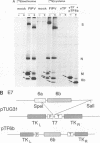
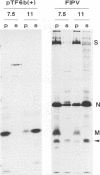
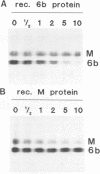
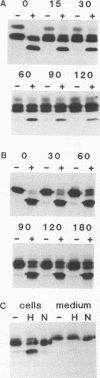
Abstract
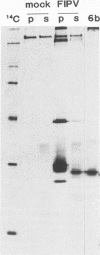
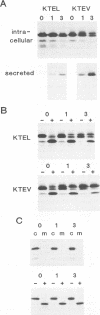
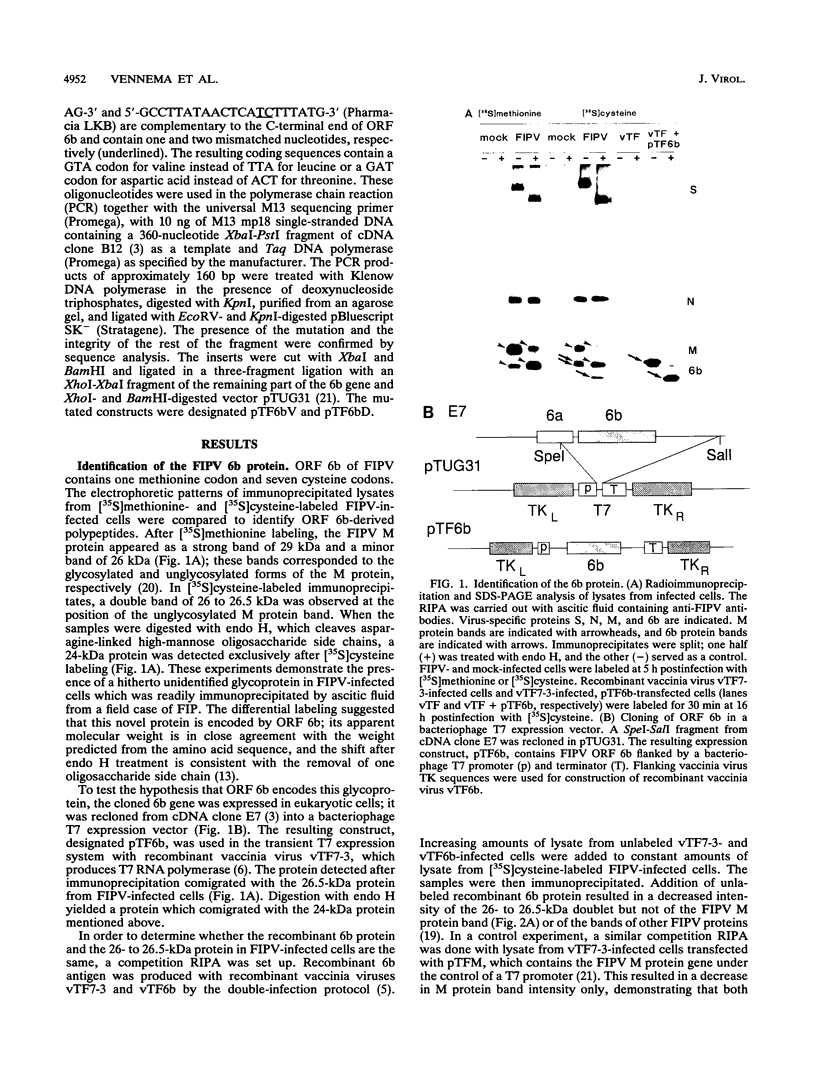
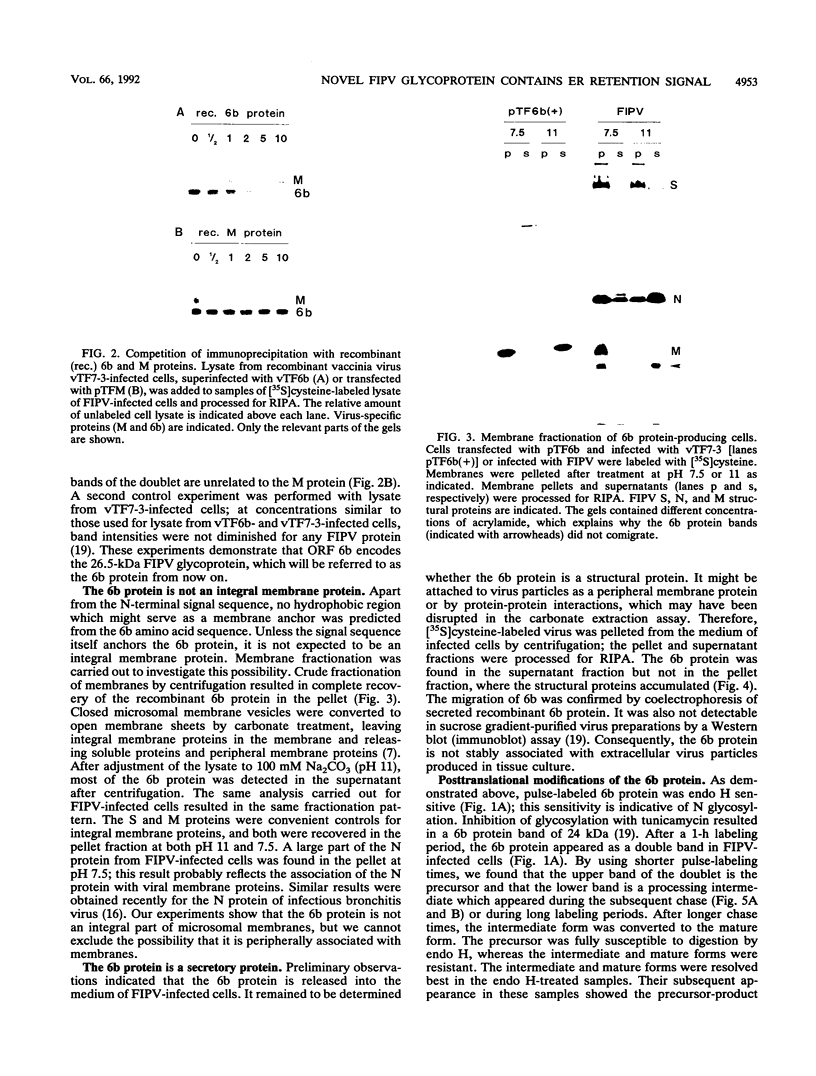
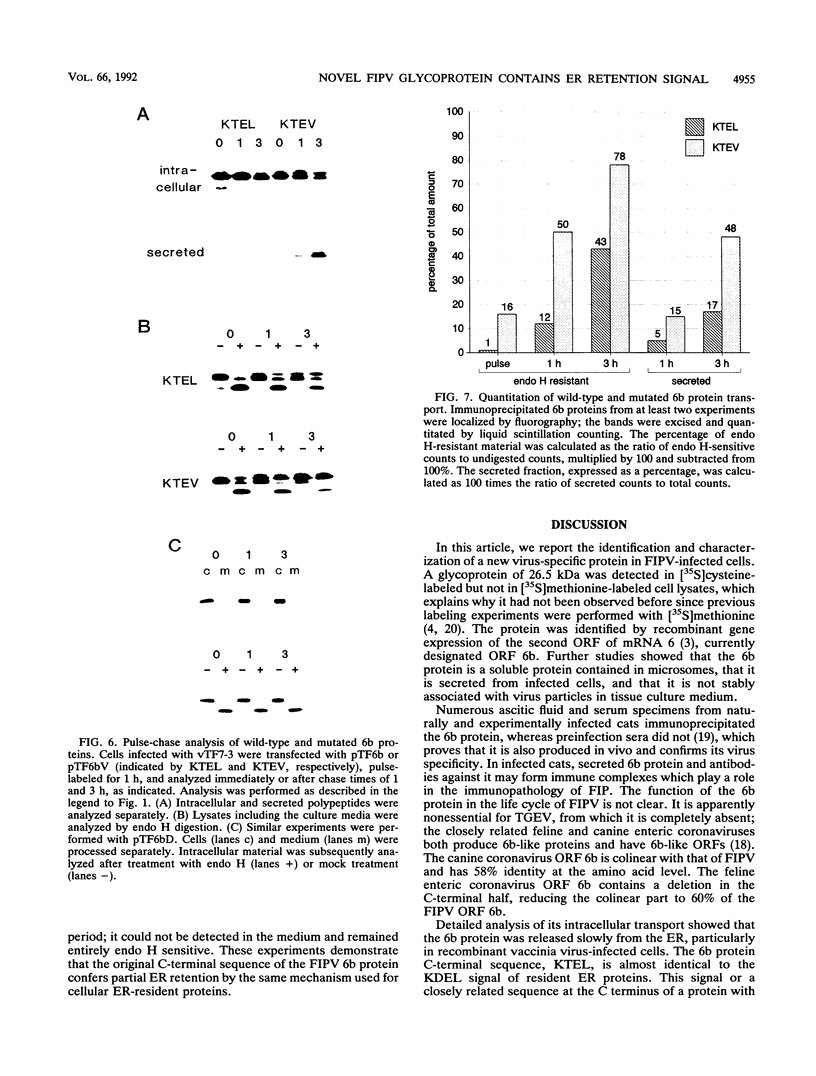
A new protein of feline infectious peritonitis coronavirus (FIPV) was discovered in lysates of [35S]cysteine-labeled infected cells. Expression of open reading frame (ORF) 6b of FIPV in recombinant vaccinia virus-infected cells was used to identify it as the 6b protein. Further characterization revealed that it is a novel type of viral glycoprotein whose function is not clear. It is a soluble protein contained in microsomes; its slow export from the cell is caused by the presence of an endoplasmic reticulum (ER) retention signal at the C terminus. This amino acid sequence, KTEL, closely resembles the consensus KDEL signal of soluble resident ER proteins. A mutant 6b protein with the C-terminal sequence KTEV became resistant to digestion by endo-beta-N-acetylglucosaminidase H with a half-time that was reduced threefold. In contrast, a mutant with the sequence KDEL was completely retained in the ER. The FIPV 6b protein is the first example of a viral protein with a functional KDEL-like ER retention signal.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres D. A., Rhodes J. D., Meisel R. L., Dixon J. E. Characterization of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J Biol Chem. 1991 Aug 5;266(22):14277–14282. [PubMed] [Google Scholar]
- Cavanagh D., Brian D. A., Enjuanes L., Holmes K. V., Lai M. M., Laude H., Siddell S. G., Spaan W., Taguchi F., Talbot P. J. Recommendations of the Coronavirus Study Group for the nomenclature of the structural proteins, mRNAs, and genes of coronaviruses. Virology. 1990 May;176(1):306–307. doi: 10.1016/0042-6822(90)90259-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot R. J., Andeweg A. C., Horzinek M. C., Spaan W. J. Sequence analysis of the 3'-end of the feline coronavirus FIPV 79-1146 genome: comparison with the genome of porcine coronavirus TGEV reveals large insertions. Virology. 1988 Dec;167(2):370–376. doi: 10.1016/0042-6822(88)90097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwes D. J., Stewart F., Britton P. The polypeptide of Mr 14,000 of porcine transmissible gastroenteritis virus: gene assignment and intracellular location. J Gen Virol. 1989 Sep;70(Pt 9):2495–2499. doi: 10.1099/0022-1317-70-9-2495. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990 Dec;15(12):483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Smith A. R., Boursnell M. E., Binns M. M., Brown T. D., Inglis S. C. Identification of a new membrane-associated polypeptide specified by the coronavirus infectious bronchitis virus. J Gen Virol. 1990 Jan;71(Pt 1):3–11. doi: 10.1099/0022-1317-71-1-3. [DOI] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Heijnen L., Zijderveld A., Horzinek M. C., Spaan W. J. Intracellular transport of recombinant coronavirus spike proteins: implications for virus assembly. J Virol. 1990 Jan;64(1):339–346. doi: 10.1128/jvi.64.1.339-346.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Rijnbrand R., Heijnen L., Horzinek M. C., Spaan W. J. Enhancement of the vaccinia virus/phage T7 RNA polymerase expression system using encephalomyocarditis virus 5'-untranslated region sequences. Gene. 1991 Dec 15;108(2):201–209. doi: 10.1016/0378-1119(91)90435-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagouras P., Rose J. K. Carboxy-terminal SEKDEL sequences retard but do not retain two secretory proteins in the endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2633–2640. doi: 10.1083/jcb.109.6.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., ter Haar R. J., Horzinek M. C., van der Zeijst B. A. Intracellular RNAs of the feline infectious peritonitis coronavirus strain 79-1146. J Gen Virol. 1987 Apr;68(Pt 4):995–1002. doi: 10.1099/0022-1317-68-4-995. [DOI] [PubMed] [Google Scholar]