Abstract
Crystal meth is a form of the stimulant drug methamphetamine that, when smoked, can rapidly achieve high concentrations in the brain. Methamphetamine causes the release of the neurotransmitters dopamine, norepinephrine and serotonin and activates the cardiovascular and central nervous systems. The levels of dopamine are low in the brain of some drug users, but whether this represents neuronal loss is uncertain. The areas of the brain involved in methamphetamine addiction are unknown but probably include the dopamine-rich striatum and regions that interact with the striatum. There is no medication approved for the treatment of relapses of methamphetamine addiction; however, potential therapeutic agents targeted to dopamine and nondopamine (e.g., opioid) systems are in clinical testing.
Crystal meth (methamphetamine hydrochloride, “ice,” “Tina”) is a smokable, crystalline solid form of methamphetamine, a stimulant that is used for recreational purposes.1 To a recreational drug user, the advantage of the smokable form of methamphetamine over the oral form is the very rapid and intense “high.” The advantage over the intravenous form, which also has comparably high bioavailability, is the decreased risk and inconvenience associated with the use of needles. The elimination half-life of smoked crystal meth and methamphetamine administered by intranasal or intravenous routes is about 11 hours.1
Apart from the generic risks associated with all forms of methamphetamine, the special public health concern with crystal meth is that this form can cause more overall harm to the public than other forms,1,2 because it rapidly achieves a high drug concentration with a correspondingly high potential for drug addiction and other toxicities.
Therapeutic uses of methamphetamine and amphetamine
Although methamphetamine can be abused, one must appreciate that oral forms of methamphetamine and amphetamine are also used for therapeutic purposes. Methamphetamine and its metabolite amphetamine are structurally related, differing only by the presence of a methyl group (Figure 1).3 Oral methamphetamine (Desoxyn, OVATION Pharmaceuticals) is approved in the United States for the treatment of attention-deficit hyperactivity disorder in children and for the short-term treatment of obesity. In Canada, amphetamine is the active ingredient in several oral medications (Adderall XR [Shire BioChem Inc.], Dexedrine [GlaxoSmithKline]) approved for the management of attention-deficit hyperactivity disorder.4,5 Methamphetamine and amphetamine have the same mechanism of action; both cause the release of monoamine neurotransmitters and both cause the same characteristic peripheral and central stimulant behavioural effects.6,7 In a study that directly compared the effects of methamphetamine and amphetamine in humans, the behavioural consequences and potencies of the drugs were similar.6 However, some differences between the 2 drugs cannot be excluded.
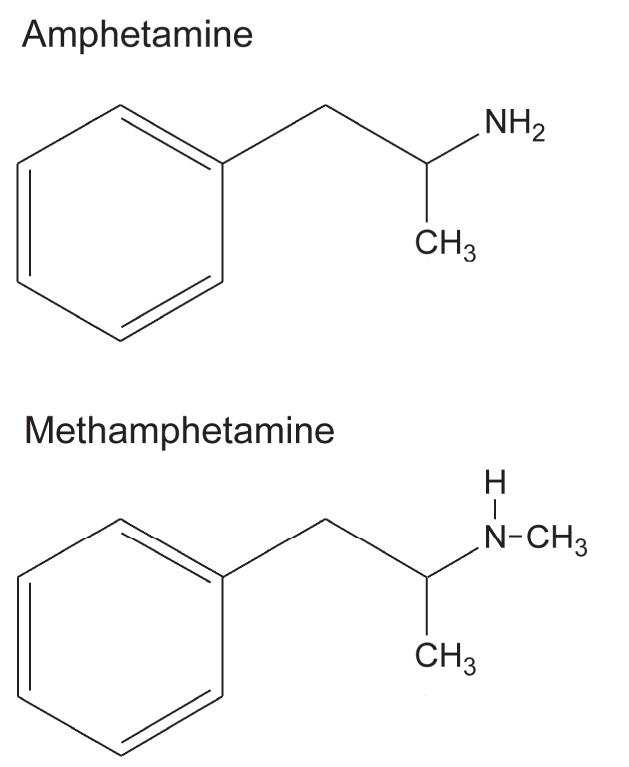
Figure 1: Comparison of chemical structures of methamphetamine and amphetamine. Methamphetamine differs from its metabolite amphetamine by the presence of a methyl group. Both produce the same stimulant behavioural effects and are used clinically for the treatment of attention-deficit hyperactivity disorder.
Because amphetamines are used for both therapeutic and recreational purposes, physicians and public health officials who advise the public about the risks of amphetamines need to acknowledge and distinguish carefully between the potential toxicity of therapeutic and recreational amphetamine use. For example, they must distinguish between the use of therapeutically effective slow-onset (e.g., 20–60 minutes) oral forms of amphetamines among medically screened patients (e.g., those with cardiac abnormalities) and the use of very fast-onset (e.g., seconds to minutes8,9) smokable forms of methamphetamine among unsupervised crystal meth users.
A typical daily dose of oral methamphetamine for the treatment of attention-deficit hyperactivity disorder in children is 20–25 mg.10 The dose of Adderall XR (a mixture of amphetamine salts) used for long-term treatment of attention-deficit hyperactivity disorder commonly ranges from 5 mg to 30 mg, which can result in peak plasma d-amphetamine levels of about 10–110 ng/mL.11 A single dose (the amount in a smoking pipe) of crystal meth sufficient to cause a “significant rush” has been reported to be about 40–60 mg;8,9 however, the actual dose is highly influenced by the pipe temperature, smoking technique, number of puffs and drug tolerance (those with a higher tolerance require higher doses). In addition, it is common for crystal meth users to take repeated doses of the drug (binge), which results in much higher drug levels. In a prospective investigation, a single 30 mg dose of crystal meth was associated with peak plasma levels of about 50 ng/mL.8 However, in a recent “real-life” study of unsupervised recreational methamphetamine users as part of a police investigation, blood levels ranged from from 15 ng/mL to 1600 ng/mL (median 190 ng/mL).12
Pharmacologic actions of methamphetamine
The typical acute behavioural effects of methamphetamine include feelings of alertness, wakefulness, energy, well-being, euphoria (at high doses) and suppression of appetite. Methamphetamine also activates the cardiovascular system (increased heart rate and blood pressure) and, for this reason, can cause death at high doses.13
In the brain, a primary action of methamphetamine is to elevate the levels of extracellular monoamine neurotransmitters (dopamine, serotonin, norepinephrine) by promoting their release from the nerve endings.14 We do not completely understand how methamphetamine causes neurotransmitter release, but it appears to involve redistribution of neurotransmitters from synaptic vesicles (via the vesicular monoamine transporter VMAT2) to the neuronal cytoplasm and the reverse transport of neurotransmitters through the plasma membrane transporter into the extracellular space.3
The cardiovascular activation caused by methamphetamine is likely explained in large part by the release of norepinephrine from sympathetic nerve endings.15 The mechanism of the alerting action and of the efficacy of amphetamines in treatment of attention-deficit hyperactivity disorder is unknown, but it probably involves, at least in part, activation of the brain noradrenergic neurotransmitter system. (Note that the selective norepinephrine transporter blocker atomoxetine has some efficacy in the treatment of attention-deficit hyperactivity disorder.16)
Is there a role for dopamine in methamphetamine-craving?
Despite much research data from animals in the literature,17 the areas of the human brain and the key neurochemicals that are responsible for the pleasurable effects of methamphetamine and for the transition from drug-liking to drug-craving are still unknown. In part, this is because of the lack of definitive data from brain lesion or deep brain stimulation studies proving that inactivation of a specific brain region influences addictive behaviour in people. There is, however, ample evidence from imaging studies of the human brain that suggest that methamphetamine increases the release of dopamine in the dopamine-rich subdivisions of the striatum (Figure 2), namely, the caudate, putamen and the ventral striatum.18 The latter area, which includes the nucleus accumbens, is a region of much interest in the study of addictions. In this regard, some preliminary neurosurgical data, which will require confirmation, suggest that lesion of the nucleus accumbens in people addicted to opiate drugs might decrease relapse in some drug users.19 In the autopsied brains of recreational methamphetamine users, we found low levels of striatal dopamine, which suggest that the doses of methamphetamine taken by recreational users are sufficient to cause depletion of this neurotransmitter.20 Low dopamine levels could explain, in part, some of the unpleasant feelings during methamphetamine withdrawal and aspects of cognitive impairment.
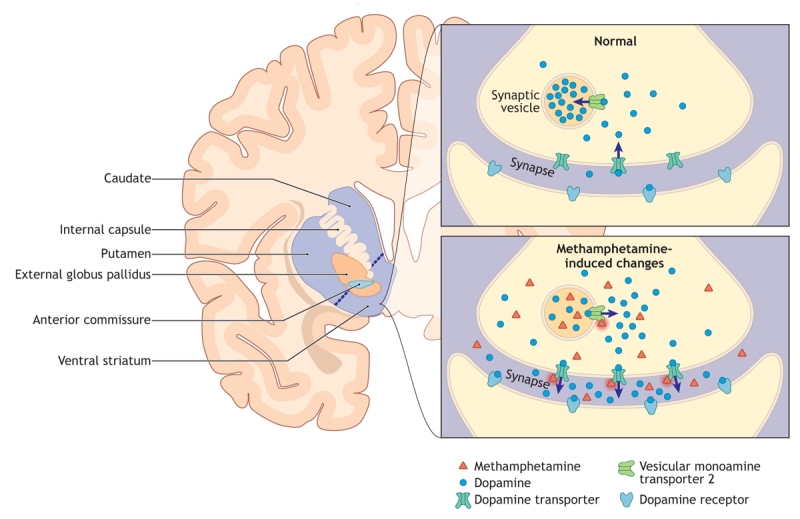
Figure 2: Figure 2: Schematic diagram of the human dopamine-rich striatum, which is made up of the caudate nucleus, putamen and ventral striatum (left), and a striatal dopamine nerve ending (right). This coronal slice is taken at the rostral tip of the anterior commissure. Methamphetamine causes dopamine release from the nerve endings. The areas of the brain responsible for methamphetamine-liking and craving are unknown but probably include the striatum and regions that provide input to the striatum. Normally, dopamine released into the synapse is taken back up into the nerve ending by the dopamine transporter and is transported into the synaptic vesicle by the vesicular monoamine transporter 2. Methamphetamine causes the release of striatal dopamine from the nerve ending into the synapse. This likely involves the translocation of dopamine from the synaptic vesicle to the neuronal cytoplasm via the vesicular monoamine transporter 2 and the reverse transport of dopamine from the cytoplasm into the synapse via the dopamine transporter.. Image by: Lianne Friesen and Nicholas Woolridge
Previously, dopamine in the ventral portion of the striatum (Figure 2) was considered to be the “pleasure” neurotransmitter involved in the action of all abused drugs.17,21,22 However, clinical findings suggest that drugs that block the dopamine receptor do not block methamphetamine “liking.” The dopamine story continues to evolve, with dopamine now viewed by some as a chemical involved in the motivational “wanting” aspect of drug-taking behaviour.23
Preliminary data show that opioid-receptor antagonists (naloxone, naltrexone) partially block some of the effects (drug-liking, arousal, craving24) of amphetamine in amphetamine-dependent people. This suggests that some of the clinically relevant actions of amphetamines are mediated by release of an endogenous opioid peptide. It is also likely that the neuronal circuitry that mediates drug-seeking behaviour includes structures and other neurotransmitter systems that interact with the dopamine-rich striatum. This might include, for example, the cerebral (especially the prefrontal and orbitofrontal) cortex, which provides glutamatergic input to striatum and the globus pallidus subdivisions and the ventral pallidum, which are innervated by striatal GABAergic and peptidergic (e.g., dynorphin, neurotensin) neurons.25
The mechanism explaining the transition from methamphetamine-liking to intense compulsive wanting (addiction) continues to be debated, but it could involve a “pathological learning” process in which dopamine facilitates learning.26,27
Does methamphetamine cause brain damage?
Through imaging studies, a variety of structural changes have been found in the brain of some methamphetamine users.28 However, the data are still too preliminary to answer the question of whether a specific pattern of brain abnormality is characteristic of chronic methamphetamine exposure.
Data from animals show that a high dose of methamphetamine damages striatal dopamine nerve terminals,29 and it is reasonable to expect from the experimental findings that such damage would also occur in people exposed to some dose of the drug.20,30 The consistent findings from animals have raised the public health concern that chronic methamphetamine exposure might damage nigrostriatal dopamine neurons to the extent that parkinsonism would develop in later life. However, there is no evidence for dopamine nerve terminal damage in humans who take therapeutic doses of amphetamines (e.g., for attention-deficit hyperactivity disorder), although this possibility has been raised as a specific concern.30 In contrast, some recreational methamphetamine users show modestly decreased levels of the dopamine transporter, a marker of dopamine nerve terminals, in the striatum.20 Whether this represents actual loss of dopamine nerve terminals or has clinical consequences continues to be debated.
Treatment of methamphetamine addiction
There are currently no medications approved by Health Canada for the treatment of of methamphetamine addiction (Dr. Cathy Peterson, Health Canada, Ottawa, Ont.: personal communication, 2008). Current treatments to prevent relapse include psychosocial approaches.31 It is probably not realistic to expect that a single medication will have high efficacy in preventing drug relapse in the majority of methamphetamine users. It can even be expected that in this chronic condition, the “memories” of addiction might be “hard-wired” and involve actual structural changes to brain neurons (e.g., in dendritic spine density) that make the addiction resistant to therapeutic intervention.32
Nevertheless, there is room for optimism that new medications can be developed that act on diverse targets and assist some drug users to maintain abstinence. This is suggested by the very preliminary findings that bupropion (which has dopaminergic and nondopaminergic actions) and the opioid-receptor antagonist naltrexone can influence aspects of amphetamine cravings.24,33 More generally, the efficacy of varenicline for tobacco cessation provides hope that a therapeutic approach that uses a partial agonist (a single compound having both agonist and antagonist properties) might be helpful in treating other addictions.34 It has also been proposed that “drug substitution therapy” (low dose oral amphetamine) might be useful in treatment of methamphetamine addiction and some very preliminary data, requiring confirmation, support this possibility.35 However, there is significant concern with the use of an abused drug for the treatment of stimulant addiction. Because some drug rehabilitation centres suggest that cognitive impairment decreases the likelihood of retention in drug rehabilitation programs,20 other strategies that might be considered include the use of cognitive-enhancing agents during methamphetamine withdrawal and the use of deep brain stimulation36,37 aimed at reversible inactivation of brain areas suspected of being involved in drug addiction and relapse.
Finally, it is important to be aware of medications that might not be effective in the treatment of methamphetamine addiction or that might worsen the condition. Data from a recent clinical trial evaluating efficacy of the antidepressant sertraline (Zoloft, Pfizer Canada) in abstinent methamphetamine users suggest that this selective serotonin reuptake inhibitor is not effective in reducing methamphetamine relapse and might even decrease the likelihood of maintaining abstinence.38
Research into the pharmacologic treatment of methamphetamine addiction has largely been limited to studies in animals. Surprisingly, there are very limited data from clinical trials of new therapies to prevent methamphetamine addiction relapse.24,33 Although animal studies are essential to the development of new medications, given the public health importance of this worldwide problem and the existence of potential drug targets, it is obvious that the very slow pace of clinical testing of new therapies in methamphetamine addiction needs to be accelerated.
@ See related article page 1655
Supplementary Material
Acknowledgments
Dr. Kish's methamphetamine studies are supported by the US National Institutes of Health's National Institute on Drug Abuse (07186).
Footnotes
Une version française de ce résumé est disponible à l'adresse www.cmaj.ca/cgi/content/full/178/13/1679/DC1
This article has been peer reviewed.
Competing interests: None declared.
Correspondence to: Dr. Stephen J. Kish, Human Neurochemical Pathology Laboratory, Centre for Addiction & Mental Health, 250 College St., Toronto ON M5T 1R8; fax 416 979-6871; stephen_kish@camh.net
REFERENCES
- 1.Schifano F, Corkery JM, Cuffolo G. Smokable (“ice,” “crystal meth”) and nonsmokable amphetamine-type stimulants: clinical pharmacological and epidemiological issues, with special reference to the UK. Ann Ist Super Sanita 2007;43:110-5. [PubMed]
- 2.Degenhardt L, Roxburgh A, Black E, et al. The epidemiology of methamphetamine use and harm in Australia. Drug Alcohol Rev 2008;27:243-52. [DOI] [PubMed]
- 3.Sulzer D, Sonders MS, Poulsen NW, et al. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 2005;75:406-33. [DOI] [PubMed]
- 4.Center for Drug Evaluation and Research. Adderall XR Capsules. The US Food and Drug Administration. Available: www.fda.gov/cder/foi/label/2004/021303s005lbl.pdf (accessed 2008 May 7).
- 5.GlaxoSmithKline. Prescribing information: Dexedrine. Mississauga (ON): GlaxoSmithKline; 2007. Available: www.gsk.ca/english/docs-pdf/Dexedrine_PM_EN_20070924.pdf (accessed 2008 May 7).
- 6.Martin WR, Sloan JW, Sapira JD, et al. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 1971;12:245-58. [DOI] [PubMed]
- 7.Rothman RB, Baumann MH, Dersch CM, et al. Ampphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 2001;39:32-41. [DOI] [PubMed]
- 8.Perez-Reyes M, White WR, McDonald SA, et al. Clinical effects of methamphetamine vapor inhalation. Life Sci 1991;49:953-9. [DOI] [PubMed]
- 9.The Vaults of Erowid. Methamphetamine dosage. Grass valley (CA): Erowid; 2003. Available: www.erowid.org/chemicals/meth/meth_dose.shtml (accessed 2008 Apr 18).
- 10. Ovation Pharmaceuticals. Desoxyn product monograph. Available: www.ovationpharma.com/pdfs/products/product_1.pdf (accessed 2008 Apr 30).
- 11.McGough JJ, Biderman J, Greenhill LL, et al. Pharmacokinetics of SLI381 (ADDERALL XR), an extended-release formulation of Adderall. J Am Acad Child Adolesc Psychiatry 2003;42:684-91. [DOI] [PubMed]
- 12.Melega WP, Cho AK, Harvey D, et al. Methamphetamine blood concentrations in human abusers: Application to pharmacokinetic modeling. Synapse 2007;61:216-20. [DOI] [PubMed]
- 13.Karch SB. Karchs Pathology of Drug Abuse. 3rd ed. Boca Raton (FL): CRC Press; 2001.
- 14.Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 2003;479:23-40. [DOI] [PubMed]
- 15.Goldstein DS, Nurnberger J Jr, Simmons S, et al. Effects of injected sympathomimetic amines on plasma catecholamines and circulatory variables in man. Life Sci 1983;32:1057-63. [DOI] [PubMed]
- 16.Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxitene treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159:1896-901. [DOI] [PubMed]
- 17.Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav 1982;17:901-4. [DOI] [PubMed]
- 18.Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry 2003;160:1726-39. [DOI] [PubMed]
- 19.Gao G, Wang X, He S, et al. Clinical study for alleviating opiate drug psychological dependence by a method of ablating the nucleus accumbens with stereotactic surgery. Sterotact Funct Neurosurg 2003;81:96-104 [DOI] [PubMed]
- 20.Moszczynska A, Fitzmaurice P, Ang L, et al. Why is parkinsonism not a feature of human methamphetamine users? Brain 2004;127:363-70. [DOI] [PubMed]
- 21.Dackis CA, Gold MA. New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev 1985;9:469-77. [DOI] [PubMed]
- 22.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 1988;85:5274-8. [DOI] [PMC free article] [PubMed]
- 23.Salamone JD, Correa M, Farra A, et al. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461-82. [DOI] [PubMed]
- 24.Jayaram-Lindström NJ, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology DOI: 10.1038/sj.npp.1301572. Epub 2007 Oct 24 ahead of print. [DOI] [PubMed]
- 25.Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict 2007;16:71-8. [DOI] [PubMed]
- 26.Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry 2005;162:1414-22. [DOI] [PubMed]
- 27.Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 2008;33:166-80. [DOI] [PubMed]
- 28.Chang L, Alicata D, Ernst T, et al. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 2007;102(Suppl 1):16-32. [DOI] [PubMed]
- 29.Ricaurte GA, Seiden LS, Schuster CR. Further evidence that amphetamines produce long-lasting dopamine neurochemical deficits by destroying dopamine nerve fibers. Brain Res 1984;303:359-64. [DOI] [PubMed]
- 30.Ricaurte GA, Mechan AO, Yuan J, et al. Amphetamine treatment similar to that used in the treatment of adult attention-deficit/hyperactivity disorder damages dopaminergic nerve endings in the striatum of adult nonhuman primates. J Pharmacol Exp Ther 2005;315:91-8. [DOI] [PubMed]
- 31.Rawson RA, Gonzales R, Brethen P. Treatment of methamphetamine use disorders: an update. J Subst Abuse Treat 2002;23:145-50. [DOI] [PubMed]
- 32.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 1997;17:8491-7. [DOI] [PMC free article] [PubMed]
- 33.Newton TF, Roache JD, De La Garza III R, et al. Buproprion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacol 2006;31:1537-44. [DOI] [PubMed]
- 34.Rollema H, Coe JW, Chambers LK, et al. Rationale, pharmacology and clinical efficacy of partial agonists of α4β2 nACh receptors for smoking cessation. Trends Pharmacol Sci 2007;28:316-25. [DOI] [PubMed]
- 35.Shearer J, Wodak A, Mattick RP, et al. Pilot randomized controlled study of dexamphetamine substitution for amphetamine dependence. Addiction 2001;96:1289-96 [DOI] [PubMed]
- 36.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005;45:651-60. [DOI] [PubMed]
- 37.Kuhn J, Lenartz D, Huff W, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: Valuable therapeutic implications? J Neurol Neurosurg Psychiat 2007;78:1152-3. [DOI] [PMC free article] [PubMed]
- 38.Shoptaw S, Huber A, Peck J, et al. Randomized placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend 2006;85:12-18. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


