Abstract
Background
Bilateral deep brain stimulation (DBS) of the subthalamic nucleus (STN) improves motor function in Parkinson disease (PD). However, little is known about the quantitative effects on motor behavior of unilateral STN DBS.
Methods
In 52 PD subjects with STN DBS, we quantified in a double-blinded manner rigidity (n= 42), bradykinesia (n= 38), and gait speed (n= 45). Subjects were tested in four DBS conditions: both on, left on, right on and both off. A force transducer was used to measure rigidity across the elbow, and gyroscopes were used to measure angular velocity of hand rotations for bradykinesia. About half of the subjects were rated using the Unified Parkinson Disease Rating Scale (part III) motor scores for arm rigidity and repetitive hand rotation simultaneously during the kinematic measurements. Subjects were timed walking 25 feet.
Results
All subjects had significant improvement with bilateral STN DBS. Contralateral, ipsilateral and bilateral stimulation significantly reduced rigidity and bradykinesia. Bilateral stimulation improved rigidity more than unilateral stimulation of either side, but there was no significant difference between ipsilateral and contralateral stimulation. Although bilateral stimulation also increased hand rotation velocity more than unilateral stimulation of either side, contralateral stimulation increased hand rotation significantly more than ipsilateral stimulation. All stimulation conditions improved walking time but bilateral stimulation provided the greatest improvement.
Conclusions
Unilateral STN DBS decreased rigidity and bradykinesia contralaterally as well ipsilaterally. As expected, bilateral DBS improved gait more than unilateral DBS. These findings suggest that unilateral STN DBS alters pathways that affect rigidity and bradykinesia bilaterally but do not support the clinical use of unilateral STN DBS since bilateral DBS clearly provides greater benefit.
Keywords: Parkinson disease, deep brain stimulation, subthalamic nucleus, kinematics, bradykinesia, rigidity
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) provides substantial relief of motor manifestations in selected people with Parkinson disease (PD) (Deuschl et al., 2006; Kumar et al., 1998; Limousin et al., 1998; Moro et al., 1999; Rodriguez-Oroz et al., 2000; Volkmann et al., 2001). STN DBS not only improves movement but also permits reduction of dopaminergic medications, leading to fewer drug-induced adverse events (Deuschl et al., 2006; Kumar et al., 1998; Limousin et al., 1998; Moro et al., 1999; Nutt et al., 2001; Volkmann et al., 2001). Although there are several reports demonstrating the effectiveness of bilateral and contralateral STN DBS there is no peer-reviewed report comparing the effects of bilateral, contralateral and ipsilateral STN DBS on quantified measures of rigidity and bradykinesia.
Several reports have demonstrated that bilateral STN DBS improves gait, tremor and bradykinesia (Bastian et al., 2003; Deuschl et al., 2006; Kumar et al., 1998; Kumar et al., 1999; Rizzone et al., 2002). However, few of studies have explored the effects of unilateral stimulation (Germano et al., 2004; Kumar et al., 1999; Linazasoro et al., 2003). One study measured the effects of ipsilateral, contralateral or bilateral STN DBS and found that contralateral and bilateral stimulation provided significant benefit in UPDRS ratings and timed hand movement tasks (Purdue pegboard and timed hand tapping). Ipsilateral stimulation did not significantly improve bradykinesia, tremor or rigidity as measured with the UPDRS or by Purdue pegboard test (Kumar et al., 1999). In that study, ipsilateral stimulation did improve timed hand tapping by 19% compared to 29% with contralateral stimulation and 54% with bilateral stimulation. A recent study used kinematic measures of gait and reaching and found that bilateral STN DBS improved walking speed and stride length more than unilateral stimulation (Bastian et al., 2003). Reaching speed increased with bilateral or contralateral stimulation but was not measured with only ipsilateral DBS (Bastian et al., 2003). Rigidity was not measured in that study.
The purpose of this study is to determine the differences among bilateral, contralateral and ipsilateral STN stimulation on quantified measures of rigidity and bradykinesia as well as the effects of unilateral versus bilateral STN DBS on a timed-gait task. We also compare these measures to a widely-used clinical rating scale (UPDRS).
Methods
Subjects
Fifty-two subjects (16 women, age 61.0 ± 0.2 [mean ± standard error], range 35 to 79 years) with clinically definite PD (Calne et al., 1992; Hughes et al., 1992; Racette et al., 1999) and previously implanted bilateral STN stimulators were studied (Medtronic Model 3387 DBS Lead and Model 7424 ITREL II or Model 7426 Soletra implantable pulse generators, Medtronic, Inc, Minneapolis, MN). Targeting of STN was performed under T2-weighted MRI guidance and confirmed by electrophysiologic mapping. All subjects had kinematic measurements at least 4 months after electrode implantation to permit optimization of stimulator settings and to diminish the potential motor effects of a microsubthalamotomy. The mean duration between electrode implantation and kinematic measurements was 15.6 months (median 8.7 months, range 4 to 76 months). A lesion effect, however, is not likely to be critical for interpretation of this study since this effect would be constant across the different stimulation conditions during the few hours of the study. Subjects were chosen for their ability to complete the motor protocol in each test condition. Average DBS variables (± standard error) were voltage = 3.0 ± 0.01 volts, pulse width = 66.3 ± 0.1 μs, and frequency = 185 ± 0 Hz, with 88% of stimulators set with the stimulator case as the anode (monopolar or multipolar mode). These studies were approved by the Human Studies Committee at Washington University in St. Louis, and each subject provided written informed consent.
Motor testing methods
Each subject refrained from PD medication overnight (Langston et al., 1992). Subjects were tested in four conditions: both stimulators on (BOTH ON), both stimulators off (BOTH OFF), only left stimulator on (LEFT ON) and only right stimulator on (RIGHT ON). The two unilateral stimulation conditions were designated as either contralateral (CONTRA ON) or ipsilateral (IPSI ON) with respect to the tested extremity for rigidity and bradykinesia testing. An investigator not involved in the ratings changed the DBS settings using the transcutaneous Medtronic Physician Programmer Model 7432 or 8840 (Medtronic, Inc, Minneapolis, MN). The examiner and the subjects were blinded to the condition. The order of conditions was randomized for each subject to reduce any bias introduced by progressive worsening of parkinsonian signs that may occur due to potential stress of prolonged testing or longer withholding of medication. Each testing condition took approximately 1 hour, and there was at least a 30 to 42 minute delay before motor testing began after each change of settings (Temperli et al., 2003). Timed-gait testing was performed at the end of each testing condition at least 1 hour after changing DBS settings.
UPDRS subscale III ratings for upper extremity rigidity were done for each of the four conditions in the second half of the subjects during the kinematic measurements to allow comparison between the UPDRS and the kinematic measures of rigidity and bradykinesia. Correlations between UPDRS and kinematic scores were not used to screen subjects. A full UPDRS subscale III motor examination was performed in both on and both off conditions to establish that the subjects had substantial clinical benefit from STN DBS (at least a 35% decrease in UPDRS motor subscale score).
Quantitative measures of motor behavior
Rigidity
Rigidity during the four conditions was measured using the Rigidity Analyzer (Neurokinetics, Inc, Edmonton, AB). The validity and reliability of the device and its use in measuring parkinsonian rigidity have been described (Patrick et al., 2001; Prochazka et al., 1997). Briefly, the device consists of an inflatable cuff force transducer and a gyro-based velocity sensor. To quantify rigidity across the elbow, the cuff was placed around the forearm just proximal to the wrist crease. The forearm was passively flexed and extended across the elbow joint (comparable to that done for the UPDRS ratings) for 50 seconds for each of three trials. The R1.1 software package (Neurokinetics, Inc, Edmonton, AB) was used to collect the data and allowed viewing for on-line quality control. Data were stored and analyzed off-line.
Bradykinesia
Bradykinesia was measured using the gyro-based G1 Motion Analysis System (Neurokinetics, Inc, Edmonton, AB) in each of the four conditions. The G1 Motion Analysis System consists of gyro sensors for monitoring motion in limb segments. The motion analyzer measures angular velocity and displacement. Gyro sensors were mounted on the ventral aspects of the left and right forearms proximal to the wrist crease to optimally capture hand rotation velocity placing the alignment of the gyros in the axis of motion. Each subject was asked to maximally rotate the hands in a pronation-supination motion as fast as possible with each hand separately (simulating the hand pronation/supination UPDRS task). Data were collected using the G1.1 Software package (Neurokinetics, Inc, Edmonton, AB) during three fifteen-second trials separated by at least twenty seconds of rest. Data were stored and analyzed offline.
Timed gait
Each subject had a verbal start cue to walk 25 feet until they crossed a line on the floor. This was repeated three times and each was videotaped. The walking time was measured by stopwatch at the time of the task and confirmed by videotape analysis. Any start hesitation or freezing was timed separately and then subtracted from the total walking time.
Data Analysis
Rigidity
The R1.1 Software uses the direct measures of applied force (F), angular displacement (θ) and arm length (d) to compute impedance (Z) to a passive range of motion based upon the previous 5 seconds of collected data (Z = Fd/θ). Thus, a 50-second trial yields a 46-second recording of impedance, that reliably reflects clinically rated parkinsonian rigidity (Patrick et al., 2001). Impedance data were analyzed offline using Origin statistical software (OriginLab, Northampton, MA). All trials were averaged to provide a mean score for each condition.
Subjects without sufficient rigidity in at least one limb in the BOTH OFF condition were excluded from the analysis to avoid flooring effects and noise with trying to measure changes from low baseline values. Sufficient rigidity for this study was defined as a UPDRS score >1, signifying rigidity present without movement of the opposite limb (Fahn et al., 1987). Only the extremity with the greatest amount of rigidity in the BOTH OFF condition was used in the analysis for each individual.
The Spearman rank order correlation coefficient (rs) was used to compare measured limb impedance and UPDRS rigidity scores in the BOTH ON condition in 24 subjects.
Bradykinesia
The root-mean-square (rms) velocity was calculated online from the previous four seconds of data acquisition using the G1.1 software package. Bradykinesia was quantified using the average of the middle ten seconds of rms velocity calculations for each fifteen-second trial (Pollo et al., 2002) using Origin statistical software (OriginLab, Northampton, MA). The rms velocity then was averaged for all three trials.
Subjects that did not have sufficient bradykinesia for reliable measurements in the DBS OFF condition were excluded from the analysis. Sufficient baseline bradykinesia was defined as UPDRS score > 1 for hand rotation (greater than a mild slowing or reduction in amplitude) (Fahn et al., 1987) which corresponded consistently to a measured hand rotation velocity < 500 deg/s. Subjects with hand action tremor rated as 4/4 on the UPDRS in any DBS condition were also excluded from the analysis.
The Spearman rank order correlation coefficient (rs) was used to compare measured hand rotation velocity and UPDRS bradykinesia scores in the BOTH ON condition in 25 subjects.
Timed Gait
The recorded time reflected solely the time for each subject to walk 25 feet. Trials that were not completed were excluded from analysis. Three trials for each person were averaged for each different condition.
For each task the data were also normalized to the findings in BOTH OFF condition to reduce individual variability (i.e., change in motor performance between DBS conditions was defined as change from baseline, using DBS condition BOTH OFF as baseline).
Statistical Analyses
Repeated measures analysis of variance (ANOVA) in SPSS software, version 12.0 (SPSS Inc, Chicago, Illinois) was used to determine whether there was a significant change in each measure across the four conditions. If a significant ANOVA was found, a pair-wise comparison was done with post hoc t-tests. The same software was also used to calculate the Spearman rank order correlation coefficients.
Results
Clinical Rating
STN DBS improved UPDRS subscale III motor ratings by 48.0 ± 0.4 % (mean ± standard error) in all 52 subjects.
Rigidity
Forty-two (81%) of the 52 subjects that participated in the study had at least mild-to-moderate rigidity in at least one limb (13 women, mean age = 60, range 35 to 78 years). Bilateral STN DBS decreased rigidity, and unilateral stimulation reduced rigidity bilaterally as reflected by decreased impedance (Fig. 1). A repeated measures ANOVA reveals a main effect of condition (F(2,82) value = 5.08, p = 0.008). BOTH ON (-35.4 ± 5.7 %, p < 0.00001), CONTRA ON (-29.0 ± 4.5 %, p < 0.00001) and IPSI ON (-18.5 ± 5.0 %, p < 0.001) decreased rigidity compared to BOTH OFF. BOTH ON reduced rigidity more than IPSI ON (p <0.005) but did not reduce it significantly more than CONTRA ON (p= 0.16). We did not find a significant difference between the effects of CONTRA ON versus IPSI ON (p = 0.08).
Fig. 1.

Effect of bilateral, contralateral and ipsilateral STN DBS on rigidity expressed as mean ± standard error percent change in impedance in all eligible subjects (N=42).
In a subset of 24 subjects, impedance-based quantitative measures of rigidity correlated with UPDRS scores of limb rigidity in the BOTH ON condition (rs = 0.61, p < 0.002, Fig. 2). Percent change in UPDRS rigidity scores among the various DBS conditions was similar to the percent change in impedances (Fig. 3a and 3c). BOTH ON (-58.7 ± 7.2 %, p < 0.00001), CONTRA ON (-48.6 ± 7.1 %, p < 0.00001) and IPSI ON (-16.1 ± 5.2 %, p < 0.05) decreased UPDRS rigidity scores (Fig. 3b). BOTH ON reduced rigidity more than IPSI ON (p <0.00001) but did not reduce it significantly more than CONTRA ON (p= 0.27). In this smaller number of subjects, CONTRA ON also reduced rigidity more than IPSI ON (p <0.002).
Fig. 2.

Comparison of impedance with rigidity UPDRS score in the BOTH ON condition in the more affected limb (N=24).
Fig. 3.
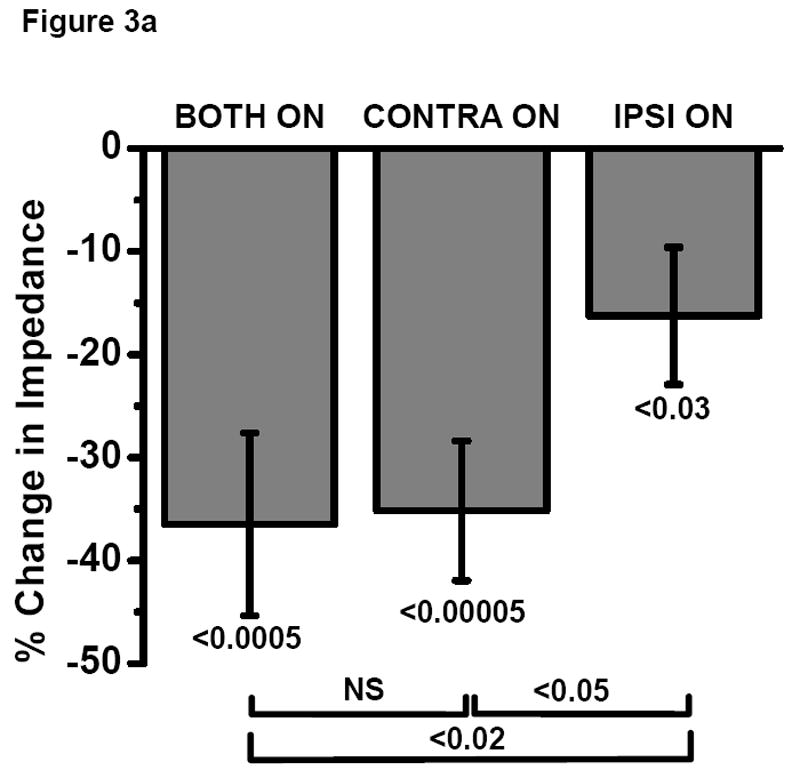

a and b Comparison of the effect of bilateral, contralateral and ipsilateral STN DBS on rigidity as measured by (a) impedance vs (b) UPDRS rigidity score in a subset of subjects (N=24).
Bradykinesia
Fourteen of the 52 subjects were excluded from the bradykinesia analysis for the following reasons: eight subjects had a bradykinesia UPDRS equal or less than 1 in the BOTH OFF state, two had severe action tremor (rated 4/4 on the UPDRS) and four had incomplete kinematic data. This left 38 participants (73%) for bradykinesia analysis (13 women, mean age = 60, range 35 to 79 years). A repeated measures ANOVA reveals a main effect of condition (F(2,74) value = 5.06, p = 0.009). BOTH ON (-193 ± 67 %, p < 0.01), CONTRA ON (-136 ± 39 %, p < 0.01) and IPSI ON (-52 ± 24 %, p < 0.05) increased hand rotation velocity compared to BOTH OFF (Fig. 4). BOTH ON reduced hand rotation velocity more than IPSI ON (p <0.05) but did not reduce it significantly more than CONTRA ON (p= 0.14).
Fig. 4.
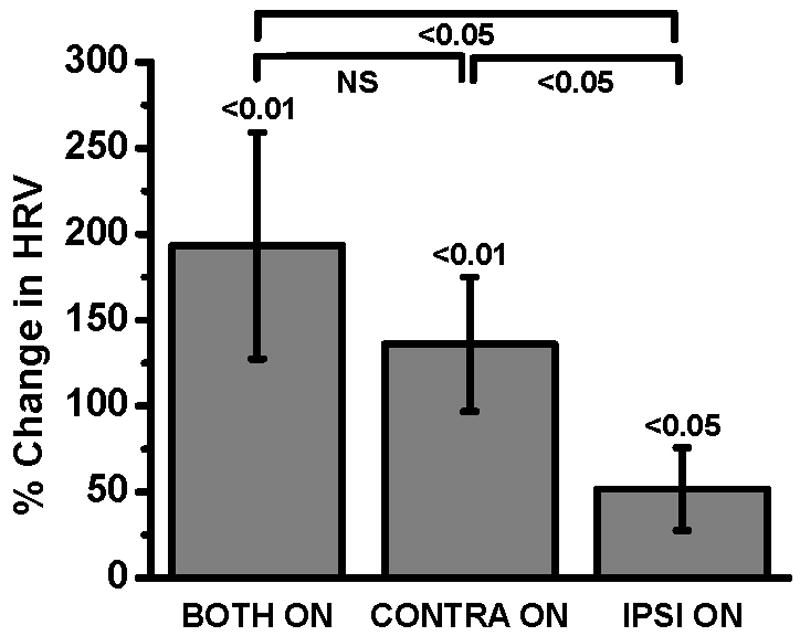
Effect of bilateral, contralateral and ipsilateral STN DBS on bradykinesia expressed as mean ± standard error percent change in hand rotation velocity (HRV) from baseline in all eligible subjects (N=38).
In a subset of 25 subjects, quantitative measures of hand rotation inversely correlated with UPDRS scores of limb bradykinesia in the BOTH ON condition (r = -0.59, p < 0.002, Fig. 5). UPDRS bradykinesia scores agreed with hand rotation velocity measurements that BOTH ON, CONTRA ON and IPSI ON improved bradykinesia compared to BOTH OFF and that CONTRA ON does so significantly more than IPSI ON (Fig. 6a and 6b). However, UPDRS bradykinesia scores revealed that BOTH ON improved bradykinesia significantly more than CONTRA ON (p<0.03), while hand rotation velocity measurements did not (p=0.24). Conversely, hand rotation velocity measurements revealed that CONTRA ON improved bradykinesia significantly more than IPSI ON (p<0.05), while UPDRS bradykinesia scores did not (p=0.24).
Fig. 5.

Comparison of measured hand rotation velocity with bradykinesia UPDRS score in the BOTH ON condition in the more affected limb (N=25).
Fig. 6.

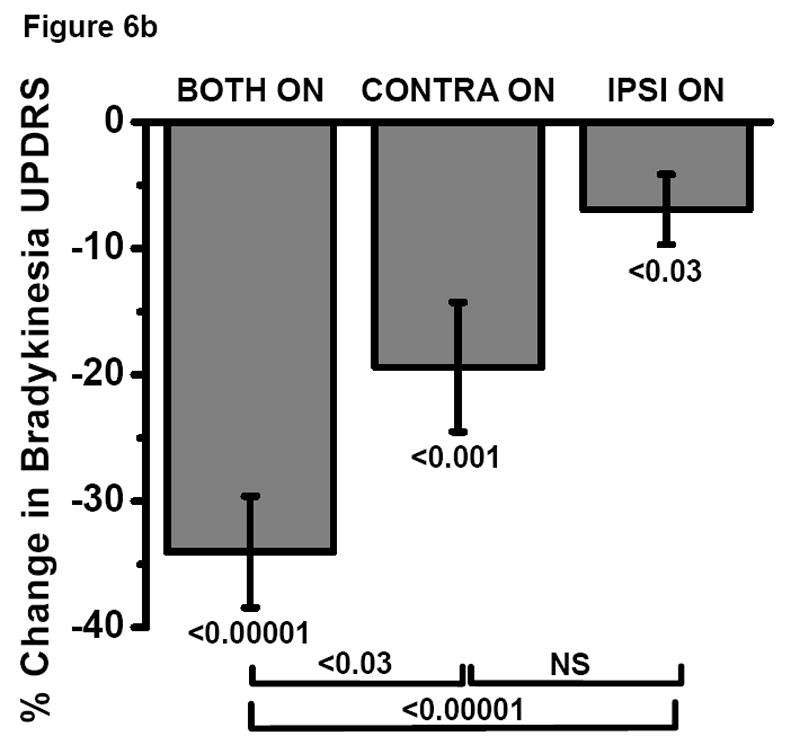
a and b Comparison of the effect of bilateral, contralateral and ipsilateral STN DBS on bradykinesia as measured by (a) quantified hand rotation velocity (HRV) vs (b) UPDRS bradykinesia score in a subset of subjects (N=25).
Timed Gait
Forty-five of the 52 (87%) subjects completed the timed gait task in each condition (15 women, mean age = 61, range 35 to 79 years). Bilateral STN DBS decreased the time to walk 25 feet with both the right and left side stimulators contributing equally to the improvement (Fig. 7). A repeated measures ANOVA revealed a main effect of condition on gait speed (F(2,88)= 7.01, p = 0.001). Turning both stimulators on (-25.9 ± 4.7 %, p = 0.00001), only the left stimulator on (-17.6 ± 4.4 %, p = 0.0005), or only the right stimulator on (-18.6 ± 4.2 %, p = 0.0001) produced a decrease in the time to walk 25-feet compared to BOTH OFF. The improvement seen with the left stimulator alone was not different from that seen with the right stimulator alone (p = 0.72), while the decreased walking time with bilateral stimulation was greater than either left or right sided stimulation alone (p = 0.0005 and 0.005 respectively).
Figure 7.

Effect of bilateral and unilateral STN DBS on gait expressed in mean ± standard error percent change in the time to walk 25-feet from baseline in all eligible subjects (N=45).
Discussion
We demonstrated using quantitative measures that unilateral STN DBS reduces rigidity and bradykinesia contralaterally as well as ipsilaterally. This suggests that unilateral stimulation affects pathways that reduce rigidity and bradykinesia bilaterally. Consistent with previous reports, bilateral STN DBS improves gait more than either left or right STN DBS separately. However, it is important to be clear that our study does not provide evidence for the clinical use of unilateral STN DBS since we found that bilateral STN DBS provided much greater benefit.
Control of Rigidity
Several reports demonstrate that bilateral STN DBS reduces rigidity as measured by clinical ratings in PD patients (Deuschl et al., 2006; Kumar et al., 1998; Limousin et al., 1998; Moro et al., 1999; Rodriguez-Oroz et al., 2000; Volkmann et al., 2001), and two other studies suggest that unilateral stimulation reduces rigidity only in the contralateral limb with no ipsilateral benefit (Kumar et al., 1999; Limousin et al., 1998). Studies in patients with unilateral subthalamotomy confirmed contralateral benefit and lack of reduction of ipsilateral rigidity as measured by UPDRS (Patel et al., 2003; Vilela et al., 2002). Unilateral pallidotomy is also effective in reducing contralateral rigidity with an ipsilateral effect ranging from none (Lang et al., 1999; Lang et al., 1997) to a 55% reduction of rigidity measured with UPDRS at 24 months post-pallidotomy (Vitek et al., 2003). However, these previous reports relied on clinical rigidity rating scales and did not employ objective, quantitative measures of rigidity. We measured rigidity using a validated analog scale (rigidity analyzer) that was demonstrated to be more sensitive and more reproducible than the rank-order scale widely used in clinical practice and studies (UPDRS) (Prochazka et al., 1997). In that validation study, under different testing conditions (such as on and off medication), mean UPDRS-rigidity ratings (R) of 4 experienced raters were plotted against the corresponding mean impedance measurements (Z) obtained using a rigidity analyzer in 7 PD patients with a wide range of rigidity severity. Clinical ratings and impedances were highly correlated (r2 = 0.84) even though UPDRS ratings differed at times by up to 1 full point among raters and R/Z ratios varied significantly (Student t-test, p<0.05) between subjects as well as between raters. The variability in the R/Z ratios suggested that raters interpreted the UPDRS differently and that clinical ratings of a specific patient may be biased by patient characteristics other than rigidity (such as expectations based on subject's overall clinical features). The authors also concluded that the mean R/Z ratio could be used to convert measured impedance into standardized clinical rigidity ratings that would reflect a consensus rating among a group of experienced raters. Finally, the rigidity analyzer more consistently detected the reduction in rigidity induced by medication than UPDRS-based measures, possibly because such reductions were in some instances quite small. The authors also demonstrated that impedances as well as clinical ratings varied substantially over a 25-second measurement interval, therefore questioning the reliability of a typical abbreviated clinical examination of rigidity lasting for a few seconds. Using the rigidity analyzer, our results confirm that unilateral STN stimulation reduces upper extremity rigidity contralaterally but also demonstrates an ipsilateral reduction.
Control of Bradykinesia
Several studies demonstrate that bilateral STN DBS reduces UPDRS ratings (Burchiel et al., 1999; Kleiner-Fisman et al., 2003; Kumar et al., 1999) or Purdue Pegboard measures (Kumar et al., 1999) of bradykinesia associated with PD. These studies found a 25 – 83% decrease in UPDRS bradykinesia ratings with bilateral STN DBS, and the performance on the Purdue Pegboard test improved by 75%. Furthermore, a recent study with objective, quantitative measures demonstrated that unilateral STN DBS reduced contralateral bradykinesia (Bastian et al., 2003) subsequently confirmed by the gyro-based velocity measurements for bradykinesia in that study. Reduction in ipsilateral UPDRS bradykinesia score varies across different studies from no effect to 33% (Bastian et al., 2003; Fine et al., 2000; Herrera et al., 2000; Lang et al., 1997; Ondo et al., 1998; Samuel et al., 1997; Vitek et al., 2003). Interestingly, the effects of unilateral pallidotomy on ipsilateral bradykinesia in one study (Vitek et al., 2003) was significant at 24 months post-operatively when using a timed tasks (hand movement between two points) but was not significant when measured by UPDRS bradykinesia scores. This further supports our notion that kinematic measures are more sensitive than UPDRS scores for identification of subtle changes in motor behavior in PD, such as motor changes induced by unilateral DBS or ablative surgeries. Using gyro-based kinematic measures of bradykinesia, we demonstrate that unilateral STN DBS significantly reduces bradykinesia ipsilaterally although contralateral stimulation provides significantly greater benefit. Although the small ipsilateral effects may not be functionally important, they carry important implications for the neurophysiology of the circuitry of motor control.
STN DBS may reduce bradykinesia and rigidity by altering the function of basal ganglia-thalamo-cortical circuits. Although such circuits primarily influence contralateral motor function (Mink, 1996; Parent et al., 1995), their ipsilateral motor projections may be sufficient to mediate the ipsilateral effects of DBS that our study demonstrated. An ipsilateral alteration of STN function could lead to downstream changes in ipsilateral GPi, thalamus and cortical areas including supplementary motor area (SMA). The SMA projects bilaterally to STN and may mediate a bilateral effect from unilateral STN DBS (Parent et al., 1995). Indeed, by recording from implanted STN DBS electrodes, the STN or nearby structures were shown to be active before self-paced movement in PD patients and such activity was induced by both ipsilateral and contralateral hand movement (Paradiso et al., 2003). This may also be the functional basis for the ipsilateral reduction of bradykinesia and rigidity by unilateral pallidotomy (Vitek et al., 2003).
Unilateral STN stimulation may also influence rigidity and possibly bradykinesia via descending pathways that ultimately connect to bilateral spinal motor neurons (Garcia-Rill et al., 2004; Hammond et al., 1983; Jackson et al., 1981; Jackson et al., 1983; Parent et al., 1995; Potter et al., 2004; Takakusaki et al., 1996). STN projects directly not only to substantia nigra pars reticulata (SNr) and the internal segment of the globus pallidus (GPi) (Hoover et al., 1993; Mink, 1996; Parent et al., 1995; Parent et al., 1995) but also to pedunculopontine nucleus (PPN) (Nakano, 2000; Potter et al., 2004). Stimulation in the region of STN may reduce rigidity and bradykinesia by directly affecting PPN or projections neurons from SNr and GPi to midbrain structures such as reticular formation or periaquaductal gray (Pahapill et al., 2000; Schwarz et al., 1984). Furthermore, PPN neurons project to contralateral PPN and to reticular formation and nucleus gigantocellularis that in turn project bilaterally to spinal cord (Delwaide, 2001; Garcia-Rill et al., 2004; Hammond et al., 1983; Jackson et al., 1981; Jackson et al., 1983; Parent et al., 1995; Potter et al., 2004; Takakusaki et al., 1996). These abundant cross connections may account for the bilateral effects of unilateral stimulation. For instance, these pathways may influence resting tone or rigidity since downward projections through PPN and reticulospinal tracts can lead to a decrease in the tonic static fusimotor activity of muscle spindles (Schwarz et al., 1984). Additionally, lesions of PPN may produce parkinsonism in non-human primates (Kojima et al., 1997; Munro-Davies et al., 1999; Nandi et al., 2002) that may be improved by PPN DBS (Nandi et al., 2002). The neurophysiological relevance and clinical usefulness of PPN DBS in humans has not been established (Mazzone et al., 2005; Plaha et al., 2005) although one preliminary study suggests that PPN DBS may selectively improve gait and postural features (Stefani et al., 2007).
It is important to appreciate that the behavioral effects of stimulation in the region of the STN may be mediated through STN as well as other neighboring structures (Dormont et al., 2004; Hamel et al., 2003; Lanotte et al., 2002; Saint-Cyr et al., 2002; Zonenshayn et al., 2004).
Control of Gait Speed
Several reports have shown that bilateral and unilateral STN DBS increase walking speed (Allert et al., 2001; Bastian et al., 2003; Bejjani et al., 2000; Faist et al., 2001; Germano et al., 2004; Stolze et al., 2001; Xie et al., 2001). This current report confirms that bilateral STN DBS provides the greatest increase in walking speed, again supporting the clinical need for bilateral STN DBS in most people with PD. It is possible that STN DBS improves gait by influencing function of the PPN either through direct connections from STN or indirectly through GPi or SNr (Hammond et al., 1983; Jackson et al., 1981; Jackson et al., 1983). The PPN is a component of the mesencephalic locomotor reticular region and has been implicated in the control of locomotion, posture and muscle tone (Garcia-Rill, 1986; Garcia-Rill et al., 2004; Jordan, 1998; Stefani et al., 2007).
DBS in the region of the subthalamic nucleus may also affect gait by stimulation of the subthalamic locomotor region (SLR) that includes the zona incerta and the Fields of Forel (Garcia-Rill, 1986; Narita et al., 2002). Stimulation of this region in cats produces locomotion, and SLR also has been implicated in postural control. Therefore, stimulation of either STN or SLR may mediate the effects of STN DBS on gait.
The residual effect of DBS after turning off the stimulators is highly unlikely to contaminate our findings that STN DBS has an ipsilateral effect on rigidity and bradykinesia. The most compelling argument that makes this point extremely unlikely is that our analysis compares the various DBS settings with respect to the bilateral DBS OFF condition (namely, percent change with respect to the bilateral DBS OFF condition). If there is any residual DBS effect after turning off DBS, this residual effect will affect equally the ipsilateral limb in the bilateral DBS OFF and contralateral DBS ON conditions (ensured by randomization of the order of DBS conditions). Consequently, any effect measured in any of the DBS ON conditions is an effect above any potential residual effects of the bilateral DBS OFF condition.
In conclusion, the main finding of this study is that unilateral STN DBS reduces rigidity and bradykinesia ipsilaterally as well as contralaterally. Stimulation in the region of the STN must affect pathways that influence rigidity and bradykinesia on both sides of the body. However, our study does not support the clinical utility of unilateral STN DBS since bilateral STN DBS clearly provided greater benefit than unilateral DBS. But it is important to note that our study was designed to investigate the mechanism of action of STN DBS rather than its clinical usefulness. Finger tapping, arm rigidity and speed of gait alone may not reflect faithfully the clinical benefits of DBS therapy.
Acknowledgments
We thank W. Thomas Thach, M.D., for support and helpful discussions, Tamara Hershey, Ph.D., for statistical consultations and Patricia Schneider-Gibson for assistance with some of the kinematic studies.
Grants: This work has been supported by National Institute of Neurological Disease and Stroke grants NS41509, NS39821 and P30 NS048056, National Institute of Health's Medical Scientist Training Program grant T32 GM07200-29, the American Parkinson's Disease Association (APDA) Advanced Research Center at Washington University, the Greater St. Louis Chapter of the APDA, the Barnes-Jewish Hospital Foundation (Jack Buck Fund for PD Research and the Elliot H. Stein Family Fund) and the Kopolow Fund.
Abbreviations
- ANOVA
repeated measures analysis of variance
- BOTH OFF
both stimulators turned off
- BOTH ON
both stimulators turned on
- DBS
deep brain stimulation
- GPi
internal globus pallidus
- HRV
hand rotation velocity
- LEFT ON
only left stimulator turned on
- PD
Parkinson disease
- PPN
pedunculopontine nucleus
- RIGHT ON
only right stimulator turned on
- rs
Spearman rank order correlation coefficient
- SLR
subthalamic locomotor region
- SMA
supplementary motor area
- SNr
substantia nigra pars reticulate
- STN
subthalamic nucleus
- UPDRS
Unified Parkinson Disease Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Allert N, Volkmann J, Dotse S, Hefter H, Sturm V, Freund HJ. Effects of bilateral pallidal or subthalamic stimulation on gait in advanced Parkinson's disease. Mov Disord. 2001;16:1076–1085. doi: 10.1002/mds.1222. [DOI] [PubMed] [Google Scholar]
- 2.Bastian AJ, Kelly VE, Revilla FJ, Perlmutter JS, Mink JW. Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson's disease. Mov Disord. 2003;18:1000–1007. doi: 10.1002/mds.10493. [DOI] [PubMed] [Google Scholar]
- 3.Bejjani BP, Gervais D, Arnulf I, Papadopoulos S, Demeret S, Bonnet AM, Cornu P, Damier P, Agid Y. Axial parkinsonian symptoms can be improved: the role of levodopa and bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2000;68:595–600. doi: 10.1136/jnnp.68.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burchiel KJ, Anderson VC, Favre J, Hammerstad JP. Comparison of pallidal and subthalamic nucleus deep brain stimulation for advanced Parkinson's disease: results of a randomized, blinded pilot. Neurosurgery. 1999;45:1375–1382. doi: 10.1097/00006123-199912000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol. 1992;32:S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 6.Delwaide PJ. Parkinsonian rigidity. Funct Neurol. 2001;16:147–156. [PubMed] [Google Scholar]
- 7.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, Daniels C, Deutschlander A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 8.Dormont D, Ricciardi KG, Tande D, Parain K, Menuel C, Galanaud D, Navarro S, Cornu P, Agid Y, Yelnik J. Is the subthalamic nucleus hypointense on T2-weighted images? A correlation study using MR imaging and stereotactic atlas data. AJNR Am J Neuroradiol. 2004;25:1516–1523. [PMC free article] [PubMed] [Google Scholar]
- 9.Fahn S, Elton RL . Members of the UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson's disease. Macmillan; New York: 1987. pp. 153–163. [Google Scholar]
- 10.Faist M, Xie J, Kurz D, Berger W, Maurer C, Pollak P, Lucking CH. Effect of bilateral subthalamic nucleus stimulation on gait in Parkinson's disease. Brain. 2001;124:1590–1600. doi: 10.1093/brain/124.8.1590. [DOI] [PubMed] [Google Scholar]
- 11.Fine J, Duff J, Chen R, Chir B, Hutchison W, Lozano AM, Lang AE. Long-term follow-up of unilateral pallidotomy in advanced Parkinson's disease. N Engl J Med. 2000;342:1708–1714. doi: 10.1056/NEJM200006083422304. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rill E. The basal ganglia and the locomotor regions. Brain Res. 1986;396:47–63. [PubMed] [Google Scholar]
- 13.Garcia-Rill E, Homma Y, Skinner RD. Arousal mechanisms related to posture and locomotion: 1. Descending modulation. Prog Brain Res. 2004;143:283–290. [PubMed] [Google Scholar]
- 14.Germano IM, Gracies JM, Weisz DJ, Tse W, Koller WC, Olanow CW. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: a double-blind 12-month evaluation study. J Neurosurg. 2004;101:36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- 15.Hamel W, Fietzek U, Morsnowski A, Schrader B, Herzog J, Weinert D, Pfister G, Muller D, Volkmann J, Deuschl G, Mehdorn HM. Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: evaluation of active electrode contacts. J Neurol Neurosurg Psychiatry. 2003;74:1036–1046. doi: 10.1136/jnnp.74.8.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond C, Rouzaire-Dubois B, Feger J, Jackson A, Crossman AR. Anatomical and electrophysiological studies on the reciprocal projections between the subthalamic nucleus and nucleus tegmenti pedunculopontinus in the rat. Neuroscience. 1983;9:41–52. doi: 10.1016/0306-4522(83)90045-3. [DOI] [PubMed] [Google Scholar]
- 17.Herrera EJ, Viano JC, Caceres M, Costello G, Suarez M, Suarez JC. Posteroventral pallidotomy in Parkinson's disease. Acta Neurochir (Wien) 2000;142:169–175. doi: 10.1007/s007010050020. [DOI] [PubMed] [Google Scholar]
- 18.Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- 19.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson A, Crossman AR. Subthalamic projection to nucleus tegmenti pedunculopontinus in the rat. Neurosci Lett. 1981;22:17–22. doi: 10.1016/0304-3940(81)90278-0. [DOI] [PubMed] [Google Scholar]
- 21.Jackson A, Crossman AR. Nucleus tegmenti pedunculopontinus: efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience. 1983;10:725–765. doi: 10.1016/0306-4522(83)90213-0. [DOI] [PubMed] [Google Scholar]
- 22.Jordan LM. Initiation of locomotion in mammals. Ann N Y Acad Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003;99:489–495. doi: 10.3171/jns.2003.99.3.0489. [DOI] [PubMed] [Google Scholar]
- 24.Kojima J, Yamaji Y, Matsumura M, Nambu A, Inase M, Tokuno H, Takada M, Imai H. Excitotoxic lesions of the pedunculopontine tegmental nucleus produce contralateral hemiparkinsonism in the monkey. Neurosci Lett. 1997;226:111–114. doi: 10.1016/s0304-3940(97)00254-1. [DOI] [PubMed] [Google Scholar]
- 25.Kumar R, Lozano AM, Kim YJ, Hutchison WD, Sime E, Halket E, Lang AE. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson's disease. Neurology. 1998;51:850–855. doi: 10.1212/wnl.51.3.850. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;53:561–566. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- 27.Lang AE, Duff J, Saint-Cyr JA, Trepanier L, Gross RE, Lombardi W, Montgomery E, Hutchinson W, Lozano AM. Posteroventral medial pallidotomy in Parkinson's disease. J Neurol. 1999;246 2:II28–II41. doi: 10.1007/BF03161079. [DOI] [PubMed] [Google Scholar]
- 28.Lang AE, Lozano AM, Montgomery E, Duff J, Tasker R, Hutchinson W. Posteroventral medial pallidotomy in advanced Parkinson's disease. New Engl J Med. 1997;337:1036–1042. doi: 10.1056/NEJM199710093371503. [DOI] [PubMed] [Google Scholar]
- 29.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 30.Lanotte MM, Rizzone M, Bergamasco B, Faccani G, Melcarne A, Lopiano L. Deep brain stimulation of the subthalamic nucleus: anatomical, neurophysiological, and outcome correlations with the effects of stimulation. J Neurol Neurosurg Psychiatry. 2002;72:53–58. doi: 10.1136/jnnp.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 32.Linazasoro G, Van Blercom N, Lasa A. Unilateral subthalamic deep brain stimulation in advanced Parkinson's disease. Mov Disord. 2003;18:713–716. doi: 10.1002/mds.10407. [DOI] [PubMed] [Google Scholar]
- 33.Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport. 2005;16:1877–1881. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- 34.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 35.Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson's disease. Neurology. 1999;53:85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- 36.Munro-Davies LE, Winter J, Aziz TZ, Stein JF. The role of the pedunculopontine region in basal-ganglia mechanisms of akinesia. Exp Brain Res. 1999;129:511–517. doi: 10.1007/s002210050921. [DOI] [PubMed] [Google Scholar]
- 37.Nakano K. Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev. 2000;22 1:S5–16. doi: 10.1016/s0387-7604(00)00139-x. [DOI] [PubMed] [Google Scholar]
- 38.Nandi D, Aziz TZ, Liu X, Stein JF. Brainstem motor loops in the control of movement. Mov Disord. 2002;17 3:S22–S27. doi: 10.1002/mds.10139. [DOI] [PubMed] [Google Scholar]
- 39.Nandi D, Liu X, Winter JL, Aziz TZ, Stein JF. Deep brain stimulation of the pedunculopontine region in the normal non-human primate. J Clin Neurosci. 2002;9:170–174. doi: 10.1054/jocn.2001.0943. [DOI] [PubMed] [Google Scholar]
- 40.Narita K, Murata T, Honda K, Nishihara M, Takahashi M, Higuchi T. Subthalamic locomotor region is involved in running activity originating in the rat ventromedial hypothalamus. Behav Brain Res. 2002;134:275–281. doi: 10.1016/s0166-4328(02)00041-4. [DOI] [PubMed] [Google Scholar]
- 41.Nutt JG, Rufener SL, Carter JH, Anderson VC, Pahwa R, Hammerstad JP, Burchiel KJ. Interactions between deep brain stimulation and levodopa in Parkinson's disease. Neurology. 2001;57:1835–1842. doi: 10.1212/wnl.57.10.1835. [DOI] [PubMed] [Google Scholar]
- 42.Ondo WG, Jankovic J, Lai EC, Sankhla C, Khan M, Ben Arie L, Schwartz K, Grossman RG, Krauss JK. Assessment of motor function after stereotactic pallidotomy. Neurology. 1998;50:266–270. doi: 10.1212/wnl.50.1.266. [DOI] [PubMed] [Google Scholar]
- 43.Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson's disease. Brain. 2000;123(Pt 9):1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- 44.Paradiso G, Saint-Cyr JA, Lozano AM, Lang AE, Chen R. Involvement of the human subthalamic nucleus in movement preparation. Neurology. 2003;61:1538–1545. doi: 10.1212/01.wnl.0000096021.28967.57. [DOI] [PubMed] [Google Scholar]
- 45.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 46.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 47.Patel NK, Heywood P, O'Sullivan K, McCarter R, Love S, Gill SS. Unilateral subthalamotomy in the treatment of Parkinson's disease. Brain. 2003;126:1136–1145. doi: 10.1093/brain/awg111. [DOI] [PubMed] [Google Scholar]
- 48.Patrick S, Denington K, Gauthier M, Gillard D, Prochazka A. Quantification of the UPDRS rigidity scale. IEEE Trans Neural Sys Rehab Engineering. 2001;9:31–41. doi: 10.1109/7333.918274. [DOI] [PubMed] [Google Scholar]
- 49.Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport. 2005;16:1883–1887. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- 50.Pollo A, Torre E, Lopiano L, Rizzone M, Lanotte M, Cavanna A, Bergamasco B, Benedetti F. Expectation modulates the response to subthalamic nucleus stimulation in Parkinsonian patients. Neuroreport. 2002;13:1383–1386. doi: 10.1097/00001756-200208070-00006. [DOI] [PubMed] [Google Scholar]
- 51.Potter M, Illert M, Wenzelburger R, Deuschl G, Volkmann J. The effect of subthalamic nucleus stimulation on autogenic inhibition in Parkinson disease. Neurology. 2004;63:1234–1239. doi: 10.1212/01.wnl.0000140287.42535.37. [DOI] [PubMed] [Google Scholar]
- 52.Prochazka A, Bennett DJ, Stephens MJ, Patrick SK, Sears-Duru R, Roberts T, Jhamandas JH. Measurement of rigidity in Parkinson's disease. Mov Disord. 1997;12:24–32. doi: 10.1002/mds.870120106. [DOI] [PubMed] [Google Scholar]
- 53.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson's disease. Am J Med Genet. 1999;88:539–543. [PubMed] [Google Scholar]
- 54.Rizzone M, Ferrarin M, Pedotti A, Bergamasco B, Bosticco E, Lanotte M, Perozzo P, Tavella A, Torre E, Recalcati M, Melcarne A, Lopiano L. High-frequency electrical stimulation of the subthalamic nucleus in Parkinson's disease: kinetic and kinematic gait analysis. Neurol Sci. 2002;23 2:S103–S104. doi: 10.1007/s100720200090. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Oroz MC, Gorospe A, Guridi J, Ramos E, Linazasoro G, Rodriguez-Palmero M, Obeso JA. Bilateral deep brain stimulation of the subthalamic nucleus in Parkinson's disease. Neurology. 2000;55:S45–S51. [PubMed] [Google Scholar]
- 56.Saint-Cyr JA, Hoque T, Pereira LC, Dostrovsky JO, Hutchison WD, Mikulis DJ, Abosch A, Sime E, Lang AE, Lozano AM. Localization of clinically effective stimulating electrodes in the human subthalamic nucleus on magnetic resonance imaging. J Neurosurg. 2002;97:1152–1166. doi: 10.3171/jns.2002.97.5.1152. [DOI] [PubMed] [Google Scholar]
- 57.Samuel M, Ceballos-Baumann AO, Turjanski N, Boecker H, Gorospe A, Linazasoro G, Holmes AP, DeLong MR, Vitek JL, Thomas DG, Quinn NP, Obeso JA, Brooks DJ. Pallidotomy in Parkinson's disease increases supplementary motor area and prefrontal activation during performance of volitional movements an H2(15)O PET study. Brain. 1997;120(Pt 8):1301–1313. doi: 10.1093/brain/120.8.1301. [DOI] [PubMed] [Google Scholar]
- 58.Schwarz M, Sontag KH, Wand P. Non-dopaminergic neurones of the reticular part of substantia nigra can gate static fusimotor action onto flexors in cat. J Physiol. 1984;354:333–344. doi: 10.1113/jphysiol.1984.sp015379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007 doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 60.Stolze H, Klebe S, Poepping M, Lorenz D, Herzog J, Hamel W, Schrader B, Raethjen J, Wenzelburger R, Mehdorn HM, Deuschl G, Krack P. Effects of bilateral subthalamic nucleus stimulation on parkinsonian gait. Neurology. 2001;57:144–146. doi: 10.1212/wnl.57.1.144. [DOI] [PubMed] [Google Scholar]
- 61.Takakusaki K, Shiroyama T, Yamamoto T, Kitai ST. Cholinergic and noncholinergic tegmental pedunculopontine projection neurons in rats revealed by intracellular labeling. J Comp Neurol. 1996;371:345–361. doi: 10.1002/(SICI)1096-9861(19960729)371:3<345::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 62.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 63.Vilela FO, da Silva DJ. Unilateral subthalamic nucleus lesioning: a safe and effective treatment for Parkinson's disease. Arq Neuropsiquiatr. 2002;60:935–948. doi: 10.1590/s0004-282x2002000600010. [DOI] [PubMed] [Google Scholar]
- 64.Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, McDonald W, Haber M, Barnhart H, Wahlay N, Triche S, Mewes K, Chockkan V, Zhang JY, DeLong MR. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol. 2003;53:558–569. doi: 10.1002/ana.10517. [DOI] [PubMed] [Google Scholar]
- 65.Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001;56:548–551. doi: 10.1212/wnl.56.4.548. [DOI] [PubMed] [Google Scholar]
- 66.Xie J, Krack P, Benabid AL, Pollak P. Effect of bilateral subthalamic nucleus stimulation on parkinsonian gait. J Neurol. 2001;248:1068–1072. doi: 10.1007/s004150170027. [DOI] [PubMed] [Google Scholar]
- 67.Zonenshayn M, Sterio D, Kelly PJ, Rezai AR, Beric A. Location of the active contact within the subthalamic nucleus (STN) in the treatment of idiopathic Parkinson's disease. Surg Neurol. 2004;62:216–225. doi: 10.1016/j.surneu.2003.09.039. [DOI] [PubMed] [Google Scholar]


