Abstract
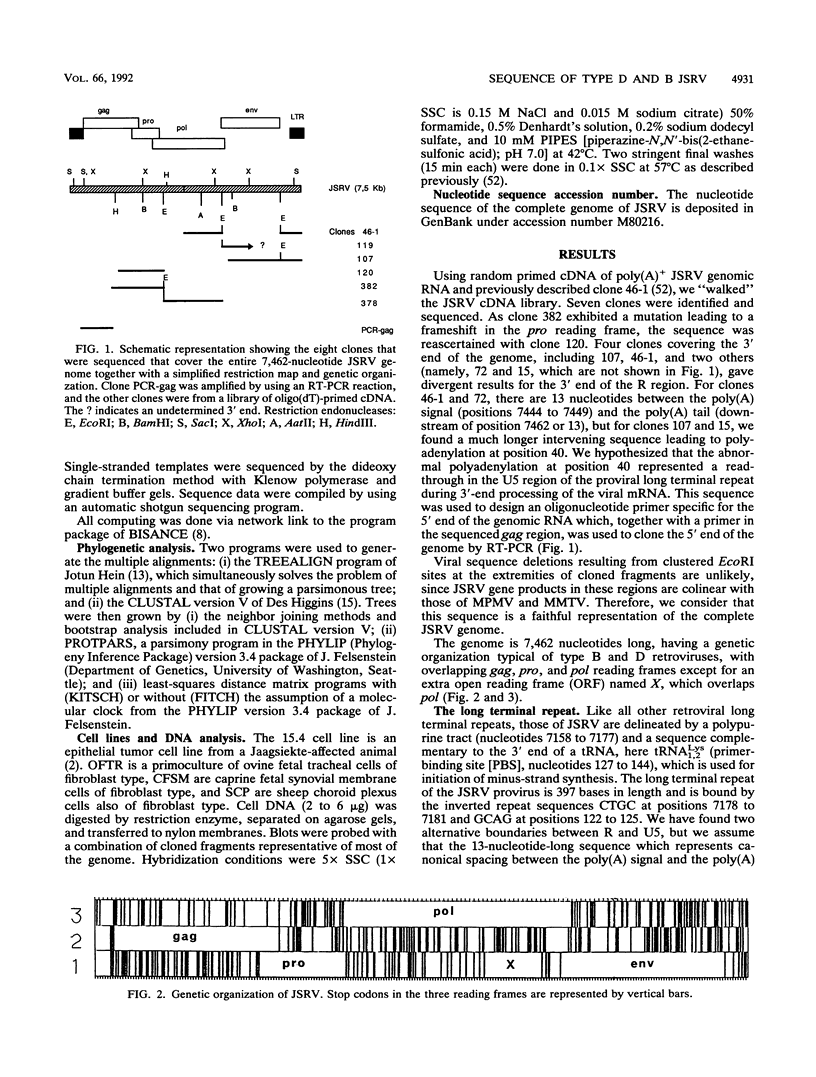
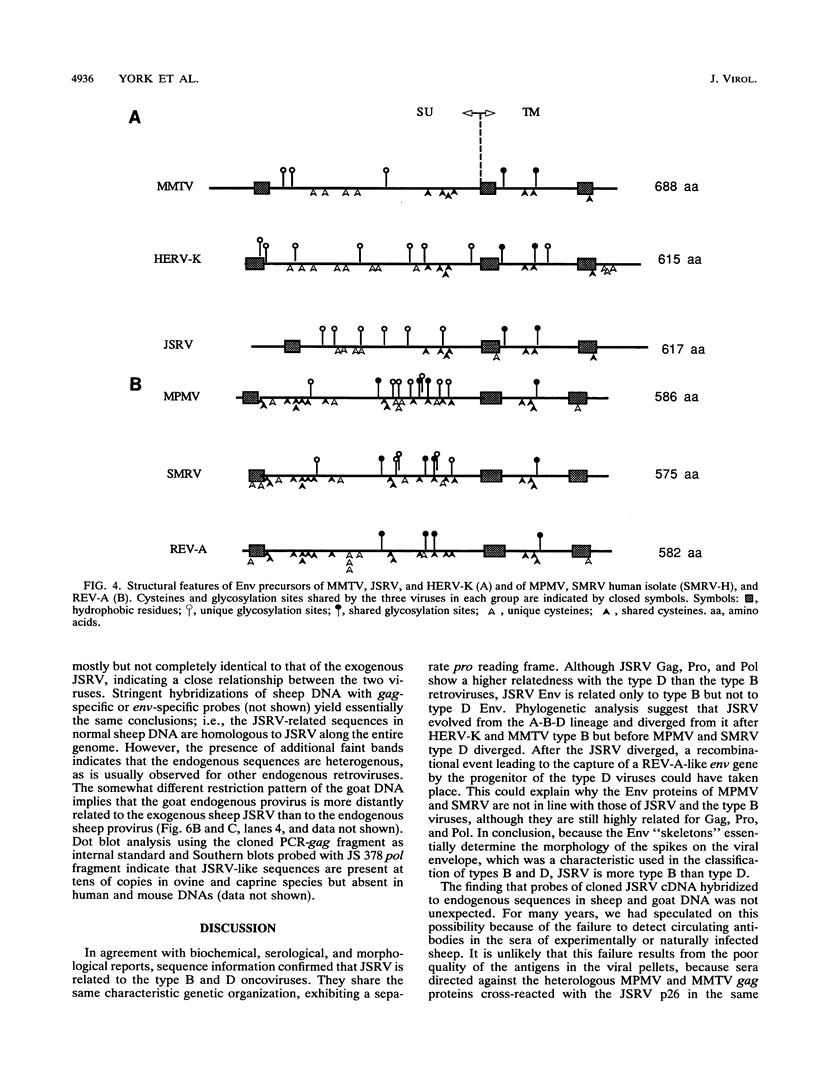
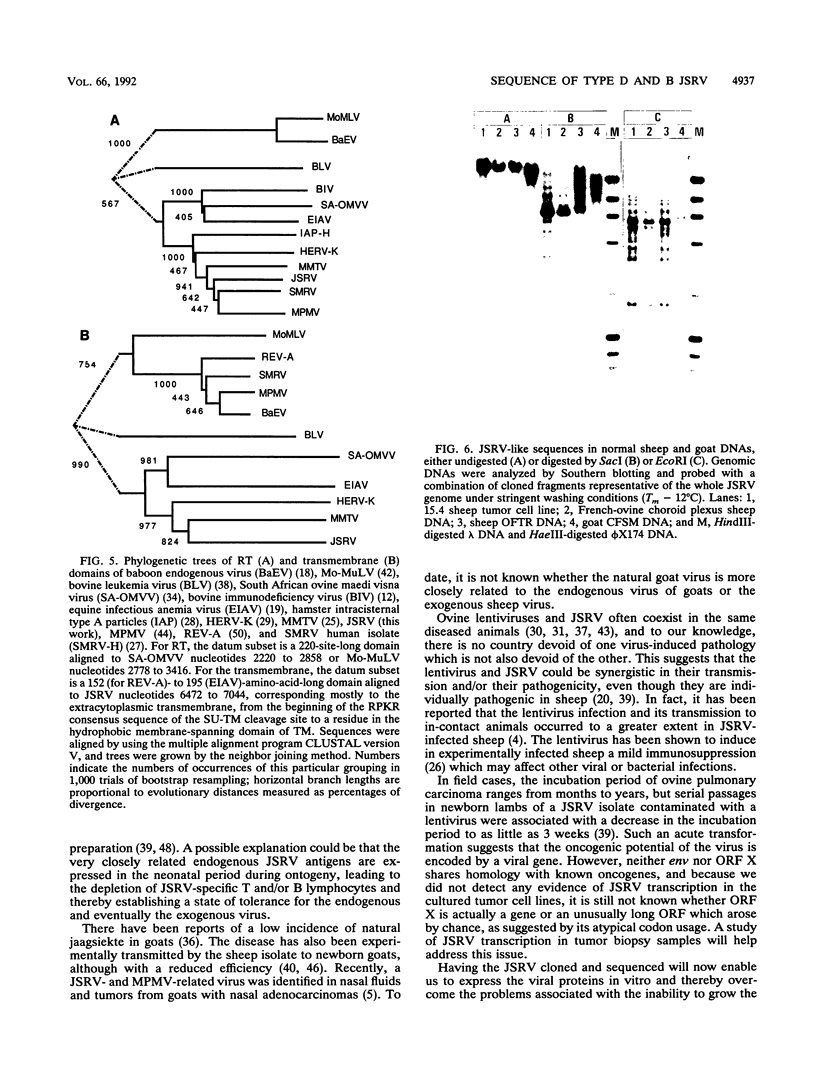
The complete genome of the jaagsiekte sheep retrovirus (JSRV), the suspected etiological agent of ovine pulmonary carcinoma, has been cloned from viral particles secreted in lung exudates of affected animals and sequenced. The genome is 7,462 nucleotides long and exhibits a genetic organization characteristic of the type B and D oncoviruses. Comparison of the amino acid sequences of JSRV proteins with those of other retrovirus proteins and phylogenetic studies suggest that JSRV diverged from its type B and D lineage after the type B mouse mammary tumor virus but before the type D oncoviruses captured the env gene of a reticuloendotheliosislike virus. Southern blot studies show that closely related sequences are present in sheep and goat normal genomic DNA, indicating that JSRV could be endogenous in ovine and caprine species.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Dawson M., Done S. H., Venables C., Jenkins C. E. Maedi-visna and sheep pulmonary adenomatosis: a study of concurrent infection. Br Vet J. 1990 Nov-Dec;146(6):531–538. doi: 10.1016/0007-1935(90)90056-9. [DOI] [PubMed] [Google Scholar]
- De las Heras M., Sharp J. M., Garcia de Jalon J. A., Dewar P. Enzootic nasal tumour of goats: demonstration of a type D-related retrovirus in nasal fluids and tumours. J Gen Virol. 1991 Oct;72(Pt 10):2533–2535. doi: 10.1099/0022-1317-72-10-2533. [DOI] [PubMed] [Google Scholar]
- DeMartini J. C., Rosadio R. H., Sharp J. M., Russell H. I., Lairmore M. D. Experimental coinduction of type D retrovirus-associated pulmonary carcinoma and lentivirus-associated lymphoid interstitial pneumonia in lambs. J Natl Cancer Inst. 1987 Jul;79(1):167–177. [PubMed] [Google Scholar]
- Demartini J. C., Rosadio R. H., Lairmore M. D. The etiology and pathogenesis of ovine pulmonary carcinoma (sheep pulmonary adenomatosis). Vet Microbiol. 1988 Jul;17(3):219–236. doi: 10.1016/0378-1135(88)90067-3. [DOI] [PubMed] [Google Scholar]
- Dessen P., Fondrat C., Valencien C., Mugnier C. BISANCE: a French service for access to biomolecular sequence databases. Comput Appl Biosci. 1990 Oct;6(4):355–356. doi: 10.1093/bioinformatics/6.4.355. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Lerner D. L., Hasselkus-Light C. S., Fontenot D. J., Hunter E., Luciw P. A., Montelaro R. C., Phillips T. R. Distinct subsets of retroviruses encode dUTPase. J Virol. 1992 Mar;66(3):1791–1794. doi: 10.1128/jvi.66.3.1791-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey K. J., Oberste M. S., Elser J. E., Braun M. J., Gonda M. A. Nucleotide sequence and genome organization of biologically active proviruses of the bovine immunodeficiency-like virus. Virology. 1990 Apr;175(2):391–409. doi: 10.1016/0042-6822(90)90424-p. [DOI] [PubMed] [Google Scholar]
- Hein J. Unified approach to alignment and phylogenies. Methods Enzymol. 1990;183:626–645. doi: 10.1016/0076-6879(90)83041-7. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Smythers G., Benveniste R. E., Oroszlan S. Purification and N-terminal amino acid sequence comparisons of structural proteins from retrovirus-D/Washington and Mason-Pfizer monkey virus. J Virol. 1985 Sep;55(3):778–787. doi: 10.1128/jvi.55.3.778-787.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hizi A., Henderson L. E., Copeland T. D., Sowder R. C., Krutzsch H. C., Oroszlan S. Analysis of gag proteins from mouse mammary tumor virus. J Virol. 1989 Jun;63(6):2543–2549. doi: 10.1128/jvi.63.6.2543-2549.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Townsley K., Varmus H. E., Majors J. Two efficient ribosomal frameshifting events are required for synthesis of mouse mammary tumor virus gag-related polyproteins. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4298–4302. doi: 10.1073/pnas.84.12.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T., Sherman L., Dahlberg J., Gazit A., Yaniv A., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of equine infectious anemia virus proviral DNA. Virology. 1987 Jun;158(2):300–312. doi: 10.1016/0042-6822(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Lairmore M. D., Rosadio R. H., DeMartini J. C. Ovine lentivirus lymphoid interstitial pneumonia. Rapid induction in neonatal lambs. Am J Pathol. 1986 Oct;125(1):173–181. [PMC free article] [PubMed] [Google Scholar]
- Lennarz W. J. Lipid linked sugars in glycoprotein synthesis. Science. 1975 Jun 6;188(4192):986–991. doi: 10.1126/science.167438. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Johnson M. S., Doolittle R. F. Relocation of a protease-like gene segment between two retroviruses. Proc Natl Acad Sci U S A. 1987 May;84(9):2693–2697. doi: 10.1073/pnas.84.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. A., Johnson M. S., Feng D. F., Doolittle R. F. Sequence comparisons of retroviral proteins: relative rates of change and general phylogeny. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2469–2473. doi: 10.1073/pnas.85.8.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J. Protein sequence comparisons show that the 'pseudoproteases' encoded by poxviruses and certain retroviruses belong to the deoxyuridine triphosphatase family. Nucleic Acids Res. 1990 Jul 25;18(14):4105–4110. doi: 10.1093/nar/18.14.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer M. S., Huchzermeyer H. F., York D. F., Hunter P., Verwoerd D. W., Garnett H. M. The possible involvement of immunosuppression caused by a lentivirus in the aetiology of jaagsiekte and pasteurellosis in sheep. Onderstepoort J Vet Res. 1988 Sep;55(3):127–133. [PubMed] [Google Scholar]
- Oda T., Ikeda S., Watanabe S., Hatsushika M., Akiyama K., Mitsunobu F. Molecular cloning, complete nucleotide sequence, and gene structure of the provirus genome of a retrovirus produced in a human lymphoblastoid cell line. Virology. 1988 Dec;167(2):468–476. [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Yasunaga T., Miyata T., Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 1986 Nov;60(2):589–598. doi: 10.1128/jvi.60.2.589-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne A. L., Verwoerd D. W., Garnett H. M. The morphology and morphogenesis of jaagsiekte retrovirus (JSRV). Onderstepoort J Vet Res. 1983 Dec;50(4):317–322. [PubMed] [Google Scholar]
- Payne A., York D. F., de Villiers E. M., Verwoerd D. W., Quérat G., Barban V., Sauze N., Vigne R. Isolation and identification of a South African lentivirus from jaagsiekte lungs. Onderstepoort J Vet Res. 1986 Mar;53(1):55–62. [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Querat G., Audoly G., Sonigo P., Vigne R. Nucleotide sequence analysis of SA-OMVV, a visna-related ovine lentivirus: phylogenetic history of lentiviruses. Virology. 1990 Apr;175(2):434–447. doi: 10.1016/0042-6822(90)90428-t. [DOI] [PubMed] [Google Scholar]
- Quérat G., Barban V., Sauze N., Vigne R., Payne A., York D., De Villiers E. M., Verwoerd D. W. Characteristics of a novel lentivirus derived from South African sheep with pulmonary adenocarcinoma (jaagsiekte). Virology. 1987 May;158(1):158–167. doi: 10.1016/0042-6822(87)90249-2. [DOI] [PubMed] [Google Scholar]
- Rosadio R. H., Sharp J. M., Lairmore M. D., Dahlberg J. E., De Martini J. C. Lesions and retroviruses associated with naturally occurring ovine pulmonary carcinoma (sheep pulmonary adenomatosis). Vet Pathol. 1988 Jan;25(1):58–66. doi: 10.1177/030098588802500108. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. M., Angus K. W., Gray E. W., Scott F. M. Rapid transmission of sheep pulmonary adenomatosis (jaagsiekte) in young lambs. Brief report. Arch Virol. 1983;78(1-2):89–95. doi: 10.1007/BF01310861. [DOI] [PubMed] [Google Scholar]
- Sharp J. M., Angus K. W., Jassim F. A., Scott F. M. Experimental transmission of sheep pulmonary adenomatosis to a goat. Vet Rec. 1986 Sep 6;119(10):245–245. doi: 10.1136/vr.119.10.245. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Snyder S. P., DeMartini J. C., Ameghino E., Caletti E. Coexistence of pulmonary adenomatosis and progressive pneumonia in sheep in the central sierra of Peru. Am J Vet Res. 1983 Jul;44(7):1334–1338. [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Tustin R. C., Williamson A. L., York D. F., Verwoerd D. W. Experimental transmission of jaagsiekte (ovine pulmonary adenomatosis) to goats. Onderstepoort J Vet Res. 1988 Mar;55(1):27–32. [PubMed] [Google Scholar]
- Verwoerd D. W., Payne A. L., York D. F., Myer M. S. Isolation and preliminary characterization of the jaagsiekte retrovirus (JSRV). Onderstepoort J Vet Res. 1983 Dec;50(4):309–316. [PubMed] [Google Scholar]
- Wandera J. G. Sheep pulmonary adenomatosis (Jaagsiekte). Adv Vet Sci Comp Med. 1971;15:251–283. [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990 Oct;9(10):3353–3362. doi: 10.1002/j.1460-2075.1990.tb07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York D. F., Vigne R., Verwoerd D. W., Querat G. Isolation, identification, and partial cDNA cloning of genomic RNA of jaagsiekte retrovirus, the etiological agent of sheep pulmonary adenomatosis. J Virol. 1991 Sep;65(9):5061–5067. doi: 10.1128/jvi.65.9.5061-5067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]