Abstract
During floral induction and flower development plants undergo delicate phase changes which are under tight molecular control. MADS-box transcription factors have been shown to play pivotal roles during these transition phases. SHORT VEGETATIVE PHASE (SVP) and AGAMOUS LIKE 24 (AGL24) are important regulators both during the transition to flowering and during flower development. During vegetative growth they exert opposite roles on floral transition, acting as repressor and promoter of flowering, respectively. Later during flower development they act redundantly as negative regulators of AG expression. In rice, the orthologues of SVP and AGL24 are OsMADS22, OsMADS47, and OsMADS55 and these three genes are involved in the negative regulation of brassinosteroid responses. In order to understand whether these rice genes have maintained the ability to function as regulators of flowering time in Arabidopsis, complementation tests were performed by expressing OsMADS22 and OsMADS47 in the svp and agl24 mutants. The results show that the rice genes are not able to complement the flowering-time phenotype of the Arabidopsis mutants, indicating that they are biologically inactive in Arabidopsis. Nevertheless, they cause floral reversions, which mimic the SVP and AGL24 floral overexpressor phenotypes. Yeast two-hybrid analysis suggests that these floral phenotypes are probably the consequence of protein interactions between OsMADS22 and OsMADS47 and other MADS-box proteins which interfere with the formation of complexes required for normal flower development.
Keywords: Arabidopsis, floral reversion, floral transition, MADS, rice
Introduction
Functional analysis by molecular genetic studies in model eudicots such as Arabidopsis thaliana, have shown that MADS-box transcription factors are essential for the regulation of various aspects of plant development. MADS-box transcription factors controlling flower development have been intensively studied and it has been proposed that they are the major driving force behind floral diversity (Kater et al., 2006; Theissen et al., 2000).
A conserved set of MADS-box proteins regulate flower development in monocots and dicots and the development of the floral organs is probably controlled by common underlying molecular mechanisms. For this reason, it is interesting to study MADS-box gene functions in species that are distantly related to Arabidopsis, since this will provide information about functional conservation and diversification. The monocot species rice is a good model for these comparative studies since Arabidopsis and rice separated about 150 million years ago (Theissen et al., 2000). Furthermore, the rice genome sequence is available and an extensive set of tools for functional genomics has been developed (Theissen et al., 2000; Goff et al., 2002; J Yu et al., 2002; Kater et al., 2006).
One of the most important developmental switches, during the plant life-cycle is the floral transition. Induction of the inflorescence fate at the shoot apical meristem must be tightly controlled to coincide with conditions that are favourable for the production of seeds and fruits. Therefore, flowering at the right time requires the perception and processing of a diverse range of environmental and internal signals (daylength, light-quality, temperature, hormones), which must be integrated in order to decide the appropriate time for flowering. The interaction of the plant with the environment and the interpretation of the signals at the molecular level have been well analysed in Arabidopsis using molecular genetic approaches. Genetic analysis resulted in the identification of flowering time mutants that were subsequently assigned to four major genetic pathways: the photoperiod and vernalization pathways mediate the response to environmental cues and the autonomous and the gibberellin (GA) pathways act largely independently from these external signals (see reviews by Komeda, 2004; Putterill et al., 2004; He and Amasino, 2005). These pathways quantitatively regulate a set of common targets, the floral pathway integrators, whose activities are required at the shoot apical meristem to regulate a correct transition to the reproductive phase (reviewed in Parcy, 2005).
The two closely related Arabidopsis MADS-box genes AGL24 and SVP are examples of genes controlling the floral transition. Despite their extensive sequence similarity, they have opposite roles in the control of the floral transition. While AGL24 acts as a promoter of this process (Michaels et al., 2003; H Yu et al., 2002), SVP is a dosage-dependent repressor of flowering (Hartmann et al., 2000; Lee et al., 2007). Consistent with its role as a promoter of floral transition, expression of AGL24 under the control of the CaMV 35S promoter accelerates flowering. However, these transgenic plants also display floral abnormalities including alterations in floral organ number and the appearance of chlorophyll-containing petals (Michaels et al., 2003; H Yu et al., 2004). The most striking feature of these plants is the partial reversion of floral meristems into inflorescence shoots which mimics the ap1 phenotype. The floral meristem that in wild-type plants develops into a single flower, in the ap1 mutant often generates extra secondary flowers in the axils of the sepals (Mandel et al., 1992). The observed phenotypes in the 35S::AGL24 plants suggest that AGL24 promotes inflorescence identity when it is expressed out of its correct spatial and temporal domains.
Functional analysis of the Arabidopsis SVP gene has shown that its ectopic expression causes late flowering (Masiero et al., 2004). SVP represses FLOWERING LOCUS T (FT) expression by directly binding to a CArG box motif in the FT promoter, thus blocking the expression of this floral stimulus in the leaf (Lee et al., 2007). During inflorescence and flower development overexpression of SVP causes the conversion of sepals and petals into leaf-like structures and the formation of inflorescence-like shoots within flowers in a manner similar to that of 35S::AGL24 plants (Masiero et al., 2004; Liu et al., 2007).
Recently it has been shown that AGL24 and SVP redundantly regulate AGAMOUS (AG) expression during the early stages of flower development (Gregis et al., 2006). The repression of AG during the initial phases of flower development is probably retained by a higher-order complex which contains AGL24 (or SVP), AP1, LEUNIG, and SEUSS. These data show that AGL24 and SVP have multiple functions, regulating both the timing of floral transition and, later, a correct flower development.
Phylogenetic analyses showed that AGL24 and SVP belong to the StMADS11 clade of the MADS-box gene family (Becker and Theissen, 2003; Garcia-Maroto et al., 2000). Several other MADS-box factors are part of this subfamily including (i) the widely expressed tomato gene JOINTLESS which suppresses the formation of pedicel abscission zones (Mao et al., 2000), (ii) the barley gene BM1 whose transcripts accumulate at high levels in both internodes and nodes (Schmitz et al., 2000), and (iii) INCO from Antirrhinum majus which is required for prophyll and floral meristem development (Masiero et al., 2004). Interestingly, ectopic expression of INCO, BM1 or BM10 in Arabidopsis causes floral reversion and enhancement of vegetative traits in the flower similar to those observed in 35S::SVP and 35S::AGL24 plants. StMADS11-like genes from dicot and monocot species seem, therefore, to be capable of interfering with normal flower development, converting flower primordia into inflorescence-like structures via a so-far unknown mechanism.
In rice, there are three StMADS11-like genes which are OsMADS22, OsMADS47, and OsMADS55. Recently, the function of these three genes has been assessed and it has been shown that they are negative regulators of brassinosteroid responses (Duan et al., 2006; Lee et al., 2008). Despite the fact that these genes do not seem to be involved in flowering time in rice, we were, based on their similarity to the Arabidopsis AGL24 and SVP genes, curious whether they could still complement for loss of SVP and AGL24 function. The complementation tests showed that OsMADS22 and OsMADS47 are unable to reverse the flowering-time phenotypes of the svp and agl24 mutants. However, the expression of these genes in Arabidopsis resulted in alterations in flower development similar to those observed in SVP and AGL24 overexpressing lines. Furthermore, yeast two-hybrid analyses to test MADS-box protein interactions showed that the rice proteins interacted with the same Arabidopsis proteins as SVP and AGL24. These data indicate that, although the overexpression of OsMADS22 and OsMADS47 causes the same floral phenotypes as the overexpression of orthologous Arabidopsis proteins, the altered floral morphology does not reflect the underlying biological function of these proteins, but is caused by inappropriate protein–protein interactions that disturb normal flower development.
Materials and methods
Sequence analysis
Protein sequences were aligned according to Fornara et al. (2004).
Plasmids construction
OsMASD22 cDNA was amplified with primers OL622 and OL624 (OL622 CCATGGGGCGGGAGAGGCGGGAGATAAAGAGGAT; OL624 GGATCCTTACTTCCATGCACCACAAGG) to introduce the cloning sites NcoI and BamHI. Amplification was followed by digestion, and the DNA fragment was sequenced and cloned in a modified version of the pUCAP19, in which are present the double CaMV 35S promoter and the 265 bp CaMV 35S terminator sequence fragment at the 3′ end. The 35S::OsMADS22 gene was introduced into the plant transformation vector pCAMBIA 1300.
To construct the 35S::OsMADS47 overexpression plasmid, the cDNA was amplified with primers OsP22 and OsP31 (OsP22 GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGAAGGAGATAGAACCATGGCTGGCGGCGGCGGTGGCGGG, OsP31 GGGGACCACTTTGTACAAGAAAGCTGGGTCAGTTTTAGTTTCCAGCCATCAC) containing the GATEWAY® attB sites and subsequently sequenced. The binary vector for the overexpression of OsMADS47 was obtained by LR reaction between the destination vector pGD12 and the entry clone pDONR207 carrying the OsMADS47 open reading frame.
Genetic stock and plant material
Overexpression of OsMADS22 and OsMADS47 in Arabidopsis was carried out in the Columbia (Col-0) ecotype. The Arabidopsis agl24-2 and svp-41 mutants (ecotype Columbia) have been kindly given by RM Amasino and P Huijser, respectively. The agl24-2 allele is an En transposon line, and genotyping of the alleles was performed as described previously (Michaels et al., 2003). In the svp-41 mutant, a 2 bp deletion causes a frame shift (Hartmann et al., 2000). Genotyping of SVP alleles was performed as described previously (Gregis et al., 2006).
Production and growth of transgenic plants
Arabidopsis was transformed according to the floral dip method as described by Clough and Bent (1998). The plants were grown at 22 °C under long-day conditions (16/8 h light/dark).
Scanning electron microscopy
Samples were prepared and analysed as described previously by Favaro et al. (2003). Samples were covered with gold using a sputter coater (Nanotech, SEMPREP2) and observed with a LEO 1430 scanning electron microscope (LEO Electron Microscopy, Thornwood, NY).
In situ hybridization and northern blotting
Total RNA was extracted from Arabidopsis and rice tissues as described by Verwoerd et al. (1989). For northern blot analysis 10 μg of glyoxal-denatured total RNA was electrophorized and blotted onto Hybond N+ membranes (Amersham, Buckinghamshire, UK). A 3′ gene-specific fragment of OsMADS22 and OsMADS47 was used for hybridization. Probes were labelled using the Random Priming DNA labelling kit supplied by Roche (Basel). For the in situ hybridization, Arabidopsis flowers were fixed and embedded in paraffin as described previously (Lopez-Dee et al., 1999). Digoxigenin-labelled gene-specific antisense RNA probes were generated by in vitro transcription following the instructions of the in vitro transcription kit (Roche). The probes used to detect SVP and AP1 have been described previously by Hartmann et al. (2000) and Mandel et al. (1992), respectively. Hybridization and immunological detection were performed as described previously (Fornara et al., 2004).
Yeast two-hybrid assay
The OsMADS22 and OsMADS47 open reading frames were generated by polymerase chain reaction (PCR) and subsequently cloned into pDONR201 (Invitrogen). Each clone was recombined with the GATEWAY® destination vectors pDEST™32 (pBDGAL4, bait) and pDEST™22 (pADGAL4, prey) from Invitrogen. All the generated bait vectors were transformed into yeast strain PJ69-4a and all prey vectors into strain PJ69-4α (James et al., 1996) and the transformants selected on SD plates lacking Leu and Trp, respectively. Subsequently, the obtained bait plasmids containing the full-length sequences of OsMADS22 and OsMADS47 were tested for autoactivation of the yeast reporter genes. All OsMADS proteins were tested for heterodimerization capacity in a yeast two-hybrid assay against some of the clones of the ‘pBDGAL4-Arabidopsis MADS’ collection (de Folter et al., 2005). AP1 shows high autoactivation activity when used as bait in the BD-vector, making it impossible to test these interactions. To overcome this problem a deletion construct encoding 1–196 bp of AP1 (AP1Δ1), eliminating the trans-activating terminus (Pelaz et al., 2001) was tested. Two-hybrid interactions were assayed on selective YSD media lacking Leu, Trp, and Adenine, and selective YSD media lacking Leu, Trp, and His supplemented with different concentrations of 3-AT (1, 3, or 5 mM).
Results
Rice MADS-box genes belonging to the StMADS11 clade
OsMADS22 and OsMADS47 are MADS-box genes initially isolated during the screening of a rice cDNA library as previously described by Pelucchi et al. (2002). Sequence comparison of the putative protein sequences with other published MADS-domain proteins indicates that OsMADS22 and OsMADS47 show extensive similarity to StMADS11-like proteins. High sequence similarity (around 65%) is observed with SVP of Arabidopsis (see Supplementary Table S1 at JXB online).
OsMADS55, a third rice protein belonging to the StMADS11 subfamily has been identified more recently (Arora et al., 2007; Lee et al., 2003) and shares 60% amino acid similarity to SVP and AGL24. OsMADS22 shares high homology with OsMADS55, namely 71% amino acid identity and 80% similarity, whereas OsMADS47 shares 48% amino acid identity and 64% similarity. Figure 1 shows an alignment for the predicted amino acid sequences of OsMADS22, OsMADS47, and OsMADS55, the Arabidopsis proteins SVP and AGL24, the potato proteins StMADS11 and StMADS16, and BM1 and BM10, two barley MADS-box factors falling in the StMADS11 clade (Carmona et al., 1998; Garcia-Maroto et al., 2000). The analysis was focused on OsMADS22 and OsMADS47, since they represent members of the two subclades of the monocot StMADS11 genes, one subclade comprises OsMADS47 and the other OsMADS22 and OsMADS55 (Sentoku et al., 2005). Furthermore, OsMADS22 and OsMADS47 are most similar to SVP and AGL24 (see Supplementary Table S1 at JXB online).
Fig. 1.
Sequence alignment of MADS-box proteins belonging to the StMADS11 subclade. Sequences of SVP and AGL24 of Arabidopsis, OsMADS22, OsMADS47, and OsMADS55 of rice, BM1 and BM10 of barley, and StMADS11 and StMADS16 of potato were used. Black boxes indicate fully conserved residues; shaded boxes indicate similar and partially conserved residues. The MADS-box region spans amino acids 1 to 58.
The expression profile of OsMADS47 was analysed in different tissues and it was found that its transcripts are restricted to mature leaves (Fig. 2A). According to published data, OsMADS22 is expressed in leaves and reproductive tissues (Lee et al., 2003; Arora et al., 2007). However, for Sentoku et al. (2005), OsMADS22 expression was excluded from expanding leaf blades, although a weak expression was still detected in tissues enriched in vegetative shoot meristems that presumably contained developing leaves.
Fig. 2.
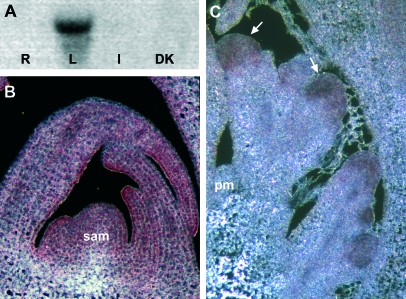
Expression of OsMADS47 and OsMADS22 in rice. (A) Northern blot hybridized using an OsMADS47 specific probe. The RNA samples are derived from wild-type Nipponbare roots (R), mature leaves (L), inflorescences (I), and developing kernels (DK). (B, C) In situ hybridizations using wild-type Nipponbare apical sections probed with an OsMADS22 antisense probe. (B) Expression in a vegetative shoot apical meristem (sam). (C) Expression in an early inflorescence meristem (pm, panicle shoot meristem). The signal is detected throughout the shoot apex and in primary rachis–branch meristems (arrows).
To investigate the OsMADS22 expression in more detail, in situ analysis was performed on vegetative tissues which confirmed that OsMADS22 is expressed in the vegetative meristem and young developing leaves (Fig. 2B). Furthermore, at the early stages of inflorescence development, OsMADS22 transcripts can be detected in primary rachis-branch meristems (Fig. 2C).
Expression of OsMADS22 and OsMADS47 in Arabidopsis does not rescue the svp and agl24 mutant phenotypes
To investigate whether there is conservation of the biological activities between rice and Arabidopsis StMADS11 subfamily members, complementation tests were performed using the well-characterized Arabidopsis svp and agl24 flowering time mutants. For these experiments the open reading frames of OsMADS22 and OsMADS47 were fused to the 35S CaMV promoter and used to transform the Arabidopsis svp-41 and agl24-2 mutants (see Materials and methods). Mutations in svp cause early flowering compared with Arabidopsis wild-type plants, whereas agl24 is a late-flowering mutant (Hartmann et al., 2000; Michaels et al., 2003; Liu et al., 2007). Both phenotypes are evident under LD or SD conditions.
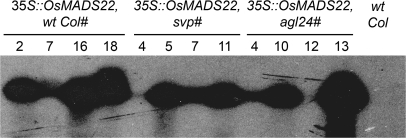
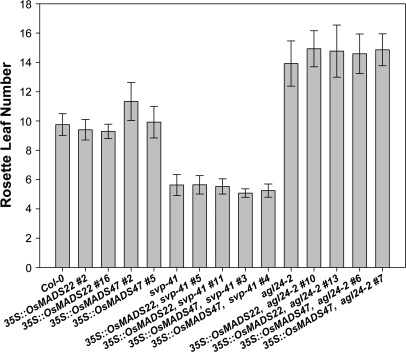
For each construct (35S::OsMADS22 and 35S::OsMADS47) about 10 T1 transgenic lines were generated in the wild-type, agl24, and svp mutant backgrounds. These primary transformants were tested for the presence of the transgene by PCR and expression levels of OsMADS22 and OsMADS47 were analysed by northern blot analysis (see Fig. 3 as an example of this analysis). Subsequently, at the T2 generation, those lines segregating the transgene in a 3:1 ratio were selected. Flowering time was determined at the T3 generation by scoring the total leaf number of at least 12 individuals homozygous for the overexpression cassette. Data for two independent transgenic lines are shown (Fig. 4). Neither OsMADS22 nor OsMADS47 are able to complement the flowering time phenotypes of the svp and agl24 mutant alleles.
Fig. 3.
Northern blot analysis of plants expressing 35S::OsMADS22 in wt Col-0 and the svp and agl24 mutants. The RNA samples were prepared from young leaves of four independent T1 transgenic plants for each genotype and tested for the expression level of OsMADS22. Numbers on the top of each lane indicate the transgenic line number. Note the variability in expression level, ranging from plants that do not express OsMADS22 to plants that express it at a very high level. Only plants expressing the transgene at a high level were used in subsequent analyses.
Fig. 4.
Flowering time of Arabidopsis plants overexpressing OsMADS22 and OsMADS47 in wt Col-0, svp-41, and agl24-2. Two independent transgenic lines were tested for each construct. Flowering was determined as the rosette leaf number at bolting of at least 12 individuals per genotype. Error bars indicate the standard deviation from the mean.
Overexpression of OsMADS22 and OsMADS47 in Arabidopsis phenocopies SVP and AGL24 overexpression
Plants overexpressing SVP or AGL24 develop aberrant flowers in which the vegetative traits are enhanced (Liu et al., 2007). 35S::AGL24 plants often show floral reversion with the central primary flower forming extra secondary flowers in the axils of leaf-like sepals (H Yu et al., 2002, 2004; Liu et al., 2007). In addition, 35S::SVP plants form shoot-like structures which occasionally develop stamens or carpelloid leaves (Masiero et al., 2004; Liu et al., 2007).
Analysis of the flowers of Arabidopsis plants expressing OsMADS22 and OsMADS47 showed that they caused severe floral abnormalities similar to those observed in plants that overexpress SVP or AGL24 (Fig. 5). The severity of the observed floral phenotypes correlated well with the level of OsMADS22 and OsMADS47 expression and, in the rest of this description, we refer to those plants that showed the most severe phenotypes.
Fig. 5.
Morphological analysis of OsMADS22 and OsMADS47 expressing plants. (A, B) Wild-type Columbia inflorescence and flower at anthesis. (C, F) 35S::OsMADS22 (C) and 35S::OsMADS47 (F) inflorescences. (D, G) Flowers of 35S::OsMADS22 (D) and 35S::OsMADS47 (G) at anthesis. Note the abundance of trichomes on sepals and the presence of an ectopic flower inside the 35S::OsMADS47 flower (indicated with an arrow). A sepal was removed in (G) to visualize the inner organs better. (H) A mature 35S::OsMADS47 flower producing extra flowers from the axil of leaf-like sepals. The primary flower is indicated by an arrow. Extra flowers are developing on long pedicels and secondary flowers reiterate the pattern shown by the primary one. (E, I) Mature inflorescences of 35S::OsMADS22 (E) and 35S::OsMADS47 (I), respectively. The sepals converted into leaves are retained on the flower after fertilization. (J–L) SEM analysis on the epidermis of a wild-type sepal (J), a wild-type cauline leaf (K) and a 35S::OsMADS22 sepal (L), respectively. The epidermis of 35S::OsMADS22 sepals resembles that of wild-type leaves (bars = 50 μm). (M) Graph showing the maturation of the siliques of 35S::OsMADS22 and 35S::OsMADS47 as determined by the number of days from anthesis to the beginning of senescence of the valves (mean ±standard deviation).
Young flower buds that develop on wild-type plants are, prior to anthesis, completely enclosed by sepals that bear few or no trichomes on their epidermis (Fig. 5A, B). By contrast 35S::OsMADS22 plants form leaf-like sepals which develop several trichomes on their adaxial side and these leaf-like sepals are not able to enclose the inner developing organs completely (Fig. 5C, D). Petals are initially green and after anthesis become purple in colour. In contrast to wild-type petals, they do not detach from the flower but remain attached until the late stages of flower development (Fig. 5E).
To investigate the leaf-like sepals in more detail, SEM analysis was performed using flowers of 35S::OsMADS22 plants (Fig. 5J–L). In wild-type plants, the sepal epidermis is characterized by different cell types, some of which are very elongated (Fig. 5J). Cells on the adaxial surface of a wild-type leaf show a characteristic interlocked and sinuous shape (Fig. 5K). In 35S::OsMADS22 plants the cells were identical in shape to those of the leaf epidermis (Fig. 5L). From these observations it can be concluded that the first and second whorl organs of flowers expressing OsMADS22 show vegetative characters indicating that floral identity is not completely established in the first two whorls. Overexpression of OsMADS47 causes similar alterations in flower development as observed in OsMADS22 expressing plants (Fig. 5F–H). However, some transgenic lines develop aberrant flowers in which the reversion effect is more severe (Fig. 5G). These plants generated a central primary flower with extra secondary flowers in the axils of the leaf-like sepals and often extra flowers reiterated this pattern producing a highly branched floral structure (Fig. 5H).
In both 35S::OsMADS22 and 35S::OsMADS47 the leaf-like structures subtending the flower did not senesce after fertilization, but rather remained attached at the base of the developing silique (Fig. 5E, I). Furthermore, these leaf-like organs continue to expand during flower development and after anthesis.
Interestingly, plants overexpressing the rice genes show a delay in senescence, which was clearly observed in flowers in which was measured a slower maturation rate, as determined by the number of days from anthesis to senescence of the siliques (Fig. 5M).
These phenotypes could indicate that either a partial conversion of the floral meristem into an inflorescence meristem takes place or that failure to establish floral identity results in the enhancement of the vegetative traits of the flower.
AP1 expression is not affected in reverted flowers
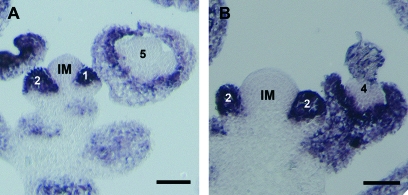
The floral phenotypes observed in plants expressing OsMADS22 or OsMADS47 suggest that in these transgenic plants flower identity is not fully maintained, resulting in perianth organs showing vegetative characters and flowers developing inflorescence-like structures. Several genes are required for the proper establishment of the flower primordium. Among these, AP1 plays a major role in determining the identity of the floral meristem and ap1 mutants fail to establish the floral primordium completely, developing shoot features (Mandel et al., 1992). The observed reversion to the inflorescence state as observed in our Arabidopsis lines expressing OsMADS22 and OsMADS47 are very similar to those observed in the ap1 mutant. Therefore, these phenotypes could be explained by the down-regulation of AP1 in the emerging flower primordia. This was investigated by in situ hybridizations using inflorescences of plants expressing OsMADS47, since the expression of this gene induced the most dramatic phenotypes in Arabidopsis flowers. AP1 transcripts are detectable throughout the floral meristem and in sepals and petals (Fig. 6A). Similarly to the wild-type situation, in 35S:OsMADS47 plants AP1 retains a normal expression profile (Fig. 6B). These data indicate that the floral reversion phenotype is not caused by an incorrect expression of AP1.
Fig. 6.
In situ hybridizations on sections of wild-type Columbia (A) and 35S::OsMADS47 (B) inflorescence sections probed with AP1. Numbers refer to the stage of flower development according to Smyth et al. (1990). IM, inflorescence meristem. Bar 50 μm.
OsMADS22 and OsMADS47 interact with Arabidopsis MADS-box proteins that interact with AGL24 and SVP
MADS-box proteins have the potential to form homo- or heterodimers and to build higher-order complexes with other MADS and non-MADS proteins (Egea-Cortines et al., 1999; Honma and Goto, 2001; Theissen and Saedler, 2001; de Folter et al., 2005; Gregis et al., 2006; Brambilla et al., 2007). Differential expression profiles of putative interacting MADS-box protein partners is probably one of the mechanisms which establish particular developmental and tissue-specific interactions to regulate specific target genes (de Folter et al., 2005). Our experiments showed that OsMADS22 and OsMADS47 are not able to complement the svp and agl24 mutant phenotypes, although their expression in Arabidopsis causes floral phenotypes that closely resemble those of SVP and AGL24 ectopic expression. Furthermore, these phenotypes do not seem to be the consequence of AP1 deregulation. Therefore, it was investigated whether the floral phenotypes are due to interactions between OsMADS22 or OsMADS47 and endogenous MADS-box proteins that also interact with AGL24 and SVP. To test this, GAL4-based yeast two-hybrid experiments were performed to assay interactions between OsMADS22 and OsMADS47, and MADS-box proteins that are known to interact with SVP and AGL24 (de Folter et al., 2005). This experiment might not only provide a molecular explanation for the floral phenotypes that were observed in OsMADS22- and OsMADS47-expressing Arabidopsis plants but will also explore the evolutionary conservation of the ability to interact with specific MADS-box proteins.
The coding sequences of OsMADS22, OsMADS47, SVP, AGL24, and the putative partners of the latter two, which are AP1, FUL, SEP3, AGL14, AGL15, SOC1, and AGL21, were fused to the BD and AD domains and tested for their ability to interact (Table 1). These assays showed that both OsMADS22 and OsMADS47 are able to interact with all the putative Arabidopsis partners of SVP and AGL24, suggesting that the domains in the rice and Arabidopsis StMADS11 proteins that are important for the interactions have been conserved during evolution. Furthermore, the fact that expression of the OsMADS22 and OsMADS47 proteins in Arabidopsis causes similar phenotypic effects as those caused by ectopic AGL24 and SVP expression suggests that, by interacting with the same partner proteins, they might disturb proper flower development.
Table 1.
Heterologous yeast two-hybrid analysis
| AGL24 | SVP | OsMADS47 | OsMADS22 | |||||
| AD | BD | AD | BD | AD | BD | AD | BD | |
| AP1 | +/− | +/− | + | + | ++ | + | + | + |
| FUL | + | − | + | + | ||||
| SEP3 | + | + | ++ | ++ | ||||
| AGL14 | + | +/− | − | − | ++ | ++ | + | ++ |
| AGL15 | − | − | − | + | ++ | + | + | + |
| SOC1 | + | ++ | − | ++ | + | ++ | + | ++ |
| AGL21 | +/− | + | +/− | + | + | + | +/− | + |
| SVP | +/− | − | − | − | − | − | + | − |
| AGL24 | + | + | − | +/− | + | + | + | + |
| OsMADS22 | + | + | − | + | + | + | + | + |
| OsMADS47 | + | + | − | − | + | + | + | + |
Interactions between OsMADS22 and OsMADS47 and the Arabidopsis MADS-box proteins able to interact either with SVP or AGL24. All interaction events resulted in yeast growth on selective medium lacking adenine and/or histidine, supplemented with 3-AT at 3 mM and 5 mM. –, No interaction; +/–, weak interaction; +, strong interaction; ++, very strong interaction.
Discussion
Functional divergence of StMADS11 subfamily members
Members of the StMADS11 subfamily have been found in a wide variety of species, including Arabidopsis, tomato, Antirrhinum, rice, barley, wheat, and Lolium, which makes it plausible that a common ancestor was present before the separation of monocots and dicots (Masiero et al., 2004; Kane et al., 2005; Ciannamea et al., 2006; Quinet et al., 2006). Phylogenetic analysis shows that SVP and AGL24 are most likely paralogous genes. SVP and AGL24 have divergent functions during the floral transition. AGL24 is a promoter of flowering gradually activated in the apical meristem upon transition but repressed in the emerging flower primordium by AP1 (H Yu et al., 2002, 2004). SVP is a floral repressor that negatively regulates the expression of FT in the leaf by directly binding to CArG motifs present in the promoter and first intron regions of the FT locus (Lee et al., 2007). Interestingly, functional analysis has shown that during early stages of flower development SVP and AGL24 act redundantly in the repression of AG (Gregis et al., 2006).
Multiple functions for StMADS11 clade genes from other species have been described as well. JOINTLESS from tomato has a role as a constitutive flowering promoter and suppresses sympodial identity in inflorescence meristems (Quinet et al., 2006; Szymkowiak and Irish, 2006). INCOMPOSITA (INCO) from Antirrhinum controls prophyll development but also functions as a repressor of the floral transition and in the control of Antirrhinum floral meristem identity (Masiero et al., 2004). In monocot plant species, functions for StMADS11 clade genes have also been proposed. Based on expression analyses and protein–protein interaction data it was proposed that in wheat the TaVRT-2 gene encodes a putative repressor of floral transition (Kane et al., 2005). In Lolium perenne the product of the StMADS11-like gene LpMADS10, binds together with LpMADS1 to the LpMADS1 promoter of winter varieties, but not of spring varieties, indicating that LpMADS10 is involved in the vernalization-dependent regulation of the LpMADS1 gene (Ciannamea et al., 2006).
The function of the three rice StMADS11 genes was recently reported (Duan et al., 2006; Lee et al., 2008). OsMADS55 is a major negative regulator of brassinosteroid responses and OsMADS22 functions to support OsMADS55. Whereas single OsMADS55 RNAi plants display weak brassinosteroid responses in the lamina joint, OsMADS22/OsMADS55 double and OsMADS22/OsMADS47/OsMADS55 triple RNAi plants manifest dramatic brassinosteroid responses with regard to lamina joint inclination, coleoptile elongation, and senescence. Furthermore, stem elongation is also notably reduced in the double and triple RNAi lines.
Our protein interaction data show that the Arabidopsis MADS-box proteins that interact in a yeast two-hybrid assay with AGL24 and SVP also interact with OsMADS22 and OsMADS47 of rice. Furthermore, Ciannamea et al. (2006) showed that LpMADS10 is able to interact with the Arabidopsis partners of AGL24 and SVP. This strongly suggests that the interaction domains are conserved in these proteins and the formation of specific interactions with related partners is a conserved evolutionary feature. This conservation of interactions between orthologous MADS-box proteins has been observed before for the ovule identity genes of Arabidopsis, Petunia, and rice (Favaro et al., 2003, 2002), and shows that orthologous MADS-box factors maintain their protein interaction profile, irrespective of function.
To test for functional conservation between Arabidopsis and rice StMADS11 genes, complementation tests were performed in Arabidopsis. The Arabidopsis agl24 and svp mutants were transformed with constructs containing the OsMADS22 and OsMADS47 genes under the control of the CaMV 35S promoter. These experiments clearly showed that the rice proteins are not functional in Arabidopsis since complementation of the flowering-time phenotype in these mutants was not observed, even though the rice genes were expressed at high levels. This suggests that there is conservation of the interaction domains, but other domains important for functionality have diverged between rice and Arabidopsis, which might reflect the functional diversification of these factors in the two species. The variability that can be observed in the C-terminal region of these proteins could be responsible for such a diversification. However, further experiments will be needed to elucidate the relative importance of the different domains in determining protein function.
Ectopic and heterologous expression of StMADS11 genes in Arabidopsis and other species cause similar floral phenotypes
Accumulating data suggest that ectopic expression of StMADS11-like genes affect flower development. In barley, overexpression of BM1 and BM10 confers vegetative characteristics to the spikes of barley (Trevaskis et al., 2007). Similarly, overexpression of LpMADS10 in Lolium resulted in an aberrant, bushy inflorescence architecture on which elongated and sterile spikelets develop (Petersen et al., 2006). In the tunicate mutant of maize, kernels are entirely wrapped by glumes and this phenotype is associated with ectopic expression of the StMADS11-like gene ZMM19 in the flower (He et al., 2004). Sentoku et al. (2005) reported that pUbi::OsMADS22 rice plants exhibit altered spikelet morphology such as abnormal lodicule development, fusion of lodicules and stamens and, rarely, a two-floret spikelet, produced at the axil of the glume on the palea side. Furthermore, OsMADS22 overexpression lines produce shortened panicles and stems (Lee et al., 2008).
Interestingly, the expression of monocot StMADS11 genes in Arabidopsis caused similar floral phenotypes as observed in this study with OsMADS22 and OsMADS47, in particular, leaf-like characteristics of sepals and petals and floral reversion. However, the expression of none of these monocot genes influenced the floral transition in Arabidopsis (He et al., 2004; Trevaskis et al., 2007; this study).
Interestingly the floral phenotype caused by overexpression of INCO in Arabidopsis was also similar (Masiero et al., 2004). However, in Arabidopsis this protein is also functionally active as a floral repressor, since 35S::INCO Arabidopsis plants were delayed in flowering. Finally, ectopic expression of the endogenous Arabidopsis genes AGL24 and SVP using the 35S promoter causes the same floral phenotypes (Masiero et al., 2004; Yu et al., 2004). In addition, in the ap1 mutant it was shown that AGL24 and SVP are up-regulated in the floral meristem and this ectopic expression probably causes the floral reversion observed in the ap1 mutant (Masiero et al., 2004; Yu et al., 2004; Liu et al., 2007). This was supported by the fact that, in the agl24 ap1 and the svp ap1 double mutants, the floral reversions were significantly reduced.
The fact that overexpression of all the StMADS11-like genes, including active endogenous and biologically inactive heterologous (at least in Arabidopsis), cause the same floral phenotypes in Arabidopsis, suggests that these phenotypic effects might not reflect the function (as suggested by Yu et al., 2004) of these genes during plant development. A more plausible explanation might come from the yeast two-hybrid studies that showed that OsMADS22 and OsMADS47 interact with all the MADS-box proteins that also interact with AGL24 and SVP. The constitutively expressed rice and Arabidopsis StMADS11-like proteins might therefore interact with partner proteins (AP1 might be a good candidate) at an inappropriate moment of flower development and therefore compete for these factors with other MADS-box factors that normally form complexes to control correct flower development.
Supplementary data
Supplementary data can be found at JXB online. Table S1 shows the percentages of identity and similarity between the rice and Arabidopsis StMADS11-like proteins.
Supplementary Material
Acknowledgments
We thank Ludovico Dreni and Silvia Cristini for their help with some of the experiments. SEM analysis were done with the help of Giulio Melone in Centro Interdipartimentale Microscopia Avanzata (CIMA), an advanced microscopy laboratory established by the University of Milan. This work was supported by the EU-projects: CerealTag QLRT-2000–01453 and CONFLOW QLK5-CT-2001–01412.
References
- Arora R, Agarwal P, Ray S, Singh AK, Singh VP, Tyagi AK, Kapoor S. MADS-box gene family in rice: genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genomics. 2007;8:242. doi: 10.1186/1471-2164-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Theissen G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Molecular Phylogenetic Evolution. 2003;29:464–489. doi: 10.1016/s1055-7903(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Brambilla V, Battaglia R, Colombo M, Masiero S, Bencivenga S, Kater MM, Colombo L. Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis. The Plant Cell. 2007;19:2544–2556. doi: 10.1105/tpc.107.051797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona MJ, Ortega N, Garcia-Maroto F. Isolation and molecular characterization of a new vegetative MADS-box gene from Solanum tuberosum L. Planta. 1998;207:181–188. doi: 10.1007/s004250050471. [DOI] [PubMed] [Google Scholar]
- Ciannamea S, Kaufmann K, Frau M, Tonaco IA, Petersen K, Nielsen KK, Angenent GC, Immink RG. Protein interactions of MADS box transcription factors involved in flowering in Lolium perenne. Journal of Experimental Botany. 2006;57:3419–3431. doi: 10.1093/jxb/erl144. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method forAgrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- de Folter S, Immink RG, Kieffer M, et al. Comprehensive interaction map of the Arabidopsis MADS box transcription factors. The Plant Cell. 2005;17:1424–1433. doi: 10.1105/tpc.105.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K, Li L, Hu P, Xu SP, Xu ZH, Xue HW. A brassinolide suppressed rice MADS-box transcription factor, OsMDP1, has a negative regulatory role in BR signaling. The Plant Journal. 2006;47:519–531. doi: 10.1111/j.1365-313X.2006.02804.x. [DOI] [PubMed] [Google Scholar]
- Egea-Cortines M, Saedler H, Sommer H. Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS, and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO Journal. 1999;18:5370–5379. doi: 10.1093/emboj/18.19.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro R, Immink RG, Ferioli V, Bernasconi B, Byzova M, Angenent GC, Kater M, Colombo L. Ovule-specific MADS-box proteins have conserved protein–protein interactions in monocot and dicot plants. Molecular and General Genomics. 2002;268:152–159. doi: 10.1007/s00438-002-0746-6. [DOI] [PubMed] [Google Scholar]
- Favaro R, Pinyopich A, Battaglia R, Kooiker M, Borghi L, Ditta G, Yanofsky MF, Kater MM, Colombo L. MADS-box protein complexes control carpel and ovule development in Arabidopsis. The Plant Cell. 2003;15:2603–2611. doi: 10.1105/tpc.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornara F, Parenicova L, Falasca G, Pelucchi N, Masiero S, Ciannamea S, Lopez-Dee Z, Altamura MM, Colombo L, Kater MM. Functional characterization of OsMADS18, a member of the AP1/SQUA subfamily of MADS box genes. Plant Physiology. 2004;135:2207–2219. doi: 10.1104/pp.104.045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Maroto F, Ortega N, Lozano R, Carmona MJ. Characterization of the potato MADS-box gene STMADS16 and expression analysis in tobacco transgenic plants. Plant Molecular Biology. 2000;42:499–513. doi: 10.1023/a:1006397427894. [DOI] [PubMed] [Google Scholar]
- Goff SA, Ricke D, Lan TH, et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica) Science. 2002;296:92–100. doi: 10.1126/science.1068275. [DOI] [PubMed] [Google Scholar]
- Gregis V, Sessa A, Colombo L, Kater MM. AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis. The Plant Cell. 2006;18:1373–1382. doi: 10.1105/tpc.106.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann U, Hohmann S, Nettesheim K, Wisman E, Saedler H, Huijser P. Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. The Plant Journal. 2000;21:351–360. doi: 10.1046/j.1365-313x.2000.00682.x. [DOI] [PubMed] [Google Scholar]
- He Y, Amasino RM. Role of chromatin modification in flowering-time control. Trends in Plant Science. 2005;10:30–35. doi: 10.1016/j.tplants.2004.11.003. [DOI] [PubMed] [Google Scholar]
- He C, Munster T, Saedler H. On the origin of floral morphological novelties. FEBS Letters. 2004;567:147–151. doi: 10.1016/j.febslet.2004.02.090. [DOI] [PubMed] [Google Scholar]
- Honma T, Goto K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature. 2001;409:525–529. doi: 10.1038/35054083. [DOI] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane NA, Danyluk J, Tardif G, Ouellet F, Laliberte JF, Limin AE, Fowler DB, Sarhan F. TaVRT-2, a member of the StMADS-11 clade of flowering repressors, is regulated by vernalization and photoperiod in wheat. Plant Physiology. 2005;138:2354–2363. doi: 10.1104/pp.105.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater MM, Dreni L, Colombo L. Functional conservation of MADS-box factors controlling floral organ identity in rice and Arabidopsis. Journal of Experimental Botany. 2006;57:3433–3444. doi: 10.1093/jxb/erl097. [DOI] [PubMed] [Google Scholar]
- Komeda Y. Genetic regulation of time to flower in Arabidopsis thaliana. Annual Review of Plant Biology. 2004;55:521–535. doi: 10.1146/annurev.arplant.55.031903.141644. [DOI] [PubMed] [Google Scholar]
- Lee S, Choi SC, An G. Rice SVP-group MADS-box proteins, OsMADS22 and OsMADS55, are negative regulators of brassinosteroid responses. The Plant Journal. 2008 doi: 10.1111/j.1365-313X.2008.03406.x. 10.1111/j.1365-313X.2008.03406.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes and Development. 2007;21:397–402. doi: 10.1101/gad.1518407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim J, Son JS, et al. Systematic reverse genetic screening of T-DNA tagged genes in rice for functional genomic analyses: MADS-box genes as a test case. Plant and Cell Physiology. 2003;44:1403–1411. doi: 10.1093/pcp/pcg156. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhou J, Bracha-Drori K, Yalovsky S, Ito T, Yu H. Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development. 2007;134:1901–1910. doi: 10.1242/dev.003103. [DOI] [PubMed] [Google Scholar]
- Lopez-Dee ZP, Wittich P, Enrico Pe M, Rigola D, Del Buono I, Gorla MS, Kater MM, Colombo L. OsMADS13, a novel rice MADS-box gene expressed during ovule development. Developmental Genetics. 1999;25:237–244. doi: 10.1002/(SICI)1520-6408(1999)25:3<237::AID-DVG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature. 1992;360:273–277. doi: 10.1038/360273a0. [DOI] [PubMed] [Google Scholar]
- Mao L, Begum D, Chuang HW, Budiman MA, Szymkowiak EJ, Irish EE, Wing RA. JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature. 2000;406:910–913. doi: 10.1038/35022611. [DOI] [PubMed] [Google Scholar]
- Masiero S, Li MA, Will I, Hartmann U, Saedler H, Huijser P, Schwarz-Sommer Z, Sommer H. INCOMPOSITA: a MADS-box gene controlling prophyll development and floral meristem identity in Antirrhinum. Development. 2004;131:5981–5990. doi: 10.1242/dev.01517. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Ditta G, Gustafson-Brown C, Pelaz S, Yanofsky M, Amasino RM. AGL24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. The Plant Journal. 2003;33:867–874. doi: 10.1046/j.1365-313x.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- Parcy F. Flowering: a time for integration. International Journal of Developmental Biology. 2005;49:585–93. doi: 10.1387/ijdb.041930fp. [DOI] [PubMed] [Google Scholar]
- Pelaz S, Gustafson-Brown C, Kohalmi SE, Crosby WL, Yanofsky MF. APETALA1 and SEPALLATA3 interact to promote flower development. The Plant Journal. 2001;26:385–394. doi: 10.1046/j.1365-313x.2001.2641042.x. [DOI] [PubMed] [Google Scholar]
- Pelucchi N, Fornara F, Favalli C, Masiero S, Lago C, Colombo L, Kater M. Comparative analysis of MADS-box genes expressed during flower development. Sexual Plant Reproduction. 2002;15:113–122. [Google Scholar]
- Petersen K, Kolmos E, Folling M, Salchert K, Storgaard M, Jensen CS, Didion T, Nielsen KK. Two MADS-box genes from perennial ryegrass are regulated by vernalization and involved in the floral transition. Physiologia Plantarum. 2006;126:268–278. [Google Scholar]
- Putterill J, Laurie R, Macknight R. It's time to flower: the genetic control of flowering time. Bioessays. 2004;26:363–373. doi: 10.1002/bies.20021. [DOI] [PubMed] [Google Scholar]
- Quinet M, Dielen V, Batoko H, Boutry M, Havelange A, Kinet JM. Genetic interactions in the control of flowering time and reproductive structure development in tomato (Solanum lycopersicum) New Phytologist. 2006;170:701–710. doi: 10.1111/j.1469-8137.2006.01717.x. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Franzen R, Ngyuen TH, Garcia-Maroto F, Pozzi C, Salamini F, Rohde W. Cloning, mapping and expression analysis of barley MADS-box genes. Plant Molecular Biology. 2000;42:899–913. doi: 10.1023/a:1006425619953. [DOI] [PubMed] [Google Scholar]
- Sentoku N, Kato H, Kitano H, Imai R. OsMADS22, an STMADS11-like MADS-box gene of rice, is expressed in non-vegetative tissues and its ectopic expression induces spikelet meristem indeterminacy. Molecular and General Genomics. 2005;273:1–9. doi: 10.1007/s00438-004-1093-6. [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. The Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkowiak EJ, Irish EE. JOINTLESS suppresses sympodial identity in inflorescence meristems of tomato. Planta. 2006;223:646–658. doi: 10.1007/s00425-005-0115-x. [DOI] [PubMed] [Google Scholar]
- Theissen G, Becker A, Di Rosa A, Kanno A, Kim JT, Munster T, Winter KU, Saedler H. A short history of MADS-box genes in plants. Plant Molecular Biology. 2000;42:115–149. [PubMed] [Google Scholar]
- Theissen G, Saedler H. Floral quartets. Nature. 2001;409:469–471. doi: 10.1038/35054172. [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Tadege M, Hemming MN, Peacock WJ, Dennis ES, Sheldon C. Short vegetative phase-like MADS-box genes inhibit floral meristem identity in barley. Plant Physiology. 2007;143:225–235. doi: 10.1104/pp.106.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Research. 1989;17:23–62. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Ito T, Wellmer F, Meyerowitz EM. Repression of AGAMOUS-LIKE 24 is a crucial step in promoting flower development. Nature Genetics. 2004;36:157–161. doi: 10.1038/ng1286. [DOI] [PubMed] [Google Scholar]
- Yu H, Xu Y, Tan EL, Kumar PP. AGAMOUS-LIKE 24, a dosage-dependent mediator of the flowering signals. Proceedings of the National Academy of Sciences, USA. 2002;99:16336–16341. doi: 10.1073/pnas.212624599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu S, Wang J, et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica) Science. 2002;296:79–92. doi: 10.1126/science.1068037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.