Abstract
Previous experiments demonstrated that second-based transient increases in choline concentrations measured by electrodes coated with choline oxidase (ChOx) and the amperometric detection of hydrogen peroxide validly indicate the depolarization-dependent release of acetylcholine (ACh) and its hydrolysis by endogenous acetylcholinesterase (AChE). Therefore, choline-sensitive microelectrodes have become valuable tools in neuropharmacological and behavioral research. The present experiments were designed to test the possibility that co-immobilization of ChOx plus AChE on recording sites increases the level of detection for evoked ACh release in the brain. If newly released ACh is not completely hydrolyzed by endogenous AChE and capable of reaching the extracellular space, currents recorded via sites equipped with both enzymes should be greater when compared with sites coated with ChOx only. Pairs of Platinum-recordings sites were coated either with AChE plus ChOx or ChOx alone. Potassium or nicotine-evoked currents were recorded throughout the entire dorsal-ventral extent of the medial prefrontal cortex (mPFC). The amplitudes of evoked cholinergic signals did not differ significantly between AChE+ChOx and ChOx-only coated recording sites. Additional experiments controlling for several potential confounds suggested that, in vivo, ACh levels ≥150 fmol were detected by recordings sites featuring dual enzyme coating. Collectively, these results indicate that co-coating of microelectrodes with AChE does not enhance the detection of cholinergic activity in the cortex compared with measurements via recording sites coated only with ChOx.
Keywords: Acetylcholine, Enzyme-selective microelectrodes, Acetylcholinesterase, Choline, Amperometry
1. Introduction
The most rostral component of the brain's cholinergic system consists of neurons that originate in the basal forebrain and project to the entire cortical mantle. A substantial amount of evidence supports the hypothesis that the basal forebrain-cortical cholinergic projection system mediates fundamental aspects of attentional information processing (Everitt and Robbins, 1997; Yu and Dayan, 2002; Hasselmo and McGaughy, 2004; Parikh et al., 2007; Sarter et al., 2001, 2005, 2006).
Choline-selective microelectrodes for the amperometric measurement of acetylcholine (ACh) release allow monitoring ACh release at a subsecond resolution (Burmeister et al., 2000, 2003; Parikh et al., 2004, 2006; Bruno et al., 2006; Parikh and Sarter, 2006) and therefore have yielded new insights in the regulation and function of cortical ACh release in task-performing animals (Parikh et al., 2007). In these studies, Platinum-recording sites lithographed onto ceramic-based microlectrodes were coated with choline oxidase (ChOx) in order to measure increases in choline resulting from hydrolysis of newly released ACh by endogenous acetylcholinesterase (AChE). On the recording site, oxidation of choline produces hydrogen peroxide that is detected amperometrically. Previous studies (references above) demonstrated that blockade of voltage-regulated sodium channels with tetrodotoxin or inhibition of endogenous AChE with an AChE-inhibitor attenuated evoked cholinergic signals recorded via ChOx-coated sites, confirming the validity of the methods. Likewise, the selectivity of this method was demonstrated in these prior studies; collectively selectivity is a combined result of coating the recording sites with a polymer to prevent other electroactive analytes from reaching the Pt-surface, the application of a voltage that optimally oxidizes peroxide, in vitro calibration against choline as well as potential interferents (such as ascorbic acid and dopamine), and self-referencing against recording sites not coated with enzyme.
The possibility that recording sites coated with AChE+ChOx detect lower levels of ACh release has gained interest (Schuvailo et al., 2005; Bruno et al., 2006). Although the possibility that ACh can escape hydrolysis by endogenous AChE and reaches the extracellular space (volume transmission; Fuxe et al., 2005; Agnati et al., 2006) has remained a subject of debate (Umbriaco et al., 1994; Descarries et al., 1997; Descarries and Mechawar, 2000; Mechawar et al., 2000, 2002; Mrzljak et al., 1993; Smiley et al., 1997; Turrini et al., 2001), the recovery of ACh by in vivo microdialysis traditionally has been considered evidence for the presence of ACh in the extracellular space. Extracellular ACh would be hydrolyzed on recording sites coated with AChE, thereby increasing local choline concentrations and therefore resulting in greater levels of peroxide when compared with recording sites coated with ChOx alone. However, given the exceptional catalytic power of AChE (one molecule of AChE can hydrolyze 5000 molecules of ACh per second; Cooper et al., 2003), spillover of ACh into the extracellular space may be extremely limited (see also Massoulie et al., 1996; Zimmerman and Soreq, 2006). The present experiments were designed to test the hypothesis that in response to a robust depolarization stimulus (KCl; Herzog et al., 2003), as well as to a less potent and more physiological stimulus (nicotine), greater currents are recorded via AChE+ChOx-coated recording sites when compared with sites coated only with ChOx. The results do not support this hypothesis and have implications concerning the concentrations of ACh that may be present in the extracellular space following a massive depolarization event such as caused by KCl.
2. Methods
2.1. Animals
Adult male Fischer-344/Brown-Norway F1 hybrid rats (FBNF1; Harlan, Indianapolis, IN, USA; N=122), weighing 250-300 g at the beginning of the experiments were used. Animals were treated in accordance with protocols approved by the University Committee on the Use and Care of Animals (UCUCA) of the University of Michigan and housed in facilities accredited by the American Association of Accreditation of Laboratory Animal Care (AAALAC). Food and water were made available ad libitum (Rodent Chow; Harlan Teklad, Madison, WI, USA).
2.2. Preparation and in vitro calibration of enzyme-selective microelectrodes
Ceramic-based, multi-site microelectrodes featuring four rectangular Platinum (Pt)-recording sites arranged in side-by-side pairs (Quanteon LLC, Nicholasville, KY; Figure 1) were coated with acetylcholinesterase (AChE) and/or choline oxidase (ChOx; Sigma Chemical Co., St. Louis, MO) as described earlier (Bruno et al., 2006; Parikh et al., 2004, 2006, 2007; Parikh and Sarter, 2006). Individual recording sites were 15×333 μm, the upper and lower pairs were 100 μm apart, individual sites per pair were separated horizontally by 30 μm and the ceramic base was 100 μm thick. The width of the ceramic base was 150 μm measured across the center of the lower pair and 200 μm measured across the center of the upper pair.
Figure 1.
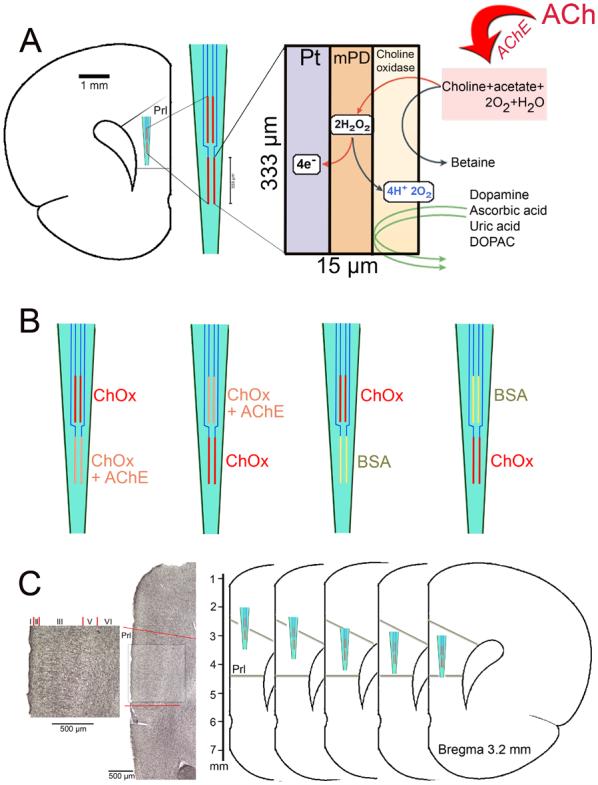
(A) Schematic illustration depicting the main features, measurement scheme and dimensions of choline-sensitive microelectrode recording sites. Choline is generated by hydrolysis of newly released acetylcholine (ACh) by acetylcholinesterase (AChE). Choline is oxidized by choline oxidase (ChOx) immobilized onto the recording site. The resulting peroxide is detected amperometrically on Pt sites. m-PD electropolymerization serves to repel electroactive interferents including DA, AA, uric acid and DOPAC from the surface of the platinum recording sites. The left insert illustrates the placement and the relative dimensions of the four recording sites when placed into the medial prefrontal cortex (mPFC). (B) Schematic illustration of the enzyme coating combinations used in the present experiments. Recording sites were coated either with AChE+ChOx or with ChOx only. The position of the coating type on the electrode (upper versus lower pair) was systematically varied. For control experiments, recording sites on individual electrodes were coated with ChOx and BSA, respectively. (C) On the left, a coronal section stained for the visualization of AChE-positive fibers is shown. The prelimbic cortex (Prl) is indicated and shown at higher magnification (scales are 500 μm). The greater density of AChE-positive fibers in layer III is apparent. As shown on the schematic drawings on the right, KCl-evoked cholinergic signals were measured in layer III of the Prl in five places, spanning the dorsal-ventral extension of this region.
Briefly, AChE/ChOx was cross-linked with a BSA-glutaraldehyde mixture and immobilized either onto the bottom or upper pair of recording sites. The remaining two recording sites were coated only with the ChOx-BSA-glutaraldehyde solution (Parikh et al., 2004). Control experiments were conducted using electrodes with recording sites coated with ChOx and BSA in order to determine potential placement-dependent variations in background current (see also Parikh et al., 2004; Parikh and Sarter, 2006).
Enzyme-coated microelectrodes were air-dried for 48-72 hrs prior to use. Afterwards, meta-Phenylenediamine (m-PD; Fluka Biochemika, Buchs, Switzerland) was electropolymerized onto the microelectrodes to prevent access of potential electroactive interferents, including ascorbic acid (AA) and catecholamines, to the Pt recording sites. m-PD electroplating was carried out by applying a constant voltage of +0.5 V to the microelectrode against a Ag/AgCl reference electrode (Bioanalytical Systems, Inc., West Lafayette, IN) in a beaker containing a solution of 5mM m-PD and 10 μM AA (in 0.05 M phosphate-buffered saline, PBS), bubbled with nitrogen gas and maintained at 37°C for 60 min. The m-PD plated, enzyme-coated microelectrodes were soaked in 0.05M PBS for 30 min prior to in vitro calibration. Calibrations were performed using fixed potential amperometry by applying a constant voltage of +0.7 V versus Ag/AgCl reference electrode in a beaker containing a stirred solution of 0.05M PBS (40 mL) maintained at 37°C using a FAST-16 electrochemical system (LLC; Quanteon, Nicholasville, KY, USA). Amperometric currents were digitized at a frequency of 5 Hz. After achieving a stable baseline current, aliquots of stock solutions of AA (20 mM), choline (20 mM) and dopamine (DA; 2 mM) were added to the calibration beaker such that the final concentrations were 250 μM AA, 20, 40, 60, and 80 μM choline and 2 μM DA.
To determine the efficacy of AChE+ChOx-coated recording sites for the measurement of choline generated by immobilized AChE-induced hydrolysis of ACh, an additional in vitro calibration was carried out. This calibration was similar to the former except that four aliquots of ACh (20 mM) solution, in place of choline, were added to achieve a concentration of 20-80 μM of ACh in the calibration beaker. The slope (sensitivity), limit of detection (LOD) and linearity (R2) for choline/ACh, as well as selectivity ratio for AA, were calculated for individual recording sites. To be used in subsequent in vivo experiments, electrodes were required to meet the following characteristics: sensitivity for detecting choline on ChOx- or ChOx+AChE-coated channels and ACh on AChE+ChOx-coated sites was >3 pA/μM, with a background current of <200 pA on all recording sites; LOD < 300 nM choline/ACh; ratio of selectivity for choline/ACh to AA, >80:1; detection of increasing choline concentrations (20-80 μM) on all Pt recording sites and ACh on AChE+ChOx-coated channels, R2 >0.98; negligible changes in current on all recording channels after DA addition (<3 pA); and the slopes of current produced by increasing choline concentrations measured via ChOx-coated channels deviated from slopes measured via ChOx+AChE-coated channels by <30%. Immersion of ChOx- and ChOx+AChE-coated channels in KCl (70 mM) and nicotine 100 (mM) for 20 min did not affect the sensitivity of the recording sites to choline or ACh, respectively. Likewise, addition of 100 μL of KCl (70 mM) and nicotine (100 mM) to the calibration bath did not increase current.
2.3. In vivo recordings
Animals were anesthetized with urethane (1.25-1.5 g/kg; i.p.) and placed in a stereotaxic frame (David Kopf Instruments, Model # 962, Tujunga, CA, USA). The body temperature of animals was maintained at 37°C using isothermal deltaphase pad (Braintree Scientific, Braintree, MA). Single-barrel glass capillaries (1.0 mm × 0.58 mm, 6 in., A-M systems, Everett, WA, USA) were pulled using a micropipette puller (Model # 51210, Stoelting, Wood Dale, IL) and then bumped against a glass slide to attain an inner tip diameter of ∼15 μm. The micropipette attached to the ceramic platform of the calibrated CO-coated microelectrode using sticky wax (Kerr, Romulus, MI, USA) such that the tip of the micropipette was placed between the lower and upper pair of recording sites. The spacing between the tip of the micropipette and the microelectrode was maintained at 70-100 μm. An Ag/AgCl reference electrode prepared from a miniature silver wire (200 μm diameter) was implanted at a site remote from the recording area. The micropipette was loaded with KCl or nicotine (concentrations below) prior to microelectrode implantation. The microelectrode/ micropipette assembly was slowly lowered into the medial prefrontal cortex (mPFC; AP, +3.2 mm; ML, −0.7 mm; DV, −2.3 mm, measured from Bregma) using a microdrive (MO-10; Narishige International, East Meadow, NY, USA; see Figure 1A for an illustration of the microelectrode placement in the mPFC).
A fixed potential of +0.7 V was applied to the microelectrode and amperometric recordings were collected at 1 Hz and digitized using the FAST-16 system. Baseline currents were allowed to stabilize for 60 min following microelectrode implantation. KCl (70 mM) was ejected from the micropipettes using PTFE tubing connected to a pressure ejection system (Picospritzer III, Parker Hannifin, Fairfield, NJ, USA), at 2-10 psi (200 nL/ejection). The volume of locally applied solutions was monitored using a stereomicroscope (Meiji Techno America, San Jose, CA, USA) fitted with a reticule (Friedemann and Gerhardt, 1992). The electrode/pipette assembly was lowered by 200 μm after each series of pressure ejection of KCl (see Experimental Design), for a total of 5 measures spanning the dorsal-ventral extension of the prelimbic region (2.3 - 3.1 mm, ventral from dura; Figure 1).
2.4. KCl-evoked signals
These experiments were designed to determine whether potassium-evoked signals recorded via AChE+ChOx-coated recording sites were larger than those recorded via ChOx-coated recording sites. A secondary goal of these experiments was to determine variations in cholinergic responses within a cortical region; such a finding would indicate the presence of functionally variable densities of cholinergic inputs and/or AChE. As a corollary, these experiments were also designed to evaluate the significance of AChE−ChOx coatings relative to ChOx-coatings. Therefore, AChE+ChOx were immobilized on the bottom pair of the recording sites and ChOx on the upper pair, and vice versa (n=3 per arrangement). Electrode/pipette assemblies were lowered by 200 μm after each series of KCl pressure ejections, for a total of 5 measures spanning the dorsal-ventral (D-V) extension of the prelimbic region (Figure 1). The effects of three KCl ejections were recorded per D-V point. Following each electrode movement, recordings were delayed by 6 min in order to allow for tissue stabilization.
A separate series of control experiments (n=6) was conducted using microelectrodes coated with ChOx on the lower pair and BSA on the upper pair of recording sites or vice versa (n=3 for each arrangement) to determine the possibility that background currents differ across placements and that potential placement-associated differences in cholinergic signals were confounded by variable background currents. Finally, a control experiment was conducted in order to exclude the possibility that endogenous AChE accumulated at ChOx-coated recording sites, thereby inadvertently transforming these sites into AChE+ChOx sites and thus attenuating the potential for demonstrating differential responses based on the two coating types (Xin and Wightman 1997). For this purpose, we implanted electrodes equipped with ChOx- and BSA-coated recording sites into the mPFC for 1 or 4 hours (n=4 per time point). Thereafter, electrodes were removed from the brain and calibrated in vitro to determine their sensitivity to ACh. If endogenous AChE accumulated on these recordings sites, exposure to ACh should elicit current.
2.5. Nicotine-evoked signals
An additional series of experiments (n=4) was conducted to determine cholinergic signals that were evoked by nicotine (Sigma Chemical Co., St. Louis, MI, USA). For these experiments, microelectrodes were coated with ChOx on the lower pair and AChE+ChOx on the upper pair of recording sites or vice versa (n=2 per arrangement). Microelectrodes were implanted in mPFC using the coordinates described above, except that measures were not taken across the entire ventral-dorsal extension but only at one D-V point (2.7 mm). The effects of nicotine (4 nmol; based on separate dose-response studies showing near maximum signal amplitudes recorded via ChOx-coated sites; Parikh et al.; unpubl. evidence) were determined. To confirm that nicotine-evoked signals required neuronal depolarization in the recording region, the effects of TTX on nicotine-evoked signals were assessed by slowly infusing 80 pmol of TTX (800 nL; Sigma) over 5 s into the vicinity of the recording sites (Parikh et al., 2004).
2.6. In vivo detection of pressure-ejected ACh at AChE+ChOx recording sites
This experiment represented an attempt to determine the minimal concentration of ACh that could be detected via AChE+ChOx recording sites in vivo (see above for the determination of the LOD in vitro). Although there is no information about the volume, velocity and concentrations of a putative extracellular wave of ACh after massive depolarization, pressure ejections of ACh into the vicinity of recordings sites were thought to provide insights into the potential amounts of extracellular ACh that can be detected by this method. For these experiments, electrodes with recording sites coated with AChE+ChOx (as described above) were implanted into the mPFC. Control recording sites for self-referencing were coated with BSA alone. The tip of the pipettes was positioned at 100 μm away from the center of the pair of AChE/ChOx recording sites. 10, 50 and 100 nL of 10 μM ACh, or 100, 500 and 1000 fmol, were pressure-ejected at 2-5 psi for 2 s (n=4-6 animals per concentration). Signals were recorded at 1 Hz sampling rate. ACh-evoked signal amplitudes that were greater than 3× level of electrode noise were considered detectable. Baseline electrode noise levels were determined by averaging the differences between two successive data points over 10 data points taken prior to the application of ACh.
2.7. Signal analyses
KCl-evoked cholinergic signal amplitudes were recorded and converted into μM equivalents of choline based on in vitro calibration slopes. As it is currently not possible to coat differentially the two Pt-recording sites that form a pair, electrodes equipped with AChE+ChOx- and ChOx-coated recording sites did not feature recording sites coated only with BSA. Therefore, self-referencing could not be conducted for these electrodes. In contrast, self-referencing was conducted for cholinergic signals recorded by using electrodes with BSA- and ChOx-coated recordings sites (Parikh et al., 2004, 2006; Parikh and Sarter, 2006). As m-PD electropolymerized electrodes completely do not respond to the electroactive DA (see calibrations), currents recorded from BSA-coated sites were subtracted from currents recorded via ChOx-coated sites and represented as equivalents of choline. The averages of three responses per pressure ejection and per animal were used for statistical analyses (below).
2.8. Histochemical analysis
After the completion of recordings, animals were transcardially perfused with 100 mL of ice-cold heparinised saline followed by 300 mL of 4% paraformaldehyde in 0.1M PBS (pH 7.4). The brains were removed and post-fixed overnight at 4°C and stored in 30% sucrose in 0.1M PBS for 72 hrs. Coronal sections (50 μm) were cut using a freezing microtome (Leica CM 2000R, Leica Microsystems Inc., Chantilly, VA) and stored in cryoprotectant solution (30% glucose, 60% ethylene glycol and 0.04% sodium azide in 0.05M PBS, pH 7.4) at −20°C until further processing. Serial sections from mPFC (between 2.7 and 3.2 mm anterior to bregma) were either Nissl-stained or processed for the histochemical visualization of acetylcholinesterase (AChE)-positive fibers. The distribution of cortical AChE-positive fibers generally parallels the pattern observed on ChAT-immunostained sections (Lysakowski et al., 1989) and therefore represents a valid method for the demonstration of cholinergic innervation patterns. Moreover, AChE-stained sections typically reveal more detail about cholinergic fiber arrangements when compared with ChAT-immuno-stained sections (see Figure 1C) and therefore was the preferred method. A modification of Tago's method (Tago et al., 1986), involving tetraisopropylphosphoramide to inhibit the visualization of non-specific, butyrylcholinesterase-positive elements (Burk et al., 2002), was used. Nissl-stained sections were used to verify microelectrode placements.
2.9. Statistical analyses
Statistical analyses were conducted using SPSS/PC+ V.13.0 (SPSS, Chicago, IL). Two-tailed unpaired Student's t-tests were employed to compare choline calibration parameters obtained via AChE+ChOx- versus ChOx-coated Pt-recordings sites. Repeated measure mixed-factor ANOVAs were employed to analyze the effects of enzyme coatings (AChE+ChOx and ChOx; two-levels) and placement of microelectrode (five-levels) on KCl-evoked cholinergic signal amplitudes. The effects of signal correction via self-referencing (raw and self-referenced; two-levels) and placement (five-levels) on choline signal amplitudes recorded via ChOx and BSA-coated recording sites were analyzed using repeated measure ANOVAS. For these statistical analyses, the data obtained from the two identically-coated recording sites that formed a pair (upper or lower pair) were averaged for each animal. Mixed factor ANOVAs were used to compare the effects of the placement of ChOx-coated sites on the microelectrode (upper versus lower pair; two-levels) and the placement of microelectrode (five-levels) on self-referenced choline signals (against BSA-coated sites). The data from individual ChOx-coated recording sites was used for this analysis. Post-hoc multiple comparisons were conducted using the Bonferroni test (α=0.05 for all tests). The effects of coating type on nicotine-evoked signals in the presence and absence of TTX were analyzed by planned two-tailed unpaired Student's t-tests. Exact P-values are reported as recommended by Greenwald and colleagues (Greenwald et al., 1996). The reported statistical results from repeated measure ANOVAs reflect Huyn–Feldt-corrected degrees of freedom (Vasey and Thayer. 1987).
3. Results
3.1. In vitro calibration of AChE/ChOx- and ChOx-coated recording sites
Currents obtained via Pt-recording sites coated with AChE+ChOx or only ChOx increased linearly to successive additions of choline (Figure 2A). Addition of AA and DA did not affect currents, indicating the effectiveness of m-PD electroplating. Sensitivity to choline did not differ between the two coating types (AChE+ChOx: 9.60±1.55 pA/μM; ChOx: 8.19±1.02 pA/μM; t(28)= 0.81; P=0.42; Table 1). Likewise, LODs, R2-values and selectivity (AA:choline) did not differ between the two types of recording sites (all P>0.05; see Table 1).
Figure 2.
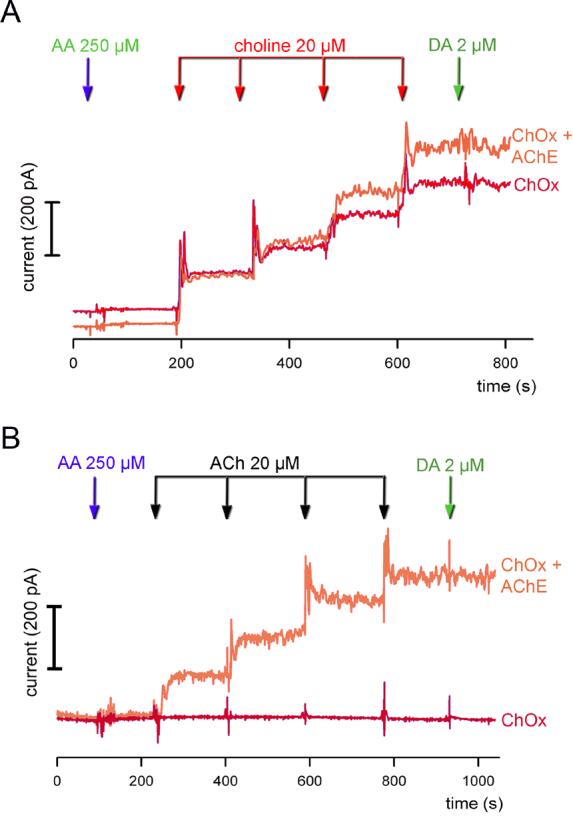
In vitro calibration of ChOx- and AChE+ChOx-coated recording sites. (A) Raw current traces depicting the responses to increasing concentrations of choline are shown, obtained from ChOx-only (red color) and AChE+ChOx-coated (orange color) recording sites. Addition of ascorbic acid (AA) and dopamine (DA) did not elicit current, reflecting the efficacy of the m-PD barrier. The responses to choline did not differ between the two coating types, indicating that co-coating with AChE did not affect the potency of ChOx. (b) Raw current traces depicting the responses to increasing concentrations of ACh. As expected, recording sites coated with ChOx only did not respond to ACh.
Table 1.
Electrode properties (choline calibration data)
| Enzyme | Sensitivity (pA/μM) | LOD (nM) | R2 | Choline:AA |
|---|---|---|---|---|
| AChE+ChOx | 9.60 ± 1.55 | 236.75 ± 46.48 | 0.992 ± 0.004 | 289.32 ± 94.40 |
| ChOx | 8.19 ± 1.02 | 231.91 ± 17.69 | 0.993 ± 0.002 | 366.14 ± 81.79 |
Data are based on 15 Pt- recording sites per enzyme coating type [Mean ± S.E.M]; LOD, limit of detection; R2, linearity of the response of the microelectrode to increasing concentrations of choline; AA, ascorbic acid
Figure 2B shows representative currents recorded via AChE+ChOx- and ChOx-coated recordings sites in response to the administration of ACh. As expected, currents recorded via sites coated with ChOx only were not affected by the addition of ACh. AChE+ChOx-coated recording sites were characterized by a sensitivity of 9.36±1.17 pA/μM, R2 of 0.989±0.003 and an LOD of 191.19±51.28 nM for ACh.
Finally, recording sites coated with ChOx only exhibited a sensitivity of 7.42±1.11 pA/μM for choline, with a background current of 139.72±38.46 pA. The LOD for choline was 232.01±6.04 nM, R2 was 0.99±0.003 and selectivity ratio for choline:AA was 429.09±100.53.
3.2. Effects of coating type and placement on KCl-evoked cholinergic signals
KCl-evoked choline signals were recorded simultaneously via recording sites coated with AChE+ChOx or ChOx alone, at 5 different placements spanning the dorsal-ventral extension of layer III of the mPFC (Figure 1). Layer III was selected for these studies as it is characterized by a particularly high density of cholinergic fibers (see Figure 1C). KCl-evoked choline signal amplitudes did not differ by enzyme coating type (AChE+ChOx vs. ChOx; F(1, 5)=0.38, P=0.40; Figure 3).
Figure 3.
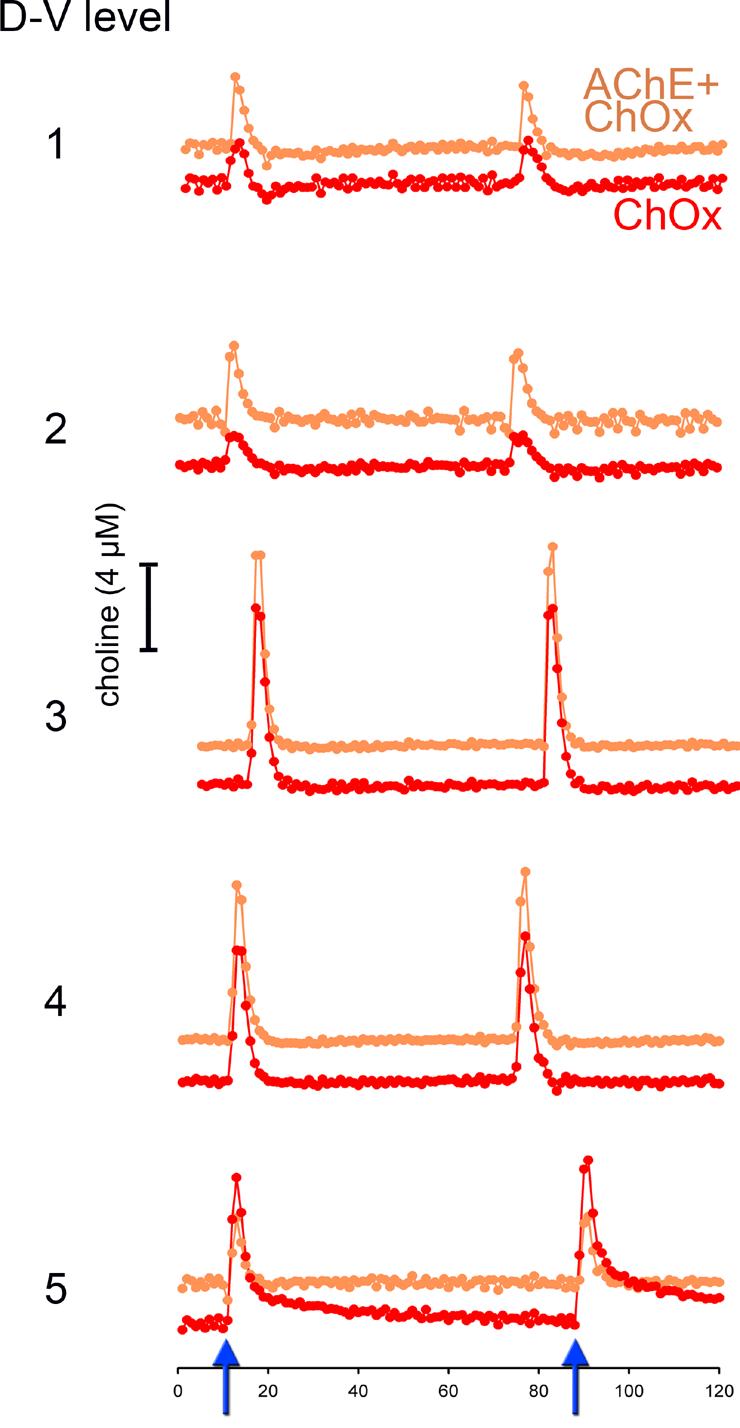
Choline signals evoked by pressure-ejection of KCl (70 mM, 200 nL) via a micropipette whose tip was placed within 70-100 μm of the four recording sites. The orange trace shows choline signals recorded via AChE+ChOx-coated sites, while the red trace depicts responses via ChOx only sites. The effects of two successive pressure ejections of KCl are depicted (see arrows on abscissa on the bottom of the figure). Electrode/pipette assemblies were lowered by 200 μm after each series of KCl pressure ejections, for a total of 5 measures spanning the dorsal-ventral (D-V) extension of the prelimbic region. The response to KCl did not differ between the two types of recording sites or between placements (see Results for statistical findings).
The position of the enzyme-coated recording sites on the ceramic surface (one pair per enzyme coating type, upper pair vs. lower pair; see Figure 1) along the dorsal-ventral extension of the mPFC did not affect the amplitudes of KCl-evoked cholinergic signals (Figure 3; F(4, 20)=1.04, P=0.40). Furthermore, coating type did not interact with placement (F(4, 20)=0.17, P=0.79).
3.3. Nicotine-evoked signals
As exemplified in Figure 4, nicotine-evoked signal amplitudes did not differ between ChOx and AChE+ChOx-coated recording sites (AChE+ChOx: 2.36±0.13 μM; ChOx: 2.09±0.13 μM; t(6)=0.53, P=0.62). Furthermore, infusions of TTX robustly attenuated nicotine-evoked signals, irrespective of whether they were recorded via sites coated with AChE+ChOx or ChOx alone. Residual signal amplitudes after TTX administration did not differ by coating type (AChE+ChOx: 0.50±0.16 μM; ChOx: 0.57±0.19 μM; t(6)=0.25, P=0.81).
Figure 4.
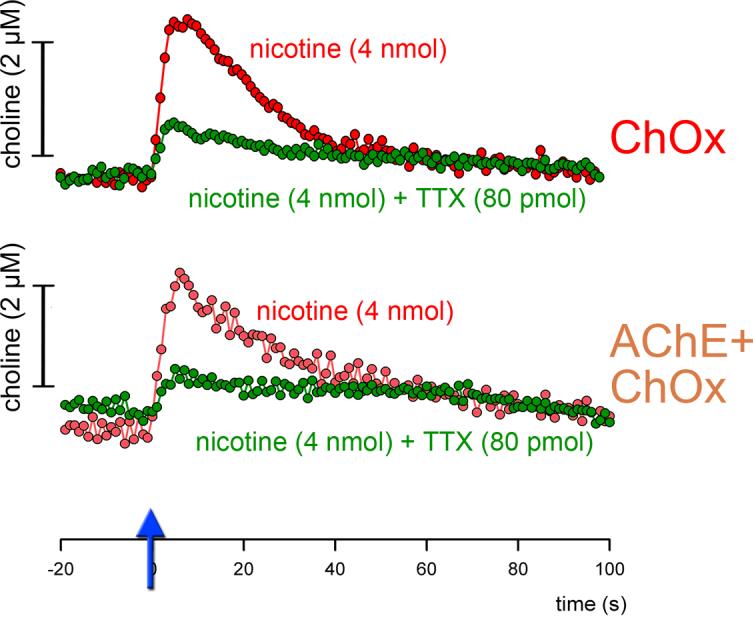
Examples of nicotine-evoked signals recorded via recording sites coated only with ChOx (top) or AChE+ChOx (bottom). Similar to KCl-evoked signals, signal amplitudes did not differ by coating type. Furthermore, the attenuation of signals by TTX did not differ by coating type (see Results for statistical findings).
3.4. Self-referenced recordings
We also measured KCl-evoked signals using electrodes equipped with pairs of recording sites coated with ChOx or BSA, respectively. These experiments were conducted in order to exclude the possibility that the measures generated via AChE+ChOx/ChOx electrodes, which did not feature a true control recording site coated only with BSA), were confounded by placement-dependent variations in electroactive species able to reach the Pt-sites despite m-PD electropolymerization. Although such a confound seemed very unlikely, we were concerned about the potential effects of “dopaminergic hotspots” and/or substantial extra-synaptic concentrations of dopamine (Sesack et al., 1998) on recordings using electrodes not featuring BSA-only recording sites and thus not allowing for self-referencing.
Correction of raw choline signals, recorded via ChOx-coated sites, by self-referencing against currents obtained via BSA-coated sites (see Methods) did not significantly alter KCl-evoked cholinergic signal amplitudes (F(1, 5)=1.58, P=0.27). Similar to the recordings obtained by using AChE+ChOx/ChOx electrodes (above), the amplitudes of self-referenced cholinergic signals were neither influenced by the placement of the electrodes (F(4, 32)=1.22, P=0.32) nor by the location of ChOx-coated and BSA only-coated sites on the electrode (lower vs. upper pair: F(1, 8)= 0.02, P=0.92).
3.5. No contamination of ChOx-only recording sites by endogenous AChE
We examined the possibility that endogenous AChE accumulates on the recording sites, thereby inadvertently transforming ChOx-only coated recording sites into AChE+ChOx sites (Xin and Wightman, 1997) and thus preventing the potential demonstration of larger KCl-evoked currents on sites pre-coated with AChE+ChOx when compared with ChOx-only coated recordings sites. Implantation of ChOx-only coated recording sites into the mPFC for 1 or 4 hours did not result in a significant increase in the sensitivity of these recording sites to ACh in vitro (0.87 ± 0.15 pA/μM and 0.69± 0.06 pA/μM, respectively; t(6) = 0.51, P = 0.63). Therefore, Pt-recording sites did not accumulate endogenous AChE and thus, the absence of KCl-evoked differences in current measured via recording sites equipped with the two coating types was not confounded by such contamination.
3.6. Detection of pressure-ejected ACh
The results described above indicate that evoked signals recorded via AChE−ChOx-coated sites matched the amplitude of signals recorded via sites coated only with ChOx, suggesting that signal amplitudes recorded via either site solely reflected transient increases in choline. To determine the concentration of an evoked “wave” of extracellular ACh that could be detected by the present method, ACh was pressure-ejected into the vicinity of AChE+ChOx-coated recording sites (see Methods). Significant increases in signal amplitudes, relatively to electrode noise were observed following the administration of 500 and 1000 fmol, but not 100 fmol of ACh (Figure 5). Assuming linearity of the response of the electrode (see in vitro calibration), ACh levels ≥150 fmol were detected following pressure ejections into the vicinity of the recording sites in the brain.
Figure 5.
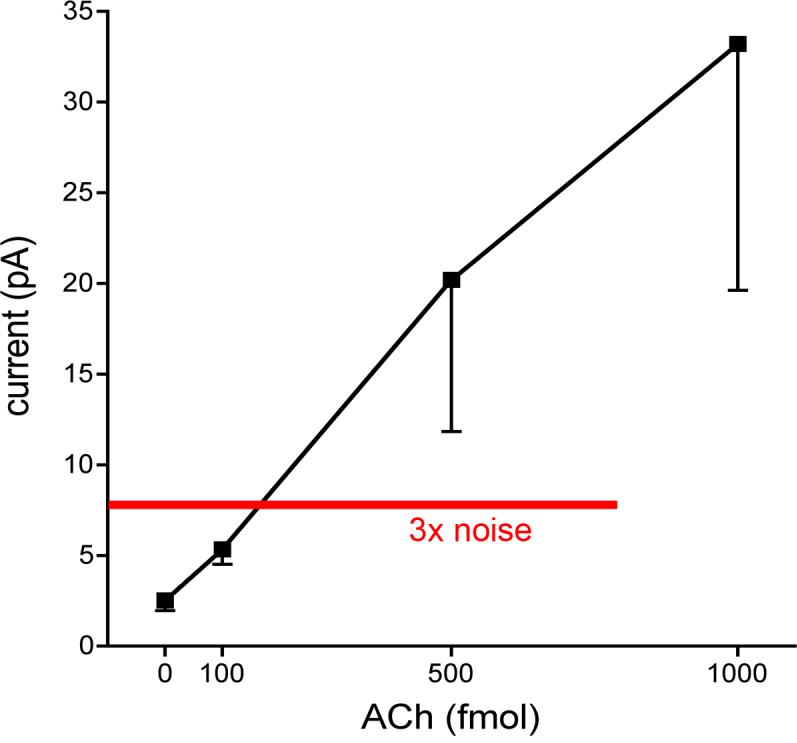
Signal amplitudes evoked by pressure ejections of ACh (0-1000 fmol) recorded via sites coated with AChE+ChOx (the data point at zero ACh indicates the baseline electrode noise; see Methods). Significant electrode response was defined as signal amplitudes greater than 3× the baseline noise levels. Linear regression analysis indicated that pressure ejections of <150 fmol of ACh were detected.
4. Discussion
We measured potassium- or nicotine-evoked cholinergic signals via recording sites sensitive to transient increases in choline resulting from hydrolysis of newly released ACh by endogenous AChE (recording sites coated only with ChOx), and via recording sites sensitive to such choline and, in addition, to choline resulting from hydrolysis of ACh by AChE immobilized on the recording site (recording sites coated with AChE+ChOx). As the amplitude of evoked signals recorded via the two types of recordings sites did not differ significantly, transient increases in choline concentrations account fully for signals recorded via both types of enzyme coating.
KCl-evoked ACh release represents a particularly effective experimental manipulation to produce potential ACh spillover and, as a result of the orchestrated depolarization of presumably thousands of cholinergic terminals, a putative wave of extra-synaptic ACh with initially high concentrations. For this reason, KCl was pressure ejected over 1-2 s, to evoke a precisely timed and orchestrated wave of ACh spillover. The present results do not indicate if such spillover occurred or remained below the detection limit of our method. ACh levels recovered by microdialysis traditionally have been thought to indicate the presence of ACh in the extracellular space. However, it is not readily possible to convert concentrations measured by microdialysis, collected over minutes and with probes exhibiting recovery rates of 10-15% into putative second-to-second based changes in extracellular concentrations following stimulation with KCl or nicotine. Thus, collectively the nature and concentration of waves of extracellular ACh following substantial release events remains unclear, although our results suggest that local levels remain below 150 fmol.
The present results indicate that co-coating ChOx-recording sites with AChE does not appreciably enhance the measurement of phasic cholinergic activity using enzyme-selective microelectrodes. Although we occasionally recorded greater currents via one relative to the other coating type (Bruno et al., 2006), these differences varied across placements and did not indicate a systematically greater sensitivity of one particular coating type over the other. In this context, it should also be mentioned that our prior research did not substantiate potential concerns suggesting that extracellular choline levels vary independently of changes in ACh release. Various manipulations, including complete cholinergic deafferentation of the recording field, did not affect basal extracellular choline levels (Parikh & Sarter, 2006; Parikh et al., 2006), indicating an extraordinary stability of extracellularcholine levels in the intact brain (Loffelholz et al., 1993; see also Hartmann et al., 2007). Finally, as pressure ejections and other manipulations occasionally produce transient amperometric artifacts, the presence of a recording site coated only with BSA is essential. Thus, microelectrodes equipped with recording sites coated only with ChOx as well as with BSA are sufficient to generate valid and sufficiently sensitive (Parikh et al., 2007) measures of synaptic ACh release.
Acknowledgements
This research was supported by NIH Grants RO3 MH073600 and KO2 NH01072 (MS). The experiment that tested the possibility that endogenous AChE accumulates at ChOx-only recording sites, thereby inadvertently transforming such sites into AChE−ChOx recording sites was originally suggested to us by Dr. R. Mark Wightman (University of North Carolina, Chapel Hill). We thank Matt Howe (University of Michigan) for helpful comments on a draft of this manuscript.
Abbreviations
- ACh
acetylcholine
- AChE
acetycholinesterase
- ChAT
choline acetyltransferase
- ChOx
choline oxidase
- BSA
bovine serum albumin
- mPFC
medial prefrontal cortex
- Prl
prelimbic cortex
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnati LF, Leo G, Zanardi A, Genedani S, Rivera A, Fuxe K, Guidolin D. Volume transmission and wiring transmission from cellular to molecular networks: history and perspectives. Acta Physiol. 2006;187:329–344. doi: 10.1111/j.1748-1716.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- Bruno JP, Gash C, Martin B, Zmarowski A, Pomerleau F, Burmeister J, Huettl P, Gerhardt GA. Second-by-second measurement of acetylcholine release in prefrontal cortex. Eur. J. Neurosci. 2006;24:2749–2757. doi: 10.1111/j.1460-9568.2006.05176.x. [DOI] [PubMed] [Google Scholar]
- Burk JA, Herzog CD, Porter MC, Sarter M. Interactions between aging and cortical cholinergic deafferentation on attention. Neurobiol. Aging. 2002;23:467–477. doi: 10.1016/s0197-4580(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Moxon K, Gerhardt GA. Ceramic-based multisite microelectrodes for electrochemical recordings. Analytical Chemistry. 2000;72:187–192. doi: 10.1021/ac9907991. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Palmer M, Gerhardt GA. Ceramic-based multisite electrode array for rapid choline measures in brain tissue. Analytica Chimica Acta. 2003;481:65–74. [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. Eighth Edition Oxford University Press; Oxford: 2003. [Google Scholar]
- Descarries L, Mechawar N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 2000;125:27–47. doi: 10.1016/S0079-6123(00)25005-X. [DOI] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Ann. Rev. Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Friedemann MN, Gerhardt GA. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol. Aging. 1992;13:325–332. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Rivera A, Jacobsen KX, Hoistad M, Leo G, Horvath TL, Staines W, De la Calle A, Agnati LF. Dynamics of volume transmission in the brain. Focus on catecholamine and opioid peptide communication and the role of uncoupling protein 2. J. Neural Transm. 2005;112:65–76. doi: 10.1007/s00702-004-0158-3. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Gonzalez R, Harris RJ, Guthrie D. Effect sizes and p values: what should be reported and what should be replicated? Psychophysiology. 1996;33:175–183. doi: 10.1111/j.1469-8986.1996.tb02121.x. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Kiewert C, Duysen EG, Lockridge O, Klein J. Choline availability and acetylcholine synthesis in the hippocampus of acetylcholinesterase-deficient mice. Neurochem. Int. 2007 doi: 10.1016/j.neuint.2007.10.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Progr. Brain Res. 2004;145:201–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Nowak KA, Sarter M, Bruno JP. Microdialysis without acetylcholinesterase inhibition reveals an age-related attenuation in stimulated cortical acetylcholine release. Neurobiol. Aging. 2003;24:861–863. doi: 10.1016/s0197-4580(02)00226-9. [DOI] [PubMed] [Google Scholar]
- Loffelholz K, Klein J, Koppen A. Choline, a precursor of acetylcholine and phospholipids in the brain. Progr. Brain Res. 1993;98:197–200. doi: 10.1016/s0079-6123(08)62399-7. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Wainer BH, Bruce G, Hersh LB. An atlas of the regional and laminar distribution of choline acetyltransferase immunoreactivity in rat cerebral cortex. Neuroscience. 1989;28:291–336. doi: 10.1016/0306-4522(89)90180-2. [DOI] [PubMed] [Google Scholar]
- Massoulie J, Legay C, Anselmet A, Krejci E, Coussen F, Bon S. Biosynthesis and integration of acetylcholinesterase in the cholinergic synapse. Progr. Brain Res. 1996;109:55–65. doi: 10.1016/s0079-6123(08)62088-9. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Cozzari C, Descarries L. Cholinergic innervation in adult rat cerebral cortex: a quantitative immunocytochemical description. J. Comp. Neurol. 2000;428:305–318. doi: 10.1002/1096-9861(20001211)428:2<305::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Watkins KC, Descarries L. Ultrastructural features of the acetylcholine innervation in the developing parietal cortex of rat. J. Comp. Neurol. 2002;443:250–258. doi: 10.1002/cne.10114. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman-Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc. Nat. Acad. Sci. 1993;90:5194–5198. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Apparsundaram S, Kozak R, Richards JB, Sarter M. Reduced expression and capacity of the striatal high-affinity choline transporter in hyperdopaminergic mice. Neuroscience. 2006;141:379–389. doi: 10.1016/j.neuroscience.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cortical choline transporter function measured in vivo using choline-sensitive microelectrodes: clearance of endogenous and exogenous choline and effects of removal of cholinergic terminals. J. Neurochem. 2006;97:488–503. doi: 10.1111/j.1471-4159.2006.03766.x. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine controls cue detection on multiple time scales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur. J. Neurosci. 2004;20:1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res. Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res. Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and top-down cholinergic modulation of signal detection. Brain Res. Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Schuvailo ON, Dzyadevych SV, El'skaya AV, Gautier-Sauvigne S, Csoregi E, Cespuglio R, Soldatkin AP. Carbon fibre-based microbiosensors for in vivo measurements of acetylcholine and choline. Biosensors & Bioelectronics. 2005;21:87–94. doi: 10.1016/j.bios.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Morrell F, Mesulam MM. Cholinergic synapses in human cerebral cortex: an ultrastructural study in serial sections. Exp. Neurol. 1997;144:361–368. doi: 10.1006/exnr.1997.6413. [DOI] [PubMed] [Google Scholar]
- Tago H, Kimura H, Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J. Histochem. Cytochem. 1986;34:1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Turrini P, Casu MA, Wong TP, De Koninck Y, Ribeiro-da-Silva A, Cuello AC. Cholinergic nerve terminals establish classical synapses in the rat cerebral cortex: synaptic pattern and age-related atrophy. Neuroscience. 2001;105:277–285. doi: 10.1016/s0306-4522(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J. Comp. Neurol. 1994;348:351–373. doi: 10.1002/cne.903480304. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Xin Q, Wightman RM. Transport of choline in rat brain slices. Brain Res. 1997;776:126–132. doi: 10.1016/s0006-8993(97)00996-7. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Acetylcholine in cortical inference. Neural Netw. 2002;15:719–730. doi: 10.1016/s0893-6080(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman G, Soreq H. Termination and beyond: acetylcholinesterase as a modulator of synaptic transmission. Cell Tissue Res. 2006;326:655–669. doi: 10.1007/s00441-006-0239-8. [DOI] [PubMed] [Google Scholar]


