Abstract
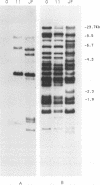
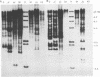
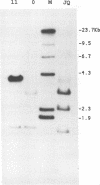
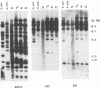
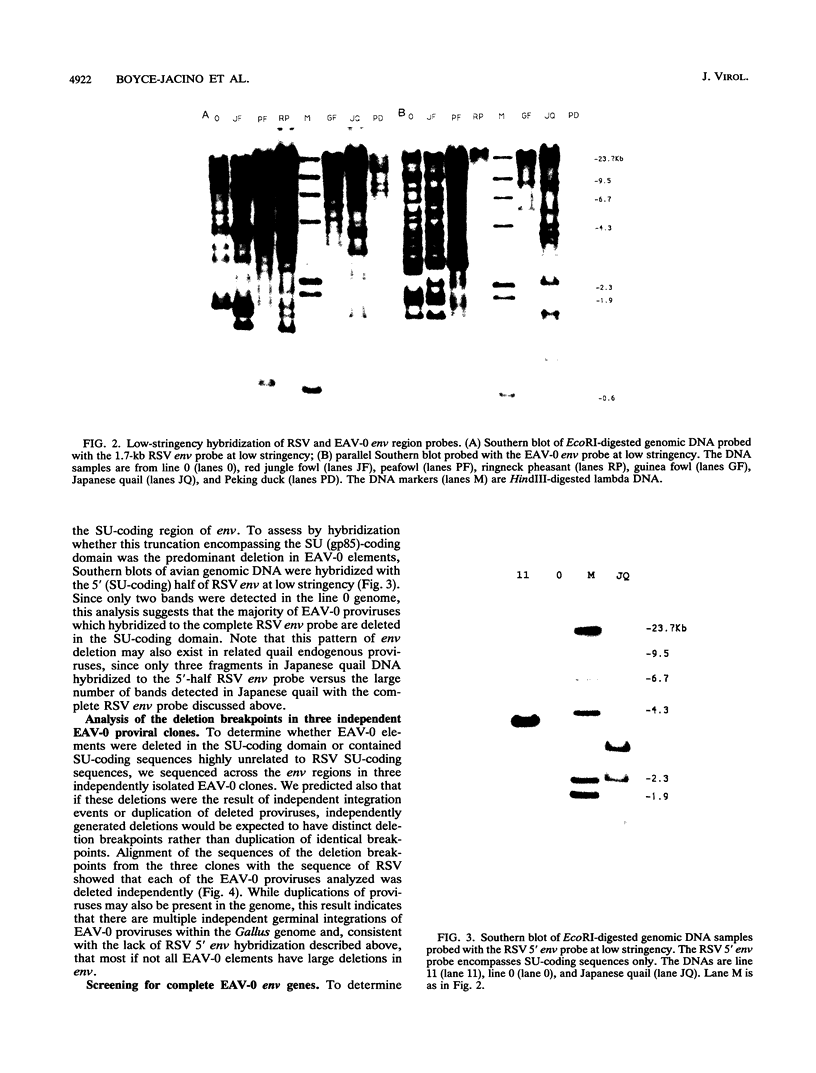
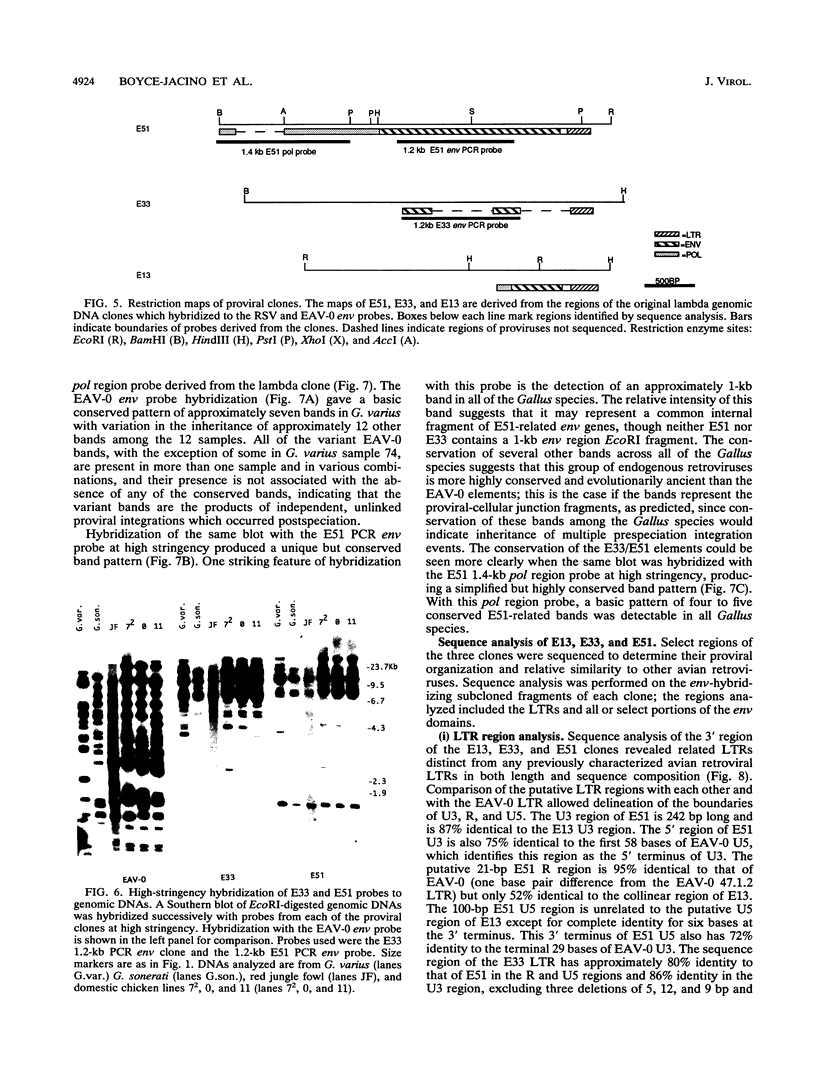
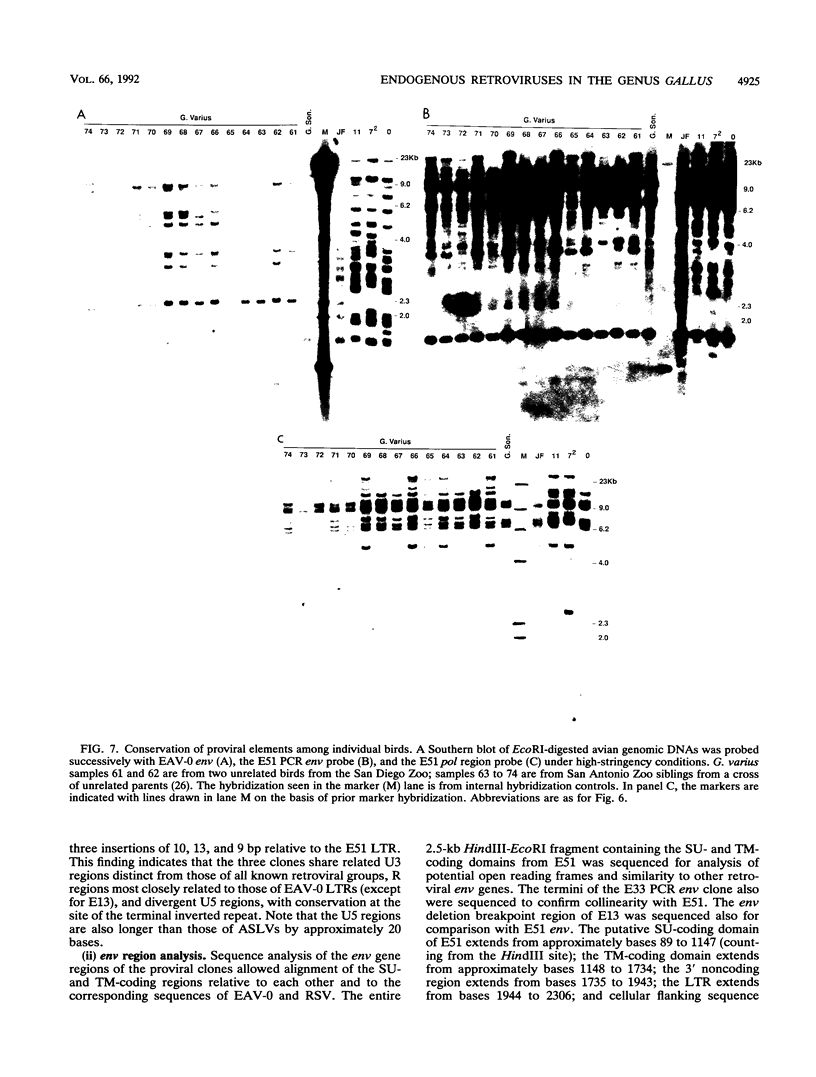
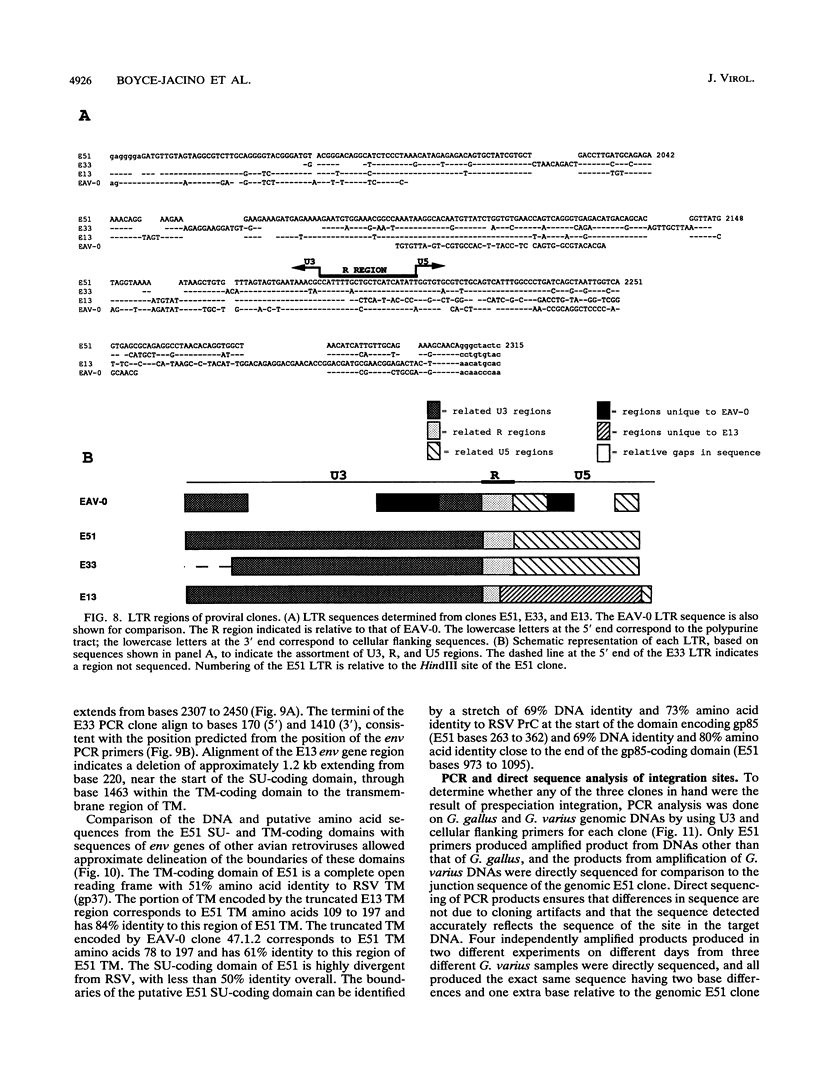
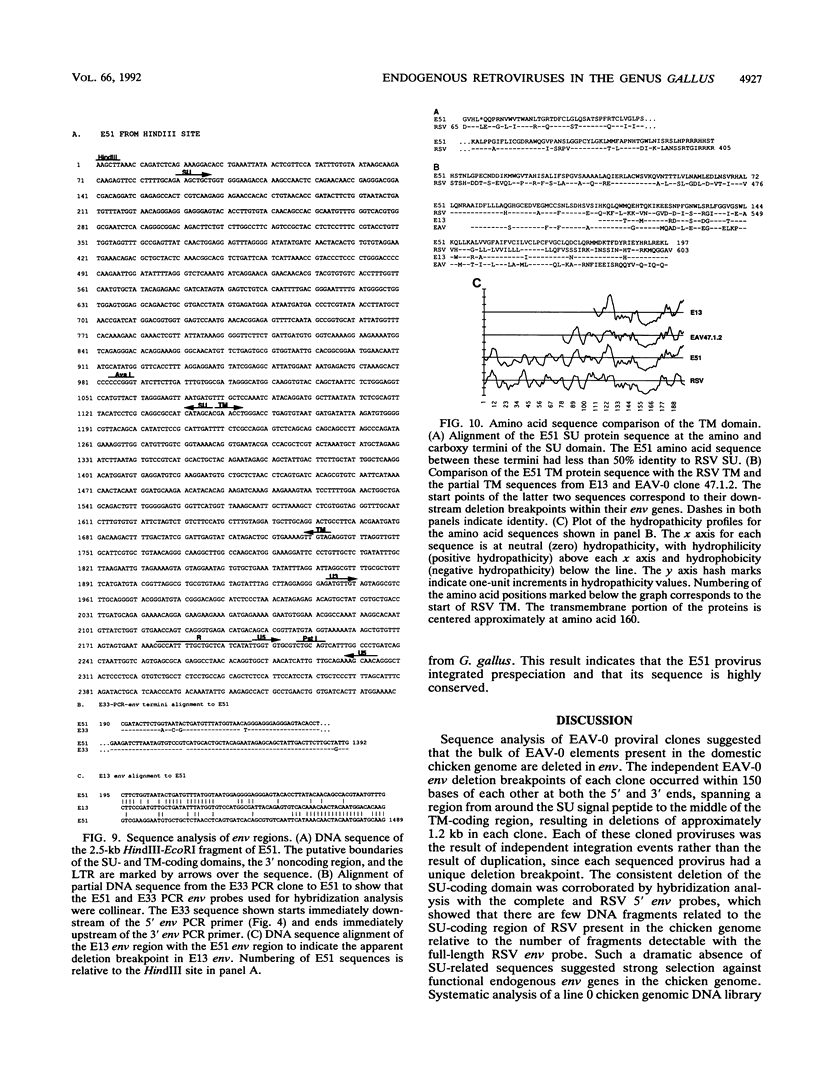
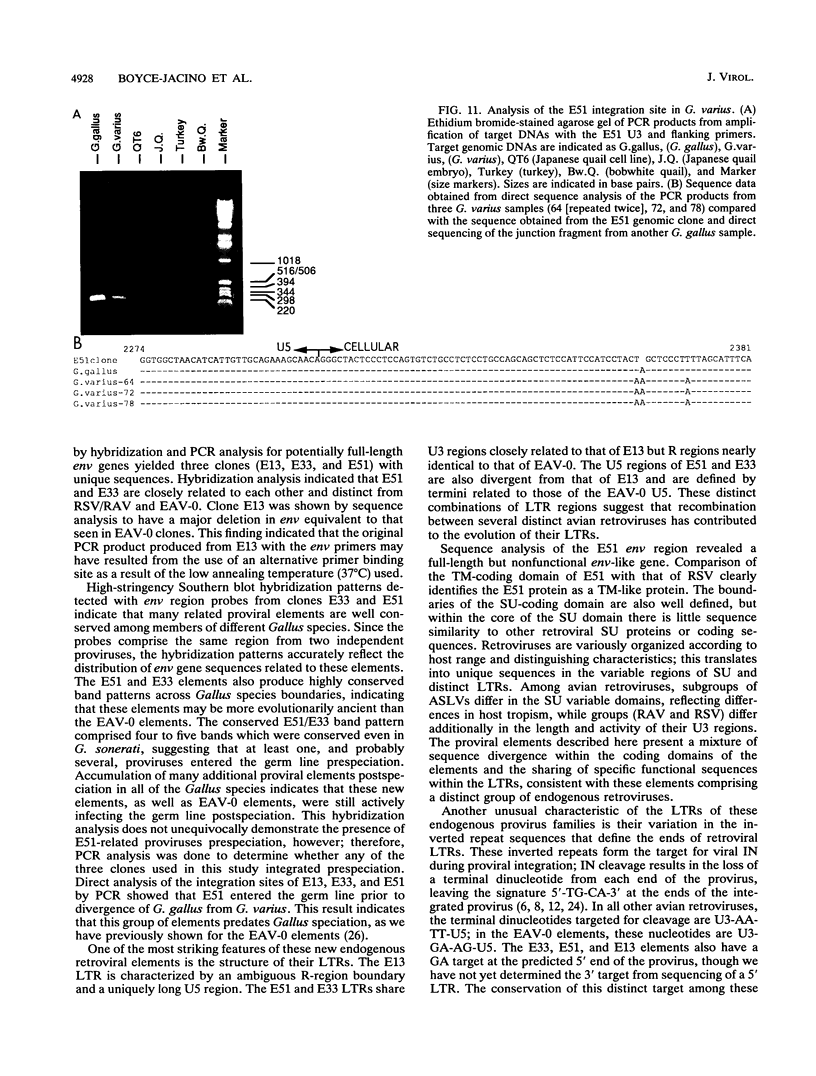
We have analyzed the genome of the domestic chicken for the presence of genetic sequences related to the envelope protein-encoding genes of avian sarcoma/leukosis retroviruses to determine the organization, structure, potential functionality, and distribution of such sequences. We have previously identified in the genus Gallus an extensive group of endogenous avian retroviruses termed EAV-0. Southern blot and sequence analysis presented here of EAV-0 elements revealed that the majority of the EAV-0 elements in the domestic chicken genome have large deletions in their env genes. Screening of a line 0 chicken genomic DNA library for potential full-length env gene-containing endogenous elements yielded three provirus clones of a previously unrecognized group of endogenous retroviruses. These three clones, E13, E33, and E51, are more closely related to each other (80% or more sequence identity) than to other avian retroviruses (70% or less sequence identity). The E13 element has a large deletion in env, but the E51 element has full-length and highly divergent SU- and TM-coding domains. Complete sequence analysis of the E51 env gene region revealed a defective SU-coding domain and an intact TM-coding domain. Sequence analysis of the E51, E33, and E13 3' termini revealed highly distinctive long terminal repeats of approximately 360 bp which appear to be the products, in part, of long terminal repeat domain shuffling. Hybridization analysis with E51 and E33 env gene probes indicated that they are members of an extensive group of elements present in all Gallus species, and at least one element, E51, could be shown by polymerase chain reaction amplification and direct sequencing to have integrated prior to Gallus speciation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Boyce-Jacino M. T., Resnick R., Faras A. J. Structural and functional characterization of the unusually short long terminal repeats and their adjacent regions of a novel endogenous avian retrovirus. Virology. 1989 Nov;173(1):157–166. doi: 10.1016/0042-6822(89)90231-6. [DOI] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Gallahan D., D'Hoostelaere L., Potter M. Novel class of mouse mammary tumor virus-related DNA sequences found in all species of Mus, including mice lacking the virus proviral genome. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4113–4117. doi: 10.1073/pnas.79.13.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Gallahan D., D'Hoostelaere L. A., Potter M. Endogenous MMTV proviral genomes in feral Mus musculus domesticus. Curr Top Microbiol Immunol. 1986;127:362–370. doi: 10.1007/978-3-642-71304-0_44. [DOI] [PubMed] [Google Scholar]
- Cobrinik D., Aiyar A., Ge Z., Katzman M., Huang H., Leis J. Overlapping retrovirus U5 sequence elements are required for efficient integration and initiation of reverse transcription. J Virol. 1991 Jul;65(7):3864–3872. doi: 10.1128/jvi.65.7.3864-3872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D., Katz R., Terry R., Skalka A. M., Leis J. Avian sarcoma and leukosis virus pol-endonuclease recognition of the tandem long terminal repeat junction: minimum site required for cleavage is also required for viral growth. J Virol. 1987 Jun;61(6):1999–2008. doi: 10.1128/jvi.61.6.1999-2008.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Craigie R., Fujiwara T., Bushman F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell. 1990 Aug 24;62(4):829–837. doi: 10.1016/0092-8674(90)90126-y. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dunwiddie C. T., Resnick R., Boyce-Jacino M., Alegre J. N., Faras A. J. Molecular cloning and characterization of gag-, pol-, and env-related gene sequences in the ev- chicken. J Virol. 1986 Sep;59(3):669–675. doi: 10.1128/jvi.59.3.669-675.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie C., Faras A. J. Presence of retrovirus reverse transcriptase-related gene sequences in avian cells lacking endogenous avian leukosis viruses. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5097–5101. doi: 10.1073/pnas.82.15.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyk G., Longiaru M., Cobrinik D., Kowal R., deHaseth P., Skalka A. M., Leis J. Circles with two tandem long terminal repeats are specifically cleaved by pol gene-associated endonuclease from avian sarcoma and leukosis viruses: nucleotide sequences required for site-specific cleavage. J Virol. 1985 Nov;56(2):589–599. doi: 10.1128/jvi.56.2.589-599.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gudkov A. V., Komarova E. A., Nikiforov M. A., Zaitsevskaya T. E. ART-CH, a new chicken retroviruslike element. J Virol. 1992 Mar;66(3):1726–1736. doi: 10.1128/jvi.66.3.1726-1736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Buckler C. E., Sears J. F., Rowe W. P., Martin M. A. Organization and stability of endogenous xenotropic murine leukemia virus proviral DNA in mouse genomes. J Virol. 1983 Jan;45(1):473–477. doi: 10.1128/jvi.45.1.473-477.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. A., Omer C. A., Weis J. H., Mitsialis S. A., Faras A. J., Guntaka R. V. Restriction endonuclease and nucleotide sequence analyses of molecularly cloned unintegrated avian tumor virus DNA: structure of large terminal repeats in circle junctions. J Virol. 1982 Apr;42(1):346–351. doi: 10.1128/jvi.42.1.346-351.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan E., Mack J. P., Katz R. A., Kulkosky J., Skalka A. M. Retroviral integrase domains: DNA binding and the recognition of LTR sequences. Nucleic Acids Res. 1991 Feb 25;19(4):851–860. doi: 10.1093/nar/19.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., O'Neill R. R. Diverse wild mouse origins of xenotropic, mink cell focus-forming, and two types of ecotropic proviral genes. J Virol. 1987 Oct;61(10):3082–3088. doi: 10.1128/jvi.61.10.3082-3088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., O'Neill R. R. Xenotropic and MCF related retroviral genes in wild mice. Curr Top Microbiol Immunol. 1986;127:349–353. doi: 10.1007/978-3-642-71304-0_42. [DOI] [PubMed] [Google Scholar]
- O'Neill R. R., Khan A. S., Hoggan M. D., Hartley J. W., Martin M. A., Repaske R. Specific hybridization probes demonstrate fewer xenotropic than mink cell focus-forming murine leukemia virus env-related sequences in DNAs from inbred laboratory mice. J Virol. 1986 May;58(2):359–366. doi: 10.1128/jvi.58.2.359-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Kitasato H., Ohishi H., Motobayashi-Nakajima Y. Molecular cloning and long terminal repeat sequences of intracisternal A-particle genes in Mus caroli. J Virol. 1984 May;50(2):352–358. doi: 10.1128/jvi.50.2.352-358.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M. Molecular biology of type A endogenous retrovirus. Kitasato Arch Exp Med. 1990 Sep;63(2-3):77–90. [PubMed] [Google Scholar]
- Panganiban A. T., Temin H. M. Circles with two tandem LTRs are precursors to integrated retrovirus DNA. Cell. 1984 Mar;36(3):673–679. doi: 10.1016/0092-8674(84)90347-7. [DOI] [PubMed] [Google Scholar]
- Resnick R. M., Boyce-Jacino M. T., Fu Q., Faras A. J. Phylogenetic distribution of the novel avian endogenous provirus family EAV-0. J Virol. 1990 Oct;64(10):4640–4653. doi: 10.1128/jvi.64.10.4640-4653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Wirth T., Kröger B., Horak I. Structure and genomic organization of a new family of murine retrovirus-related DNA sequences (MuRRS). Nucleic Acids Res. 1985 May 24;13(10):3461–3470. doi: 10.1093/nar/13.10.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]