Abstract
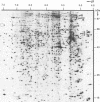
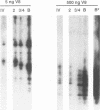
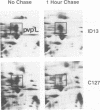
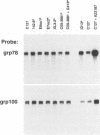
We have previously characterized five proteins induced by the presence of the E2 open reading frame (ORF) region of bovine papillomavirus type 1 (BPV-1) in C127 mouse fibroblasts (R. M. Levenson, U. G. Brinckmann, M. K. O'Banion, E. J. Androphy, J. T. Schiller, F. Tabatabai, L. P. Turek, K. Neary, M. T. Chin, T. R. Broker, L. T. Chow, and D. A. Young, Virology 172:170-179, 1989). By specific immunoprecipitation, we now find that one of the papillomavirus-associated proteins (pvp1) is a highly glycosylated form of glucose-regulated protein 100 (grp100), a major constituent of the endoplasmic reticulum. A second set of pvps (2, 3, and 4) are shown to be related precursors of another protein already present in C127 cells (protein B). Based on their induction by the calcium ionophore A23187 and their positions on giant two-dimensional gels, we have tentatively identified pvp2, -3, and -4 and B as forms of calcium-regulated protein 55, another constituent of the endoplasmic reticulum (D. R. J. Macer and G. L. E. Koch, J. Cell Sci. 91:61-70, 1988). The mechanism by which BPV-1 brings about these changes is not yet defined; however, it is unlikely to involve calcium level perturbations or transformation per se, since ionophore treatment changes other proteins in C127 cells not seen with BPV and the papillomavirus-associated proteins are found in nontransformed cells harboring the E2 ORF region. Furthermore, the BPV changes are not associated with increased grp mRNA levels, as occurs in ionophore-treated cells. Rather, it appears that BPV-1 somehow retards the normal processing of these resident endoplasmic reticulum proteins that are believed to serve as critical regulators of host protein processing and assembly.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Chang S. C., Erwin A. E., Lee A. S. Glucose-regulated protein (GRP94 and GRP78) genes share common regulatory domains and are coordinately regulated by common trans-acting factors. Mol Cell Biol. 1989 May;9(5):2153–2162. doi: 10.1128/mcb.9.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. C., Wooden S. K., Nakaki T., Kim Y. K., Lin A. Y., Kung L., Attenello J. W., Lee A. S. Rat gene encoding the 78-kDa glucose-regulated protein GRP78: its regulatory sequences and the effect of protein glycosylation on its expression. Proc Natl Acad Sci U S A. 1987 Feb;84(3):680–684. doi: 10.1073/pnas.84.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Curran T., Teich N. M. Identification of a 39,000-dalton protein in cells transformed by the FBJ murine osteosarcoma virus. Virology. 1982 Jan 15;116(1):221–235. doi: 10.1016/0042-6822(82)90415-9. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Guralski D., Schiller J. T. Translation of open reading frame E5 of bovine papillomavirus is required for its transforming activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1797–1801. doi: 10.1073/pnas.83.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Edwards D. P., Weigel N. L., Schrader W. T., O'Malley B. W., McGuire W. L. Structural analysis of chicken oviduct progesterone receptor using monoclonal antibodies to the subunit B protein. Biochemistry. 1984 Sep 11;23(19):4427–4435. doi: 10.1021/bi00314a029. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Koch G. L. Reticuloplasmins: a novel group of proteins in the endoplasmic reticulum. J Cell Sci. 1987 May;87(Pt 4):491–492. doi: 10.1242/jcs.87.4.491. [DOI] [PubMed] [Google Scholar]
- Koch G., Smith M., Macer D., Webster P., Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J Cell Sci. 1986 Dec;86:217–232. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Hubbert N. L., Howley P. M., Schiller J. T. Genetic assignment of multiple E2 gene products in bovine papillomavirus-transformed cells. J Virol. 1989 Jul;63(7):3151–3154. doi: 10.1128/jvi.63.7.3151-3154.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S., Bell J., Ting J. Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1984 Apr 10;259(7):4616–4621. [PubMed] [Google Scholar]
- Lee A. S., Delegeane A. M., Baker V., Chow P. C. Transcriptional regulation of two genes specifically induced by glucose starvation in a hamster mutant fibroblast cell line. J Biol Chem. 1983 Jan 10;258(1):597–603. [PubMed] [Google Scholar]
- Levenson R. M., Brinckmann U. G., O'Banion M. K., Androphy E. J., Schiller J. T., Tabatabai F., Turek L. P., Neary K., Chin M. T., Broker T. R. Papillomavirus-associated inductions of cellular proteins in mouse C127 cells: correlation with the presence of open reading frame E2. Virology. 1989 Sep;172(1):170–179. doi: 10.1016/0042-6822(89)90118-9. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Turco S. J., Green M. Structure and assembly of the endoplasmic reticulum. Biosynthetic sorting of endoplasmic reticulum proteins. J Biol Chem. 1985 Jun 10;260(11):6926–6931. [PubMed] [Google Scholar]
- Macer D. R., Koch G. L. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. J Cell Sci. 1988 Sep;91(Pt 1):61–70. doi: 10.1242/jcs.91.1.61. [DOI] [PubMed] [Google Scholar]
- Martin P., Vass W. C., Schiller J. T., Lowy D. R., Velu T. J. The bovine papillomavirus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell. 1989 Oct 6;59(1):21–32. doi: 10.1016/0092-8674(89)90866-0. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Neary K., DiMaio D. Open reading frames E6 and E7 of bovine papillomavirus type 1 are both required for full transformation of mouse C127 cells. J Virol. 1989 Jan;63(1):259–266. doi: 10.1128/jvi.63.1.259-266.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Infection with paramyxoviruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6120–6124. doi: 10.1073/pnas.75.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Resendez E., Jr, Attenello J. W., Grafsky A., Chang C. S., Lee A. S. Calcium ionophore A23187 induces expression of glucose-regulated genes and their heterologous fusion genes. Mol Cell Biol. 1985 Jun;5(6):1212–1219. doi: 10.1128/mcb.5.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Yang Y. C., Howley P. M. Transactivation of a bovine papilloma virus transcriptional regulatory element by the E2 gene product. Cell. 1985 Aug;42(1):183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Subjeck J. R., Shyy T. T. Stress protein systems of mammalian cells. Am J Physiol. 1986 Jan;250(1 Pt 1):C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- Tada A., Sekine H., Yamamoto T., Fuse A., Simizu B. Characterization of bovine papillomavirus type 1-transformed clones which show distinct transformed phenotypes. J Gen Virol. 1989 Jun;70(Pt 6):1593–1599. doi: 10.1099/0022-1317-70-6-1593. [DOI] [PubMed] [Google Scholar]
- Vaux D., Tooze J., Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990 Jun 7;345(6275):495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- Watowich S. S., Morimoto R. I. Complex regulation of heat shock- and glucose-responsive genes in human cells. Mol Cell Biol. 1988 Jan;8(1):393–405. doi: 10.1128/mcb.8.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Spalholz B. A., Rabson M. S., Howley P. M. Dissociation of transforming and trans-activation functions for bovine papillomavirus type 1. Nature. 1985 Dec 12;318(6046):575–577. doi: 10.1038/318575a0. [DOI] [PubMed] [Google Scholar]
- Young D. A., Voris B. P., Maytin E. V., Colbert R. A. Very-high-resolution two-dimensional electrophoretic separation of proteins on giant gels. Methods Enzymol. 1983;91:190–214. doi: 10.1016/s0076-6879(83)91017-0. [DOI] [PubMed] [Google Scholar]