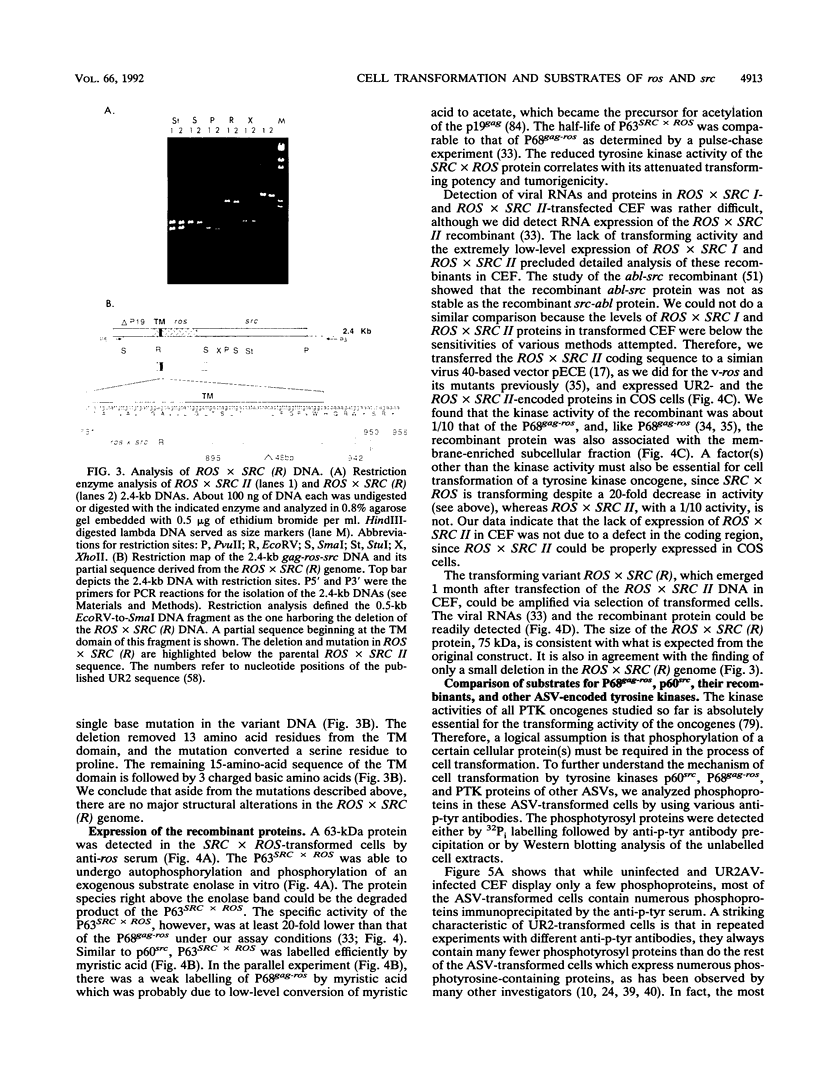
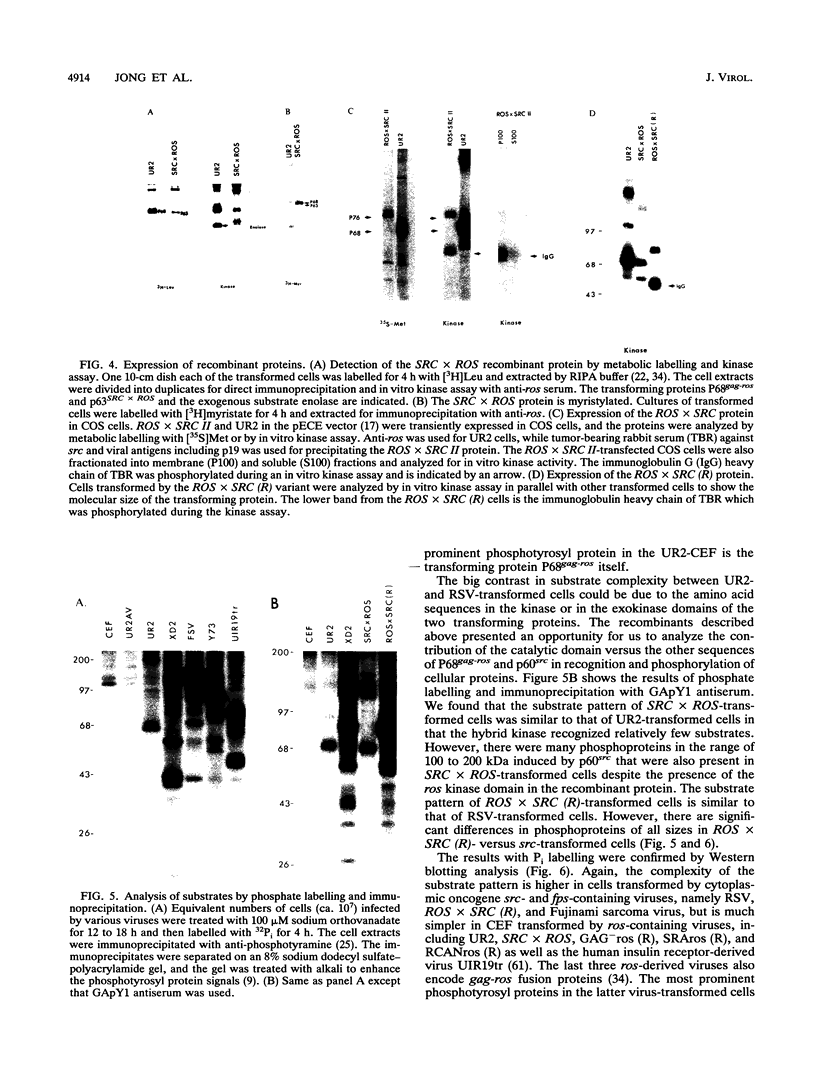
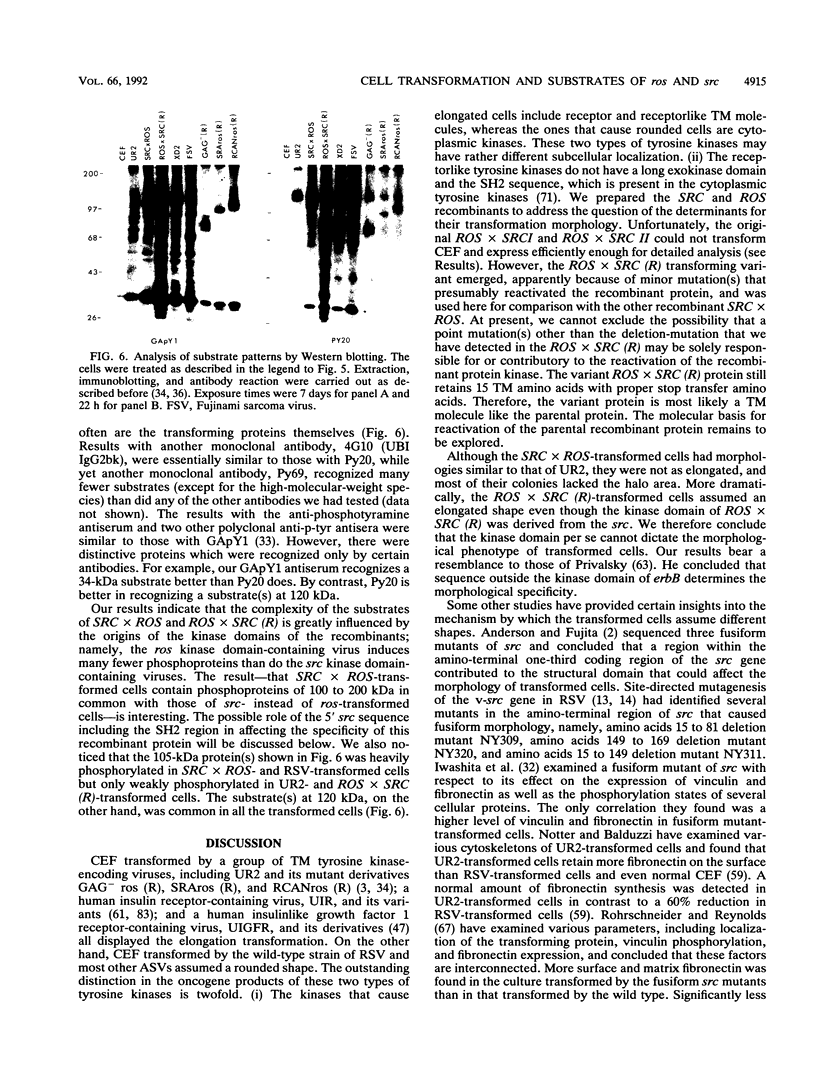
Abstract
To determine the sequences of the oncogenes src (encoded by Rous sarcoma virus [RSV]) and ros (encoded by UR2) that are responsible for causing different transformation phenotypes and to correlate those sequences with differences in substrate recognition, we constructed recombinants of the two transforming protein tyrosine kinases (PTKs) and studied their biological and biochemical properties. A recombinant with a 5' end from src and a 3' end from ros, called SRC x ROS, transformed chicken embryo fibroblasts (CEF) to a spindle shape morphology, mimicking that of UR2. Neither of the two reverse constructs, ROS x SRC I and ROS x SRC II, could transform CEF. However, a transforming variant of ROS x SRC II appeared during passages of the transfected cells and was called ROS x SRC (R). ROS x SRC (R) contains a 16-amino-acid deletion that includes the 3' half of the transmembrane domain of ros. Unlike RSV, ROS x SRC (R) also transformed CEF to an elongated shape similar to that of UR2. We conclude that distinct phenotypic changes of RSV- and UR2-infected cells do not depend solely on the kinase domains of their oncogenes. We next examined cellular proteins phosphorylated by the tyrosine kinases of UR2, RSV, and their recombinants as well as a number of other avian sarcoma viruses including Fujinami sarcoma virus Y73, and some ros-derived variants. Our results indicate that the UR2-encoded receptorlike PTK P68gag-ros and its derivatives have a very restricted substrate specificity in comparison with the nonreceptor PTKs encoded by the rest of the avian sarcoma viruses. Data from ros and src recombinants indicate that sequences both inside and outside the catalytic domains of ros and src exert a significant effect on the substrate specificity of the two recombinant proteins. Phosphorylation of most of the proteins in the 100- to 200-kDa range correlated with the presence of the 5' src domain, including the SH2 region, but not with the kinase domain in the recombinants. This corroborates the conclusion given above that the kinase domain of src or ros per se is not sufficient to dictate the transforming morphology of these two oncogenes. High-level tyrosyl phosphorylation of most of the prominent substrates of src is not sufficient to cause a round-shape transformation morphology.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. D., Beckmann R. P., Harms E. H., Nakamura K., Weber M. J. Biological properties of "partial" transformation mutants of Rous sarcoma virus and characterization of their pp60src kinase. J Virol. 1981 Jan;37(1):445–458. doi: 10.1128/jvi.37.1.445-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. K., Fujita D. J. Morphf mutants of Rous sarcoma virus: nucleotide sequencing analysis suggests that a class of morphf mutants was generated through splicing of a cryptic intron. J Virol. 1987 Jun;61(6):1893–1900. doi: 10.1128/jvi.61.6.1893-1900.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi P. C., Notter M. F., Morgan H. R., Shibuya M. Some biological properties of two new avian sarcoma viruses. J Virol. 1981 Oct;40(1):268–275. doi: 10.1128/jvi.40.1.268-275.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Bryant D. L., Parsons J. T. Amino acid alterations within a highly conserved region of the Rous sarcoma virus src gene product pp60src inactivate tyrosine protein kinase activity. Mol Cell Biol. 1984 May;4(5):862–866. doi: 10.1128/mcb.4.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. The proto-oncogene c-ets is preferentially expressed in lymphoid cells. Mol Cell Biol. 1985 Nov;5(11):2993–3000. doi: 10.1128/mcb.5.11.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Regulation of cell growth and transformation by tyrosine-specific protein kinases: the search for important cellular substrate proteins. Curr Top Microbiol Immunol. 1983;107:125–161. doi: 10.1007/978-3-642-69075-4_4. [DOI] [PubMed] [Google Scholar]
- Coulier F., Kumar R., Ernst M., Klein R., Martin-Zanca D., Barbacid M. Human trk oncogenes activated by point mutation, in-frame deletion, and duplication of the tyrosine kinase domain. Mol Cell Biol. 1990 Aug;10(8):4202–4210. doi: 10.1128/mcb.10.8.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Hanafusa H. N-terminal deletions in Rous sarcoma virus p60src: effects on tyrosine kinase and biological activities and on recombination in tissue culture with the cellular src gene. Mol Cell Biol. 1985 Oct;5(10):2789–2795. doi: 10.1128/mcb.5.10.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Hanafusa H. Local mutagenesis of Rous sarcoma virus: the major sites of tyrosine and serine phosphorylation of pp60src are dispensable for transformation. Cell. 1983 Sep;34(2):597–607. doi: 10.1016/0092-8674(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Di Marco E., Pierce J. H., Knicley C. L., Di Fiore P. P. Transformation of NIH 3T3 cells by overexpression of the normal coding sequence of the rat neu gene. Mol Cell Biol. 1990 Jun;10(6):3247–3252. doi: 10.1128/mcb.10.6.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Wang L. H., Hanafusa H., Balduzzi P. C. Avian sarcoma virus UR2 encodes a transforming protein which is associated with a unique protein kinase activity. J Virol. 1982 Apr;42(1):228–236. doi: 10.1128/jvi.42.1.228-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990 Oct 5;63(1):185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Fujita D. J., Bechberger J., Nedic I. Four Rous sarcoma virus mutants which affect transformed cell morphology exhibit altered src gene products. Virology. 1981 Oct 15;114(1):256–260. doi: 10.1016/0042-6822(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Fujita D. J., Boschek C. B., Ziemiecki A., Friis R. R. An avian sarcoma virus mutant which produces an aberrant transformation affecting cell morphology. Virology. 1981 May;111(1):223–238. doi: 10.1016/0042-6822(81)90667-x. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Hanafusa T., Hanafusa H. Membrane association of the transforming protein of avian sarcoma virus UR2 and mutants temperature sensitive for cellular transformation and protein kinase activity. J Virol. 1985 Dec;56(3):790–797. doi: 10.1128/jvi.56.3.790-797.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Grandori C., Hanafusa H. Phosphorylation of cellular proteins in Rous sarcoma virus-infected cells: analysis by use of anti-phosphotyrosine antibodies. Mol Cell Biol. 1988 Aug;8(8):3035–3042. doi: 10.1128/mcb.8.8.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Hanafusa H. Association of p60src with Triton X-100-resistant cellular structure correlates with morphological transformation. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2312–2316. doi: 10.1073/pnas.84.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Matsuda M., Hanafusa H. A glycoprotein in the plasma membrane matrix as a major potential substrate of p60v-src. Mol Cell Biol. 1990 Feb;10(2):830–836. doi: 10.1128/mcb.10.2.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E., Nocka K., Beier D. R., Chu T. Y., Buck J., Lahm H. W., Wellner D., Leder P., Besmer P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990 Oct 5;63(1):225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- Hughes S., Mellstrom K., Kosik E., Tamanoi F., Brugge J. Mutation of a termination codon affects src initiation. Mol Cell Biol. 1984 Sep;4(9):1738–1746. doi: 10.1128/mcb.4.9.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S., Kitamura N., Yoshida M. Molecular events leading to fusiform morphological transformation by partial src deletion mutant of Rous sarcoma virus. Virology. 1983 Mar;125(2):419–431. doi: 10.1016/0042-6822(83)90213-1. [DOI] [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. Role of gag sequence in the biochemical properties and transforming activity of the avian sarcoma virus UR2-encoded gag-ros fusion protein. J Virol. 1990 Dec;64(12):5997–6009. doi: 10.1128/jvi.64.12.5997-6009.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. The transforming protein P68gag-ros of avian sarcoma virus UR2 is a transmembrane protein with the gag portion protruding extracellularly. Oncogene Res. 1987 Jun;1(1):7–21. [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. Two point mutations in the transmembrane domain of P68gag-ros inactive its transforming activity and cause a delay in membrane association. J Virol. 1991 Jan;65(1):180–189. doi: 10.1128/jvi.65.1.180-189.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleko M., Rutter W. J., Miller A. D. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990 Feb;10(2):464–473. doi: 10.1128/mcb.10.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Rous sarcoma virus transforming protein lacking myristic acid phosphorylates known polypeptide substrates without inducing transformation. Cell. 1986 Apr 11;45(1):105–112. doi: 10.1016/0092-8674(86)90542-8. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Most of the substrates of oncogenic viral tyrosine protein kinases can be phosphorylated by cellular tyrosine protein kinases in normal cells. Oncogene Res. 1988 Sep;3(2):105–115. [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Kozma L. M., Reynolds A. B., Weber M. J. Glycoprotein tyrosine phosphorylation in Rous sarcoma virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1990 Feb;10(2):837–841. doi: 10.1128/mcb.10.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenberger J. A., Court D., Papas T. S. High-level expression in Escherichia coli of the carboxy-terminal sequences of the avian myelocytomatosis virus (MC29) v-myc protein. Gene. 1983 Jul;23(1):75–84. doi: 10.1016/0378-1119(83)90218-4. [DOI] [PubMed] [Google Scholar]
- Lev S., Yarden Y., Givol D. Receptor functions and ligand-dependent transforming potential of a chimeric kit proto-oncogene. Mol Cell Biol. 1990 Nov;10(11):6064–6068. doi: 10.1128/mcb.10.11.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Rutter W. J., Wang L. H. Enhancement of transforming potential of human insulinlike growth factor 1 receptor by N-terminal truncation and fusion to avian sarcoma virus UR2 gag sequence. J Virol. 1992 Jan;66(1):374–385. doi: 10.1128/jvi.66.1.374-385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Baltimore D. Recombinants within the tyrosine kinase region of v-abl and v-src identify a v-abl segment that confers lymphoid specificity. Mol Cell Biol. 1988 Jan;8(1):234–240. doi: 10.1128/mcb.8.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Mayer B. J., Fukui Y., Hanafusa H. Binding of transforming protein, P47gag-crk, to a broad range of phosphotyrosine-containing proteins. Science. 1990 Jun 22;248(4962):1537–1539. doi: 10.1126/science.1694307. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hanafusa H. Association of the v-crk oncogene product with phosphotyrosine-containing proteins and protein kinase activity. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2638–2642. doi: 10.1073/pnas.87.7.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M. F., Koch C. A., Anderson D., Ellis C., England L., Martin G. S., Pawson T. Src homology region 2 domains direct protein-protein interactions in signal transduction. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8622–8626. doi: 10.1073/pnas.87.21.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Shibuya M., Hsu M. T., Wang L. H. Proto-oncogene c-ros codes for a molecule with structural features common to those of growth factor receptors and displays tissue specific and developmentally regulated expression. Mol Cell Biol. 1986 May;6(5):1478–1486. doi: 10.1128/mcb.6.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Molecular cloning and characterization of avian sarcoma virus UR2 and comparison of its transforming sequence with those of other avian sarcoma viruses. J Virol. 1984 Jun;50(3):914–921. doi: 10.1128/jvi.50.3.914-921.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Nucleotide sequence of avian sarcoma virus UR2 and comparison of its transforming gene with other members of the tyrosine protein kinase oncogene family. J Virol. 1985 Mar;53(3):879–884. doi: 10.1128/jvi.53.3.879-884.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter M. F., Balduzzi P. C. Cytoskeletal changes induced by two avian sarcoma viruses: UR2 and Rous sarcoma virus. Virology. 1984 Jul 15;136(1):56–68. doi: 10.1016/0042-6822(84)90247-2. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E. The structure and protein kinase activity of proteins encoded by nonconditional mutants and back mutants in the sec gene of avian sarcoma virus. Virology. 1981 Jan 15;108(1):47–70. doi: 10.1016/0042-6822(81)90526-2. [DOI] [PubMed] [Google Scholar]
- Poon B., Dixon D., Ellis L., Roth R. A., Rutter W. J., Wang L. H. Molecular basis of the activation of the tumorigenic potential of Gag-insulin receptor chimeras. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):877–881. doi: 10.1073/pnas.88.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky M. L. Creation of a chimeric oncogene: analysis of the biochemical and biological properties of v-erbB/src fusion polypeptide. J Virol. 1987 Jun;61(6):1938–1948. doi: 10.1128/jvi.61.6.1938-1948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Ling H. P. Identification of a 32K plasma membrane protein that binds to the myristylated amino-terminal sequence of p60v-src. Nature. 1990 Jul 5;346(6279):84–86. doi: 10.1038/346084a0. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Specific and saturable binding of pp60v-src to plasma membranes: evidence for a myristyl-src receptor. Cell. 1989 Jul 28;58(2):281–286. doi: 10.1016/0092-8674(89)90842-8. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Roussel M. F., Ashmun R. A., Ralph P., Price K., Sherr C. J. Synthesis of membrane-bound colony-stimulating factor 1 (CSF-1) and downmodulation of CSF-1 receptors in NIH 3T3 cells transformed by cotransfection of the human CSF-1 and c-fms (CSF-1 receptor) genes. Mol Cell Biol. 1987 Jul;7(7):2378–2387. doi: 10.1128/mcb.7.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L., Reynolds S. Regulation of cellular morphology by the Rous sarcoma virus src gene: analysis of fusiform mutants. Mol Cell Biol. 1985 Nov;5(11):3097–3107. doi: 10.1128/mcb.5.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Transy C., Kato J. Y., Reinherz E. L., Sherr C. J. Antibody-induced mitogenicity mediated by a chimeric CD2-c-fms receptor. Mol Cell Biol. 1990 May;10(5):2407–2412. doi: 10.1128/mcb.10.5.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I., Stone J. C., Pawson T. A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol Cell Biol. 1986 Dec;6(12):4396–4408. doi: 10.1128/mcb.6.12.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Shu H. K., Pelley R. J., Kung H. J. Tissue-specific transformation by epidermal growth factor receptor: a single point mutation within the ATP-binding pocket of the erbB product increases its intrinsic kinase activity and activates its sarcomagenic potential. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9103–9107. doi: 10.1073/pnas.87.23.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. Separation of morphological conversion and virus production in Rous sarcoma virus infection. Cold Spring Harb Symp Quant Biol. 1962;27:407–414. doi: 10.1101/sqb.1962.027.001.038. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. The control of cellular morphology in embryonic cells infected with rous sarcoma virus in vitro. Virology. 1960 Feb;10:182–197. doi: 10.1016/0042-6822(60)90038-6. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H., Junghans R. P., Ju G., Skalka A. M. Comparison between the viral transforming gene (src) of recovered avian sarcoma virus and its cellular homolog. Mol Cell Biol. 1981 Nov;1(11):1024–1037. doi: 10.1128/mcb.1.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Verderame M. F., Kaplan J. M., Varmus H. E. A mutation in v-src that removes a single conserved residue in the SH-2 domain of pp60v-src restricts transformation in a host-dependent manner. J Virol. 1989 Jan;63(1):338–348. doi: 10.1128/jvi.63.1.338-348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y. Isolation of antibodies for phosphotyrosine by immunization with a v-abl oncogene-encoded protein. Mol Cell Biol. 1985 Dec;5(12):3640–3643. doi: 10.1128/mcb.5.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Lin B., Jong S. M., Dixon D., Ellis L., Roth R. A., Rutter W. J. Activation of transforming potential of the human insulin receptor gene. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5725–5729. doi: 10.1073/pnas.84.16.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. E., Eisenman J., Baird A., Rauch C., Van Ness K., March C. J., Park L. S., Martin U., Mochizuki D. Y., Boswell H. S. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990 Oct 5;63(1):167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Woolford J., McAuliffe A., Rohrschneider L. R. Activation of the feline c-fms proto-oncogene: multiple alterations are required to generate a fully transformed phenotype. Cell. 1988 Dec 23;55(6):965–977. doi: 10.1016/0092-8674(88)90242-5. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Ikawa Y. Induction of some transformation-related properties by a transformation-defective mutant of avian sarcoma virus. Virology. 1977 Dec;83(2):444–448. doi: 10.1016/0042-6822(77)90192-1. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Williams D. A., Geissler E. N., Broudy V. C., Martin F. H., Atkins H. L., Hsu R. Y., Birkett N. C., Okino K. H., Murdock D. C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990 Oct 5;63(1):213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]