Abstract
It has been hypothesized that the substantial reductions in xylemic water flow occurring at veraison are due to physical disruption (breaking) of the xylem as a result of renewed berry growth. In a companion paper, evidence was presented that the vast majority of xylem tracheary elements remained intact despite the growth of the berry, and it was proposed that existing tracheary elements stretch to accommodate growth and that additional elements may also differentiate after veraison. Measurements of the intergyre distance of tracheary elements in macerated tissue were used to test for stretching, and the numbers of tracheary elements per vascular bundle and of branch points of the peripheral xylem network were analysed to test for continued differentiation from 18 to 120 d after anthesis in Chardonnay berries. The distance between the epidermis and the vasculature increased substantially from pre- to post-veraison, potentially increasing the amount of skin available for analysis of compounds important for winemaking. Tracheary elements continued to differentiate within the existing vascular bundles throughout berry development. Additional vascular bundles also appeared until after veraison, thereby increasing the complexity of the peripheral vascular network. The results also confirmed that tracheary elements stretched by ∼20%, but this was not as much as that predicted based on the growth of the vascular diameter (40%). These results complete a comprehensive evaluation of grape berry peripheral xylem during its development and show that tracheary development continues further into berry maturation than previously thought.
Keywords: Tracheary element, vasculature, vessel, water movement
Introduction
Water flow in many fleshy fruits changes from being predominantly xylemic to being predominantly phloemic during development. In grape (Vitis vinifera L.), this transition appears to occur around veraison (Lang and Thorpe, 1989; Greenspan et al., 1994, 1996; Rogiers et al., 2001; Matthews and Shackel, 2005). Although in some species increased phloem flux is accompanied by xylem recycling (Pate et al., 1985), the most common hypothesis in grape is that the growth of the berry stretches and breaks the peripheral xylem (Coombe and McCarthy, 2000). Photomicrographs of broken xylem (Düring et al., 1987; Findlay et al., 1987) and the absence of passive dye uptake into the xylem of post-veraison berries (Düring et al., 1987; Findlay et al., 1987; Creasy et al., 1993; Rogiers et al., 2001; Dichio et al., 2003) have been used as evidence in support of this hypothesis. However, recent studies (Bondada et al., 2005; Keller et al., 2006) have shown that movement of apoplastic dye in the xylem of post-veraison berries was possible if an appropriate driving force was applied. This implies that the xylem remains intact throughout berry development.
In a companion paper (Chatelet et al., 2008), xylem structure and integrity in developing Chardonnay berries was investigated to confirm the results of Bondada et al. (2005). Most tracheary elements remained intact post-veraison, with only a small fraction appearing ruptured, supporting the conclusion that the xylem remains physically functional in post-veraison Chardonnay berries. Since most of the xylem was intact despite the growth of the berry, it was hypothesized that the annular and helical thickenings of the tracheary elements allowed them to stretch to accommodate the berry growth. It was also found that Chardonnay berry xylem was not primarily composed of tracheids, as the literature indicates (Pratt, 1971), but rather vessels.
Based on their microscopic observations of the xylem before and after veraison, the presence of breaks or gaps in vascular bundles (During et al., 1987) or gaps in the helical thickenings of berry tracheary elements (Findlay et al., 1987) was attributed to the inability of the lignified xylem to increase in length during the second phase of berry growth. This interpretation was supported by the previous results of Kollmann and Côté (1968) that mature xylem elements should not stretch very easily. In addition, Nobel (1974) estimated that the primary wall of Nitella cells was relatively inelastic and would stretch by only ∼4% when subjected to an internal pressure of 6 bars. However, more recent studies (MacAdam and Nelson, 2002; Paolillo, 1995) showed that protoxylem vessels in expanding leaves were able to stretch to >10 times their initial length and still remained functional. In the case of the grape berry, the diameter typically increases by 25–35% from pre-veraison to post-veraison (Coombe and Bishop, 1980; Huang and Huang, 2001), implying that the peripheral tracheary elements, which are close to the surface and parallel to it, should have about the same relative increase in length. Since the xylem was continuous despite the berry growth, a potential stretching of these elements needs to be investigated.
If tracheary elements cannot stretch enough to accommodate the growth of the berry, then the addition of new tracheary elements to the xylem to maintain its integrity needs to be considered. This could occur by adding new elements to the existing vascular bundles or by adding new vascular bundles to the vasculature. The berry vascular system is derived from that of the carpel (Pratt, 1971; Galet, 2000), and is made up of a peripheral network in the outer part of the mesocarp and a larger axial bundle that supplies the seeds and is connected to the peripheral vasculature at the style end of the berry. There is no published information on how the vascular system grows in the berry. However, the flower is viewed as a determinate stem with appendages, and these appendages, including the carpel, are homologous with leaves (Eames, 1931; Esau, 1965; Dickinson, 2000). Leaves have shown considerable flexibility in their vascular patterning (Sachs, 1989), so comparing berry vascular system development with studies on leaf vascular patterning could provide a better understanding of the berry vascular development.
The objective of this study was to investigate the anatomy and development of the peripheral vasculature of grape berry from pre- to post-veraison. Two hypotheses were tested: (i) the vessels are stretched in response to the growth of the berry; and (ii) vascular bundles are added to the vascular network.
Materials and methods
Plant material
Berries were obtained before and after the onset of fruit ripening (veraison) from 1-year-old grapevines (V. vinifera L. cv. Chardonnay), that were planted in 7.5 l plastic pots filled with a mixture of GrowCoir™ (Greenfire Co., Ltd, Sacramento, CA, USA), clay pellets, and perlite (4:1:1 by vol.) and grown in a greenhouse at UC Davis (30/20±3 °C; 40/70±10% relative humidity; natural light with a daily maximum of ∼1200 μmol photons m−2 s−1 PAR). The vines were pruned to two shoots, and the shoots were vertically trained to ∼2 m. Vines were fully watered daily with a modified Hoagland's nutrient solution (in mM: NO3–, 6.85; NH4+, 0.43; PO43–, 0.84; K+, 3.171; Ca2+, 2.25; Mg2+, 0.99; SO42–, 0.50; and in μM: Fe2+, 28.65; Mn2+, 4.91; BO33–, 24.05; Zn2+, 1.83; MoO42–, 0.17; Cu2+, 2.52) with EC 1.00 dS m−1 at pH 5.75. Berries were collected before 10.00 h, placed in a plastic bag, and transported to the laboratory within 15 min. The developmental age of the berries was determined by the number of days after anthesis (DAA) and by the concentration of soluble solids in the berry juice (°Brix, measured with a refractometer). Veraison occurred consistently at ∼7 °Brix.
Dye infusion and peripheral vasculature network
Berries free of blemishes were infused with basic fuchsin using the wicking system described by Bondada et al. (2005) to establish a matric potential gradient across the berry. The berries were prepared in the same manner as for the pressure membrane method (Bondada et al., 2005), but in this case the berry cut surface was placed in direct contact with a single layer of lint-free paper (Kimwipe, Kimberly-Clark, Irving, TX, USA), on a feminine hygiene pad (‘always’ thin ultra regular, Proctor and Gamble, Toronto, Canada) with the external netting removed, as a wick material. Berries were incubated in a 100% relative humidity environment for 10–14 h. After incubation, the stained berry was peeled and cut longitudinally. The tissues surrounding the vasculature were carefully removed to expose the stained peripheral vasculature.
Berry diameter and epidermis–vasculature distance
From the same cluster, 10 berries were collected at each of eight different stages of development between 12 and 120 DAA, and brought to the laboratory. First, their diameter was measured, and then a slice of ∼1 mm thick was cut transversally near the pedicel, equator, and style. The slices were then observed with a dissecting microscope linked to a digital camera. From the resulting images, the distance between the epidermis and the peripheral vasculature was measured with ImageJ software (National Institutes of Health, Bethesda, MD, USA). Each image was divided into quadrants and two vascular bundles perpendicular to the surface of the slice were randomly selected in each quadrant. A total of eight measurements were made for each slice and averaged. The vascular diameter was calculated from the berry diameter minus the distance between the epidermis and the vasculature.
In addition, an expected value for the distance between the peripheral vasculature and the epidermis was calculated for each DAA assuming that expansion was uniform across the berry, i.e. the ratio of berry diameter to vascular diameter remained constant. The ratio at DAA 12 was used to calculate the expected vascular diameter at each subsequent DAA, and the expected distance between the vasculature and the epidermis was calculated by subtracting the expected vascular diameter from the measured berry diameter.
Maceration
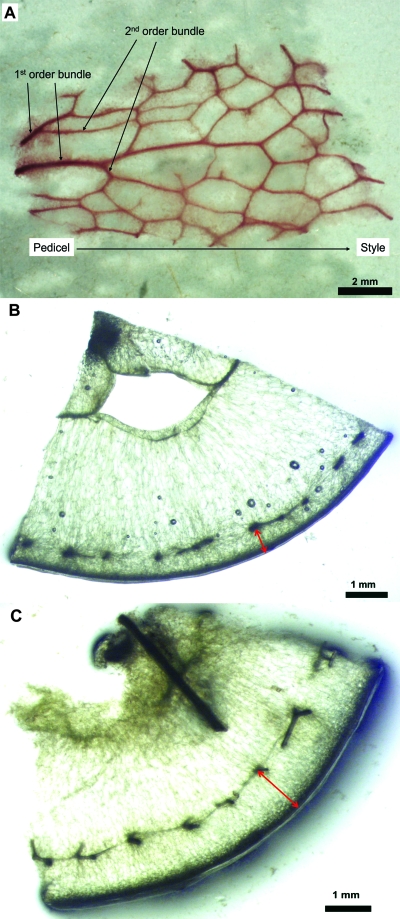
Several berries were collected at different stages of development as indicated by their °Brix and DAA. Cross-sections ∼2 mm thick were made with a razor blade near the pedicel, at the berry equator, and near the style. The axial xylem was removed to leave only the peripheral xylem. Cross-sections from each location were then immersed separately in capped vials containing a maceration solution (1:4:5 by vol., 30% hydrogen peroxide:distilled water:glacial acetic acid) and placed in the oven at 57 °C until the tissues became translucent, usually overnight. After washing with water several times, sections were stained with 0.1% aqueous Safranin O (w/v) for several hours. The vials were then strongly shaken to loosen the tissues. When the tissues had settled to the bottom of the vial, a drop was pipetted out, deposited onto a slide, and scanned with an Olympus Vanox-AHBT (Olympus America, Melville, NY, USA) compound light microscope. Digital images of macerated xylem consisting of intact radial files of tracheary elements, presumably arranged in developmental sequence (Fig. 3B, C; Chatelet et al., 2008), were taken with a Pixera 600ES digital camera (Pixera Corporation, Los Gatos, CA, USA).
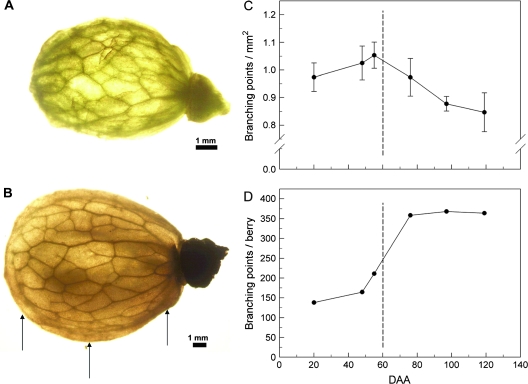
Fig. 3.
Backlit photograph revealing the peripheral vascular network in (A) pre- and (B) post-veraison peeled bisected berries. The number of branch points visible in a sample area of one-half of the peeled berry was used to calculate the number of branch points per unit area (C) and the number of branch points per berry (D). Arrows indicates the relative position of sections taken for the data of Fig 4. Data are means ±SD, n=10 berries.
Intergyre distance
When following the helix of a particular vessel within a radial file, the intergyre distance represented the distance from one point of the helix to the corresponding point along the long axis of the vessel after one complete revolution of the helix. This distance was measured using ImageJ 1.34s software (National Institutes of Health) by counting the number of gyres over a defined length of a tracheary element within the same plane of focus. Each xylem element of a radial file was assigned a rank number from 1 (the earliest developing element, always with annular secondary thickenings) to n (the latest developing element with the smallest intergyre distance; Paolillo, 1995), and only images of intact radial files (starting with rank #1) were used. Intergyre distances were measured in each rank in samples from the brush, equator, and style: 21, 46, 57, 76, 86, 98, 108, 128, 138, and 148 DAA.
In addition, a predicted intergyre distance for DAA 138 was calculated for each rank between DAA 57 and DAA 138. DAA 57 was chosen as the initial time point for this analysis because the lower ranked tracheary elements present at this stage would be expected to be the same ones as those found at a later stage, even if new tracheary elements were formed after veraison. In addition, to ensure further that the tracheary elements were of the same developmental stage, only radial files with ≥5 ranks were used to measure the intergyre distances. The predicted intergyre distance was calculated based on the increase of the vascular berry diameter from DAA 57 to DAA 138 and on the assumption that the berry is a sphere. This assumption implies that the peripheral length from the pedicel to the style increases by the same proportion as the vascular diameter of the berry. The vascular berry diameter is the diameter of the berry minus the distance from its epidermis to the vasculature. Therefore, the predicted intergyre distance of tracheary elements at DAA 138 was calculated as the intergyre distance of tracheary elements at DAA 57 multiplied by the fraction increase of the observed vascular diameter between DAA 57 and 138.
Tracheary element length
The brush, equator, and style of five berries at DDA 30 and DAA 94 were macerated as explained above. After shaking the vial containing the stained macerated berry tissue thoroughly, the solution was examined with a microscope for the presence of intact tracheary elements (tracheids or vessel members) and their length was measured and compared.
Plastic-embedded vascular bundles
Pieces of berry of different ages containing peripheral vascular bundles were cut and most of the mesocarp tissue surrounding the vascular bundles was carefully removed. The resulting bundles were stained with toluidine blue O (0.1% aqueous, w/v) for several hours to be able to see them, and separated into first- and second-order bundles (see Results). After rinsing them several times with water, the stained vascular bundles were fixed in formaldehyde–glutaraldehyde fixative and embedded in glycol methacrylate (JB-4 Plus) by standard methods (Ruzin, 1999). The vascular bundles were sectioned transversally at 2.5 μm with a Microm HM 304E rotary microtome (Microm, Walldorf, Germany). Sections were stained with 0.1% toluidine blue O (aqueous, w/v) and prepared slides were observed with an Olympus Vanox-AHBT (Olympus America) compound light microscope linked to a Pixera 600ES digital camera (Pixera Corporation). For each bundle, the numbers of tracheary elements and radial files were counted and the diameter of the elements was measured with ImageJ 1.34s software (National Institutes of Health).
Vasculature: number of bundles, branching
The images obtained from measuring the distance between the epidermis and vasculature were also used to count the number of vascular bundles viewed in cross-section of the berry brush, equator, and style during berry development. Another set of berries at different stages of development was peeled with a scalpel and cut in half from pedicel to style. Each half was observed with a dissecting microscope linked to a digital camera. Illuminating the sections from below allowed the visualization of most of the peripheral vasculature. On every image, an area was chosen centred on the equator of the berry, where distortion by surface curvature would be minimal, and the number of branching points in this area was counted. The berry vasculature surface area was then used to estimate the number of branching points per berry.
Results
Peripheral vasculature
Dye infusion through the pedicel using the wicking system resulted in the movement of basic fuchsin within the berry peripheral vascular system of post-veraison berries (Fig. 1A). The peripheral vasculature formed a network of bundles oriented longitudinally from the pedicel to the stylar end and transversally across the berry. The bundles starting from the pedicel until they branched were termed first-order bundles. All the other bundles were termed second-order bundles.
Fig. 1.
(A) Post-veraison peripheral vasculature network showing first-order vascular bundles (before any branching) and second-order vascular bundles. Basic fuchsin was infused using the wicking method, and the stained peripheral vasculature was dissected from the surrounding berry tissues. (B, C) Cross-sections near the berry equator at pre-veraison (DAA 48; B) and post-veraison (DAA 97; C) showing the distance between the epidermis and the peripheral vasculature (arrows).
Berry and vasculature diameter
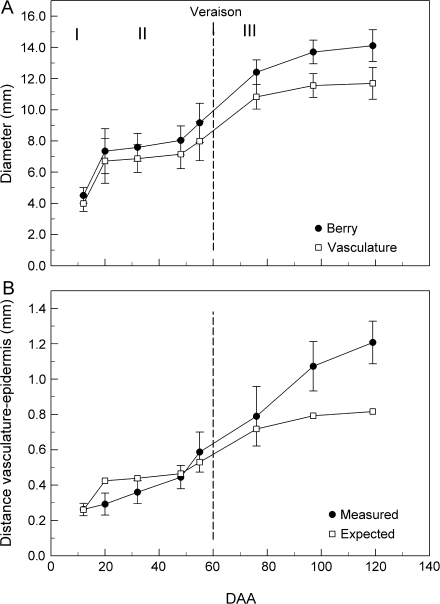
Berry transverse sections showed a clear increase in the distance between the vasculature and the epidermis from pre- to post-veraison (Fig. 1B, C). Overall berry diameter increased 3.5-fold between 12 and 120 DAA, and followed a double sigmoidal shape (Fig. 2A) with a strong increase in diameter early (stage I), a slower phase (stage II), and a strong increase at veraison (stage III) which gradually slowed. The diameter delimited by the vasculature also followed this double sigmoid curve (Fig. 2A). In contrast to the whole berry (Fig. 2A), the distance between the epidermis and the vasculature increased more or less linearly from pre- to post-veraison (Fig. 2B). The distance was less than expected for uniform growth during the pre-veraison period, but much more than expected by the end of berry development (Fig. 2B).
Fig. 2.
Diameter of the berry and the peripheral vasculature (A) and the distance between the berry surface and the peripheral vasculature (B) at various DAA. In (A), I, II, and III represent the three stages of berry development. Data are means ±SD, n=10 berries.
Vasculature branching
The peripheral vasculature networks of early pre-veraison berries (DAA 20) and post-veraison berries were similar morphologically (Fig. 3A, B). In the brush area, the peripheral vasculature was composed of vascular bundles aligned in the pedicel–style direction (data not shown). After only a few millimetres, these bundles branched in different directions with no discernible pattern. Near the style, the number of vascular bundles was reduced to a few bundles aligned in the pedicel–style direction, which fused at the stylar end. Very few free vascular bundle endings were observed.
The number of branching points mm−2 increased slightly (∼8%) until veraison and then decreased by ∼25% from the onset of veraison (DAA 55) to post-veraison (DAA 119) (Fig. 3C). On a whole-berry basis, the number of branching points appeared to follow a double sigmoid curve, although the first point (DAA 12) is missing (Fig. 3D). From DAA 20 to DAA 48, the number of points increased by 19%. From DAA 48 to DAA 76, the number of branching points increased by 118% and remained relatively constant after veraison.
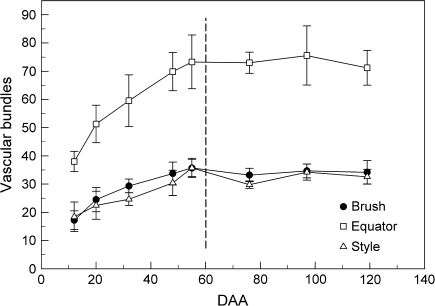
Number of vascular bundles per berry cross-section
The number of vascular bundles at the berry equator was ∼2.5 times higher than in the brush area or at the stylar end (Fig. 4). For the three locations (brush, equator, and style), the average number of vascular bundles doubled during pre-veraison and then became fairly constant during post-veraison. The brush area and the stylar end had similar numbers of bundles from pre- (59 DAA) to post-veraison.
Fig. 4.
Number of vascular bundles per berry at various DAA. Bundles were counted in fresh cross-sections near the brush area, equator, and style of berries (see Fig. 3B). Data are means ±SD, n=80 bundles (10 berries).
Number of tracheary elements per bundle cross-section
First-order vascular bundles had about twice the number of tracheary elements per bundle as second-order bundles (Fig. 5). The average number of elements per bundle increased by ∼200% from pre-veraison (DAA 20) to post-veraison (DAA 121). In both first- and second-order bundles, xylem differentiation was faster before veraison than after veraison.
Fig. 5.
Number of tracheary elements in first- and second-order bundles from berries at various DAA. Data are means ±SD, n=20 bundles (10 berries).
Number of tracheary elements per radial file: cross-section versus maceration
The number of tracheary elements per bundle cross-section almost tripled from pre-veraison (DAA 20) to post-veraison (DAA 121), while the number of radial files per bundle remained fairly constant. As a result, the number of tracheary elements per radial file doubled from pre- to post-veraison (Table 1). This progressive increase in the number of tracheary elements per radial file indicated that differentiation of tracheary elements within existing bundles occurred continuously throughout berry development. Since the tracheary elements had either annular or spiral secondary wall thickenings, the xylem of grape berries may be considered as mostly composed of protoxylem pre-veraison, and then both proto- and metaxylem post-veraison (Fig. 3B, C; Chatelet et al., 2008).
Table 1.
Anatomy of peripheral vascular bundles from vascular bundle cross-sections and from macerated radial files of berries at various DAA
| DAA | Bundle cross-sectiona | Macerated bundleb |
||
| Tracheary elements/bundle | Radial files/bundle | Tracheary elements/radial file | Tracheary elements/macerated file | |
| First-order vascular bundle | ||||
| 20 | 11.34 (1.7) | 3.5 (1.8) | 3.2 | 3.3 (0.6) |
| 33 | 14.66 (0.9) | 4.0 (1.6) | 3.6 | 3.8 (0.6) |
| 58 | 22.60 (1.6) | 5.6 (1.7) | 4.0 | 4.6 (0.3) |
| 76 | 24.00 (1.9) | 4.0 (1.5) | 6.0 | 5.4 (0.2) |
| 121 | 32.50 (2.2) | 4.6 (1.4) | 7.0 | 6.6 (0.1) |
| Second-order vascular bundle | ||||
| 20 | 5.59 (1.2) | 1.9 (0.4) | 2.9 | – |
| 33 | 9.77 (0.8) | 2.5 (1.0) | 3.9 | – |
| 58 | 12.88 (1.2) | 2.7 (1.7) | 4.7 | – |
| 76 | 12.36 (1.0) | 3.0 (1.1) | 4.1 | – |
| 121 | 16.08 (1.3) | 2.9 (0.6) | 5.5 | – |
Data are means ±SE, n=20 bundles (10 berries).
Data are means ±SE, n=30 bundles (10 berries).
Additionally, the number of tracheary elements per radial file calculated from bundle cross-sections was similar to the number of elements per radial file obtained from macerated bundles (Table 1). This implied that no elements were lost during maceration and that the intergyre distances of tracheary elements of the same rank in the radial files of macerated bundles could be compared throughout development.
Tracheary elements
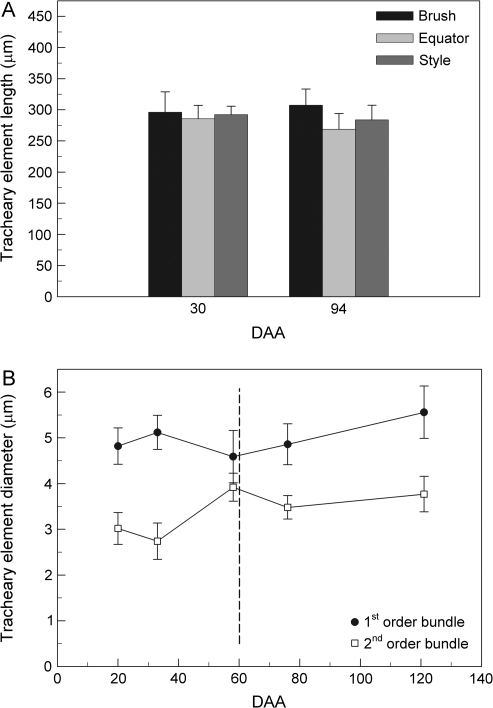
The length of individual tracheary elements following maceration was comparable between pre-veraison (DAA 30) and post-veraison (DAA 94, Fig. 6A). Also the tracheary elements collected from the brush, the equator, and the style were comparable in length at ∼250–300 μm. Most of them were vessel members, as indicated by the presence of perforation plates (Fig. 4; Chatelet et al., 2008).
Fig. 6.
Vessel member dimensions. (A) Length measured from the macerated peripheral vasculature of berries at 30 and 94 DAA. Data are means ±SE, n=30 vessels (five berries). (B) Diameter in first- and second-order bundles from berries at various DAA. Data are means ±SE, n = 20 bundles (10 berries).
The tracheary elements from the first-order vascular bundles had a larger diameter compared with second-order bundles (Fig. 6B). The average diameter was fairly constant from pre-veraison to post-veraison, ∼5 μm in first-order bundles and ∼3.5 μm in second-order bundles.
Gyre stretching
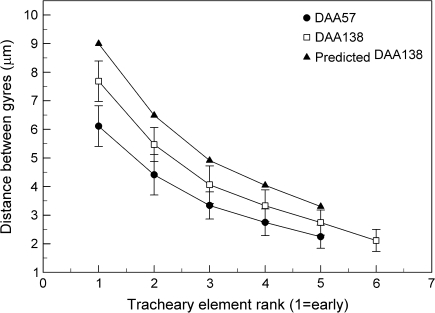
At every stage of berry development (DAA 21–148), a consistent difference in the average intergyre distance appeared from old to young tracheary elements, with a greater distance in the old elements (6–10 μm, rank 1) and a lower value (∼2 μm, rank 5) in the young ones (Fig. 7). Variability was high, but the intergyre distance of tracheary elements from post-veraison berries (DAA 138) was ∼20% longer in each rank than at the onset of veraison (DAA 57, Fig. 7). Based on the increase in vascular diameter from DAA 57 to DAA 138 (47%), the intergyre distance for DAA 138 should have been ∼20% higher than that observed for every rank, assuming that all tracheary elements stretched uniformly (Fig. 7).
Fig. 7.
Distance between gyres of tracheary elements as a function of rank (early to late) within peripheral bundles at 57 DAA and 138 DAA and a predicted value for DAA 138 based on berry diameter increase from DAA 57 (Fig. 2A). Data are means ±SD, n=30 bundles.
Discussion
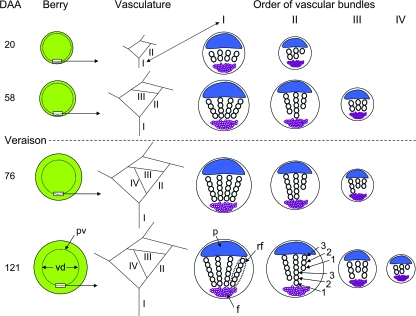
In the companion paper by Chatelet et al. (2008), it was concluded that the peripheral xylem remained essentially intact during the growth of the berry at veraison and therefore was still potentially functional. The results of this study, summarized in Fig. 8, showed (i) new, lower order, vascular bundles differentiated until the end of veraison, but not afterwards; (ii) new tracheary elements differentiated within the vascular bundles continuously during berry development; (ii) the tracheary elements present at veraison stretched by ∼20% from veraison to post-veraison; and (iv) the distance between the epidermis and the peripheral vasculature increased continuously during berry development.
Fig. 8.
Diagram summarizing the development of the vascular network and the vascular bundles of berries at various DAA. Starting at veraison, the distance between the berry surface and the peripheral vasculature (pv) increased. This distance is the difference between the berry surface and the vascular diameter (vd). As the berry expanded, new vascular bundles differentiated, increasing the order of bundles (I–IV). Within each vascular bundle, the number of radial files (rf) remained unchanged as the berry aged. However, the ranks (1, 2, 3,…) of tracheary elements within the radial files increased throughout berry development, rank 1 being the first differentiated element. p = phloem, f = fibres.
The substantial increase (∼5-fold) in distance between the epidermis and the peripheral vasculature system that was found during berry development is particularly noteworthy. Two mechanisms are involved in berry growth: first cell division, then, with some overlap, cell enlargement, both of them starting from the centre of the berry and progressing toward the outside (Coombe, 1960; Pratt, 1971; Ojeda et al., 1999; Galet, 2000). Until the berry is 30–40 d old (stage I and II), berry growth was accompanied by cell division with little enlargement (Harris, 1968; Jona and Botta, 1988). This was confirmed by measurements of the epidermis–vasculature distance which increased by ∼1.6-fold in the first 50 d. As the cell divisions progressively stop near the epidermis, cell enlargement became the sole growth factor. When the enlargement reached the vasculature, about half the cells between the vasculature and the epidermis that were previously rectangular greatly enlarged and became progressively polygonal, similar to mesocarp cells (Ollat et al., 2002) and resulting in the greater increase of the epidermis–vasculature distance. This was also confirmed by measurements showing that the distance tripled from the onset of ripening to the mature stage.
The marked increase in the epidermis–vasculature distance over development may be relevant to the definition of ‘skin’ in the grape berry. The skin is made of the cuticle, the epidermis, and the hypodermis, which consists of two series of cells (Considine and Knox, 1979), and is limited by the peripheral vascular network (Considine and Knox, 1979; Galet, 2000). It is generally assumed that the skin separates at the peripheral vasculature where a transition occurs between the small and rectangular hypodermal cells and the large, round mesocarp cells. However, as the distance between epidermis and vasculature increases between pre- and post-veraison, this zone of transition may change. Because the skin contains a high proportion of substances responsible for coloration (flavonols and anthocyanins), flavour, and aroma (Winkler et al., 1962) that are important for winemaking, it may thus be important to clarify what the term ‘skin’ encompasses, to standardize the analyses of the compounds present.
Even though the vasculature diameter increased by ∼40% from post-veraison to maturity, the intergyre distance of tracheary elements in macerated samples only indicated an average element stretching of ∼20%. The predicted intergyre distance represents the distance exhibited by tracheary elements of the same rank if their stretching was entirely due to berry growth (Harris et al., 1968; Considine and Knox, 1979; Gillaspy et al., 1993). One reason for the discrepancy between observed and predicted stretching could be that measurements were made on tracheary elements of different ages, i.e. with different degrees of stretching. However, this seems unlikely as the measurements compared DAA 57 and DAA 138, between which there is very little differentiation of new vascular bundles. Furthermore, the measurements were only made on radial files possessing an element with annular thickening (rank 1) and only the radial files with ≥5 tracheary elements were considered. However, as new vascular bundles still differentiated until the end of veraison, it is possible that systematically smaller intergyre distances of elements from vascular bundles that differentiated near veraison were included in the measurements, resulting in an underestimate for the average intergyre distance at DAA 138.
Another hypothesis to explain the lack of stretching in response to berry growth could derive from the maceration method. The purpose of maceration is to separate the xylem from the surrounding parenchyma. One effect of the maceration could be that some tracheary elements were lost. However, this seems unlikely as the data show a similar number of tacheary elements per radial file between macerated bundles and cross-sectioned bundles (Table 1). Another possibility could be that the tracheary elements were stretched and under tension in situ, but relaxed following maceration because the surrounding tissue was removed. Testing this possibility will probably require the use of cryo-microscopy. While it was not possible to confirm the hypothesis that the xylem stretches to accommodate berry growth fully, the results did show that the existing xylem does stretch.
In the companion paper (Chatelet et al., 2008), the concept was presented that grape berry xylem was composed of protoxylem before veraison and metaxylem after ripening started. Regardless of terminology, however, it was found that all tracheary elements had either annular or spiral secondary wall thickenings, and these should allow for some longitudinal stretching as measured by the increase in distance between rings in older xylem elements, the tilting of the rings, and the uncoiling of the helices (Esau, 1965; Barnett, 1981; Mauseth, 1988). MacAdam and Nelson (2002) showed, although not quantitatively, that the distance between annular wall thickenings of protoxylem from tall fescue (Festuca arundinacea Schreb.) leaves increased as the leaves continued to elongate. In the same species, Martre et al. (2000) showed, through xylem microcasting, that the protoxylem was able to stretch ∼10-fold without rupturing. During the elongation of wheat leaves, Paolillo (1995), through clearing and paraffin sectioning, also observed an increase in distance between secondary wall thickenings. According to his study, protoxylem cells collapsed when the rings of the annular secondary walls were completely tilted and the distance between gyres reached ∼50 μm. In the grape berry, the increase in intergyre distance never exceeded 15 μm and was always measured in the older tracheary elements. Incidentally, these stretched elements were the same ones that were broken in the vascular bundles of post-veraison berries (Chatel et al., 2008), indicating that the breaking threshold of berry tracheary elements may be lower than that of the tracheary elements in leaves.
In addition to the stretching of tracheary elements, the berry also apparently adapts the morphology of its peripheral vascular network to accommodate growth by the differentiation of new vascular bundles until veraison and of new tracheary elements within bundles throughout development (until 120 DAA). As a result, the peripheral vascular network is comprised of short, interconnected bundles aligned in multiple directions (transversally, oblique, and longitudinally).
This increase in vascular network complexity by the addition of vascular bundles is also observed in the development of dicotyledonous angiosperm plants. During leaf elongation, the vasculature is laid down progressively and sequentially. The procambial bundles of the primary and secondary veins are first laid down, followed by the progressive acropetal and basipetal differentiation of vessels (Chatelet et al., 2006). The reticulum of tertiary and higher order veins is established by intercalary growth and usually proceeds in a basipetal direction (Esau, 1965). Tertiary vein provascular bundles form in a simultaneous pattern, i.e. a complete bridge between two secondary veins appears at once rather than developing progressively across the areole (Pray, 1955; Lersten, 1965; Herbst, 1972; Merrill, 1979). As the leaf lamina expands, areoles formed by tertiary veins are subdivided by the next vein order, and this process is reiterated by higher order minor veins until the leaf is mature and stops expanding.
In the berry, however, while the vascular network increased in complexity by the addition of new vascular bundles during the growth of the berry, there appeared to be no vascular hierarchy as in the leaf. The first-order vascular bundles leaving the pedicel branched almost immediately and could not be followed to the style. However, the present investigation of the vasculature was limited to berries ≥20 d old. It is possible that, at earlier stages, the vasculature was formed of single vascular bundles running from the pedicel to the style and that connecting bundles appeared later and formed the reticulum.
In conclusion, this study confirmed the hypotheses that the xylem of post-veraison grape berries retains structural integrity during berry growth because: (i) the vessels are able to stretch, although not as much as was expected; and (ii) tracheary elements continually differentiate and new vascular bundles are added to the vascular network until most of the growth has ceased. The mechanisms by which the berry is able to differentiate new xylem are as yet unknown. Finally, an important and still unanswered question arises as to why the berry would invest resources to maintain functional xylem after veraison in the apparent absence of its use for water transport. Keller et al. (2006) have suggested that excess water delivered to the fruit by the phloem causes a positive hydrostatic ‘back-pressure’ in the fruit apoplast, analogous to root pressure, and that this pressure is responsible for returning the excess water back to the plant via the berry xylem. A positive pressure is possible in roots because the endodermis functions as a membrane separating soil water from the water within the stele; however, an analogous structure has not been suggested as occurring in grape berries. If more phloem water arrives at the fruit than is required for growth and berry transpiration, and the berry xylem remains connected to the plant, then backflow to the plant would be expected, even in the absence of a positive pressure in the berry xylem. The only requirements are that the hydrostatic pressure in the plant xylem (which is negative and can be called a tension) is lower than the pressure in the berry xylem, and that the two are connected. However, Keller et al. (2006) correctly point out that backflow of excess phloem-delivered water would cease if the phloem were girdled, and hence that any estimate of the direction and magnitude of xylemic flow based on the girdling approach of Lang and Thorpe (1989) would be erroneously low. Since essentially all the evidence for reduced post-veraison xylem flow comes from the use of this method (Greenspan et al., 1994), it is appropriate to re-evaluate the interpretation of this evidence. Based on the anatomical evidence that has been presented here, the many lines of evidence for berry hydraulic isolation around veraison, such as reduced diurnal size fluctuations (Greenspan et al., 1994) and independence of cell turgor from plant water status (Thomas et al., 2006), cannot simply be attributed to structural changes in the berry xylem. An alternative hypothesis for the apparent independence of post-veraison berry water relations from the water relations of the parent plant is that the rate of water import from the phloem simply compensates for diurnal or drought-induced changes in xylem tension that occur in the parent plant, such that the tension in the berry xylem is held relatively constant (perhaps close to zero). Fruit cell turgor may additionally be controlled by active regulation of the level of solutes in the fruit apoplast, as suggested by Matthews and Shackel (2005). Further research in this area is needed, but it is clear that an increase in phloem water and sugar transport to the berry at the onset of ripening should have a substantial influence both on berry composition and on berry water relations.
Acknowledgments
The authors thank Cal-Western Nurseries, Visalia, CA, USA, for providing Chardonnay grapevines. This work was funded by the California Department of Food and Agriculture, Agreement no. 01-0712 and the USDA-CREES grant no. 2005-3442-15841.
References
- Barnett JR. Xylem cell development. Van Nuys, CA: Castle House Publications Ltd; 1981. [Google Scholar]
- Bondada BR, Matthews MA, Shackel KA. Functional xylem in the post-veraison grape berry. Journal of Experimental Botany. 2005;56:2949–2957. doi: 10.1093/jxb/eri291. [DOI] [PubMed] [Google Scholar]
- Chatelet DS, Rost TL, Shackel KA, Matthews MA. The peripheral xylem of grapevine (Vitis vinifera). 1. Structural integrity in post-veraison berries. Journal of Experimental Botany. 2008;59:XXX–XXX. doi: 10.1093/jxb/ern060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelet DS, Matthews MA, Rost TL. Xylem structure and connectivity in grapevine (Vitis vinifera) shoots provides a passive mechanism for the spread of bacteria in grape plants. Annals of Botany. 2006;98:483–494. doi: 10.1093/aob/mcl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine JA, Knox RB. Development and histochemistry of the cells, cell walls, and cuticle of the dermal system of fruit of the grape, Vitis vinifera L. Protoplasma. 1979;99:347–465. [Google Scholar]
- Coombe BG. Relationship of growth and development to changes in sugars, auxins, and gibberellins in fruit of seeded and seedless varieties of Vitis vinifera. Plant Physiology. 1960;35:241–250. doi: 10.1104/pp.35.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe BG, Bishop GR. Development of the grape berry. II. Changes in diameter and deformability during veraison. Australian Journal of Agricultural Research. 1980;31:499–509. [Google Scholar]
- Coombe BG, McCarthy MG. Dynamics of grape berry growth and physiology of ripening. Australian Journal of Grape and Wine Research. 2000;6:131–135. [Google Scholar]
- Creasy GL, Price SF, Lombard PB. Evidence for xylem discontinuity in Pinot Noir and Merlot: dye uptake and mineral composition during berry maturation. American Journal of Enology and Viticulture. 1993;44:187–192. [Google Scholar]
- Dichio B, Picaud S, Lombard PB. Developmental changes in xylem functionality in kiwifruit: implications for fruit calcium accumulation. Acta Horticulturae. 2003;610:191–195. [Google Scholar]
- Dickinson WC. Integrative plant anatomy. San Diego: Academic Press; 2000. [Google Scholar]
- Düring H, Lang A, Oggionni F. Patterns of water flow in Riesling berries in relation to developmental changes in their xylem morphology. Vitis. 1987;26:123–131. [Google Scholar]
- Eames AJ. The vascular anatomy of the flower with refutation of the theory of carpel polymorphism. American Journal of Botany. 1931;18:147–188. [Google Scholar]
- Esau K. Plant anatomy. 2nd edn. NewYork: Wiley; 1965. [Google Scholar]
- Findlay N, Olivier KJ, Nii N, Coombe BG. Solute accumulation by grape pericarp cells. IV. Perfusion of pericarp apoplast via the pedicel and evidence for xylem malfunction in ripening berries. Journal of Experimental Botany. 1987;38:668–679. [Google Scholar]
- Galet P. General viticulture. France: Oenoplurimedia and P. Galet; 2000. [Google Scholar]
- Gillaspy G, David H, Gruissem W. Fruits: a developmental perspective. The Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan MD, Shackel KA, Matthews MA. Developmental changes in the diurnal water budget of the grape berry exposed to water deficits. Plant, Cell and Environment. 1994;17:811–820. [Google Scholar]
- Greenspan MD, Schultz HR, Matthews MA. Field evaluation of water transport in grape berries during water deficit. Physiologia Plantarum. 1996;97:55–62. [Google Scholar]
- Harris JM, Kriedemann PE, Possingham JV. Anatomical aspects of grape berry development. Vitis. 1968;7:106–119. [Google Scholar]
- Herbst D. Ontogeny of foliar venation in Euphorbia forbesii. American Journal of Botany. 1972;59:843–850. [Google Scholar]
- Huang X-M, Huang H-B. Early post-veraison growth in grapes: evidence for a two-step mode of berry enlargement. Australian Journal of Grape and Wine Research. 2001;7:132–136. [Google Scholar]
- Jona R, Botta R. Fruit set and early berry development in two grapevine cultivars. Israel Journal of Botany. 1988;37:203–216. [Google Scholar]
- Keller M, Smith JP, Bondada BR. Ripening grape berries remain hydraulically connected to the shoot. Journal of Experimental Botany. 2006;57:2577–2587. doi: 10.1093/jxb/erl020. [DOI] [PubMed] [Google Scholar]
- Kollmann FFP, Cõté WA. Principles of wood science and technology. I. Sold wood. London: George Allen & Unwin Ltd; 1968. [Google Scholar]
- Lang A, Thorpe MR. Xylem, phloem and transpiration flows in a grape: application of a technique for measuring the volume of attached fruits using Archimedes’ principle. Journal of Experimental Botany. 1989;40:1069–1078. [Google Scholar]
- Lersten N. Histogenesis of leaf venation in Trifolium wormskioldii (Leguminosae) American Journal of Botany. 1965;52:767–774. [Google Scholar]
- MacAdam JW, Nelson CJ. Secondary cell wall deposition causes radial growth of fibre cells in the maturation zone of elongating tall fescue leaf blades. Annals of Botany. 2002;89:89–96. doi: 10.1093/aob/mcf010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre P, Durand JL, Cochard H. Changes in axial hydraulic conductivity along elongating leaf blades in relation to xylem maturation in tall fescue. New Phytologist. 2000;146:235–247. doi: 10.1046/j.1469-8137.2000.00641.x. [DOI] [PubMed] [Google Scholar]
- Matthews MA, Shackel KA. Growth and water transport in fleshy fruit. In: Holbrook NM, Zwieniecki MA, editors. Vascular transport in plants. Burlington, CA: Academic Press; 2005. pp. 181–197. [Google Scholar]
- Mauseth JD. Plant anatomy. Menlo Park, CA: The Benjamin/Cummings Publishing Company, Inc; 1988. [Google Scholar]
- Merrill EK. Comparison of ontogeny of three types of leaf architecture in Sorbus L. (Rosaceae) Botanical Gazette. 1979;140:328–337. [Google Scholar]
- Nobel PS. Introduction to biophysical physiology. San Franscisco: W.H. Freeman and Company; 1974. [Google Scholar]
- Ojeda H, Deloire A, Carbonneau A, Ageorges A, Romieu C. Berry development of grapevines: relations between the growth of berries and their DNA content indicate cell multiplication and enlargement. Vitis. 1999;38:145–150. [Google Scholar]
- Ollat N, Diakou-Verdin P, Carde J-P, Barrieu F, Gaudillère J-P, Moing A. Revue bibliographique: développement de la baie de raisin. Journal International des Sciences de la Vigne et du Vin. 2002;36:109–131. [Google Scholar]
- Pate JS, Peoples MB, Vanbel AJE, Kuo J, Atkins CA. Diurnal water balance of the cowpea fruit. Plant Physiology. 1985;77:148–156. doi: 10.1104/pp.77.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolillo DJ., Jr Protoxylem maturation in the seedling leaf of wheat. American Journal of Botany. 1995;82:337–345. [Google Scholar]
- Pratt C. Reproductive anatomy in cultivated grapes—a review. American Journal of Enology and Viticulture. 1971;22:92–109. [Google Scholar]
- Pray TR. Foliar venation of angiosperms. II. Histogenesis of the venation of Liriodendron. American Journal of Botany. 1955;42:18–27. [Google Scholar]
- Rogiers SY, Smith JA, White R, Keller M, Holzapfel BP, Virgona JM. Vascular function in berries of Vitis vinifera (L.) cv. Shiraz. Australian Journal of Grape and Wine Research. 2001;7:46–51. [Google Scholar]
- Ruzin SE. Plant microtechnique and microscopy. Oxford: Oxford University Press; 1999. [Google Scholar]
- Sachs T. The development of vascular networks during leaf development. Current Topics in Plant Biochemistry and Physiology. 1989;8:168–183. [Google Scholar]
- Thomas TR, Matthews MA, Shackel KA. Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant, Cell and Environment. 2006;29:993–1001. doi: 10.1111/j.1365-3040.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Winkler AJ, Cook JA, Kliewer WM, Lider LA. General viticulture. University of California Press. 1962 [Google Scholar]