bind to the heat shock element (HSE), which is located in the promoter region on the Hsp genes (Pelham, 1982). The mRNA is then transcribed and translated, which leads to increased levels of Hsps in the cytosol. As such, they function as chaperones of denatured proteins as well as assisting in the translocation and/or degradation of damaged proteins (Bukau and Horwich, 1998). Within the diverse Hsp family, Hsp70 is the most widely studied member, and a highly conserved 70 kDa protein that plays a key role in the stress response in plants (Vierling, 1991), as it does in mammals (Ohtsuka and Hata, 2000).
To investigate the mechanism of how SA influences the HSR in plants, the focus of the present investigation was on the events preceding Hsp70 protein accumulation. Tomato seedlings were treated with SA alone, heat-shocked, or exposed to a combination of both SA and heat shock, before analyses of levels of hsp70 mRNA, Hsf–DNA binding, and the gene expression of hsp70, hsfA1, hsfA2, and hsfB1.
Materials and methods
Plant material and treatments
Tomato seeds (S. lycopersicum cv. UC82B) were germinated on agar-supplemented Gamborg's medium [3.19 g l−1 Gamborg's B-5 (Highveld Biologicals, Gauteng, South Africa), 10 g l−1 sucrose, 6.5 g l−1 agar, pH 5.83]. Three-week-old seedlings were placed in liquid Gamborg's medium for at least 8 h to allow for equilibration. Seedlings were treated with 0.1 mM SA for 17 h, followed by a heat shock for 30 min at 40 °C. After heat shock, the seedlings were allowed to recover at room temperature before the isolation of total proteins, nuclear proteins, or RNA at different time intervals. RNA was also isolated during the heat shock treatment (10, 20, and 30 min after commencing heat shock treatment). Non-heat-treated control seedlings were kept at room temperature in liquid Gamborg's for the duration of the treatments.
Immunoblotting of isolated proteins with Hsp/Hsc70 antibody
After treatments, proteins were extracted in SDS–PAGE sample buffer [0.1 M TRIS-HCl, 20% glycerol (w/v), 4% SDS (w/v), 2% (v/v) 2-mercaptoethanol, and 0.001% bromophenol blue]. Samples were denatured at 95 °C for 10 min and centrifuged at 14 000 g for 10 min. Protein concentration was determined as described by Sheffield et al. (1987). Equal quantities of protein and an appropriate molecular weight marker (Roche, South Africa) were loaded and separated by SDS–PAGE (10%). Proteins in gels were transferred to a PVDF membrane (MSI, Westboro, MA, USA). Following transfer, the membranes were blocked with 1% casein in TRIS-buffered saline (TBS; pH 7.5) (20 mM TRIS, 500 mM NaCl) for 1 h before incubation (1 h at 25 °C) with a mouse monoclonal antibody (1:5000) (SPA820, StressGen, Victoria, Canada) directed against both constitutive and inducible isoforms of the 70 kDa HSP family. The membrane was then rinsed, blocked, and incubated with a goat anti-mouse peroxidase-labelled secondary antibody (1:100 000) (Pierce, Rockford, IL, USA). Secondary antibody was visualized with a SuperSignal West Pico™ Chemiluminescent Substrate (Pierce).
Electrophoretic mobility shift assay
Nuclear protein isolation:
After differential treatments, seedlings were crushed with liquid nitrogen and dissolved in 2 ml of HEPES nuclear buffer (HNB) to isolate nuclear proteins. HNB consisted of 25 mM HEPES, 25 mM NaCl, 25 mM MgCl2, 1 mM EDTA, 2 mM CaCl2, 10 mM NaF, 0.2 ml of β-mercaptoethanol, 5% sucrose, 30% glycerol, 0.1% Triton X-100, and protease inhibitors [including Pefabloc (0.1 mg ml−1), pepstatin (5 μg ml−1), chymostatin (6 μg ml−1), leupeptin (5 μg ml−1), aprotinin (1 μg ml−1), bestatin (1 μg ml−1)] (Roche Diagnostics, Germany). The crude homogenate was filtered through two layers of a nylon mesh (28 μm) and loaded onto a cushion of 2.5 ml of HNB buffer containing additional 1% sucrose. The sample was then centrifuged at 4500 g for 10 min. The pellet was resuspended in 0.5 ml of NEB500 buffer (nuclear extraction buffer) (25 mM HEPES, 500 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 10 mM NaF, 10 μl of β-mercaptoethanol, 10% sucrose, 0.1% Triton X-100 and protease inhibitors). The extract was sonicated gently (60% power for 30 s each) to disrupt the DNA and incubated on ice for 20 min. Thereafter the extracts were centrifuged at 15 000 g for 15 min. The supernatant was incubated with 1.5 vol. of 80% ice-cold acetone overnight at –20 °C. Proteins were precipitated via centrifugation at 16 000 g for 20 min and resuspended in 50 μl of NEB500 buffer before labelling (protocol kindly provided by K-D Scharf).
Labelling of HSE3:
Heat shock element 3 [HSE3; 5′ GCCAGAAGCTTCTAGAAAGC 3′ (wild type)] was used, as described by Mishra et al. (2002). This oligo was biotin labelled using a Biotin 3’ End DNA Labeling Kit (Pierce).
Gel shift assay:
The binding of the Hsf to the HSE was determined by using the LightShift® Chemiluminescent EMSA kit (Pierce) and the Chemiluminescent Nucleic Acid Detection Module™ (Pierce). Briefly, isolated proteins were incubated with the biotin-labelled HSE which allowed the binding reaction to occur, after which the sample was loaded on a native polyacrylamide gel (5%) with 0.5×TBE buffer. The gel was transferred onto a nylon membrane, and detection of biotin-labelled HSE was achieved using the Chemiluminescent Nucleic Acid Detection Module™.
Quantitative real-time PCR
Total RNA was isolated using the Qiagen RNeasy® Plant Mini Kit (Qiagen Inc., Valencia, CA, USA). cDNA was synthesized from the RNA with the ImProm-II™ Reverse Transcription System (Promega, Madison, WI, USA). Quantitative PCR (qPCR) was performed making use of the SensiMix (dT) DNA Kit (Quantace, London, UK) and RotorGene qPCR instrumentation (Corbett Research, Sydney, Australia). The housekeeping gene, tubulin, was tested by comparing all the ΔCt values of this gene during different treatments, and was found not to vary between treatments. Gene expression was determined with the two standard curve method as described by Ramakers et al. (2003) and using the RotorGene software (version 1.7). In all cases the efficiency was ∼100% (±1%) and the R2 values equal to 0.9. The following primers were used for real-time PCR: tubulin (β tubulin, accession no. BH012320) Tubulin-F, 5′ TGCTCAGCAC AAACAACCTC 3′, Tubulin-R, 5′ CCTTTCTCCGTCCCTACACA 3′; Hsp70 (from cDNA—2162 bp DNA linear) Hsp70-F, 5′ TGCTGGAGGTGTT ATGACCA 3′, Hsp70-R, 5′ GACTCCTCTTGGTGCTGGAG 3′; HsfA1 (LeHsfA1a, accession no. TC128701) HsfA1-F, 5′ GGGATAAATGAGGCAGC AAA 3′, HsfA1-R, 5′ TTGACCTGCAATTGCTGAAG 3′; HsfA2 (LeHsfA2a, accession no. TC131560) HsfA2-F, 5′ TTCCACCACATTGTTGCCTA 3′, HsfA2-R, 5′ GCAAGCACCAGATCCTTGTT 3′; HsfB1 (LeHsfB1, accession no.TC119447) HsfB1-F, 5′ GTCCCAGTTCACGGACTTGT 3′, HsfB1-R, 5′ TTGGCTCATGATACGGTTGA 3′.
Statistical analysis
Univariate analysis of variance (ANOVA) was performed on all treatments as complete randomized blocks where there were two or more factors. The least significant difference (LSD) test was used to determine which means differ using the statistical package SPSS 14.0. Differences were considered significant if the LSD was calculated from the pooled variance at P <0.05, P <0.01, or P <0.001. The SEM was used as a measurement of reproducibility between replicates within a treatment and is shown as error bars on the graphs.
Results
Salicylic acid potentiates the accumulation of Hsp/Hsc70 during heat stress
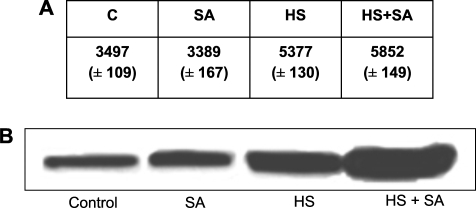
The ability of SA to enhance heat-induced Hsp/Hsc70 accumulation was demonstrated in 3-week-old tomato seedlings (Fig. 1: HS versus HS+SA). SA (0.1 mM) on its own did not significantly affect the levels of Hsp/Hsc70 when compared with the control (Fig. 1: control versus SA). As expected, heat shock significantly increased the levels of Hsp/Hsc70 accumulation (Fig. 1: HS versus control), while the exogenous addition of SA during HS significantly potentiated the Hsp/Hsc70 accumulation (Fig. 1: HS versus HS+SA). Extensive pilot time and dose studies (results not shown) revealed that 0.1 mM SA exposure for 17 h resulted in the most significant (P <0.001) potentiation of heat-induced Hsp/Hsc70 accumulation in the tomato seedlings and was used throughout this study.
Fig. 1.
Salicylic acid (SA) treatment followed by a heat shock (HS) leads to potentiation of Hsp/Hsc70 accumulation in seedlings. (A) The table represents relative Hsp/Hsc70 accumulation. Values represent the average of four biological repeats, SEM (P <0.001). (B) Representative western blot analysis of tomato seedlings, left untreated at room temperature (Control); or treated with SA (0.1 mM) for 17 h (SA); heat shocked at 40 °C for 30 min followed by a 2.5 h recovery (HS); or treated with both SA and HS (0.1 mM SA for 17 h followed by HS at 40 °C for 30 min) (HS+SA), using a mouse anti-Hsp/Hsc70 monoclonal antibody. SA alone did not induce Hsp/Hsc70 accumulation. SA in conjunction with HS was able to potentiate Hsp/Hsc70 accumulation significantly when compared with the HS treatment alone.
Salicylic acid enhances Hsf–DNA binding when administered in combination with a heat shock
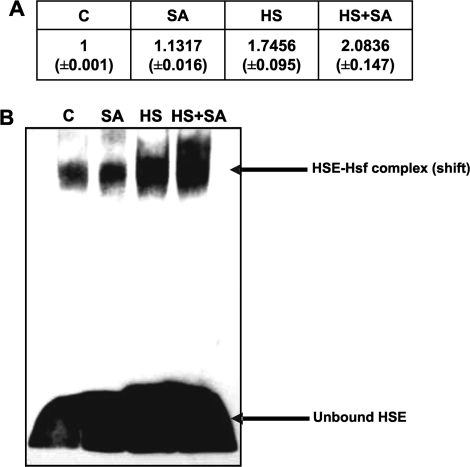
To determine whether the observed potentiation of heat-induced Hsp/Hsc70 protein accumulation was accompanied by an enhanced Hsf–DNA binding influenced by SA, EMSAs (gel retardation assays) were done (Fig. 2). In SA-treated plants, there was a very slight, albeit noticeable, degree of Hsf–DNA binding when compared with the control (Fig. 2: C versus SA). The Hsf–DNA binding was significantly enhanced in heat-shocked seedlings when compared with non-heat-shocked controls (Fig. 2: C versus HS). A more prominent shift (significant; P <0.001) associated with a higher degree of Hsf–DNA binding was observed in the seedlings treated with heat shock in the presence of SA (Fig. 2: HS+SA) when compared with heat-treated seedlings in the absence of SA (Fig. 2: HS).
Fig. 2.
Tomato seedlings showed enhanced Hsf–DNA binding when treated with SA in combination with heat shock. (A) Table representing the relative density of Hsf–DNA binding. Values represent the average of four repeats, SEM (P <0.001). (B) Representative EMSA of seedlings maintained at room temperature (C); treated with 0.1 mM SA for 17 h (SA); heat shocked [40 °C, 30 min (HS)]; and treated with SA and heat shock (heat shocked after 17 h SA exposure). Nuclear proteins were isolated directly after the heat shock treatment. The most significant band shift was observed in seedlings treated with SA in combination with heat shock when compared with all of the respective controls.
Salicylic acid potentiates the expression levels of the hsp70 gene during and after HS
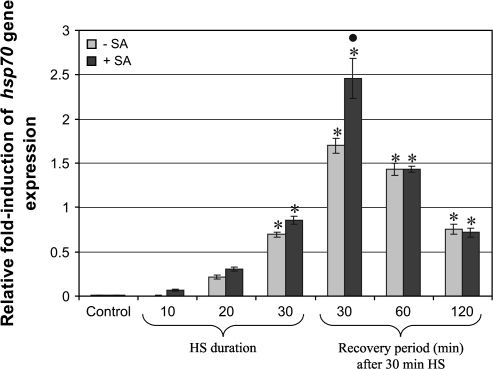
Since Hsp accumulation commences after the transcription of the hsp70 gene, the level of expression of this gene was quantified during the heat stress, as well as at time points following heat shock. In the absence of heat shock, the presence of SA had no effect on the expression of hsp70 (Fig 3: Control+SA). The expression of the hsp70 gene was shown to increase almost immediately after the onset of heat shock (Fig. 3: 10 min and 20 min during HS). hsp70 gene levels increased with time, reaching a peak at 30 min after heat shock (Fig. 3: 30 min after HS); thereafter hsp70 gene expression started to subside (Fig. 3: 60 min and 120 min after HS). Seedlings exposed to SA during heat shock showed an enhanced hsp70 gene expression at all the time points (Fig 3: –SA versus +SA), and most significantly (P <0.001) at time 30 min after heat shock, when compared with the respective control.
Fig. 3.
SA potentiates hsp70 gene expression at different time intervals during and after heat shock (HS). Real-time PCR analysis of RNA isolated from tomato seedlings maintained at room temperature (Control, –SA) or treated with 0.1 mM SA (Control, +SA), HS (40 °C, 30 min) in the presence (+SA) or absence (–SA) of SA. RNA was isolated during HS (10, 20, and 30 min, no recovery period), or after 30 min HS followed by a 30, 60, or 120 min recovery period. SA alone had no effect on hsp70 gene expression (Control, +SA). Increased hsp70 gene expression was already observed after 10 min HS, reaching a maximum after 1 h (30 min HS+30 min recovery) followed by a decline during the 60 min recovery period. The presence of SA caused increased heat-induced expression of hsp70 during and following HS to be significantly potentiated. Bars represent average values (n=10) and the error bars indicate the SEM. Symbols indicate significant differences (least significant difference) at P <0.001: * indicates a difference between each treated sample and the non-treated control (Control –SA); the filled circle • indicates the difference between the SA treated sample versus the non-SA treated sample at that specific time point (HS plus recovery period).
Salicylic acid influenced the expression of the heat shock factors HsfA1, HsfA2, and HsfB1
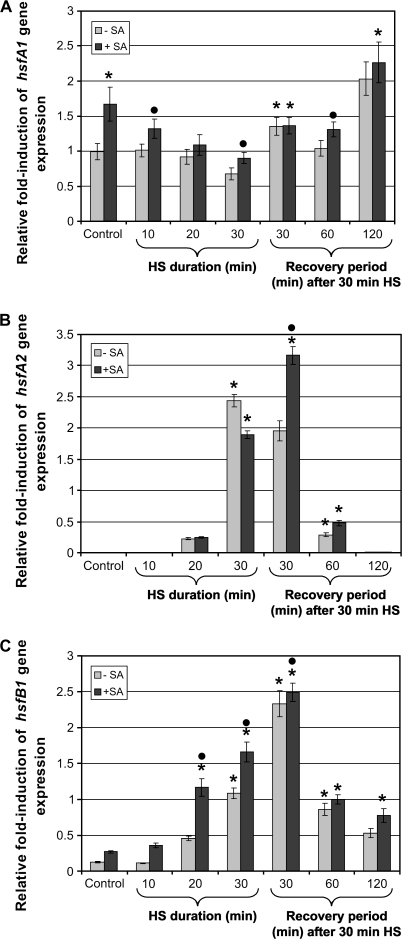
Since the expression of hsp70 genes is regulated by Hsfs, the gene expression of three plant Hsfs was investigated by qRT-PCR. HsfA1 is a constitutively expressed Hsf and levels were therefore elevated during control conditions (Fig. 4A: Control). These expression levels remained constant during initial heat shock treatment, decreased significantly after 30 min heat shock, then increased significantly after 1 h (30 min heat shock+30 min recovery), thereafter decreasing slightly at 60 min after heat shock and then increasing to the highest induced levels at time 120 min after heat shock, when compared with the control (Fig. 4A: –SA). In the non-heat-shocked (i.e. control) samples, the addition of SA caused a significant 2-fold increase in hsfA1 expression. The exposure of seedlings to SA during heat shock caused a significant (LSD: P <0.05) increase of hsfA1 expression after 10 min and 30 min heat shock, as well as after 30 min heat shock plus a 60 min recovery period when compared with each non-SA-treated control (Fig. 4A: –SA versus +SA).
Fig. 4.
Expression levels of hsfA1, hsfA2, and hsfB1 in tomato seedlings. Real-time PCR analysis of RNA isolated from tomato seedlings maintained at room temperature (Control, –SA) or treated with SA (0.1 mM) (Control, +SA), or heat shocked (40 °C, 30 min) in the presence (+SA) or absence (–SA) of SA. RNA was isolated after heat shock (HS; 10, 20, and 30 min, no recovery period), or after 30 min HS followed by a 30, 60, or 120 min recovery period. (A) SA on its own was able to enhance hsfA1 gene expression on its own. In heat-treated seedlings, the presence of SA was able to potentiate hsfA1 gene expression at all time intervals except after the 30 min recovery period. (B) The heat-inducible hsfA2 remained unaffected under control conditions (Control, –SA and +SA). In the absence of SA, maximum induction of hsfA2 expression was observed after 30 min HS (no recovery period). During the recovery period, hsfA2 gene expression started to decline and no expression was observed after a 120 min recovery period. The presence of SA caused a significant potentiation of hsfA2 in heat-treated seedlings after the 30 min recovery period. (C) In heat-treated seedlings (no SA), hsfB1 showed increased gene expression at early intervals followed by a sharp decline during the 60 min and 120 min recovery period. The presence of SA was able to potentiate the expression of hsfB1 in control and heat-treated seedlings when compared with the respective controls (–SA). Bars represent average values (n=7) and error bars indicate SEM. Symbols indicate significant differences (least significant difference) at P <0.001: * indicates a difference between each treated sample (SA and/or HS) and the non-treated control (Control, –SA); the filled circles indicates a difference between the SA-treated sample versus the non-SA-treated sample at that specific time point (HS±recovery period).
The heat-inducible Hsf, HsfA2, seemed to be activated at a later time period after heat shock, but is also active for a shorter period (Fig. 4B). The level of hsfA2 was elevated slightly at 20 min during heat shock, but became pronounced after 30 min heat shock then decreased after 1 h (30 min heat shock+30 min recovery) before dropping significantly (LSD: P <0.001) in gene expression at 60 min and 120 min after heat shock. SA affected hsfA2 gene expression in a similar manner as the heat-shocked samples (Fig. 4B: –SA versus +SA), but the potentiation was the most prominent after 1 h (30 min heat shock+30 min recovery).
The second heat shock-induced Hsf investigated in this study, HsfB1, was significantly up-regulated within 20 min of heat shock and increased over time, reaching a maximum after 1 h (30 min heat shock+30 min recovery period) (Fig. 4C). These induced levels declined significantly (LSD: P <0.001) 30 min later and further declined 2 h after heat shock (Fig. 4C: 60 min and 120 min after HS, –SA). The presence of SA in non-heat-shocked seedlings resulted in increased expression of hsfB1. SA was also able to enhance significantly the heat-induced expression of hsfB1, during both heat shock treatment (at 10, 20, and 30 min) and the 120 min recovery period, when compared with their relative controls (Fig. 4C: –SA versus +SA). Although there was an increase in hsfB1 at 30 min after heat shock with the addition of SA, it was not significant at P <0.001 (LSD) (Fig. 4C: 30 min after HS, +SA versus –SA), while heat-induced potentiation of hsfB1 expression in the presence of SA was most prominent after 20 min heat shock. Thus, in almost all instances, SA was able to increase significantly the expression of hsfB1, both at normal temperature and following heat shock.
Discussion
Hsps induced in response to elevated temperatures are not considered as part of the classical defence responses launched by plants in recognition of pathogens. However, it is known that heat shock survival takes priority over defence responses, and it is probably related to heat-induced disease susceptibility (Walter, 1989; Malamy et al., 1992). It was previously shown that SA-mediated potentiation of Hsp70 is associated with decreased cell death events (Cronjé et al., 2004). Considering the important signalling role of SA in the activation of various plant defence responses, as well as the ability of SA to enhance the expression of Hsp70, it was felt to be important to investigate the mechanism whereby SA is able to modulate the HSR in tomato seedlings.
It was observed that exogenous, non-phytotoxic levels of SA did not have an effect on the accumulation of Hsp/Hsc70 (Fig. 1). The failure of 0.1 mM SA to induce Hsp/Hsc70 accumulation relates to several reports in various organisms indicating that salicylates, in moderation, do not activate hsp gene transcription (Jurivich et al., 1992; Amici et al., 1995; Giardina and Lis, 1995; Winegarden et al., 1996). Furthermore, the findings agree with results previously demonstrated by Jurivich et al. (1992), showing enhanced Hsf–DNA binding in the presence of SA that was not accompanied by expression of hsp70. Treating seedlings with a combination of SA and heat shock produced increased levels of the Hsp/Hsc70 compared with seedlings exposed to a heat shock only. This potentiation of Hsp/Hsc70 by SA was also observed in tomato cell suspension cultures (Cronjé and Bornman, 1999), tobacco protoplasts (Cronjé et al., 2004), Arabidopsis seedlings (Cronjé and Berger, unpublished), as well as in mammalians (Fawcett et al., 1997).
SA has been shown to affect oxidative phosphorylation and cause decreased levels of ATP in tobacco cells (Xie and Chen, 1999). In mammalian cells, Hsp70 and altered ATP levels function to maintain cell homeostasis (Mallouk et al., 1999). The cumulative effects of SA and heat shock on Hsp70 levels were therefore investigated in the presence of ATP inhibitors and reactive oxygen species (ROS) scavengers. It was possibble to rule out the possibility that the secondary effects of SA (including changes in ATP and ROS levels) in combination with heat shock (including increased ROS levels) were responsible for the potentiating effect (unpublished data). The ability of SA to potentiate heat-induced accumulation of Hsp70 was next investigated by studying Hsf–DNA binding, hsp70 mRNA levels, and the quantitative gene expression of Hsp70 and three different Hsfs.
Treating seedlings with SA at normal temperatures caused partial Hsf–DNA binding (Fig. 2), which was not accompanied by activation of hsp70 transcription nor hsp70 gene expression (Fig. 3). The observed heat-induced potentiation of Hsp70/Hsc70 accumulation in the presence of SA, however, did coincide with significantly enhanced Hsf–DNA binding, events which were preceded by elevated levels of hsp70 gene transcription. It has been established that the hyperphosphorylation of the mammalian Hsf1 by SA is different from that during heat shock, the latter resulting in activation of hsp70 transcription (Jurivich et al., 1995; Cotto et al., 1996). Whether plant Hsfs are similarly phosphorylated remains to be determined.
Various studies reveal the multiplicity and the complex nature of the plant Hsf family (Scharf et al., 1998a, b; Nover et al., 2001; Baniwal et al., 2004). This makes the study of the effects of SA on the heat shock response much more complex. The three best studied tomato Hsfs, HsfA1, HsfA2 and HsfB1, were selected.
The enhanced DNA binding in the presence of SA alone (Fig. 2) was accompanied by elevated hsfA1 gene expression (Fig. 4A). In fact, hsfA1 gene expression was also observed under control conditions in the absence of SA. Since HsfA1 is considered to be the master regulator and is constitutively present (Mishra et al., 2002), it is possible that HsfA1 is involved in enhanced binding to the HSE. Not surprisingly, heat shock alone resulted in the expression of hsfA2 and hsfB1 (Fig. 4B, C), since they are heat inducible (Scharf et al., 1990), and was accompanied by enhanced Hsf–DNA binding immediately after heat shock (Fig. 2: time 0 after HS).
When seedlings were exposed to SA in combination with heat shock, hsfA1 was potentiated rapidly after only 10 min heat shock (Fig. 4A), while a marked increase in hsfA2 and hsfB1 gene expression was observed later, 30 min after heat shock (Fig. 4B, C). Interestingly, after an early increase, HsfA1 expression started to decline over time while the other genes investigated (hsp70, hsfA2, and hsfB1) increased in expression. Then, as gene expression of hsfA2 and hsfB1 started to subside (60 min and 120 min after heat shock), hsfA1 was once again up-regulated (120 min after heat shock). Since increased hsp70 and hsf gene expression coincide with increased levels of Hsp70 accumulation, it therefore seems highly likely that SA-mediated potentiation of Hsp70 is due to modulation of these Hsfs by SA.
It has been shown that in heat-shocked tomato cell cultures (S. peruvianum) (Mishra et al., 2002; Baniwal et al., 2004), the constitutively expressed HsfA1 is complemented by the two heat shock-inducible forms, HsfA2 and HsfB1. HsfA2 has a functional nuclear localization signal (NLS) but is defective in nuclear import, forming cytoplasmic granules. HsfB1 represents a novel type of co-activator cooperating with class A Hsfs and appears to form an enhanceosome-like complex. Considering that HsfA1 is constitutively expressed and has a functional NLS, it would appear that the enhanced binding observed after SA exposure in the present studies (Fig. 2) is represented by HsfA1. Furthermore, since HsfA2 is heat inducible and only translocates to the nucleus after having a direct physical interaction with HsfA1 (Scharf et al., 1998a), it is possible that the potentiated levels of Hsf–DNA binding in the tomato seedlings exposed to SA and heat shock is due to the presence of a hetero-oligomer complex consisting of HsfA1 and HsfA2. Given the fact that HsfB1 expression was also up-regulated by SA and heat shock, it is possible that the enhanced binding might have existed as an ‘enhanceosome-like’ complex. Future protein–protein interaction or yeast-two hybrid studies could clarify the composition of such a possible complex.
Apart from the role of SA in the HSR, the SA pathway has been shown to play a very important role in acquired thermotolerance (Clarke et al., 2004; Larkindale et al., 2005). In fact, in has been shown that exogenously applied SA during heat acclimation ultimately results in increased heat tolerance (Dat et al., 2000; Larkindale and Huang, 2004). potentiation of Hsp70 by exogenous SA was observed at similar concentrations. For example, mustard seedlings were treated with 100 μM SA (Dat et al., 1998b); Kentucky bluegrass with ≥0.25 mmol SA (He et al., 2005), and potato microplants with 10−6–10−3 M SA (Lopez-Delgado et al., 1998). It would be interesting to evaluate whether the potentiated Hsp70 levels by SA can contribute to thermotolerance, since previous studies have revealed other plant Hsps, i.e. Hsp101 and class I and II small Hsps, to be involved in the acquisition of thermotolerance (Larkindale et al., 2005). Interestingly, Charng et al. (2007) showed that the heat-inducible HsfA2 in Arabidopsis sustained the expression of Hsp genes and extended the duration of acquired thermotolerance, although this Hsf was not required for initial regulation of the HSR genes. Mishra et al. (2002) and Baniwal et al. (2004), who provided evidence that HsfA1 is the master regulator for the HSR, stated that this function is not replaced by any other HSF in tomatoes. They furthermore revealed that transgenic lines deficient in HsfA1 were unable to acquire thermotolerance.
In pea leaves, elevated levels of endogenous SA, leading to the acquisition or reinforced levels of thermotolerance, was preceded by the activation of PIP2-phospholipase C (PLC), resulting in the concomitant production of inositol-P3 (IP3) and diacylglycerol (DAG) following hydrolysis of PIP2 by PLC. This IP3 production causes the mobilization of calcium release (Liu et al., 2006a). It is a well-established fact that in mammalian cells, DAG results in the activation of protein kinase C (PKC). Interestingly, previous studies in peripheral blood monocytes have shown that a potent activator of PKC (phorbol 12-myristate 13-acetate) resulted in elevated expression of Hsp70 (and Hsp90), although this expression was mediated differently from heat shock (Jacquier-Sarlin et al., 1995). It would be interesting to investigate whether DAG can modulate an orthologue of PKC in plants during heat acclimation, and whether the potentiated levels of Hsp70 observed in this study in tomato seedlings exposed to exogenous SA in combination with heat shock can be correlated to elevated levels of such a possible protein kinase.
Microarray studies in Arabidopsis wild type and SA-mutant plants are currently being undertaken and in future these should underscore the role of SA in regulating the HSR. This knowledge will contribute to our overall understanding of heat-related disease susceptibility and can assist with the development of plant defence strategies in temperate or hot climates. It should furthermore prove to be interesting to unravel further the degree of cross-talk between the pathways in which SA is involved, inter alia, the plant defence response, the HSR, and thermotolerance.
Acknowledgments
We thank IA Dubery for critical reading of the manuscript and H-A Byth for assistance with statistical analysis. This work was financially supported by the National Research Foundation of South Africa and the University of Johannesburg.
Glossary
Abbreviations
- Hsf
heat shock factor
- Hsp
heat shock protein
- Hsp/Hsc70
inducible and constitutive heat shock protein 70
- HSR
heat shock response
- SA
salicylic acid
- qRT-PCR
quantitative real-time PCR
References
- Amici C, Rossi A, Santoro GM. Aspirin enhances thermotolerance in human erythroleukemic cells: an effect associated with the modulation of the heat shock response. Cancer Research. 1995;55:4452–4457. [PubMed] [Google Scholar]
- Baniwal SK, Bharti K, Chan KY, et al. Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. Journal of Bioscience. 2004;29:471–487. doi: 10.1007/BF02712120. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiology. 2007;143:251–262. doi: 10.1104/pp.106.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SM, Mur LA, Wood JE, Scott IM. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. The Plant Journal. 2004;38:432–447. doi: 10.1111/j.1365-313X.2004.02054.x. [DOI] [PubMed] [Google Scholar]
- Cotto JJ, Kline M, Morimoto RI. Activation of heat shock factor 1 DNA-binding precedes stress-induced serine phosphorylation. Journal of Biological Chemistry. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- Cronjé MJ, Bornman L. Salicylic acid influences Hsp70/Hsc70 expression in Lycopersicon esculentum: dose- and time-dependent induction or potentiation. Biochemical and Biophysical Research Communications. 1999;265:422–427. doi: 10.1006/bbrc.1999.1692. [DOI] [PubMed] [Google Scholar]
- Cronjé MJ, Weir IE, Bornman L. Salicylic acid-mediated potentiation of Hsp70 induction correlates with reduced apoptosis in tobacco protoplasts. Cytometry. 2004;61A:76–87. doi: 10.1002/cyto.a.20036. [DOI] [PubMed] [Google Scholar]
- Dat JF, Foyer CH, Scott IM. Changes in salicylic acid and antioxidants during induction of thermotolerance in mustard seedlings. Plant Physiology. 1998a;118:1455–1461. doi: 10.1104/pp.118.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiology. 1998b;116:1351–1357. doi: 10.1104/pp.116.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM. Effects of salicylic acid on oxidative stress and thermotolerance in tobacco. Journal of Plant Physiology. 2000;156:659–665. [Google Scholar]
- Dempsey DA, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Critical Reviews in Plant Science. 1999;18:547–575. [Google Scholar]
- Fawcett TW, Qingbo X, Holbrook NJ. Potentiation of heat stress-induced hsp70 expression in vivo by aspirin. Cell Stress and Chaperones. 1997;2:104–109. doi: 10.1379/1466-1268(1997)002<0104:pohsih>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina C, Lis JT. Sodium salicylate and yeast heat shock gene transcription. Journal of Biological Chemistry. 1995;271:10368–10372. doi: 10.1074/jbc.270.18.10369. [DOI] [PubMed] [Google Scholar]
- He Y, Liu Y, Cao W, Huai M, Xu B, Huang B. Effects of salicylic acid on heat tolerance associated with antioxidant metabolism in Kentucky Bluegrass. Crop Science. 2005;45:988–995. [Google Scholar]
- Howarth C, Ougham HJ. Gene expression under temperature stress. New Phytologist. 1993;125:1–26. doi: 10.1111/j.1469-8137.1993.tb03862.x. [DOI] [PubMed] [Google Scholar]
- Jacquier-Sarlin MR, Jornot L, Polla BS. Differential expression and regulation of hsp70 and hsp90 by phorbol esters and heat shock. Journal of Biological Chemistry. 1995;270:14094–14099. doi: 10.1074/jbc.270.23.14094. [DOI] [PubMed] [Google Scholar]
- Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- Jurivich DA, Pachetti C, Qiu L, Welk JF. Salicylate triggers heat shock factor differently than heat. Journal of Biological Chemistry. 1995;270:24489–24495. doi: 10.1074/jbc.270.41.24489. [DOI] [PubMed] [Google Scholar]
- Klessig DF, Malamy J. The salicylic acid signal in plants. Plant Molecular Biology. 1994;26:1439–1458. doi: 10.1007/BF00016484. [DOI] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiology. 2005;138:882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Huang B. Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. Journal of Plant Physiology. 2004;161:405–413. doi: 10.1078/0176-1617-01239. [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiology. 2002;128:682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H-T, Huang W-D, Pan Q-H, Weng F-H, Zhan J-C, Liu Y, Wan S-B, Liu Y-Y. Contributions of PIP2-specific-phospholipase C and free salicylic acid to heat acclimation-induced thermotolerance in pea leaves. Journal of Plant Physiology. 2006a;163:405–416. doi: 10.1016/j.jplph.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Liu H-T, Liu Y-Y, Pan Q-H, Yang H-R, Zhan J-C, Huang W-D. Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. Journal of Experimental Botany. 2006b;57:3337–3347. doi: 10.1093/jxb/erl098. [DOI] [PubMed] [Google Scholar]
- Lopez-Delgado H, Dat JF, Foyer CH, Scot IM. Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. Journal of Experimental Botany. 1998;49:713–720. [Google Scholar]
- Malamy J, Hennig J, Klessig DF. Temperature-dependent induction of salicylic acid and its conjugates during resistance responses to tobacco mosaic virus infection. The Plant Cell. 1992;4:359–366. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallouk Y, Vayssier-Taussat M, Bonventre JV, Polla BS. Heat shock protein 70 and ATP as partners in cell homeostasis. International Journal of Molecular Medicine. 1999;4:463–474. doi: 10.3892/ijmm.4.5.463. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf K-D. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes and Development. 2002;16:1555–1567. doi: 10.1101/gad.228802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Bharti K, Döring P, Mishra SK, Ganguli A, Scharf K-D. Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress and Chaperones. 2001;6:177–189. doi: 10.1379/1466-1268(2001)006<0177:aathst>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K, Hata M. Molecular chaperones function of mammalian Hsp70 and Hsp40—a review. International Journal of Hyperthermia. 2000;16:231–245. doi: 10.1080/026567300285259. [DOI] [PubMed] [Google Scholar]
- Pelham HR. A regulatory upstream promoter element in the Drosophila hsp70 heat-shock gene. Cell. 1982;30:517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Lekanne Deprez RH, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Scharf K-D, Heider H, Höhfeld I, Lyck R, Schmidt E, Nover L. The tomato Hsf system: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Molecular and Cellular Biology. 1998a;18:2240–2251. doi: 10.1128/mcb.18.4.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf K-D, Höhfeld I, Nover L. Heat stress response and heat stress transcription factors. Journal of Bioscience. 1998b;23:313–329. [Google Scholar]
- Scharf K-D, Rose S, Zott W, Schölff F, Nover L. Three tomato genes code for heat stress transcription factors with a region of remarkable homology to the DNA-binding domain of the yeast HSF. EMBO Journal. 1990;9:4495–4501. doi: 10.1002/j.1460-2075.1990.tb07900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JB, Graff D, Li HP. A solid-phase method for the quantitation of protein in the presence of sodium dodecyl sulfate and other interfering substances. Analytical Biochemistry. 1987;166:49–54. doi: 10.1016/0003-2697(87)90544-6. [DOI] [PubMed] [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1991;42:579–620. [Google Scholar]
- Walter MH. The induction of phenylpropanoids biosynthetic enzymes by ultraviolet light or fungal elicitor in cultured parsley cells is overridden by a heat-shock treatment. Planta. 1989;177:1–8. doi: 10.1007/BF00392148. [DOI] [PubMed] [Google Scholar]
- Winegarden NA, Wong KS, Sopta M, Westwood JT. Sodium salicylate decreases intracellular ATP, induces both heat shock factor binding and chromosomal puffing, but does not induce hsp 70 gene transcription in Drosophila. Journal of Biological Chemistry. 1996;271:26971–26980. doi: 10.1074/jbc.271.43.26971. [DOI] [PubMed] [Google Scholar]
- Xie Z, Chen Z. Salicylic acid induces rapid inhibition of mitochondrial electron transport and oxidative phosphorylation in tobacco cells. Plant Physiology. 1999;120:217–225. doi: 10.1104/pp.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]