Abstract
Dopamine plays an important role in modulating incentive motivation, expressed behaviorally as approach behavior. EEG studies report association between approach behavior and asymmetric pattern of activation in anterior cortical regions (as measured by the inverse of EEG alpha power). Therefore, individual differences in incentive motivation may reflect asymmetries in dopaminergic systems. We examined this hypothesis by studying the relationship between self-reported degree of incentive motivation, and asymmetry of D2 receptor availability in healthy volunteers. Nineteen healthy participants were studied with positron emission tomography (PET) and [11C]raclopride to assess the availability of dopamine D2 receptors in left and right striatum. Incentive motivation was assessed by the Achievement scale of the Multidimensional Personality Questionnaire. The Achievement score was negatively correlated with the Asymmetry Index ([R−L]/[R+L]) of D2 receptor availability (r = −.721, p =.001), suggesting that greater positive incentive motivation is associated with higher receptor availability in the left relative to the right hemisphere.
Introduction
Individual differences in personality have been suggested to reflect variation in the sensitivity to stimuli associated with positive and negative reinforcement (Gray, 1973). Depue and Collins (1999) proposed a model according to which extraversion, and more specifically the agency component of this personality trait, is based on a single neurobiological network that integrates positive incentive motivation. These authors further suggest that dopamine plays an important role in modulating incentive motivation, and individual differences in the degree of positive incentive motivation may be related to functional variation in the ventral tegmental area dopamine projections. Although Depue & Collins (1999) focused on the ventral tegmental area (VTA) dopamine projection, recent evidence from both animal studies (Robinson et al., 2006) and imaging studies in humans (Hikosaka, 2007; Delgado et al., 2004), as well as neuroanatomical findings (Haber et al., 2006) have pointed to the role of the dorsal striatum, where cortical and dopaminergic inputs converge, in mediating motivated or approach, goal-directed, behaviors.
Differential sensitivity to positive and negative stimuli has been associated with relatively asymmetric pattern of activation in anterior cortical regions (as measured most often by the power within the alpha frequency range, which is inversely related to underlying cortical processing), and approach behavior has been linked to greater activation in the left (relative to right) frontal lobe (Davidson, 2004). Support for this model is derived mostly from studies of EEG asymmetry. However, if dopamine plays a major role in modulating approach, some of the individual differences in the sensitivity to reward (as reflected in approach behaviors and agency, the personality component representing positive incentive motivation) may reflect differences in asymmetries in dopaminergic systems.
In animals, differences in dopaminergic asymmetry co-vary with, or predict, individual differences in spatial behavior, stress reactivity and drug sensitivity (Carlson & Glick, 1989). In humans, although an association between temperament and personality traits and individual differences in dopaminergic markers in the dorsal striatum has been reported previously (e.g. Farde et al., 1997; Breier et al., 1998; Laakso et al., 2000), these studies did not examine the possible role of dopaminergic asymmetry.
The present study was therefore aimed at examining the hypothesis that individual differences in the degree of incentive motivation in healthy individuals may be related to the direction and degree of asymmetry in dopamine in the dorsal striatum. As marker of dopamine neurotranmission, we use the measure of dopamine D2/D3 receptor availability obtained with PET and [11C]raclopride (a ligand that binds to dopamine D2 and D3 receptors and is sensitive to competition with endogenous dopamine). More specifically, in view of the reports relating relatively greater left hemisphere activation to approach behavior, we predicted that dopamine asymmetry favoring the left striatum would be associated with higher incentive motivation. This latter prediction was also based on our earlier finding that in patients with Parkinson’s disease, significantly reduced novelty seeking (which may be viewed as reduced approach behavior) was observed in patients with greater dopamine deficit in the left striatum (Tomer & Aharon-Peretz, 2004).
Method
Nineteen healthy right-handed participants (15 men, 4 women; age= 38.5 ± 11.4, range: 21–67; years of education: 15.9 ± 2.3, range: 11–19) were recruited by local advertisement. Exclusion criteria consisted of past or present psychiatric or neurologic disorder, alcohol or substance abuse and a history of head trauma with loss of consciousness, as verified by a psychiatric interview and a complete neurological examination. None of the subjects was taking medication at the time of the study and pre-scan urine tests were done to ensure the absence of psychoactive drug use. Informed consent was obtained following guidelines set by the Institutional Review Board at Brookhaven National Laboratory.
Imaging and image analysis
PET scans were performed with a CTI 931 scanner (Siemens, Knoxville, Tenn.). Subjects were scanned after intravenous injection of [11C]raclopride, a low affinity dopaminergic D2 receptor ligand. Details of the procedures for positioning, arterial and venous catetherization, and quantification of the radiotracer have been described by Volkow et al. (1993). Briefly, the 60-minute dynamic scans were started immediately after intravenous injection of 4–10 mCi of [11C]raclopride (specific activity >0.25 Ci/μmol at time of injection). During the study, the subject lay with eyes open in the PET camera, the room was dimly lit, and noise was kept to a minimum. Regions of interest (ROIs) in the [11C]raclopride images were obtained for the striatum (the caudate and the putamen) and for the cerebellum, used to control for non-specific effects. For every subject, the ROIs were initially selected on an averaged scan (activity from 10 to 60 minutes) and were then projected to the dynamic scans, as previously described (Volkow et al., 1993). The time-activity curves for [11C]raclopride in the striatum and the cerebellum and the time-activity curves for unchanged tracer in plasma were used to calculate distribution volumes by using a graphical analyses technique for reversible systems (Logan Plots). The parameter Bmax/Kd, obtained as the ratio of the distribution volume in the striatum to that in the cerebellum minus 1, was used as the model parameter of dopamine D2 receptor availability. To assess the degree of asymmetry of D2 receptor availability, an asymmetry index (AI= [R−L]/[R+L]) was calculated, where a positive value reflects higher levels in the right hemisphere, whereas negative values reflect higher availability in the left dorsal striatum.
Assessment of incentive motivation
The Achievement subscale of the Multidimensional Personality Questionnaire (MPQ, Tellegen & Waller, 1997) was used to assess incentive motivation. The MPQ consists of several higher-order dimensions, including the higher-order factor Agentic Positive Emotionality, which was purposefully developed to assess an emotional system based on sensitivity to signals of reward. Agency represents a more general disposition encompassing dominance, ambition, mastery, and efficacy that is manifest in a range of achievement-related, as well as interpersonal, contexts (Depue & Morrone-Strupinsky (2005), and the lower-order traits associated with the agency factor represent different manifestations of a single underlying process. Since the processing of affiliative reward involves not only dopamine but the opiate system and other hormones and neuromodulators (Depue & Morrone-Strupinsky, 2005), we chose to employ the lower-order construct Achievement, which evaluates a motivational disposition that comprises social dominance, enthusiasm, energy, assertiveness, ambitiousness, and achievement striving, and may be dissociated from affiliation trait (Depue & Collins, 1999).
Results
Achievement scores (mean score: 13.47 ± 3.96; range: 6–19) were not correlated with age (r = −066) or education (r = −044 ), and did not differ between male (13.33 ± 4.15) and female (14.0 ± 3.65) participants. D2 receptor availability was significantly correlated with age for the four striatal ROIs (left caudate: r = −565, df = 17, p=.012; right caudate: −.646, df = 17, p=.003; left putamen: −.532, df = 17, p=.019; right putamen: −.581, df = 17, p=.009). Therefore, we partialled out age effects when analyzing the relationship between the MPQ achievement score and receptor availability. Correlations between achievement score and D2 receptor availability values in the four striatal ROIs were not significant. However, consistent with the a priori hypothesis, the MPQ achievement score was negatively correlated with the Asymmetry Index of D2 receptor availability in the putamen (r = −721, df = 16, p =.001, Figure 1), suggesting that higher incentive motivation is strongly associated with relatively higher availability of D2 receptors in the left relative to the right putamen. There was no correlation between achievement and the AI in the caudate (r =.105, df = 16, n.s.). As shown in Figure 1, this relationship between higher incentive motivation and higher D2 receptor availability in the left, as compared to right, putamen, is found for both male and female participants.
Figure 1.
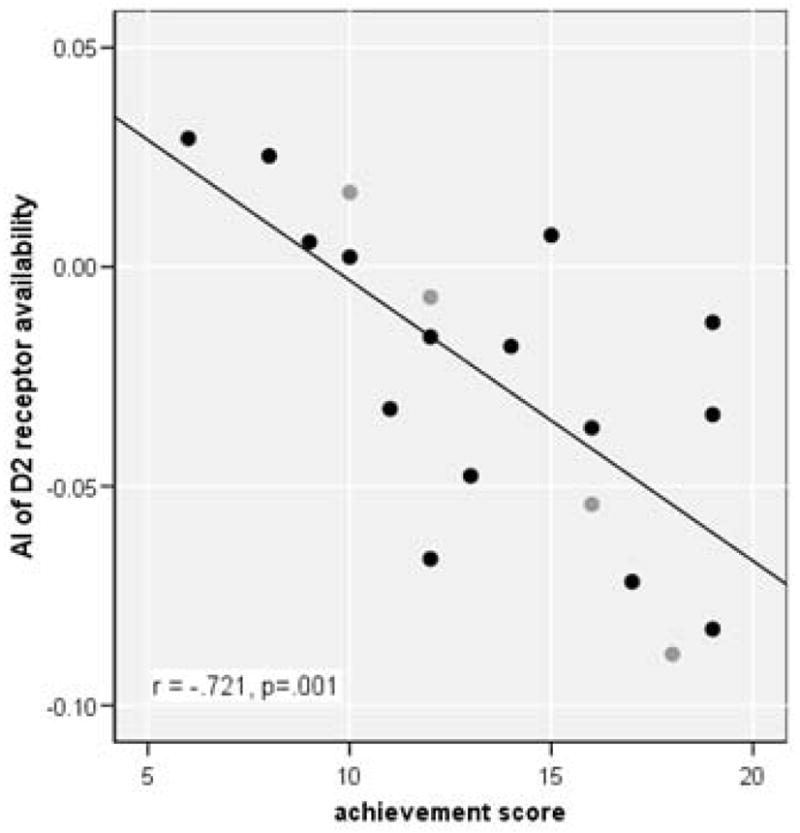
Correlation between achievement score and asymmetry of D2 receptor availability in the putamen
Legend: AI: (R−L)/(R+L); positive index score indicates higher right hemisphere availability whereas negative index score represents higher availability in the left hemisphere. Black circles: male participants; grey circles: female participants.
Discussion
The current results reveal a highly significant association between the level of incentive motivation (as measured by the MPQ Achievement subscale) and the pattern of asymmetry in D2 receptor availability in the putamen, such that greater positive incentive motivation is associated with higher receptor availability in the left relative to the right hemisphere. To the best of our knowledge, this is the first report relating variability in a personality trait to the direction and degree of asymmetry of D2 receptor availability in healthy individuals.
There is ample evidence suggesting that in animals inter-individuals differences in dopaminergic asymmetry covary with, or predict, individual differences in spatial behavior, stress reactivity and drug sensitivity (Carlson & Glick, 1989). In humans, a recent review reported a population bias of higher D2 receptor binding in the right as compared to left striatum in healthy individuals (Larisch et al., 1998). This conclusion is based on group averages. However, careful examination of studies that report individual data reveals that in each such study, some subjects had higher values in the right striatum, whereas others showed left-side preference. Thus, notwithstanding the population bias towards higher values of D2 receptor binding in the right striatum, there are considerable individual differences, not only in the direction, but also in the degree of asymmetry. Our findings suggest that the direction and degree of asymmetry in striatal dopaminergic markers may index a behavioral trait of the individual, namely incentive motivation.
As discussed in the Introduction, there is a considerable body of literature suggesting that left (relative to right) frontal and anterior temporal activation (as reflected in EEG power) is associated with positive, approach-related emotion (see Davidson, 2004, for review). The frontal lobes are extensively connected with the striatum through a series of parallel frontal-subcortical circuits (Alexander et al., 1986). Dopaminergic neurons from the substantia nigra project to the striatum and affect all frontal–subcortical functions, allowing interaction between the emotional input and motor activity, cognition and motivation (Tekin & Cummings, 2002). Volkow et al. (2001) reported a significant correlation between D2 receptor availability in the putamen and FDG metabolism in the orbitofrontal cortex and in the anterior cingulate gyrus. This association could reflect dopamine-mediated striatal regulation of orbitofrontal activity by means of striato-thalamo-cortical pathways (Haber et al., 1995). Thus, the asymmetry in D2 receptor availability in the putamen may contribute to the asymmetric pattern of activation in the frontal lobes, mentioned above. Based on the reported association between approach behavior and relatively greater left frontal activation level, we expected higher incentive motivation to be associated with a corresponding (L>R) dopaminergic asymmetry in the striatum. Our current findings appear to support this prediction. However, it is the difficult to determine whether the D2 receptor availability represents asymmetry of receptor density or of levels of endogenous dopamine, since [11C]raclopride’s binding is sensitive to competition with endogenous dopamine. Further studies are needed in order to clarify this point. Since [11C]raclopride has a greater sensitivity for detecting binding in the putamen, predominantly to D2 receptors, than in the globus pallidum, where binding is predominantly to D3 receptors (Graff Guerrero et al., 2007), the current results pertain mostly to asymmetries in dorsal putamen and caudate. Nevertheless, the importance of our findings lies in highlighting the association between asymmetry in dopamine-driven brain circuits and individual differences in motivational and reward-related processes, as reflected in a personality trait representing positive incentive motivation. Another limitation is the small sample which consisted mostly of males, thus limiting the generalizabiliy of our findings.
The current results may have important implications not only for mapping the neurobiological underpinnings of personality in healthy individuals, but also for understanding the pathophysiological correlates of abnormal behavior. Pathological manifestations of motivated behavior occur in a number of neuropsychiatric syndromes (including addiction, depression, schizophrenia and parkinson’s disease). Indeed, reduced motivation and decreased novelty seeking have been described in Parkinson’s disease and this has been shown to be related to the asymmetry of dopamine deficit (Menza et al., 1995; Tomer & Aharon-Peretz, 2004). Future studies may clarify the role of asymmetric dysregulation of dopaminergic neurotransmission in mediating behavioral pathology in these disorders.
References
- 1.Alexander G, DeLong M, Strick P. Parallel organisation of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 2.Breier A, Kestler L, Adler C, Elman I, Wiesenfeld N, Malhotra A, Pickar D. Dopamine D2 receptor density and personal detachment in healthy subjects. American Journal of Psychiatry. 1998;155:1440–1442. doi: 10.1176/ajp.155.10.1440. [DOI] [PubMed] [Google Scholar]
- 3.Carlson JN, Glick SD. Cerebral lateralization as a measure of interindividual differences in behavior. Experientia. 1989;45:788–798. doi: 10.1007/BF01954054. [DOI] [PubMed] [Google Scholar]
- 4.Davidson RJ. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology. 2004:219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Delgado MR, Stenger VA, Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex. 2004;14:1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- 6.Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- 7.Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–395. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- 8.Farde L, Gustavsson JP, Jönsson E. D2 dopamine receptors and personality traits (letter) Nature. 1997;385:590. doi: 10.1038/385590a0. [DOI] [PubMed] [Google Scholar]
- 9.Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D(2/3) agonist [(11)C]-(+)-PHNO and the D(2/3) antagonist [(11)C]raclopride in healthy humans. Human Brain Mapping. 2007 May 11; doi: 10.1002/hbm.20392. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray JA. In: Causal theories of personality and how to test them, in: Multivariate analysis and psychological theory. Royce JR, editor. Academic Press; 1973. [Google Scholar]
- 11.Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. Journal of Neuroscience. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haber SN, Ki-Sok Kim K-S, Mailly P, Calzavara R. Reward-Related Cortical Inputs Define a Large Striatal Region in Primates That Interface with Associative Cortical Connections, Providing a Substrate for Incentive-Based Learning. Journal of Neuroscience. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movements. Annals of the New York Academy of Sciences. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- 14.Laakso A, Vilkman H, Alakare B, Haaoaranta M, Bergman J, Solin O, Peurasaari J. Striatal dopamine transporter binding in neuroleptic-naïve patients with schizophrenia studies with positron emission tomography. American Journal of Psychiatry. 2000;157:269– 271. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- 15.Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Muller-Gartner HW. Left–right asymmetry of striatal dopamine D2 receptors. Nuclear Medicine Communications. 1998;19:781–787. doi: 10.1097/00006231-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Menza MA, Mark MH, Burn DJ, Brooks DJ. Personality correlates of [18F]dopa striatal uptake: results of positron-emission tomography in Parkinson’s disease. Journal of Neuropsychiatry and Clinical Neuroscience. 1995;7:176–9. doi: 10.1176/jnp.7.2.176. [DOI] [PubMed] [Google Scholar]
- 17.Robinson S, Sotak BN, During MJ, Palmiter RD. Local dopamine production in the dorsal striatum restores goal-directed behavior in dopamine-deficient mice. Behavioral Neuroscience. 2006;120:196–200. doi: 10.1037/0735-7044.120.1.000. [DOI] [PubMed] [Google Scholar]
- 18.Tekin S, Cummings JL. Frontal–subcortical neuronal circuits and clinical neuropsychiatry An update. Journal of Psychosomatic Research. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 19.Tellegen A, Waller NG. Exploring personality through test construction: Development of the multidimensional personality questionnaire. In: Briggs S, Cheek J, editors. Personality measures: Development and evaluation. Vol. 1 JAI Press; 1997. [Google Scholar]
- 20.Tomer R, Aharon-Peretz J. Novelty seeking and harm avoidance in Parkinson’s disease: effects of asymmetric dopamine deficiency. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:972–975. doi: 10.1136/jnnp.2003.024885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 22.Volkow ND, Fowler JS, Wang G-J, Dewey SL, Schlyer D, MacGregor R, Logan J, Alexoff D, Shea C, Hitzemann R, Angrist B, Wolf AP. Reproducibility of repeated measures of 11C raclopride binding in the human brain. Journal of Nuclear Medicine. 1993;34:609–613. [PubMed] [Google Scholar]


