Abstract
During the development of many fleshy fruits, water flow becomes progressively more phloemic and less xylemic. In grape (Vitis vinifera L.), the current hypothesis to explain this change is that the tracheary elements of the peripheral xylem break as a result of berry growth, rendering the xylem structurally discontinuous and hence non-functional. Recent work, however, has shown via apoplastic dye movement through the xylem of post-veraison berries that the xylem should remain structurally intact throughout berry development. To corroborate this, peripheral xylem structure in developing Chardonnay berries was investigated via maceration and plastic sectioning. Macerations revealed that, contrary to current belief, the xylem was comprised mostly of vessels with few tracheids. In cross-section, the tracheary elements of the vascular bundles formed almost parallel radial files, with later formed elements toward the epidermis and earlier formed elements toward the centre of the berry. Most tracheary elements remained intact throughout berry maturation, consistent with recent reports of vascular dye movement in post-veraison berries.
Keywords: Tracheary element, vasculature, vessel, water movement
Introduction
In fleshy fruits, water movement in xylem and phloem is dependent upon the developmental stage of the fruit (Matthews and Shackel, 2005). In tomato (Ho et al., 1987), kiwifruit (Dichio et al., 2003), apple (Lang and Ryan, 1994; Dražeta et al., 2004), and grape (Greenspan et al., 1994, 1996), the amount of water entering the fruit via the xylem decreases as fruit matures. Growth of many fleshy fruit, including Prunus sp. and Vitis sp., exhibits a double sigmoid pattern (Coombe, 1976) in which there are two periods of growth (stages I and III) separated by a lag phase (stage II). In grape, the transition from xylemic to phloemic water is apparently rapid, occurring at the transition from stage II to stage III (Greenspan et al., 1994). In their model of vascular function during growth and maturation of grape berries, Coombe and McCarthy (2000) have presented the xylem as becoming non-functional upon the transition to stage III (called veraison).
The Coombe and McCarthy model was deduced from the microscopic observations of Düring et al. (1987) and Findlay et al. (1987) that were interpreted as showing that the peripheral xylem was stretched to failure during veraison, and from passive dye uptake studies with several grape varieties: Muscat Gordo Blanco (Findlay et al., 1987), Riesling (Düring et al., 1987), Pinot noir, and Merlot (Creasy et al., 1993). Before veraison, apoplast dye was passively absorbed through a cut pedicel into the axial and peripheral xylem of a berry, whereas after veraison its uptake was limited to the basal few millimetres of vascular tissue (Findlay et al., 1987; Creasy et al., 1993; Rogiers et al., 2001). This change has been interpreted as being due to the inability of the lignified xylem to increase in length during stage III growth and that growth itself causes a physical disruption in xylem continuity (Lang and Düring, 1991; Coombe and McCarthy, 2000).
However, Bondada et al. (2005) showed that by artificially establishing an appropriate hydrostatic gradient in post-veraison berries, apoplastic dye was transported from the pedicel to the stylar end through the axial and peripheral xylem. In addition, when post-veraison growth and subsequent potential physical failure due to stretching was prevented, apoplastic dye still did not move into the peripheral xylem without an artificial gradient. They speculated that the decrease in xylem water flow at veraison was due to a reduction in the hydrostatic gradient between the xylem in the pedicel and the xylem in the fruit flesh, and not to physical disruption of xylem continuity. There is little information, however, on berry xylem anatomy. Description of berry xylem structure apparently originated when Kroemer (1923) and de Villiers (1926) described the ventral, embryonic, and peripheral vascular bundles present in the berry flesh. This was reviewed briefly in Pratt (1971) who reported that the xylem elements are exclusively tracheids. Despite the many physiological studies published in which the interpretations are structural, there is essentially no information published on the details of the xylem structure during grape berry development. Therefore, this study was conducted to describe xylem conduit structure in pre- and post-veraison berries, looking specifically for evidence of physical integrity or loss thereof. Since Bondada et al. (2005) were able to induce dye uptake in post-veraison berries of many varieties (Chardonnay, Cabernet Sauvignon, Pinot noir, Shiraz, Muscat Gordo, and Grey Riesling) with similar results, berries of the Chardonnay variety were chosen as the model as this variety was easily available.
Materials and methods
Plant material
Berries were obtained before and after the onset of fruit ripening (veraison) from 1-year-old grapevines (Vitis vinifera L. cv. Chardonnay), that were planted in 7.5 l plastic pots filled with a mixture of GrowCoir™ (Greenfire Co., Ltd, Sacramento, CA, USA), clay pellets, and perlite (4:1:1 by vol.) and grown in a greenhouse at UC Davis (30/20±3 °C; 40/70±10% relative humidity; natural light with a daily maximum of ∼1200 μmol photons m−2 s−1 PAR). The vines were pruned to two shoots, and the shoots were vertically trained to ∼2 m. Vines were fully watered daily with a modified Hoagland's nutrient solution (in mM: NO3–, 6.85; NH4+, 0.43; PO43–, 0.84; K+, 3.171; Ca2+, 2.25; Mg2+, 0.99; SO42–, 0.50; and in μM: Fe2+, 28.65; Mn2+, 4.91; BO33–, 24.05; Zn2+, 1.83; MoO42–, 0.17; Cu2+, 2.52) with EC 1.00 dS m−1 at pH 5.75. Berries were collected before 10 am, placed in a plastic bag, and transported to the laboratory within 15 min. The developmental age of the berries was determined by the number of days after anthesis (DAA) and by the concentration of soluble solids in the berry juice (°Brix, measured with a refractometer). Veraison occurred consistently at ∼7 °Brix.
Maceration
Cross-sections ∼2 mm thick were made with a razor blade near the pedicel, at the berry equator, and near the style. The axial xylem was removed to leave only the peripheral xylem. Cross-sections from each location were then immersed separately in capped vials (Wheaton Glass 20 ml Scintillation Vials, Fisher Scientific, USA) containing 15 ml of a maceration solution (1:4:5 by vol. 30% hydrogen peroxide:distilled water:glacial acetic acid) and placed in an oven at 57 °C. After 12 h or 24 h, samples were removed and washed with water several times, strongly shaken to loosen the tissues, and several drops of 0.1% aqueous Safranin O (w/v) were added to the solution. After several hours, several drops of 0.1% aqueous (v/w) Calcofluor White 2MR were added, and the vials were shaken again. When the tissues settled at the bottom of the vial, a drop was pipetted out, deposited onto a slide, and scanned with an Olympus Vanox-AHBT (Olympus America, Melville, NY, USA) epifluorescent microscope under bright light and under UV light (dichroic mirror DM400, exciter filter=UG1, barrier filter=L435). Digital images of macerated xylem were taken with a Pixera 600ES digital camera (Pixera Corporation, Los Gatos, CA, USA).
Scanning electron microscopy of individual elements
From the macerated solutions, a drop of solution was dropped on scanning electron microscope (SEM) stubs and left to dry. The dry samples were coated with gold (Denton Vacuum Desk II Cold Sputter-Etch Unit; Denton Vacuum, Moorestown, NJ, USA) and observed with an SEM (Hitachi S-3500N; Hitachi Co., Hitachi, Japan) at an accelerating voltage of 5 kV.
Plastic embedding: cross-section and longitudinal section
Pieces of berry containing peripheral vascular bundles from the brush, midsection, and the style were excised. and most of the mesocarp tissue surrounding the vascular bundles was carefully removed without damaging the xylem. The resulting bundles were fixed in formaldehyde–glutaraldehyde fixative and embedded in glycol methacrylate (JB-4 Plus) by standard methods (Ruzin, 1999). The vascular bundles were sectioned at 2.5 μm, either transversally or longitudinally, with a Microm HM 304E rotary microtome (Microm, Walldorf, Germany). Sections were stained with 0.1% toluidine blue O (aqueous, w/v), and prepared slides were observed with an Olympus Vanox-AHBT (Olympus America) compound light microscope linked to a Pixera 600ES digital camera (Pixera Corporation).
Dye uptake
Clusters of pre- and post-veraison berries, with a °Brix of ∼5 and 16, respectively, were harvested from the plant, and the peduncle was immediately immersed in a 0.1% aqueous (w/v) acid fuchsin solution. After 5 h, the clusters were brought to the laboratory and the presence or absence of the dye in the berry peripheral xylem was observed under the microscope. Even though early workers assumed that external conditions controlled dye uptake, Bondada et al. (2005) reported no difference in dye uptake at ambient or 100% humidity, and concluded that internal hydrostatic gradients and not evaporative losses were responsible for the uptake.
Pre- (5 °Brix, DAA=52) and post-veraison (16 °Brix, DAA=95) berries with uniform shape and colour were harvested under water in the greenhouse and prepared for dye uptake (basic fuchsin, 0.1% aqueous) using the pressure membrane and wicking techniques devised by Bondada et al. (2005). The berries were prepared by sealing a small piece of tubing (inner diameter of 1 mm) to the surface of the berry at the proximal (pedicel) end using lanolin. The tubing served as a reservoir for the dye solution and, while filling the tube with dye, it was made certain that the cut end of the pedicel was fully immersed. A cross-section of the berry was made, removing ∼2 mm of the pericarp at the stylar (distal) end, and the berry was placed with its flat cut surface directly on the membrane for the pressure membrane method. The berry, reservoir, and membrane were assembled into the pressure membrane apparatus, and a pressure of 0.8 MPa was applied with nitrogen gas for 24 h. For the wicking method, the flat cut surface of the berries was placed in direct contact with a single layer of lint-free paper (Kimwipe, Kimberly-Clark, Irving, TX, USA), on a feminine hygiene pad (‘always’ thin ultra regular, Proctor and Gamble, Toronto, Canada). Berries were incubated in a 100% relative humidity environment for 10–14 h. After incubation, the berries were skinned to observe the progress of the stain within the vasculature. Stained vascular bundles were also dissected from the berry flesh for closer inspection.
Bleaching
Pre- (5 °Brix, DAA=52) and post-veraison (19 °Brix, DAA=117) berries were collected from plants in the greenhouse and brought back to the laboratory for dye uptake. Basic fuchsin was passively absorbed by the pre-veraison berries by submerging their pedicel in the stain, while the post-veraison berries absorbed the red stain by using the pressure membrane apparatus (Bondada et al., 2005). The resulting red xylem was then carefully separated from the berry flesh to prevent any tearing. Individual vascular bundles were mounted with water on a slide and observed with an Olympus Vanox-AHBT (Olympus America) compound light microscope linked to a Pixera 600ES digital camera (Pixera Corporation). The water was then progressively replaced by a 1:1 water:commercial bleach solution [Clorox Regular-Bleach (sodium hypochlorite=6.15%) The Clorox Company, Oakland, CA, USA]. After a few seconds, the vascular bundle started to decolorize and as a reaction between the basic fuchsin and the bleach, some gas, possibly oxygen, appeared within several tracheary elements. As the decolorization continued, more gas was produced and its movement within the tracheary elements was observed with the microscope until the bundle was completely bleached.
Results
Dye movement in pre- and post-veraison berries
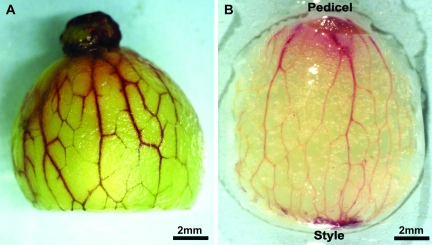
Acid fuchsin and basic fuchsin were taken up through the cut pedicel and into and throughout the peripheral xylem of pre-veraison berries using the pressure membrane, the wicking, or the passive dye infusion methods (Fig. 1A). In post-veraison berries, however, the dye did not move into the peripheral xylem using the passive dye infusion method (data not shown), but the stain was taken up into the xylem when using the wicking system or the pressure membrane technique with a hydrostatic pressure gradient of 0.8 MPa (Fig. 1B). For both pre- and post-veraison, vascular bundles stained red from the pedicel to the stylar end. However, the staining was more rapid, consistent, and extensive in pre-veraison berries.
Fig. 1.
Movement of basic fuchsin throughout the peripheral vasculature: (A) pre-veraison berry stained using the wicking method; (B) post-veraison berry stained using the pressure membrane method.
Anatomy of a typical vascular bundle
The vascular bundles had a typical collateral organization (Mauseth, 1988), with the xylem on the inner side of the bundle and the phloem on the outer side (Fig. 2A, B). The tracheary elements were more or less linearly arranged in radial files with the latest elements toward the epidermis and the earliest toward the centre of the berry (Fig. 2A–C).
Fig. 2.
Cross-section of a typical vascular bundle showing radial files of tracheary elements (red ellipses) from a pre-veraison (DAA=32) (A) and a post-veraison berry (DAA=120) (B). (C) Schematic diagram of a central longitudinal section of a berry (right) with the detailed anatomy of a vascular bundle (left).
Maceration and plastic longitudinal sections of xylem bundles
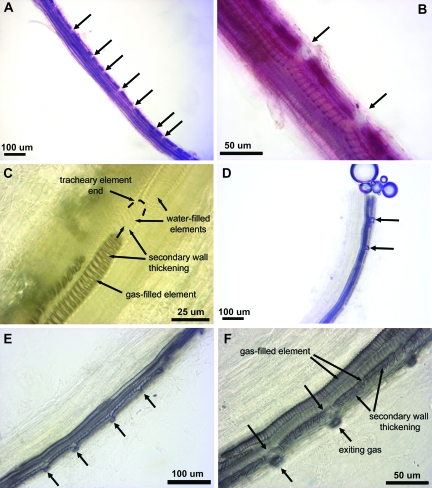
After being macerated for ∼12 h, long branched vascular bundles showed some nicks (Fig. 3A, arrows), but were never completely broken. At each of these nicks, there were always intact vessels adjacent (Fig. 3A, arrowheads). Incidently, these nicks were also observed on vascular bundles observed in situ, carefully dissected from the berry. When macerated for ∼24 h, the bundles were further separated into groups of tracheary elements appearing as a unicellular sheet (Fig. 3B, C) that presumably resulted from the separation of the radial files from one another. These elements presented a spatial arrangement reflecting the ontological development of the xylem. Initially, elements with annular secondary wall differentiated (Fig. 3B, C, arrows), followed by elements with a secondary wall forming a helix with widely spaced coils. Also, as new elements continued to differentiate, their secondary wall formed a helix that became more and more tightly coiled (Fig. 3B, C, arrowheads). The majority of the elements that were observed had helical secondary wall thickenings. Therefore, it was difficult to differentiate between proto- and metaxylem because both types can have helical thickenings. In addition, in other organs, such as leaf, stem, and roots, the protoxylem is found in elongating and sometimes in mature tissues, while the metaxylem is exclusively found in non-growing tissues. However, in the case of the berry, the growth by cell expansion is more or less continuous until late in development. As a result, the tracheary elements could be all protoxylem or some of them may be considered as metaxylem.
Fig. 3.
Images of vascular bundles and tracheary elements showing no apparent discontinuity. (A), (B) and (C) are vascular/xylem bundles at different stages of maceration from a pre-veraison berry (DAA=32) (B) and a post-veraison berry (DAA=120) (A, C). In (A), arrows indicate apparently broken areas of the vascular bundle, but arrowheads show intact xylem in the same area. (D) and (E) are longitudinal sections of plastic-embedded vascular bundles from a pre-veraison berry (D) and a post-veraison berry (E). In (E), the arrows indicate the intact primary wall.
Longitudinal sections of plastic-embedded vascular bundles showed no apparent breaks or nicks in individual tracheary elements (Fig. 3D, E). Although the helix of some post-veraison tracheary elements seemed stretched, an intact primary wall was still present between the gyres of the secondary wall helix (Fig. 3E, arrows). No evidence of tyloses was seen within the tracheary elements of the peripheral vasculature of berries at different stages of development (120 bundles were observed).
When macerated for ∼24 h, individual tracheary elements could also be observed (Fig. 4). All these elements had helical secondary wall thickenings, and it was not clear whether they had a closed end (tracheid) or a perforation plate (vessel) (Fig. 4A). When observed under UV, however, openings corresponding to perforation plates were visible in 90 tracheary elements out of 100 observed under the microscope (Fig. 4B). The presence of a scalariform perforation plate was further demonstrated using an SEM (Fig. 4C, D). However, in ∼10% of the elements, the presence of perforation plates was not conclusive; therefore, some tracheids may be present.
Fig. 4.
Vessel members from the xylem of a post-veraison berry showing perforation plates (arrows). (A) and (B) are the same vessels stained with Safranin O and Calcofluor White M2R, viewed under bright light (A) and UV light (B). (C) An isolated vessel member observed with SEM; and (D) the same sample as (C) at a higher magnification.
Continuous xylem
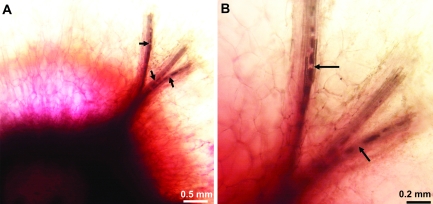
When post-veraison berries were allowed to absorb acid fuchsin passively, the dye moved into the pedicel, the receptacle, and ∼1 mm into the brush (Fig. 5). When viewed under a light microscope at ×4 magnification, the bundles were completely stained for ∼1 mm into the brush, followed by intermittent staining from 1–2 mm and no staining beyond ∼2 mm. Because of this staining pattern, the vascular bundles appeared to be broken in several places (Fig. 5A, arrows). However at a higher magnification (×10), vessels were found to be present around and through the apparently broken portions of the vascular bundle (Fig. 5B, arrows).
Fig. 5.
Images of the xylem from the brush area of a post-veraison berry passively infused with acid fuchsin from the pedicel. Arrows in (A) indicate sites where the vascular bundles appear broken. However, apparently intact xylem can be seen across and around these ‘gaps’ (B, arrows). Acid fuchsin did not move past the brush.
After post-veraison berries were stained with basic fuchsin using the pressure membrane system, vascular bundles were separated from their surrounding fleshy tissues and observed at ×10 magnification under a microscope. Due to the staining, the bundle was dark red and appeared to be partially broken on one side only (Fig. 6A). A higher magnification (×40) revealed that some of the xylem elements with loosely helical secondary walls were damaged and probably non-functional (Fig. 6B, arrows) and that the berry tissues located next to the damaged elements were also stained, seemingly due to the leaking of the dye outside of the elements. This intermittent partial breaking of the bundles appeared similar to the intermittent staining of the bundles of post-veraison berries absorbing the dye passively (Fig. 5 and previous paragraph). However, in that case, the leaking is limited to the brush area, possibly because of the loss of driving forces.
Fig. 6.
Images of two stained vascular bundles isolated from their surrounding tissue (A, B), and cleared with bleach (C–F). (A) Vascular bundle with apparent nicks on one side (arrows). (B) These nicks are located in the early elements with a loose helix (early protoxylem). (C) Gas progressively fills a tracheary element during bleaching, but does not pass into an adjacent element. (D, E) Gas forms on one side of a vascular bundle exiting from the cut end and from nicks on one side (arrows). (F) The gas exits only from tracheary elements with a loose helix, where the secondary walls are stretched.
When bleach was allowed to diffuse in the water surrounding red-stained bundles, the progressive decolorization of the bundles revealed the secondary cell wall thickenings of the tracheary elements. In addition to removing the red colour, gas formed inside the water-filled tracheary elements. The water-filled elements were pale brown and it was difficult to distinguish their walls, but the gas-filled elements were dark grey with distinct walls (Fig. 6C), presumably due to the contrast in refractive index between the gas and the liquid. The gas progressively filled the tracheary elements over a period of several minutes, eventually reaching the cut end of the bundle, and forming bubbles outside the bundle (Fig. 6D). Bubbles also formed along the length of the bundle, but only on one side. Indeed, when observed at high magnification (×40), the gas exited only from the tracheary elements having the loosest helical thickenings and, within those, only where the distance between secondary walls was wider and abnormal compared with the rest of the elements (Fig. 6E, F). At a still higher magnification (×60), the primary wall between these widely spaced secondary wall thickenings was observed to be torn apart, allowing the gas to escape (Fig. 7).
Fig. 7.
Image showing gas exiting a tracheary element between secondary wall thickenings and through broken primary walls (arrows).
Discussion
The results of this study show that tracheary elements of the peripheral xylem in the grape berry contain primarily vessels and few tracheids. Vessels were clearly predominant because in only a few elements were open and scalariform end walls not observed. This was a surprise as the current view (Coombe and McCarthy, 2000; Rogiers et al., 2001; Ollat et al., 2002; Bondada et al., 2005; Keller et al., 2006), based mostly on one review article by Pratt (1971), is that the xylem conduits of the pedicel and the fruit are comprised exclusively of tracheids. Because the present study used only one grape variety (V. vinifera cv. Chardonnay), the results cannot be generalized for all grapes. However, caution should now be used when employing the terms tracheid and vessel in the description of the grape berry xylem.
The vascular bundles were comprised of radially contiguous groups of vessels that remained continuous and hence presumably functional during berry development. This observation confirms the works by Bondada et al. (2005) and Keller et al. (2006) on the xylem functionality in post-veraison berries, and is in contrast to the often accepted idea that xylemic water transport into berries ceases at veraison because the xylem elements in the berry become physically disrupted and dysfunctional (Düring et al., 1987; Findlay et al., 1987; Creasy et al., 1993; Rogiers et al., 2001; Dichio et al., 2003) as a direct or indirect consequence of post-veraison berry growth (Coombe and McCarthy, 2000). However, this idea has been questioned by the work by Bondada et al. (2005) and Keller et al. (2006)
The dominance of vessels rather than tracheids could be of importance in relation to movement of both water and pathogens, such as the xylem-dwelling bacteria Xylella fastidiosa, a cause of Pierce's disease in grapevine. Vessels are also more susceptible to embolism and tyloses which could block water flow. Although no tyloses were observed in the fruit, Kasimatis (1957) associated the presence of tyloses in the berry pedicel with the ‘waterberry’ disorder in grapes. Vessels also present fewer barriers to pathogen movement than do tracheids and, in some cases, form extensive open conduits (Chatelet et al., 2006; Thorne et al., 2006), whereas movement through tracheids would require repeatedly traversing the primary cell wall.
The peripheral vascular bundles contained tracheary elements that appear, in both cross-section and upon partial maceration, as essentially linear files of 3–7 radially contiguous elements. The secondary wall thickenings of these elements appear in a specific ontogenetic pattern that indicates a progressive increase in the extent of the primary wall covered by secondary wall material. Thus, in the earliest tracheary elements, the secondary wall occurs as rings (annular thickenings); subsequent elements have helical thickenings; and as later elements differentiate the gyres become progressively closer to each other. Morphologically, the protoxylem commonly has annular and helical thickenings, and the metaxylem may have helical, reticulate, or pitted secondary wall thickenings (Esau, 1977). Since the tracheary elements of the berry had only annular and helical thickenings, and the berry growth transiently stops at veraison, the elements maturing before veraison may be considered protoxylem and those maturing after veraison considered metaxylem.
The present anatomical results do not contradict the data found in two widely cited studies (Düring et al., 1987; Findlay et al., 1987) purportedly showing direct evidence of broken xylem in the post-veraison berry, but the interpretation does. In the single published photomicrograph of Düring et al. (1987), the magnification is unknown and no details of the xylem can be observed. Hence, it is not clear whether the xylem bundle is entirely broken or not. In the course of the present study, similar observations of seemingly broken vascular bundles in fresh tissues were made. However, at a higher magnification, intact tracheary elements could be seen within and around each apparent gap. Therefore, these areas should be considered more as nicks than as proper gaps in the bundle. These observations of partially broken vascular bundles were further confirmed by observing stained vascular bundles dissected from the berry and cleared with bleach. Nicks appeared at small points along the primary wall of the early elements. Gas that formed inside these elements following the bleaching treatment (possibly chlorine or oxygen) emerged through the nicks to the outside of the conduit. This phenomenon occurred only on one side of a bundle, with the other tracheary elements, including all of the more recently developed elements, remaining intact and non-leaky. No xylem bundle was entirely broken.
Findlay et al. (1987) observed some irregular spacing of the gyres in clearings and longitudinal sections of plastic-embedded tissues from post-veraison berries. They interpreted this as the result of stretching during berry growth and as support for the hypothesis that the xylem in post-veraison berries is physically disrupted and non-functional. However, their images clearly show that not all tracheary elements are so affected. Most appear with uniform intergyre distances and hence should be regarded as intact and functional. Irregularities in gyre spacing of a few tracheary elements were also observed. In addition, it must be recognized that only elements in the same plane of focus will be clearly seen (Findlay et al., 1987). It is therefore possible that intact vessels were present below the seemingly broken ones, similar to the present observations here (Fig. 5).
The results of the present study and the recent literature demonstrate that the apparent hydraulic isolation of the berry after veraison is not simply due to a loss of peripheral xylem continuity as a result of growth-induced xylem disruption. Greenspan et al. (1994) and Rogiers et al. (2000) also produced evidence of xylem function after veraison and questioned the conventional interpretation of the previous passive dye uptake studies. Bondada et al. (2005) demonstrated that the changes in passive dye uptake at veraison were not dependent upon post-veraison berry growth and, hence, could not be due to physical disruption caused by berry growth. In the present study, even though dye always traversed the entire berry from stem to stylar end, a reduction in vascular dye movement was seen after veraison compared with pre-veraison. The nicks that developed in the early formed xylem may have influenced this process. As long as the water column in the xylem is maintained by connected and intact tracheary elements and subjected to an adequate driving force, water should be able to move from one intact tracheary element to another, either longitudinally or radially (Tyree and Zimmermann, 2002). Since each vascular bundle was comprised of a total of 10–15 tracheary elements, water transport could continue around the compromised elements. Nevertheless, the nicked tracheary elements may contribute to a reduced hydraulic conductivity of the vascular system because they represent compromised conduits. Thus, it is possible that part of the decrease in hydraulic conductance of berries that is suggested in several studies and measured in Tyerman et al. (2004) arises in part from loss of functional tracheary elements in the xylem network.
Other works point to additional, non-xylem factors in apparent hydraulic isolation. Bondada et al. (2005) speculated that the absence of passive dye uptake by post-veraison berries was the result of an absence of tension in the berry xylem possibly related to the presence of solutes in the berry apoplast. Consistent with this interpretation, Tyerman et al. (2004) reported an equilibrium hydrostatic pressure close to zero in the berry pedicel near veraison. In addition, the possible role of a symplastic regulation of water transport (Delrot et al., 2001; Picaud et al., 2003; Tyerman et al. 2004) awaits investigation. Picaud et al. (2003) reported an increase in expression of a group of aquaporins associated with the plasma membrane (PIP1) after veraison and at harvest, but the role of aquaporins in the regulation of water transport after veraison remains to be determined. More recently, Keller et al. (2006) showed dye movement from the style of post-veraison berries back into the shoot and adjacent transpiring leaves. They proposed that the decrease in the amount of water flow into post-veraison berries may be due to apoplastic phloem unloading and solute accumulation in the berry apoplast. They further speculated that the xylem could be used to recycle excess water from the phloem back to the shoot.
The maintenance of the physical integrity of the vascular bundles during post-veraison growth raises the question of how this growth is accommodated. Although the secondary cell wall thickenings provide support and rigidity to the xylem elements, it is presumed that xylem vessels with annular and helical secondary walls are able to stretch to a certain extent (Esau, 1965; Barnett, 1981; Mauseth, 1988). Since vascular bundles do not break as a result of berry growth, the tracheary elements may stretch to accommodate the growth, as was observed in growing leaves (Paolillo, 1995; MacAdam and Nelson, 2002).
Another mechanism that would allow the berry to grow without compromising vascular transport would be the formation of additional vessels after veraison. This could be realized by adding either new vessels in the existing vascular bundles or new vascular bundles to the xylem network, although it must also be recognized that regardless of any xylem addition, existing tracheary elements must be stretched by berry growth. The general consensus regarding vascular development in fruits is that the procambium becomes inactive upon fruit ripening (Harris et al., 1968; Considine and Knox, 1979; Gillaspy et al., 1993). However, this consensus is based only on observation of an absence of mesocarp cell divisions rather than an absence of cambial activity. The absence of breakage of the xylem by the growth of the berry leads to the formulation of two hypotheses to accommodate the growth: (i) existing vessels stretch; and/or (ii) new xylem conduits are formed after veraison. These two hypotheses will be explored in the companion paper (Chatelet et al., 2008).
Acknowledgments
The authors thank Brendan Choat for the images in Fig. 5A and B, and Cal-Western Nurseries, Visalia, CA, USA, for providing Chardonnay grapevines. This work was funded by the California Department of Food and Agriculture, Agreement no. 01-0712 and the USDA-CREES grant no. 2005-3442-15841.
References
- Barnett JR. Xylem cell development. Van Nuys, CA: Castle House Publications Ltd; 1981. [Google Scholar]
- Bondada BR, Matthews MA, Shackel KA. Functional xylem in the post-veraison grape berry. Journal of Experimental Botany. 2005;56:2949–2957. doi: 10.1093/jxb/eri291. [DOI] [PubMed] [Google Scholar]
- Chatelet DS, Matthews MA, Rost TL. Xylem structure and connectivity in grapevine (Vitis vinifera) shoots provides a passive mechanism for the spread of bacteria in grape plants. Annals of Botany. 2006;98:483–494. doi: 10.1093/aob/mcl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelet DS, Rost TL, Matthews MA, Shackel KA. The peripheral xylem of grapevine (Vitis vinifera) berries. 2. Anatomy and development. Journal of Experimental Botany. 2008;59 doi: 10.1093/jxb/ern061. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine JA, Knox RB. Development and histochemistry of the cells, cell walls, and cuticle of the dermal system of fruit of the grape, Vitis vinifera L. Protoplasma. 1979;99:347–465. [Google Scholar]
- Coombe BG. The development of fleshy fruits. Annual Review of Plant Physiology. 1976;27:207–228. [Google Scholar]
- Coombe BG, McCarthy MG. Dynamics of grape berry growth and physiology of ripening. Australian Journal of Grape and Wine Research. 2000;6:131–135. [Google Scholar]
- Creasy GL, Price SF, Lombard PB. Evidence for xylem discontinuity in Pinot Noir and Merlot: dye uptake and mineral composition during berry maturation. American Journal of Enology and Viticulture. 1993;44:187–192. [Google Scholar]
- Delrot S, Picaud S, Gaudillère JP. Water transport and aquaporins in grapevine. In: Roubelakis-Angelakis KA, editor. Molecular biology and biotechnology of the grapevine. Dordrecht: Kluwer Academic Publishers; 2001. pp. 241–262. [Google Scholar]
- de Villiers FJ. Physiological studies of the grape. Union of the South African Department of Agriculture Science Bulletin. 1926;45 [Google Scholar]
- Dichio B, Picaud S, Lombard PB. Developmental changes in xylem functionality in kiwifruit: implications for fruit calcium accumulation. Acta Horticulturae. 2003;610:191–195. [Google Scholar]
- Dražeta L, Lang A, Hall AJ, Volz RK, Jameson PE. Causes and effects of changes in xylem functionality in apple fruit. Annals of Botany. 2004;93:275–282. doi: 10.1093/aob/mch040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düring H, Lang A, Oggionni F. Patterns of water flow in Riesling berries in relation to developmental changes in their xylem morphology. Vitis. 1987;26:123–131. [Google Scholar]
- Esau K. Plant anatomy. 2nd edn. New York: Wiley; 1965. [Google Scholar]
- Esau K. Anatomy of seed plants. 2nd edn. New York: Wiley; 1977. [Google Scholar]
- Findlay N, Olivier KJ, Nii N, Coombe BG. Solute accumulation by grape pericarp cells. IV. Perfusion of pericarp apoplast via the pedicel and evidence for xylem malfunction in ripening berries. Journal of Experimental Botany. 1987;38:668–679. [Google Scholar]
- Gillaspy G, David H, Gruissem W. Fruits: a developmental perspective. The Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan MD, Schultz HR, Matthews MA. Field evaluation of water transport in grape berries during water deficits. Physiologia Plantarum. 1996;97:55–62. [Google Scholar]
- Greenspan MD, Shackel KA, Matthews MA. Developmental changes in the diurnal water budget of the grape berry exposed to water deficits. Plant, Cell and Environment. 1994;17:811–820. [Google Scholar]
- Harris JM, Kriedemann PE, Possingham JV. Anatomical aspects of grape berry development. Vitis. 1968;7:106–119. [Google Scholar]
- Ho LC, Grange RI, Picken AJ. An analysis of the accumulation of water and dry matter in tomato fruit. Plant, Cell and Environment. 1987;10:157–162. [Google Scholar]
- Kasimatis AN. Some factors influencing the development of water berries in Thompson Seedless grapes grown for table use. 1957 Thesis (M-S), UC Davis. [Google Scholar]
- Keller M, Smith JP, Bondada BR. Ripening grape berries remain hydraulically connected to the shoot. Journal of Experimental Botany. 2006;57:2577–2587. doi: 10.1093/jxb/erl020. [DOI] [PubMed] [Google Scholar]
- Kroemer K. Organographie, Anatomie und Physiologie der Rebe. In: von Babo F, Mach E, editors. Handbuch des Weinbaues und der Kellerwirtschaft. 1. Halbband, 4. Aufl. Berlin: Paul Parey; 1923. pp. 255–456. [Google Scholar]
- Lang A, Düring H. Partitioning control by water potential gradient: evidence for compartmentation breakdown in grape berries. Journal of Experimental Botany. 1991;42:1117–1122. [Google Scholar]
- Lang A, Ryan KG. Vascular development and sap flow in apple pedicels. Annals of Botany. 1994;74:381–388. [Google Scholar]
- MacAdam JW, Nelson CJ. Secondary cell wall deposition causes radial growth of fibre cells in the maturation zone of elongating tall fescue leaf blades. Annals of Botany. 2002;89:89–96. doi: 10.1093/aob/mcf010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MA, Shackel KA. Growth and water transport in fleshy fruit. In: Holbrook NM, Zwieniecki MA, editors. Vascular transport in plants. Burlington, CA: Academic Press; 2005. pp. 181–197. [Google Scholar]
- Mauseth JD. Plant anatomy. Menlo Park, CA: The Benjamin/Cummings Publishing Company, Inc; 1988. [Google Scholar]
- Ollat N, Diakou-Verdin P, Carde J-P, Barrieu F, Gaudillère J-P, Moing A. Revue bibliographique: développement de la baie de raisin. Journal International des Sciences de la Vigne et du Vin. 2002;36:109–131. [Google Scholar]
- Paolillo DJ., Jr Protoxylem maturation in the seedling leaf of wheat. American Journal of Botany. 1995;82:337–345. [Google Scholar]
- Picaud S, Becp F, Dédaldéchamp F, Ageorges A, Delrot S. Cloning and expression of two plasma membrane aquaporins expressed during ripening of grape berry. Functional Plant Biology. 2003;30:621–630. doi: 10.1071/FP02116. [DOI] [PubMed] [Google Scholar]
- Pratt C. Reproductive anatomy in cultivated grapes—a review. American Journal of Enology and Viticulture. 1971;22:92–109. [Google Scholar]
- Rogiers SY, Keller M, Holzapfel BP, Virgona JM. Accumulation of potassium and calcium by ripening berries on field vines of Vitis vinifera (L.) cv. Shiraz. Australian Journal of Grape and Wine Research. 2000;6:240–243. [Google Scholar]
- Rogiers SY, Smith JA, White R, Keller M, Holzapfel BP, Virgona JM. Vascular function in berries of Vitis vinifera (L.) cv. Shiraz. Australian Journal of Grape and Wine Research. 2001;7:46–51. [Google Scholar]
- Ruzin SE. Plant microtechnique and microscopy. Oxford: Oxford University Press; 1999. [Google Scholar]
- Thorne ET, Young BM, Young GM, Stevenson JF, Labavitch JM, Matthews MA, Rost TL. The structure of xylem vessels in grapevine and a possible passive mechanism for the systemic spread of bacterial disease. American Journal of Botany. 2006;93:497–504. doi: 10.3732/ajb.93.4.497. [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Tilbrook J, Pardo C, Kotula L, Sullivan W, Steudle E. Direct measurement of hydraulic properties in developing berries of Vitis vinifera L. cv Shiraz and Chardonnay. Australian Journal of Grape and Wine Research. 2004;10:170–181. [Google Scholar]
- Tyree MT, Zimmermann MH. Xylem structure and the ascent of sap. New York: Springer; 2002. [Google Scholar]