Summary
Salmonella typhimurium genetically modified at the purI and msbB genes to increase dependence on adenine and decrease stimulation of tumor necrosis factor-α production were injected intravenously into C57BL/6 mice bearing subcutaneous tumor or lung metastases. Decreased tumor growth and prolonged survival were seen in some, but not all of nine transplantable tumors. Salmonella increased in number in the tumor and reached levels 10,000 times higher than in the normal liver reservoir of these bacteria. Histologic studies revealed Salmonella growth in areas of the tumor although, in all cases, a viable rim of tumor survived and ultimately resulted in progressive tumor growth in all mice. These studies demonstrate that Salmonella can localize to transplantable murine tumors and partially inhibit tumor growth; however, additional modifications of the bacteria may be necessary if this approach is to develop into an effective treatment for patients with cancer.
Keywords: Cancer, Salmonella, Therapy, Mice
Anecdotal reports of the regression of cancers in humans after bacterial infections have stimulated interest in the use of infectious agents in cancer therapy. In transplantable animal tumor models, bacteria such as Clostridium, Corynebacterium, Bacillus Calmette-Guérin and Salmonella as well as Vaccinia and Newcastle disease viruses have been evaluated for their selective growth in tumors (reviewed in [1,2]). The toxicity of these infectious agents has, however, limited their clinical usefulness.
In an attempt to increase the applicability of Salmonella organisms for cancer treatment, Salmonella typhimurium was genetically engineered to improve tumor targeting as well as to reduce toxicity (3–7). Chromosomal deletion of the purI gene creates a requirement for an external source of adenine, and deletion of the msbB gene prevents the addition of a terminal myristyl group to the lipid-A domain of lipopolysaccharide and thus, markedly diminishes its capacity to induce tumor necrosis factor-α production compared with the parental Salmonella. These deletions were genetically stable and increased the LD50 in mice by approximately 10,000 fold (5). These modified bacteria were reported to selectively grow in transplanted tumors in mice and result in reduced tumor growth (3).
In the present studies, we have tested the antitumor impact of these genetically modified Salmonella typhimurium in nine different transplantable tumor models in C57BL/6 mice. Therapeutic studies have been performed in mice bearing implanted subcutaneous tumors or in lung metastases, and the selective growth of Salmonella in tumor deposits was compared with its major normal reservoir in the liver.
MATERIALS AND METHODS
Transplantable Tumors
Seven different transplantable sarcomas in C57BL/6 mice (MCA-101, 102, 105, 106, 203, 205, and 207) were induced in our laboratory by the injection of 3-methy-cholanthrene as previously described (8). The B16 melanoma and the MC-38 colon-cancer tumor have been serially transplanted in our laboratory, and cultured cell lines were established. In experiments to induce subcutaneous tumors, single-cell suspensions of 5 × 105 tumor cells from cultured lines were injected in 0.1 mL subcutaneously in the flank of mice. The perpendicular diameters of the subcutaneous tumors were serially measured using a caliper. To induce lung metastases, 5 × 105 tumors cells were injected intravenously in the tail vein.
Preparation and Administration of VNP 20009 Salmonella Typhimurium
Vion Pharmaceutical Inc. (New Haven, CT, U.S.A.) provided the VNP 20009 strain of Salmonella typhimurium. Vials contained 1.2 mL of Salmonella at greater than 2 × 109 colony forming units per mL and was stored at −80°C in 15% glycerol. This strain of bacteria was attenuated by chromosomal deletion of the purI and msbB genes (4). Vials of Salmonella were thawed at room temperature, diluted in normal saline, and intravenously injected in the tail veins of mice in volumes of 0.5–1 mL.
Quantitative bacteriologic cultures from the original injected material as well as from homogenates of tumor and liver were performed using medium conditions that were optimized for this strain of Salmonella, which grew best at 35°C in air with CO2. Good growth detectable by 24 hours was obtained on brain heart infusion agars. Serial dilutions were performed to quantitate the number of bacteria per gram of tissue.
RESULTS
Impact of the Intravenous Injection of Salmonella Typhimurium on Tumor Growth
Subcutaneous tumors of the B16 melanoma and the MCA-205 sarcoma were induced in mice by the injection of 5 × 105 viable tumor cells. Eight days later when subcutaneous tumors were about 4–5 mm in diameter, 106 colony-forming units of Salmonella typhimurium, or control saline, were injected intravenously in groups of ten mice each. Tumor growth and survival were evaluated (Fig. 1). The growth of individual tumors in control mice (upper left) and in mice receiving Salmonella (upper right) are shown as well as the average size of tumors (lower left) and the survival of mice (lower right). Mice receiving Salmonella exhibited decrease of tumor growth (p < 0.001) and prolonged survival (< 0.0001); although, all mice eventually died with progressive tumor. A greater effect of the Salmonella injection was seen in mice bearing the B16 melanoma (Fig. 1A) then the MCA-205 sarcoma (Fig. 1B). These results were replicated in multiple experiments.
FIG. 1.
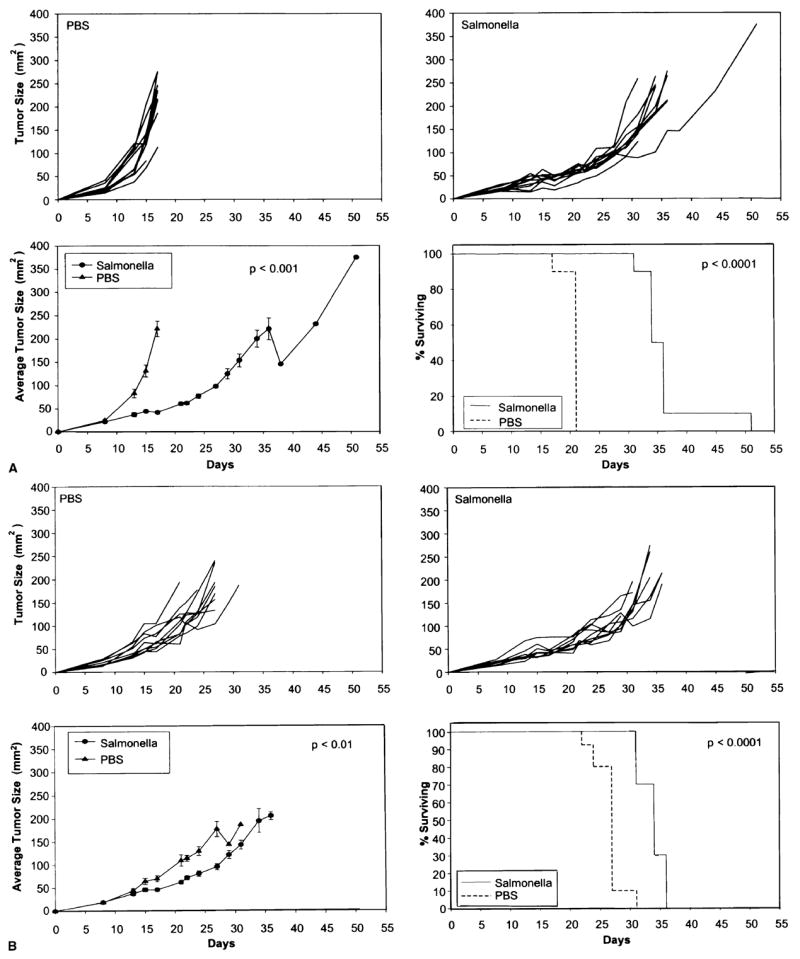
Injection of 106 Salmonella typhimurium into C57BL/6 mice (ten per group), 8 days after the subcutaneous injection of (A) B16 melanoma or (B) MCA-205 sarcoma. Tumors were approximately 21–24 mm2 at the time of injection. Growth rates of individual control mice (upper left) and mice injected with bacteria (upper right) are shown. Average growth rate of the tumors (lower left) and the survival of mice (lower right) are shown. Injection of bacteria slowed tumor growth and improved survival although, all mice eventually died of progressive tumor.
To evaluate the ability to generalize these observations, a similar experiment was performed utilizing nine different transplantable tumors treated on day eight including repeat experiments with the B16 melanoma and the MCA-205 sarcoma. These results are shown in Figure 2 and Table 1. The most profound impact of Salmonella injection was seen in the B16 tumor model (p < 0.01). Significant reduction (p < 0.05) of tumor growth was seen in mice receiving the MCA-105, MCA-203, MCA-205, and MC-38 tumor models, and no significant impact was seen in mice receiving the MCA-101, MCA-102, MCA-106, and MCA-207 tumors. The average tumor size as well as the percent decrease in the size of the tumors on day 21 and day 27 after tumor injection are shown in Table 1.
FIG. 2.
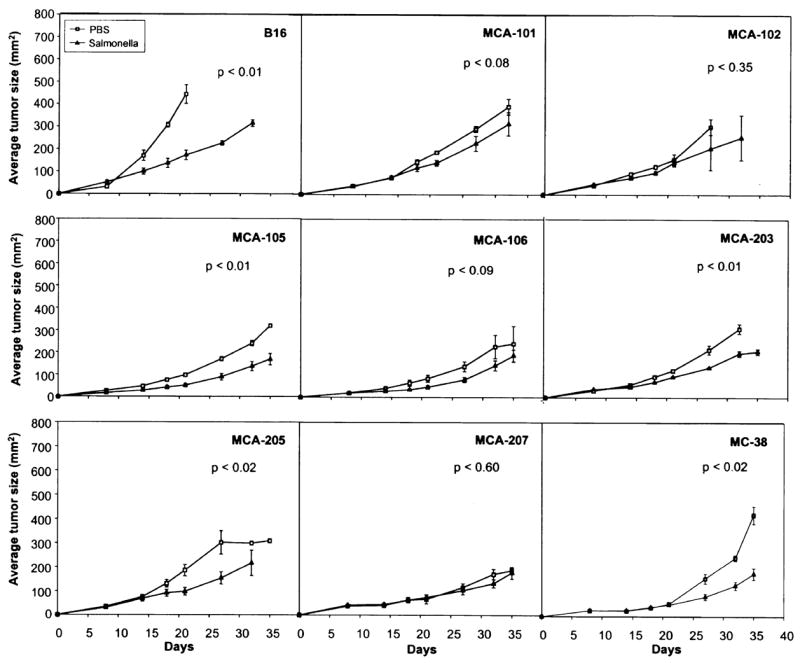
Injection of 106 Salmonella typhimurium intravenously into mice bearing each of nine different transplantable tumors. Bacteria were injected on day eight when the tumors were palpable. Varying levels of suppression of tumor growth were seen in different tumors, although, some tumors were resistant to the effects of Salmonella. All mice in all groups eventually developed progressive tumor.
TABLE 1.
Effect of salmonella injection on nine different transplantable tumors in C57BL/6 mice
| Tumor | Treatment | Day 21* average tumor size (mm2) | % Decrease | Day 27* average tumor size (mm2) | % Decrease |
|---|---|---|---|---|---|
| B16 | Salmonella | 172 ± 21 | 61.3 | 226 ± 10 | ND† |
| PBS | 444 ± 42 | ND† | |||
| MC-38 | Salmonella | 50 ± 6 | 6.8 | 83 ± 12 | 47.8 |
| PBS | 53 ± 9 | 159 ± 21 | |||
| MCA-101 | Salmonella | 140 ± 13 | 25.5 | 228 ± 34 | 22.1 |
| PBS | 188 ± 6 | 293 ± 14 | |||
| MCA-102 | Salmonella | 143 ± 16 | 8.5 | 206 ± 96 | 32.1 |
| PBS | 156 ± 25 | 304 ± 35 | |||
| MCA-105 | Salmonella | 51 ± 8 | 47.5 | 89 ± 15 | 47.7 |
| PBS | 97 ± 5 | 170 ± 10 | |||
| MCA-106 | Salmonella | 46 ± 8 | 45.2 | 80 ± 12 | 42.7 |
| PBS | 85 ± 16 | 139 ± 22 | |||
| MCA-203 | Salmonella | 94 ± 4 | 23.3 | 135 ± 2 | 37.3 |
| PBS | 123 ± 6 | 216 ± 20 | |||
| MCA-205 | Salmonella | 97 ± 16 | 47.8 | 152 ± 26 | 49.4 |
| PBS | 185 ± 24 | 301 ± 48 | |||
| MCA-207 | Salmonella | 73 ± 13 | −10.6 | 103 ± 17 | 11.1 |
| PBS | 66 ± 18 | 116 ± 16 |
Mean ± standard error.
All control B16 melanoma mice died of progressive tumor by day 27.
PBS, phosphate buffered saline.
The impact of multiple Salmonella injections as well as the treatment of larger B16 subcutaneous tumors are shown in Figure 3. Injection of Salmonella on day 7, days 7 and 14, or on days 7, 14, and 21 after tumor injection resulted in similar antitumor effects (Fig. 3 [top]) (p = 0.01). No added impact of additional Salmonella injections could be demonstrated. However, injection of Salmonella could impact the growth of fairly large murine tumors. When Salmonella injections were delayed until day 14 when tumors were approximately 100 mm2, a slowing of tumor growth could be seen (p = 0.03) that lasted approximately 10–14 days although, all tumors again resumed growth and caused the demise of all mice.
FIG. 3.
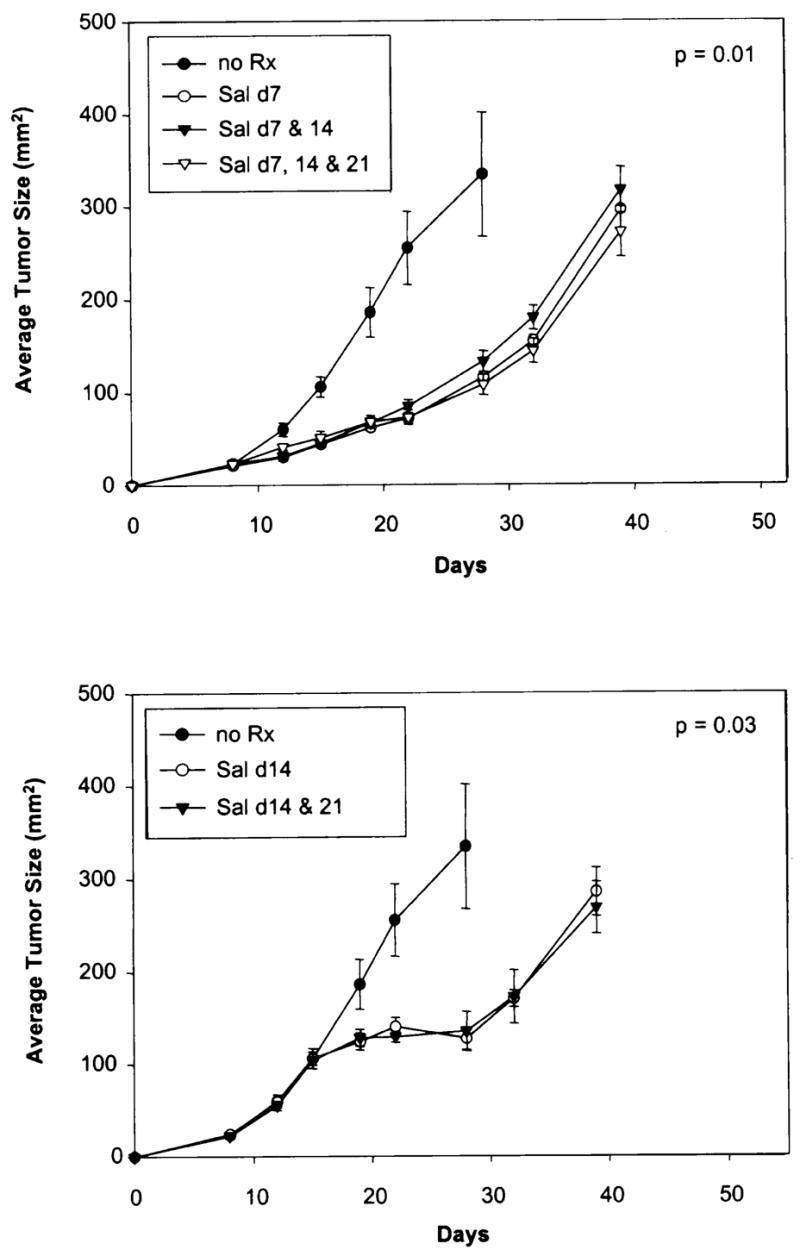
Impact of multiple injections of Salmonella typhimurium into mice bearing palpable B16 melanoma (top). Seven days after injection of tumor cells, injection of bacteria significantly impeded tumor growth, which was not impeded further by more injections on day 14 or on days 14 and 21. Injection of 106 Salmonella typhimurium on day 14 when tumors had achieved a diameter of 1 cm also resulted in slowing of tumor growth that lasted about 2 weeks before tumor growth resumed (bottom).
To evaluate the impact of Salmonella injection in mice with visible lung metastases, the B16 melanoma was injected intravenously and 14–15 days later, 106 Salmonella were injected intravenously. Results from two experiments are shown in Figure 4. Although, all mice succumbed from tumor growth in lungs, there was a small but significant prolongation of survival in mice receiving the intravenous Salmonella compared with controls (p < 0.001 in both experiments).
FIG. 4.
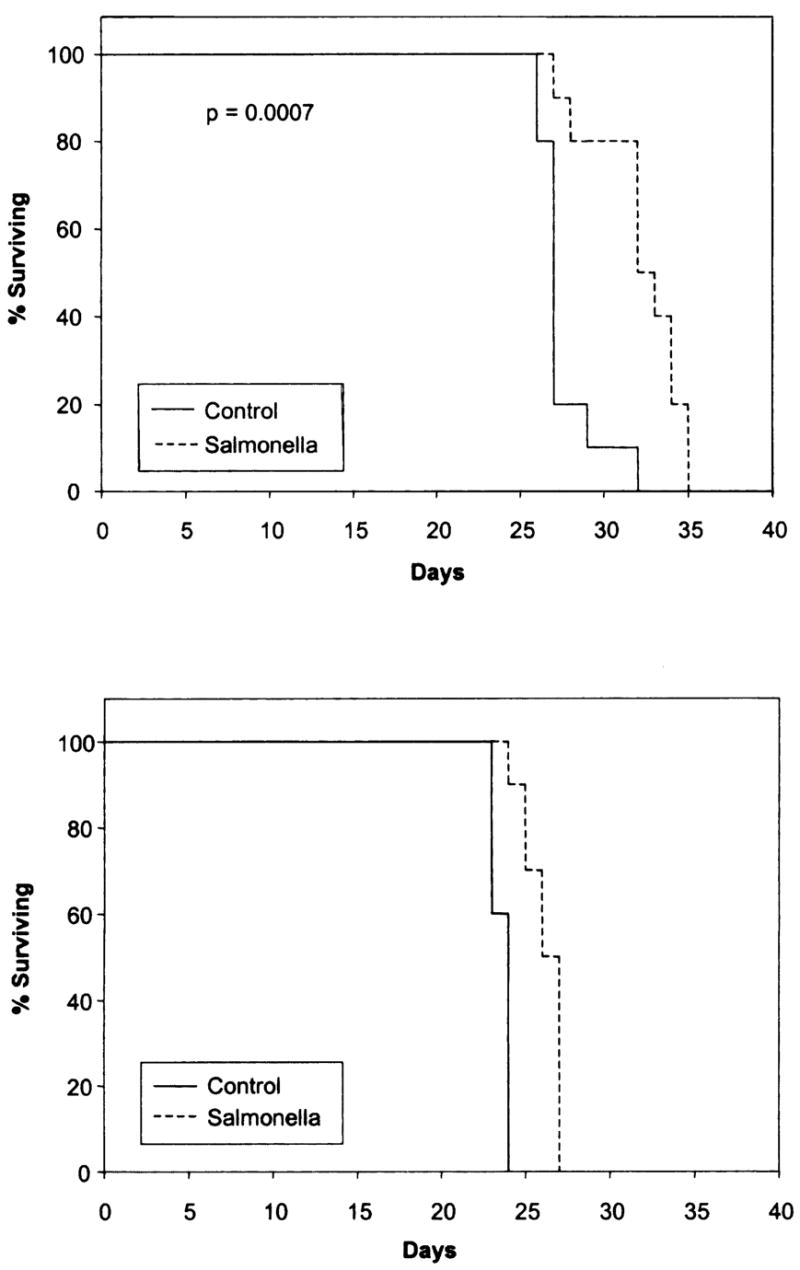
Injection of Salmonella typhimurium into mice 15 days after the injection of B16 melanoma cells intravenously. At this time, multiple lung nodules are visible in the lung. Injection of Salmonella significantly prolonged the survival of mice although, all mice eventually died of progressive tumor growth. Two independent experiments are shown.
Localization of Salmonella Typhimurium to Tumor and Liver
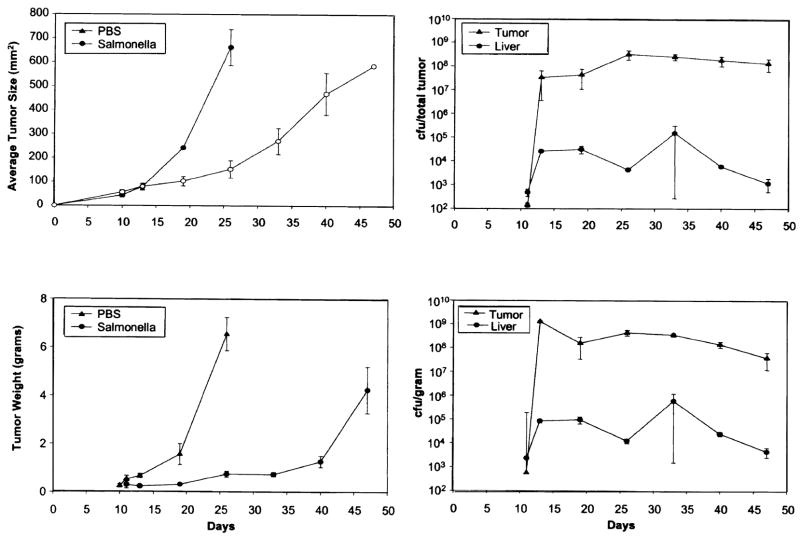
The growth of Salmonella in the liver and B16 subcutaneous tumor of mice treated on day ten after tumor injection is shown in Figure 5. Two mice in each group were killed at varying times after Salmonella injection and the tumor and liver were excised, weighed, and the number of Salmonella organisms determined. The size (Fig. 5 [upper left]) and the weight (Fig. 5 [lower left]) of tumor in mice receiving Salmonella was considerably less than that of control mice. Quantitation of the number of Salmonella organisms in the entire tumor (Fig. 5 [upper right]) and per gram of tumor tissue (Fig. 5 [lower right]) showed a four-log increase in bacteria, which persisted beyond 50 days. Salmonella could also be detected in the liver, although, the levels were much lower than in tumor.
FIG. 5.
Quantitation of bacteria growing in the tumor and liver of C57BL/6 mice injected with Salmonella typhimurium 10 days after tumor induction. Injection of Salmonella typhimurium resulted in slowing of tumor growth (upper left) and decrease in the weight of tumors excised at varying times (lower left). Salmonella rapidly replicated in tumor until a plateau was achieved in tumor and liver that was sustained for almost 50 days. The total number of Salmonella (upper right) and per gram of tissue (lower right) is shown.
Pathologic Analysis of Tumors and Liver in Mice Receiving Salmonella
Histologic sections of tumors from mice treated with Salmonella showed no difference from control animals until day three after therapy. At this time, the treated tumors developed a focal neutrophilic infiltrate associated with tumor necrosis. After day three, there was a dramatic increase in the amount of tumor necrosis and by day nine, almost total necrosis of tumor cells was seen. The zone of neutrophils surrounding the zone of tumor necrosis was similar to the cuff of inflammation that surrounds an abscess cavity (Fig. 6). At later time points, the zone of necrosis with surrounding inflammation remained, but outside the necrotic area, there was a rim of viable tumor that continued to expand with time. Acute inflammation extended outside the tumor mass to involve the surrounding adipose tissue. In contrast, tumors from control animals showed zones of necrosis that had no significant inflammatory infiltrate and seemed primarily related to distance from the vascular supply. Control tumors also showed increased necrosis as the size of the tumor increased.
FIG. 6.
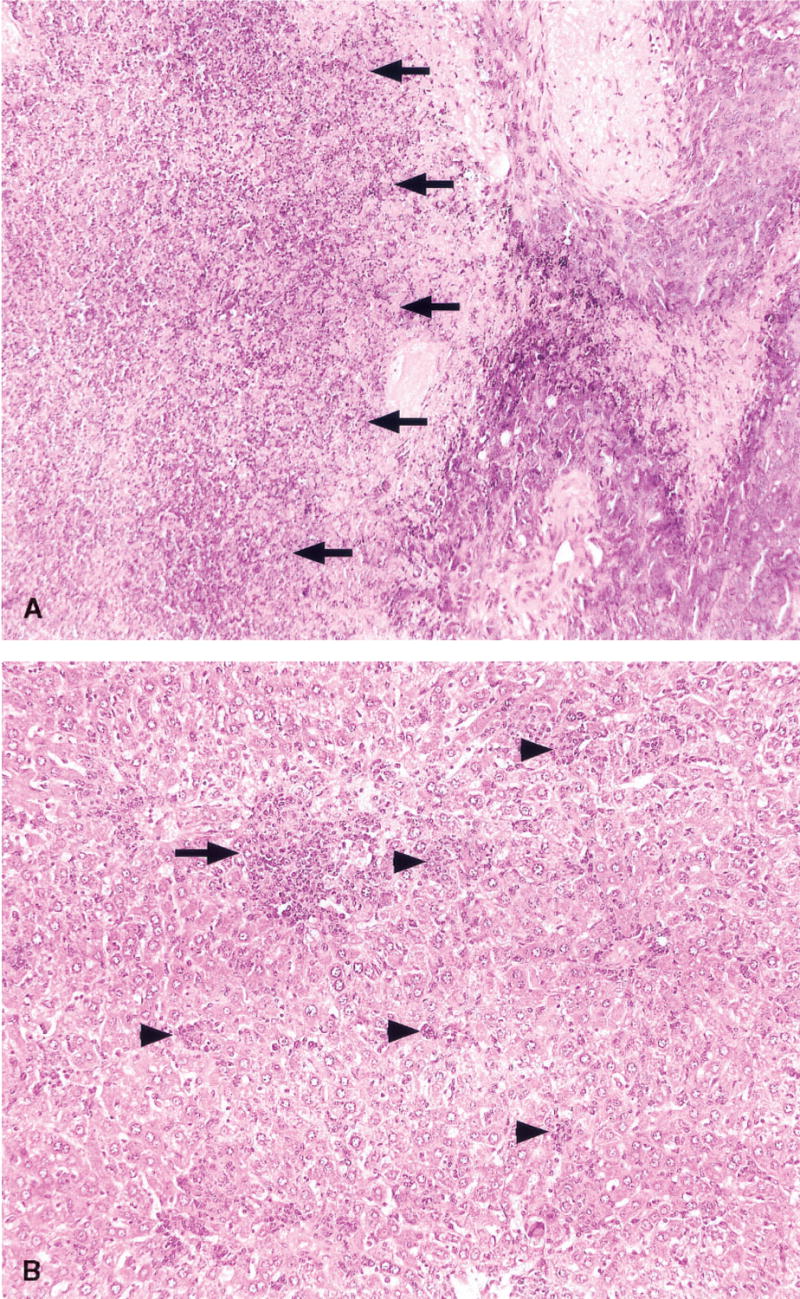
Histologic appearance of tumors and liver from mice 9 days after treatment with Salmonella typhimurium (H & E, 200×). (A) The tumor show extensive necrosis, and this necrotic zone is surrounded by a cuff of neutrophils (indicated by arrows). At the edge of the necrosis (behind the arrows), a residual rim of melanoma cells may be seen. At later time points, the tumor grew progressively from this rim of residual tumor. (B) The liver shows neutrophilic microabscesses ranging in size from small foci about the size of two–three hepatocytes (arrowheads) to larger zones of confluent necrosis (arrow). The number and size of these foci increased through day 23 before a gradual return to normal liver architecture.
Sections of liver from control animals showed only rare foci of inflammation and no abscess formation at all time points. However, by day three after therapy, concurrent with the appearance of neutrophils in tumors, the livers of treated animals showed two to three microabscesses per high power (400x) field. These microabscesses varied from two to three hepatocytes to about 30 hepatocytes in cross-sectional size and were composed of a mixture of neutrophils, lymphocytes, macrophages, and plasma cells. Some hepatocyte necrosis was seen within the microabscesses. The degree of hepatic inflammation increased through day 23 with coalescence of inflammatory foci into larger zones of inflammation and necrosis. The mice also developed a thrombophlebitis of intrahepatic portal and central veins. By day 16, there was a visible hepatic regenerative response with the formation of vague regenerative nodules. No fibrosis was seen at any time point. At the longest time point studied, (37 days after treatment), there was a decrease in the amount of hepatic inflammation with recovery of normal, nonregenerative architecture.
DISCUSSION
Salmonella contains many of the properties considered important for selective tumor targeting in vivo by infectious agents and anecdotal reports of Salmonella growing in human cancers have led to preclinical studies of the therapeutic value of Salmonella in tumor-bearing mice.
Salmonella are gram-negative bacteria that belong to the family Enterobacteriaceae (reviewed in [6]). They are highly motile with multiple flagella and except for Salmonella typhi and paratyphi they are not encapsulated. Salmonella are facultative anaerobes i.e., they can grow in aerobic and anaerobic conditions. Two of the main serogroups of Salmonella are Salmonella typhi, which is the main cause of typhoid fever and Salmonella typhimurium, which is only modestly pathogenic in humans, but has been associated with gastroenteritis. Salmonella typhimurium has several properties that are attractive for use in development of cancer treatment. It is infectious in mice and humans and thus, allows for pre-clinical evaluation of antitumor efficacy. It is highly sensitive to many commonly used antibiotics, and thus, infections that do occur can most often be successfully managed. Because it is a facultative anaerobe, it can grow in hypoxic or necrotic areas that occur in many tumors. It can be easily engineered in the laboratory to express foreign genes as well as to attenuate its pathogenicity. Pawelek et al. originally reported that Salmonella administered to tumor-bearing mice preferentially replicated in tumor tissue and could achieve tumor to normal tissue ratios in excess of 1,000 to 1 (3).
The attenuated Salmonella typhimurium used in this study contained a mutation of the msbB gene that is responsible for adding a terminal myristyl group to lipid-A, which is the glycolipid component of lipopolysaccharide responsible for endotoxin activity (4). Thus, mutants containing the msbB mutation induce significantly less tumor necrosis factor production in vitro and in vivo than does the parental strain. A second mutation, deletion of the purI gene, creates a requirement for an external source of adenine, which is thought to be present in high concentrations in the interstitial fluid of tumors because of their rapid turnover. These mutations were genetically stable in vitro for over 150 generations.
In the present studies, we reproduced the reported selective localization of the attenuated Salmonella typhimurium to transplantable B16 melanomas growing in mice (3). These bacteria slowed the growth of B16 tumor when administered as late as day 14 when the tumors achieved a diameter of 1 cm. The B16 melanoma was the most susceptible of all of nine transplantable tumors tested in C57BL/6 mice. Some tumors, such as MCA-205, experienced more modest slowing of growth whereas, other tumors, such as the MCA-207 and MCA-101, exhibited minimal if any impact of Salmonella injection. Salmonella could also slow the growth of small, but visible multiple lung metastases derived from the B16 melanoma. All tumors eventually grew and resulted in the death of mice despite attempts to repeatedly inject the Salmonella.
Visual inspection of the treated tumor-bearing mice as well as analysis of pathologic sections of tumor showed central necrosis with growth of bacteria within the tumor (Fig. 6). However, a viable rim of tumor always survived the Salmonella injection and continued to grow. Thus, it appeared that Salmonella grew in the necrotic and relatively hypoxic foci within the tumor, but did not grow in well-oxygenated tumors at the rim of the growing nodules. Quantitative analysis of bacteria within the tumor demonstrated rapid growth of bacteria within days after injection of Salmonella and remained fairly constant for up to 50 days. The normal reservoir of Salmonella in the liver also continued to harbor bacteria, but 10,000 fold less per gram of tissue than did tumor. This low level of Salmonella infection did not appear to influence the health of the mice although, all ultimately died of progressively growing tumor.
These observations support the hypothesis that increased nutrients, especially purine, near the necrotic and apoptotic tumor cells may selectively favor the growth of Salmonella. Less effective elimination of bacteria may also occur as a result of the local immunosuppressive environment of tumors. Secretion of transforming growth factor-β has been reported at tumor sites that can suppress neutrophil activation and infiltration.
Possible mechanisms to explain the slowing of tumor growth by Salmonella include the consumption of nutrients by bacteria needed for tumor growth, the production of enzymes by bacteria such as asparaginase that can deplete essential amino acids needed by the tumor, the secretion of local toxins into the extracellular environment, or the stimulation of tumor necrosis factor-α production, which can impact tumor vasculature. The non-specific inflammatory responses seen at the site of bacterial growth could also potentially activate antitumor T-cells.
A variety of attempts were made in these experiments to improve the antitumor efficacy of the Salmonella injections. Multiple experiments utilizing varying doses and schedules of interleukin-2 administered in conjunction with Salmonella injection did not result in improvement in antitumor efficacy (data not shown). Similarly, simultaneous adoptive transfer of cells with antitumor activity after Salmonella injection also did not increase antitumor efficacy (data not shown).
The dose of Salmonella we have used is near the lethal dose in mice, and thus, escalation of the number of injected Salmonella is not likely to improve the antitumor effects. The ability to genetically engineer Salmonella to secrete inflammatory or immunostimulatory cytokines at the tumor site represents a possible method for improving its antitumor efficacy although, it will depend on the fairly efficient infiltration of Salmonella throughout the growing tumor. Preliminary studies utilizing Salmonella typhimurium containing the human interleukin-2 gene were reported to improve the antitumor efficacy of Salmonella injection by stimulating natural killer and CD8+ cells (7).
These preclinical studies have led to ongoing attempts to use Salmonella injected either intravenously or intratumorally to patients with metastatic cancer (9).
References
- 1.Kirn DH. Replication-selective microbiological agents: fighting cancer with targeted germ warfare. J Clin Invest. 2000;105:837–9. doi: 10.1172/JCI9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sznol M, Lin SL, Bermudes D, et al. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest. 2000;105:1027–30. doi: 10.1172/JCI9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawelek JM, Low B, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–44. [PubMed] [Google Scholar]
- 4.Low KB, Ittensohn M, Le T, et al. Lipid a mutant Salmonella with suppressed virulence and TNF alpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 5.Clairmont C, Lee KC, Pike M, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 6.Bermudes D. Enteric gram-negative microorganisms. In: Jawetz E, Melnick JL, Adelberg EA, editors. Review of Medical Microbiology. Los Altos: Lange Medical Publications; 2001. pp. 234–7. [Google Scholar]
- 7.Saltzman DA, Kaatsanis E, Heise CP, et al. Antitumor mechanisms of attenuated Salmonella typhimurium containing the gene for human interleukin-2: a novel antitumor agent? J Ped Surg. 1997;32:301–6. doi: 10.1016/s0022-3468(97)90198-6. [DOI] [PubMed] [Google Scholar]
- 8.Wexler H, Rosenberg SA. Pulmonary metastases from autochthonous methylcholanthrene-induced murine tumors: a suggested model for evaluating cancer therapy (Brief Communication) J Natl Cancer Inst. 1979;63:1393–5. [PubMed] [Google Scholar]
- 9.Toso JF, Gill VJ, Witebsky WG, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–52. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]