Abstract
Since Src kinase inhibitors decrease brain injury produced by intracerebral hemorrhage (ICH) and thrombin is activated following ICH, this study determined whether Src kinase inhibitors decrease thrombin induced brain injury. Thrombin injections into adult rat striatum produced focal infarction and motor deficits. The Src kinase inhibitor PP2 decreased thrombin-induced Src activation, infarction in striatum and motor deficits in vivo. Thrombin applied to cultured post-mitotic striatal neurons caused: injury to axons and dendrites; many TUNEL positive neuronal nuclei; and re-entry into the cell cycle as manifested by cyclin D1 expression, induction of several other cell cycle genes and cyclin-dependent kinase 4 activation. PP2 dose-dependently attenuated thrombin-induced injury to the cultured neurons; and attenuated thrombin-induced neuronal cell cycle re-entry. These results are consistent with the hypotheses that Src kinase inhibitors decrease injury produced by ICH by decreasing thrombin activation of Src kinases and, at least in part, by decreasing Src induced cell cycle re-entry.
Keywords: thrombin, mitogenic signaling, cell cycle, neuronal apoptosis, intracerebral hemorrhage, striatum
Introduction
Intracerebral hemorrhages (ICH), which account for 10–15% of all strokes, have a very high morbidity and mortality (Ariesen et al., 2003). ICH activates thrombin, which is a major contributor to ICH induced brain injury (Xi et al., 2003a, Xi et al., 2006). Though thrombin is neuroprotective at low concentrations (Vaughan et al., 1995, Donovan and Cunningham, 1998, Striggow et al., 2000, Friedmann et al., 2001, Xi et al., 2003b), it is detrimental at high concentrations (Donovan et al., 1997, Xi et al., 2003a, Xue et al., 2006, Fujimoto et al., 2007). Direct infusion of 20U of thrombin into rat brain causes a lesion with brain edema formation and neuronal death (Fujimoto et al., 2007). Thrombin mediates the acute brain edema caused by ICH since thrombin inhibitors block the acute edema (Xi et al., 2003a, Xue et al., 2006). The mechanisms by which thrombin injures brain are still under study and are the subject of this report.
Thrombin-triggered receptor signaling is mediated in part via activation of protease-activated receptors (Biscardi et al., 2000), which contribute to the subsequent brain injury (Wang and Reiser, 2003). Inhibiting protease-activated receptors (PARs) decreases thrombin-induced brain injury (Xue et al., 2006). However, PAR inhibitors may not be good treatment targets for ICH since these might affect the clotting and hemostatic functions of thrombin needed to halt progression of ICH (Thomas and Brugge, 1997).
PARs modulate several intracellular molecules including Src kinases. The Src family of nonreceptor tyrosine kinases is represented by at least eight different protein tyrosine kinases, including c-Src, c-Yes, Fyn, Lck, Lyn, Hck, Blk and c-Fgr (Oda et al., 1999, Biscardi et al., 2000). Our genomic studies showed that the expression of Lyn, one of the Src family kinases, was up-regulated 21-fold following ICH (Tang et al., 2002, Lu et al., 2006). This led us to test the role of Src kinase inhibitors in ICH. Src kinase inhibitors, which are less likely to affect coagulation (Thomas and Brugge, 1997), decreased glucose hypermetabolism and cell death around ICH and improved behavioral deficits following ICH (Ardizzone et al., 2007). These results led to the current experiments that determined whether the improvement observed following ICH treated with Src kinase inhibitors was due, at least in part, to Src kinase inhibitors blocking thrombin and thrombin signaling.
Finally, both thrombin and Src kinases affect the cell cycle that may be important in ICH mediated cell death. Thrombin actions in brain are associated with mitogen-activated protein kinase (MAPK) signaling (Fujimoto et al., 2007). In cortical neuronal cultures, the mitogenic signaling by thrombin causes neurons to re-enter the cell cycle, and result in their apoptotic death (Rao et al., 2007). These results support a possible thrombin-induced “mitogenic signaling/cell cycle/neuronal death hypothesis.” Src kinases affect mitogenic signaling pathways and the cell cycle, and mutant Src is important in causing some cancers (Taylor and Shalloway, 1993, Mishra et al., 2005). Therefore, we tested the possibility that Src kinase antagonists might block thrombin activation of the cell cycle in mature neurons.
Materials and Methods
Unilateral injection of thrombin into rat striatum
Male Sprague-Dawley rats, weighing 300–320 g, were anesthetized with isoflurane (Minrad, New York, NY) and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA). A heating blanket maintained body temperature at 37°C. Thrombin injections were performed as previously described with minor modifications (Fujimoto et al., 2007). Briefly, 20U of thrombin (from bovine plasma, Sigma, St. Louis, MO) was dissolved in 5µl saline and injected into the right striatum (striatum coordinates: 1.0 mm anterior, 3.0 mm lateral; and 5.0 mm ventral, with respect to bregma) (Paxinos and Watson, 1998). The control group received bovine serum albumin (BSA) with equal molar amounts of protein to the thrombin- injected animals. Pharmacological injections, including the Src kinase inhibitor 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2, 0.3 or 1.0 mg/kg, Bimol International LP, Plymouth Meeting, PA) or the potent N-methyl D-aspartate (NMDA) receptor antagonist (5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cycl ohepten-5,10-imine maleate (MK801, 1.0 mg/kg, Calbiochem, San Diego, CA) and saline vehicle control, were administered intraperitoneally (i.p.) 10 minutes before the striatal thrombin injections. After closure of the operative sites, rats were allowed to recover in an incubator maintained at 37°C, and then returned to their home cages with free access to food and water. Using our previously described methods with minor modifications (Ardizzone et al., 2007), Src kinase activity was assessed at 4h, motor deficits at 23.5h and histological damage 24h after the injection of thrombin or control BSA. All experimental procedures were performed in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee, University of California at Davis.
Src Kinase Activity assay
Four hours after the injection of thrombin or control BSA, rats were euthanized with chloral hydrate (350 mg/kg, i.p., Sigma, St. Louis, MO) and the ipsilateral and contralateral striatum were dissected, homogenized, and protein isolated in lysis buffer (Ardizzone et al., 2007). Src protein was immunoprecipitated with a c-Src antibody (Delta Biolabs, Gilroy, CA), washed three times in lysis buffer, twice in kinase buffer, and resuspended in the latter, optimized to preserve the Src activity. The immunoprecipitated c-Src kinase was assayed for activity using the Kinase Activity Assay Kit (Chemicon, Temecula, CA). There were 3 animals per group. Statistical differences were determined using one-way ANOVA followed by Bonferroni’s post hoc test.
Assessment of motor deficits
The Elevated Body Swing Test (EBST) was used to examine the motor deficits 23.5 hours following thrombin injections. EBST is a behavioral test that can evaluate unilaterally injected animals in a drug-free state that can detect a natural response of the animals to a striatal lesion (Borlongan and Sanberg, 1995, Baluchnejadmojarad and Roghani, 2004). Briefly, each rat was placed into a Plexiglas box (50 × 50 × 20 cm), and allowed to habituate for 10 minutes and attain a neutral position as defined as having all four paws on the ground. Then the rat was held approximately 3 cm above the table and approximately 3 cm from the base of its tail. The left or rightward swings of each rat were recorded over 45 seconds. This was repeated 3 times with 2 minutes in between each test. Biased swinging behavior was calculated as follows: L/ (L+R) (%) for left biased swings (L, left-biased swings; R, right-biased swings). The criterion for biased swinging behavior was set at 70% or higher. Statistical differences were determined using one-way ANOVA followed by Dunnett’s post hoc test. There were 9 subjects per group. After the behavioral tests, six rats in each group were used for histological measurements; and the other 3 rats were used for Western-blotting.
Measurement of histological damage
After the behavioral tests, six rats in each group were anesthetized with chloral hydrate (350 mg/kg, i.p.) and perfused transcardially with 0.9% phosphate buffered saline (PBS), followed by 4% paraformaldehyde in PBS. Brains were removed, post-fixed overnight, and placed in 30% sucrose in PBS. Four coronal sections, approximately +2.0 mm to 0 mm from bregma, were cut on a freezing microtome and stored at −20°C in a cryoprotectant composed of 30% glycerol, 20% ethylene-glycol, and 50% PBS. Following three PBS rinses, the brain sections were stained using Hematoxylin & Eosin (Xue et al., 2006). These sections were scanned and analyzed with MCID elite software (Imaging Research, Linton, England).
Infarction volume was calculated using an “indirect method” (area of intact contralateral hemisphere minus area of intact region of the ipsilateral one) (Swanson et al., 1990, Lee et al., 2002, Liu et al., 2005). Statistical differences were determined using one-way ANOVA followed by Dunnett’s post hoc test. There were 6 subjects per group.
Rat striatal neuronal cultures
Striatal tissues, purchased from Brainbits (Springfield, IL), were dissected from embryonic day 18 Sprague Dawley rats. Striatal neuronal cultures were prepared according to the manufacturer’s directions. Briefly, individual cells were mechanically isolated from two striatal samples following trituration ten times in 1 ml B27/Hibernate (Brainbits, Springfield, IL). After the non-dispersed tissue settled by gravity for 1 minute, the supernatant was transferred to a 15 ml tube and centrifuged 1 minute at 200 × g. The pellet was gently re-suspended in B27/Neurobasal (Invitrogen, Carlsbad, CA) containing 0.5 mM glutamine. The cells were seeded into Poly-D-Lysine coated 8-well slide chambers or 6 cm dishes at a density of 20,000 cells /cm2. 50% of the medium was removed and replaced every 3 days. This method yields more than 97% pure neuronal cultures, as judged by immunocytochemistry for glial fibrillary acidic protein (GFAP) and neuron-specific microtubule-associated protein 2 (MAP-2). After the medium was changed to N-2/ Neurobasal (Invitrogen, Carlsbad, CA), thrombin was added to the differentiated neurons at 12–16 days. Unless otherwise indicated, pharmacological agents were applied 10 minutes before 30 U/ml thrombin treatments.
Cell cycle PCR-array
Four hours after thrombin incubation, total RNA from each group was isolated using the RNeasy mini plus kit according to the manufacturer’s protocol (Qiagen, Valencia, CA). Rat cell cycle RT2 Profiler PCR Array and RT2 Real-Time SyBR Green/ROX PCR Mix were purchased from SuperArray Bioscience (Frederick, MD). PCR was performed on an ABI 7900 Sequence Detector (Applied Biosystems, Foster City, CA). For data analysis the ΔΔCt method was used as previously reported (Baron et al., 2006). Fold differences were calculated for each gene as gene expression for Thrombin vs Control, PP2+Thrombin vs Thrombin, and PP2+Thrombin vs Control using the analysis: http://www.superarray.com/pcrarraydataanalysis.php.
Immunocytochemistry
Cells were fixed with ice-cooled 4% paraformaldehyde for 15 minutes or 1 part glacial acetic acid, 3 parts 95% ethanol for 12 minutes. Primary antibodies used included mouse anti-MAP-2 (1:2,000; Chemicon, Temecula, CA), rabbit anti-GFAP (1:1,000, Chemicon, Temecula, CA), and rabbit anti-cyclin D1 (1:300; Santa Cruz biotechnology, Santa Cruz, CA). These antibodies produce single bands on Western blots, and are specific for each molecule. The antibodies were incubated 1h at room temperature (RT) in PBS containing 0.1% Triton X-100 and 3% serum of the species in which the secondary antibodies were raised. Species-specific IgG, conjugated to Alexa 594 or 488 (1:1,000; Invitrogen, Carlsbad, CA), were used as secondary antibodies.
Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
TUNEL labeling was performed according to a published method (Chen et al., 1997), with minor modifications. In brief, fixed cells in slide chambers were incubated in the terminal deoxynucleotidyl-transferase (TDT) buffer (Invitrogen, Carlsbad, CA) for 10 minutes at RT and then incubated for 2h at 37°C in TDT buffer containing 10 mM biotin-aha-dUTP (Invitrogen, Carlsbad, CA) and 300 U/ml TDT (Invitrogen, Carlsbad, CA). The biotinylated dUTP 3’-OH DNA end-label was detected using the biotin-streptavidin-Alexa 594 (1:1,000; Invitrogen, Carlsbad, CA). Positive controls were obtained by pre-treating the fixed cells with 200 U/ml DNase I (Invitrogen, Carlsbad, CA) at 37°C for 30 minutes; and negative controls were checked by omission of the TDT.
Double staining for MAP-2 and TUNEL
Twenty-four hours after thrombin incubation, cells were fixed and used for MAP-2/TUNEL double staining. MAP-2 immunostaining was performed first using IgG conjugated to Alexa 488-conjugated secondary antibodies (1:1,000; Invitrogen, Carlsbad, CA). Subsequently, TUNEL labeling was performed using streptavidin-Alexa 594 (1:1,000; Invitrogen, Carlsbad, CA). Finally, slides were mounted with antifade reagent that contained 4’, 6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA).
Double staining for MAP-2 and Cyclin D1
Four hours after thrombin incubation, cells were fixed and double labeled for MAP-2 and Cyclin D1. MAP-2 labeling was performed first using streptavidin-Alexa 594 (1:1,000; Invitrogen, Carlsbad, CA). Subsequently, Cyclin D1 immunostaining was performed using IgG conjugated to Alexa 488-conjugated secondary antibodies (1:1,000; Invitrogen, Carlsbad, CA).
Cyclin-dependent kinase 4 (Cdk4) activity assay
Four hours after thrombin incubation, the Cdk4 assay was performed according to published methods (Rao et al., 2007). Briefly, the retinoblastoma (Rb) peptide is phosphorylated as a result of Cdk4 activity, which is measured using luminometric estimation of ATP depletion. Four hours after thrombin exposure, control and treated cells were lysed, and protein-extracted. Each sample was adjusted to 100 µg of total protein extract in 500 µl lysis buffer. Cdk4 was immunoprecipitated with a Cdk4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), washed three times in lysis buffer, twice in kinase buffer (Cell Signaling Technology, Danvers, MA), and re-suspended in 30 µl of kinase buffer, optimized to preserve the Cdk4 activity. To perform the kinase reaction, 20 µl of 0.1 µmol/l ATP was mixed with 2 µg Rb peptide substrate (US Biologicals, Massachusetts, MA) in kinase buffer. The reaction was incubated for 30 minutes at 30°C and then an equal volume of kinase-GLO reagent (Promega, Madison, WI) was added. Samples were then incubated for 10 minutes at RT and the resulting luminescence was measured and expressed as relative light units. Statistical differences were determined using one-way ANOVA followed by Bonferroni’s post hoc test.
Results
The effects of PP2 on thrombin-induced Src kinase activity
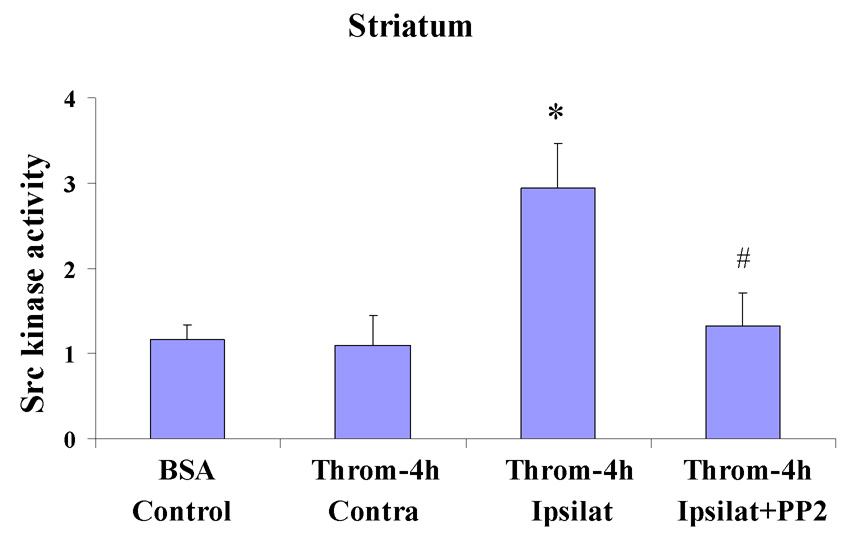
Src kinase activity assays showed similar activity in the BSA injected striatum (Fig. 1, BSA/Control) and in the striatum contralateral to the thrombin injected striatum (Fig. 1, Throm-4h/Contra). Injection of thrombin (20U) into the striatum increased Src kinase activity ~3-fold at 4h after the thrombin injection [Throm-4h/Ipsilat in Fig. 1; * p<0.05 for Throm-4h/Ipsilat (n=3) vs BSA/control (n=3) and vs Throm-4h/Contra (n=3)]. Injection of PP2 (1.0 mg/kg, i.p.) 10 minutes before the thrombin (Throm-4h/Ipsilat+PP2) blocked the increase of Src kinase activity produced by thrombin injection [Fig. 1; # p<0.05 for Throm-4h/Ipsilat+PP2 (n=3) vs Throm-4h/Ipsilat (n=3)].
Figure 1.
Src kinase activity was measured 4h after thrombin (20U) or control BSA injections into striatum of adult rats that had prior intraperitoneal administration of vehicle or PP2 (1.0 mg/kg), a Src kinase inhibitor. The Y-axis shows Src kinase activity expressed as a fold change from control (1=control levels; 3=3-fold increase in activity). Each column represents the mean ± standard error of the mean for 3 separate experiments. The BSA/Control animals had BSA injected into striatum, The next group of animals had thrombin injections into striatum and 4h later the ipsilateral striatum (Throm-4h, Ipsilat) and contralateral, un-injected striatum (Throm-4h, Contra) were removed for Src kinase activity assays. For the last group of animals PP2 was given i.p. 10 minutes before thrombin injections into striatum. Four hours after the thrombin injections this group (Throm-4h, Ipsilat+PP2) had the ipsilateral striatum removed for Src kinase activity assays. *p<0.05 for Throm-4h/Ipsilat vs Throm-4h/Contra; *p<0.05 for Throm-4h/Ipsilat vs BSA/Control. # p <0.05 for Throm-4h/Ipsilat+PP2 vs Throm-4h/Ipsilat (one-way ANOVA followed by Bonferroni’s post hoc test).
The effects of PP2 on thrombin-induced brain damage in rats
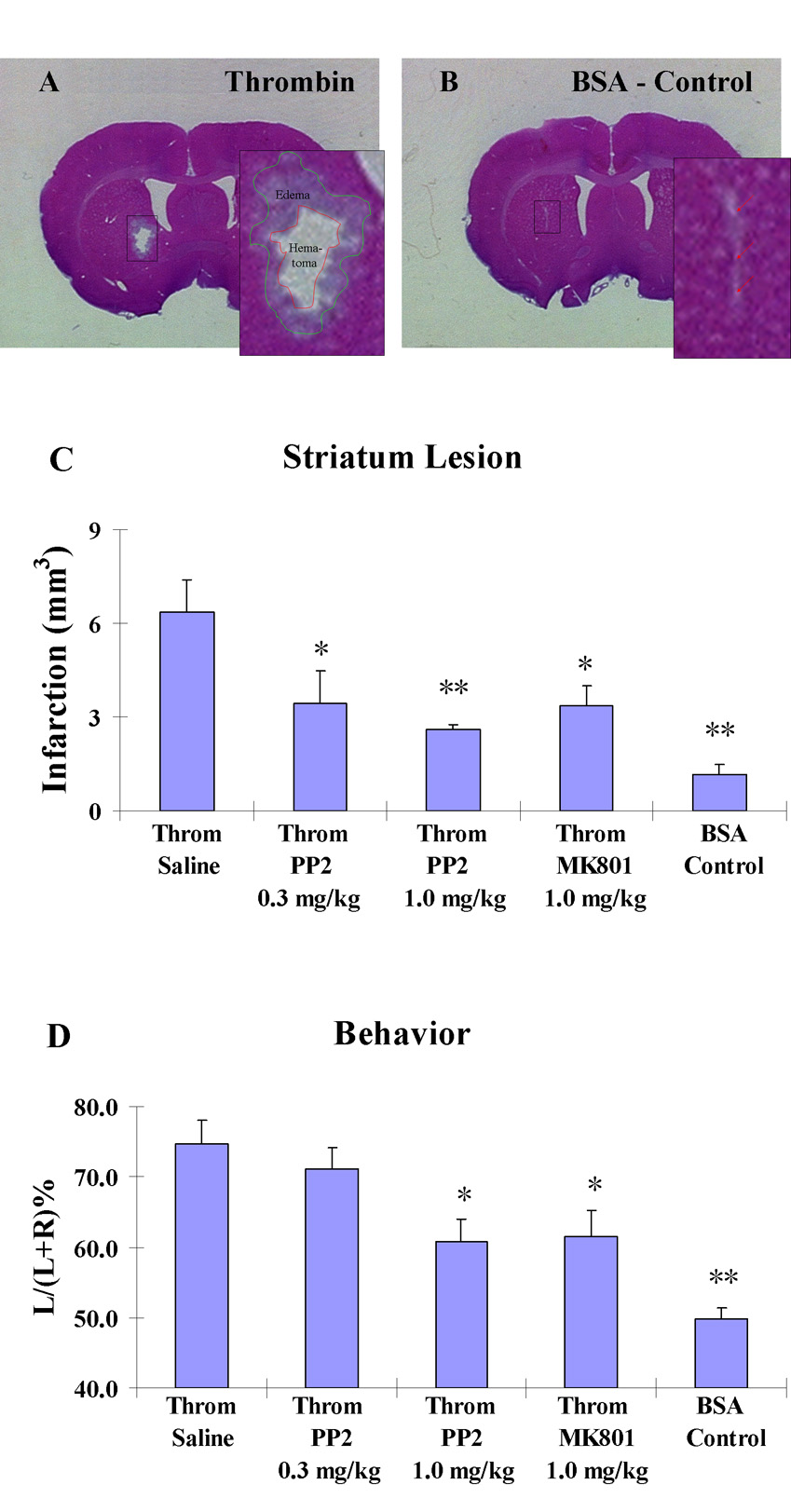
In the core of the thrombin (20U) injection site, a hematoma was observed with surrounding edema (Fig. 2A), with an infarction volume (including hematoma and edema) of 6.4±1.0 mm3 (Throm/Saline in Fig. 2C, n=6). In the BSA/Control injected group, mechanical damage was caused by needle insertion (Fig. 2B). The infarction volume was 1.1±0.3 mm3 (BSA/Control in Fig. 2C, n=6) and was significantly less than the Throm/Saline group (Fig. 2C, ** p<0.01). When pretreated with PP2 (0.3 or 1.0 mg/kg, i.p.) or MK801 (1.0 mg/kg, i.p.) 10 minutes before the injections of thrombin, there was significant reduction of infarction volume in the thrombin injected striatim (Fig. 2C). As compared to the control thrombin injected group (Throm/Saline), the infarction volume was reduced from 6.4±1.0 mm3 (n=6) to 3.5±1.0 mm3 (Throm/PP2/0.3 mg/kg, * p<0.05, n=6), to 2.6±0.2 mm3 (Throm/PP2/1.0 mg/kg, **p<0.01, n=6) and to 3.4±0.7 mm3 (Throm/MK801/1.0 mg/kg, *p<0.05, n=6), respectively (Fig. 2C).
Figure 2.
The effects of graded doses of PP2 (0.3 and 1.0 mg/kg, i.p.) and MK801 (1.0 mg/kg, i.p.) on injury produced by thrombin injections into striatum of adult rats.
A. Representative Hematoxylin-Eosin stained section shows that 20U of thrombin causes brain injury, including hematoma and edema. B. Control injections of BSA into striatum produced minor injury. C. Infarction volumes 24h following striatum injections of thrombin compared to control BSA injections (BSA/Control) (n=6). Several groups of animals received striatal injections of thrombin: just thrombin alone (Throm/Saline) (n=6); prior intraperitoneal injection of PP2 (Throm/PP2/0.3mg/kg) (n=6); prior intraperitoneal injection of a higher dose of PP2 (Throm/PP2/1.0mg/kg) (n=6); and prior intraperitoneal injection of MK801 (Throm/MK801/1.0mg/kg) (n=6). The Y-axis indicates increasing infarction volumes as described in the Materials and Methods. Each vertical bar represents the mean ± standard error of the mean. * p<0.05 and ** P<0.01 vs Throm/Saline (one-way ANOVA followed by Dunnett’s post hoc test). D. PP2 (0.3 and 1.0 mg/kg, i.p.) and MK801 (1.0 mg/kg, i.p.) decrease the thrombin-induced motor deficits (n= 9 for each group). The Y-axis indicates increasing motor deficit as described in the Materials and Methods. Each vertical bar represents the mean ± standard error of the mean. * p<0.05 and ** P<0.01 vs Throm/Saline (one-way ANOVA followed by Dunnett’s post hoc test).
The effects of PP2 on thrombin-induced motor deficits in rats
At 23.5 hours after the thrombin injections into striatum, EBST was conducted to determine the motor deficits in the unilateral striatum-injected rats. The percentage left biased swings of these lesioned rats was markedly higher than that observed in BSA/Control injected animals [Fig. 2D; 74.7±3.4% (n=9) vs. 49.8±1.7% (n = 9); ** p<0.01]. When pretreated with PP2 (0.3 or 1.0 mg/kg, i.p.) or MK801 (1.0 mg/kg, i.p.) 10 minutes before the injection of thrombin, there was a significant improvement of thrombin-induced motor deficits when compared to thrombin injected control (Throm/Saline) group injected rats (Fig. 2D). As compared to the Throm/Saline group, the percentage left biased swings was decreased from 74.7±3.4% (n=9) to 71.2±3.0% (Throm/PP2/0.3mg/kg, n=9), to 60.9±3.0% (* p<0.05 for Throm/PP2/1.0mg/kg, n=9) and 61.5±3.8% (* p<0.05 for Throm/MK801/1.0mg/kg, n=9), respectively (Fig. 2D).
Thrombin causes apoptosis in striatal neurons
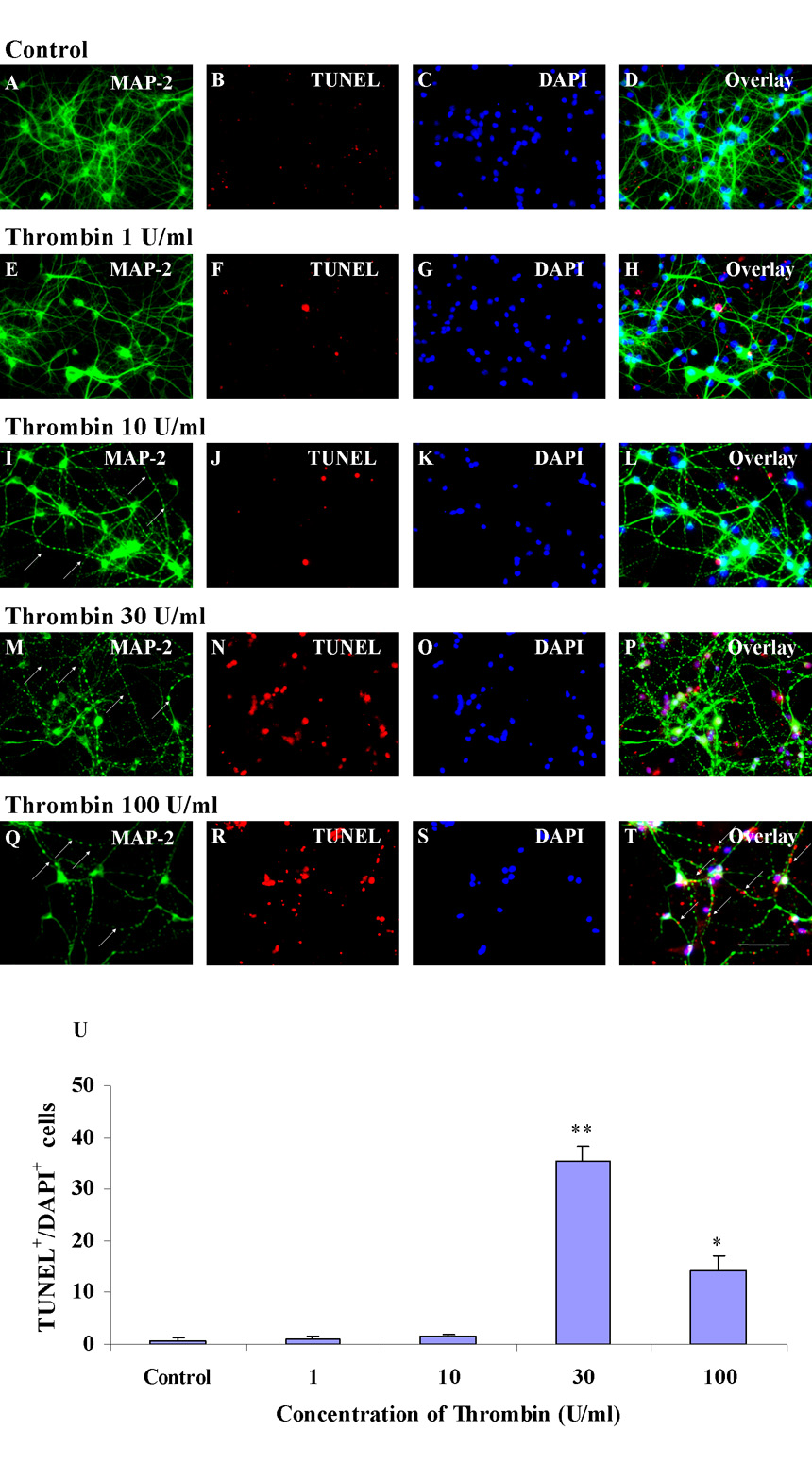
Thrombin-induced neuronal apoptosis was examined using MAP-2 and TUNEL double labeling in vitro. DAPI was used as a counter stain for nuclei. After being in culture for 12–16 days, striatal neurons were positive for MAP-2 with well-differentiated axons and dendrites (Fig. 3A). Addition of thrombin over a 1–100 U/ml range caused beading of the processes that could represent breaks or variations in the diameter of the processes (marked with arrows in Figs. 3I, M and Q) of neuronal axons and dendrites after thrombin incubation for 24h.
Figure 3.
Cultured striatal neurons die within 24h when treated with increasing doses of thrombin. Panels A–D show control striatal neurons labeled for MAP-2 (A), TUNEL (B), DAPI (C), and the overlay (D). E–H show neurons incubated with thrombin 1 U/ml. I–L show neurons incubated with thrombin 10 U/ml. M–P show neurons incubated with thrombin 30 U/ml. At this 30 U/ml dose many but not all DAPI+ /MAP-2− cells are TUNEL positive. Q–T show neurons incubated with thrombin 100 U/ml. At this 100 U/ml dose nearly all DAPI+ /MAP-2− cells are positive for TUNEL. There is TUNEL staining (arrows in T) that appears to be localized in damaged axons and dendrites. Panel U shows the quantitation of TUNEL+/DAPI+ cultured striatal neurons (Y-axis) at 24 h after exposure to increasing concentration of thrombin (X-axis). Thrombin 100 U/ml (* p<0.05) and thrombin 30 U/ml (** p<0.01) are different compared to Control whereas thrombin 1 and 10 U/ml are not statistically different from Control (one-way ANOVA followed by Bonferroni’s post hoc test). Scale bars: A–T, 100 µm.
There were very few or no MAP-2+ cells labeled for TUNEL in control cultures or following treatment with low concentrations of thrombin (Figs. 3A–L). At a thrombin concentration of 30 U/ml, a number of MAP-2 stained cells (Fig. 3M) had neuronal nuclei (Fig. 3O) that were TUNEL positive (Figs. 3N and P). When the dose of thrombin was increased to 100 U/ml, the number of MAP-2+ cells decreased (Fig. 3Q) and those left showed that the nuclei (Fig. 3S) were TUNEL positive (Figs. 3R and T). Some of the MAP-2+/ TUNEL+ profiles (marked with arrows in Fig. 3T) appeared to be located in the damaged axons and dendrites. This could suggest that damaged DNA had traveled into these neuronal processes when thrombin increased to 100 U/ml. Figure 3U shows the quantification of TUNEL+/DAPI+ cells produced by 30 U/ml and 100 U/ml thrombin. The 30 U/ml dose produced more TUNEL+ cells compared to 100U/ml. However, thrombin 100 U/ml caused much greater MAP-2+ cell death than the 30 U/ml (Fig. 3Q and M). This result is interpreted to mean that 30 U/ml thrombin injury was mostly apoptotic and 100 U/ml thrombin injury was more necrotic. Since a more moderate injury was produced by the 30 U/ml concentrations of thrombin, this concentration was selected for the subsequent experiments. Three replicates of the above experiments yielded identical results.
The effects of PP2 on thrombin-induced injury and apoptosis in striatal neurons
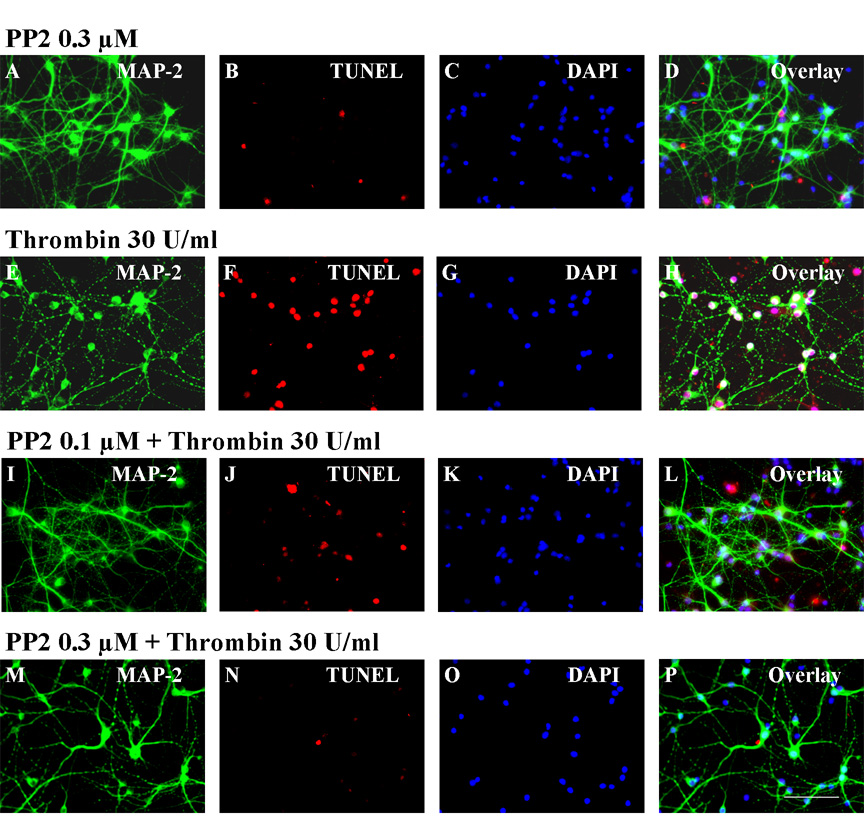
PP2, the potent Src kinase inhibitor used in the in vivo experiments, was used to test whether it would block thrombin-induced injury in vitro. In preliminary experiments we found that PP2 (0.3 µM) itself did not affect the viability of striatal neurons (Fig. 4A–D). When applied 10 minutes before thrombin treatment, PP2 slightly decreased TUNEL (Fig. 4J) positive MAP-2 labeled (Fig. 4I) neurons at a PP2 dose of 0.1 µM (Fig. 4L, overlay) as compared to control striatal neurons treated with 30U thrombin without PP2 (Figs. 4E–H). However, a PP2 dose of 0.3 µM almost completely blocked TUNEL staining (Fig. 4N) in MAP-2 positive neurons (Fig. 4M) that were treated with 30 U/ml of thrombin (Fig. 4P, overlay) as compared to striatal neurons treated with thrombin without PP2 (Figs. 4E–H). Three replicates of these experiments yielded identical results.
Figure 4.
PP2 applied just prior to thrombin decreases thrombin induced injury to cultured striatal neurons. Panels A–D show striatal neurons exposed to PP2 (0.3 µM) alone for 24h and labeled for MAP-2 (A), TUNEL (B), DAPI (C), and the overlay (D). Application of thrombin (30U/ml) produced injury to the neurons labeled for MAP-2 (E), TUNEL (F), DAPI (G) and the overlay (H). Application of 0.1 µM PP2 ten minutes prior to thrombin (30 U/ml) has a marginal effect 24h later on TUNEL stained (J) MAP-2 (I) and DAPI (K) positive striatal neurons (L, overlay). Application of 0.3 µM PP2 ten minutes prior to thrombin (30 U/ml) markedly decreases the numbers of TUNEL stained cells (N) that were MAP-2 (M) and DAPI (O) positive striatal neurons (P, overlay). Scale bar: A–P, 100 µm.
Thrombin modulates several cell cycle genes/mRNAs in striatal neurons
To begin to determine whether thrombin exerted mitogenic effects on cultured striatal neurons, a total of 84 cell cycle-related genes were evaluated with a RT-PCR array (see Materials and Methods). Thrombin tended to increase mRNA expression in as many as 78 genes as compared to the control (Supplementary Table). However, thrombin significantly increased mRNA expression in only 8 of the 84 cell cycle genes tested as compared to control (Table 1; Supplementary Table). These genes, which were significantly up-regulated at a p<0.05 or better, included: Ccnd1, Ccnd2, Cdkn2b, Chek1, Gpr132, RGD1561600, Mcm2, and LOC680111 (also known as PRB1) (Table 1; Supplementary Table).
Table 1.
mRNA expression profiling of part of cell cycle genes in striatal neurons following exposure to thrombin with or without PP2 pretreatment. For detailed information, see Supplementary Table. Striatal neurons were treated either with vehicle for 4h (Control), thrombin (30 U/ml) for 4h (Thrombin); or pretreated with PP2 (0.3 µM) for 10 minutes followed by treatment with thrombin (30 U/ml) for 4h (PP2+Thrombin). RNA was extracted from the cells and RT-PCR performed as described in the Methods. For data analysis the ΔΔCt method was used. Fold diferneces were calculated for each gene as gene expression for Thrombin vs Control, PP2+Thrombin vs Thrombin, and PP2+Thrombin vs Control using the analysis template (http://www.superarray.com/pcrarraydataanalysis.php). Rplp1 was used as one of the housekeeping genes. Three separate experiments were performed, and significant changes were determined by T-tests.
| Gene Name | Thrombin vs Control | PP2+Thrombin vs Thrombin | PP2+Thrombin vs Control | Description | |||
|---|---|---|---|---|---|---|---|
| Fold diference | t-test p value | Fold diference | t-test p value | Fold diference | t-test p value | ||
| Ccnd1 | 2.36±0.34 | ** 0.002 | 0.73±0.07 | # 0.043 | 1.74±0.33 | ^ 0.027 | Cyclin D1 |
| Ccnd2 | 2.07±0.40 | * 0.011 | 0.78±0.03 | # 0.023 | 1.64±0.38 | 0.055 | Cyclin D2 |
| Cdkn2a | 1.05±0.18 | 0.898 | 1.43±0.04 | # 0.043 | 1.50±0.23 | 0.088 | Cyclin-dependent kinase inhibitor 2A |
| Cdkn2b | 2.68±0.46 | ** 0.001 | 0.72±0.03 | ## 0.007 | 1.89±0.25 | ^^ 0.006 | Cyclin-dependent kinase inhibitor 2B (p15, inhibits CDK4) |
| Chek1 | 1.71±0.23 | * 0.024 | 1.14±0.49 | 0.928 | 2.15±1.17 | 0.294 | Checkpoint kinase 1 homolog (S. pombe) |
| Gpr132_predicted | 1.49±0.23 | * 0.034 | 0.84±0.12 | 0.213 | 1.23±0.19 | 0.235 | G protein-coupled receptor 132 (predicted) |
| RGD1561600_predicted | 1.34±0.13 | * 0.025 | 1.00±0.15 | 0.893 | 1.35±0.25 | 0.19 | Similar to E2f3 protein (predicted) |
| Mcm2_predicted | 1.67±0.27 | * 0.013 | 0.88±0.02 | 0.288 | 1.47±0.28 | 0.054 | Minichromosome maintenance deficient 2 mitotin (S. cerevisiae) (predicted) |
| Pmp22 | 2.12±0.83 | 0.137 | 0.82±0.19 | 0.451 | 1.42±0.16 | ^ 0.021 | Peripheral myelin protein 22 |
| Rad9_predicted | 1.00±0.11 | 0.931 | 1.22±0.07 | # 0.029 | 1.23±0.19 | 0.336 | RAD9 homolog (S. pombe) (predicted) |
| LOC680111 | 1.34±0.16 | * 0.038 | 0.92±0.00 | 0.505 | 1.22±0.14 | 0.103 | Similar to Retinoblastoma-like protein 1 (107 kDa retinoblastoma-associated protein) (PRB1) (P107) |
| RGD1562456_predicted | 1.53±0.34 | 0.07 | 0.97±0.11 | 0.768 | 1.41±0.11 | ^ 0.010 | Similar to S-phase kinase-associated protein 2 (F-box protein Skp2) (F-box/WD-40 protein 1) (predicted) |
| Wee1 | 1.27±0.25 | 0.234 | 1.31±0.17 | 0.215 | 1.61±0.21 | ^ 0.042 | Wee 1 homolog (S. pombe) |
| Cdk4 | 1.34±0.28 | 0.188 | 0.81±0.04 | 0.13 | 1.07±0.20 | 0.837 | Cyclin-dependent kinase 4 |
| Rplp1 | 0.97±0.16 | 0.699 | 1.16±0.12 | 0.264 | 1.09±0.11 | 0.53 | MGC72935, house keeping gene |
p<0.05
p <0.01 for Thrombin vs Control
p<0.05
p <0.01 for PP2+Thrombin vs Thrombin
p<0.05
p <0.01 for PP2+Thrombin vs Control
When compared to thrombin treated alone, PP2, applied 10 minutes prior to thrombin treatment, down-regulated mRNAs for 55 of the 84 genes (Supplementary table). However, only three of these genes were significantly down regulated at a p<0.05 level. These genes included Ccnd1, Ccnd2 and Cdkn2b, all of which were up regulated by thrombin (Table 1; Supplementary Table). Unexpectedly, PP2, applied 10 minutes prior to thrombin treatment, significantly (p<0.05) up-regulated mRNA in a few cell cycle inhibition-related genes including Cdkn2a and Rad 9 (Table 1; Supplementary Table).
Many of the genes up-regulated by thrombin compared to control were still up-regulated in the cells treated with PP2+Thrombin compared to control (Table 1; Supplementary Table). Statistically significant changes in expression (p<0.05) for the comparison of PP2+Thrombin to control (p<0.05) occurred for Ccnd1, Cdkn2b, Pmp22, RGD1562456 and Wee1 (Table 1; Supplementary Table).
PP2 blocks thrombin-induced cyclin D1 up-regulation and Cdk4 activation in cultured striatal neurons
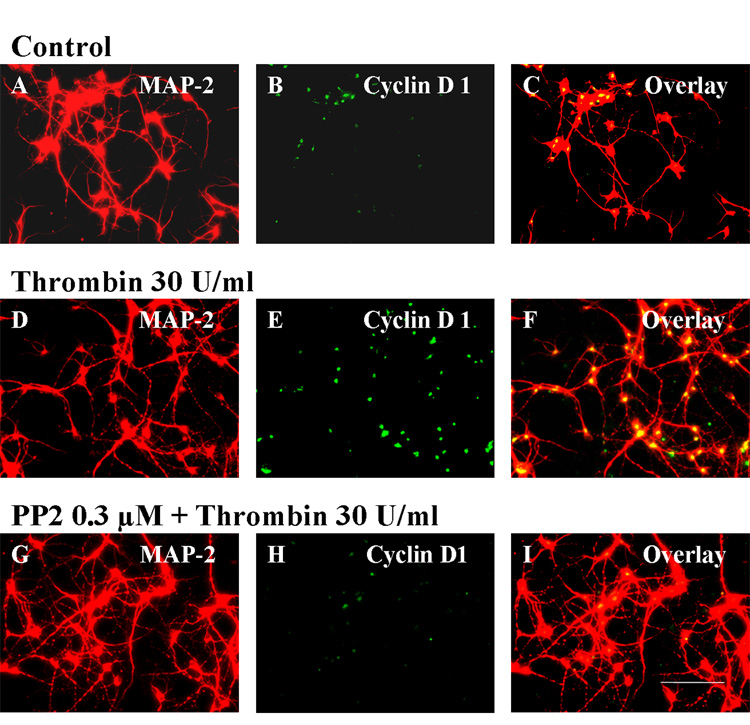
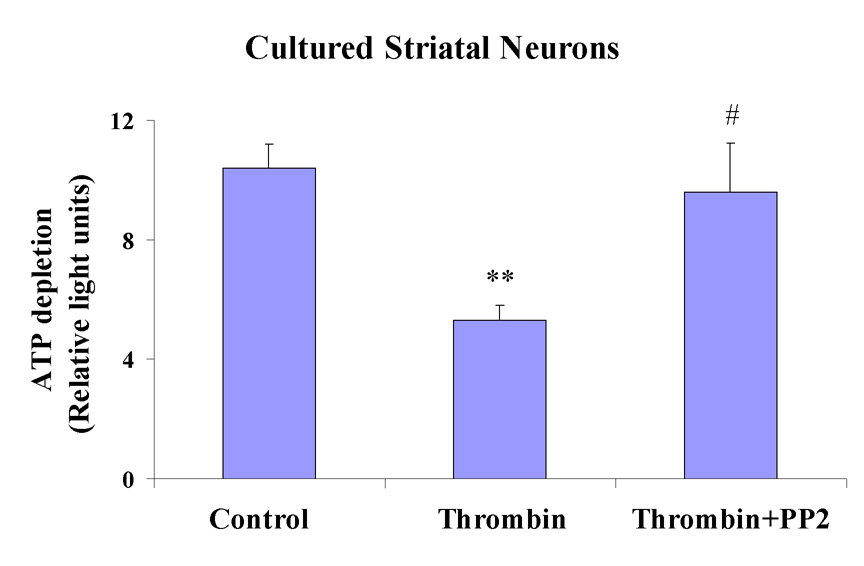
To further confirm the mitogenic effects of thrombin on cultured striatal neurons, we examined the protein expression of cyclin D1 and the activity of Cdk4, early events in cell cycle progression. After incubation with thrombin 30 U/ml for 4h, the expression of cyclin D1 (Fig. 5E) in MAP-2 positive neurons (Fig. 5D) increased markedly (Fig. 5F, overlay) compared to vehicle treated MAP-2 positive, striatal neurons (Figs. 5A–C). Similarly, after incubation in thrombin (30 U/ml) for four hours, the Cdk4 kinase was activated in cultured striatal neurons compared to vehicle control treated cells (Fig. 6). The Src kinase inhibitor, PP2, blocked both of these effects. PP2 (0.3 µM) decreased thrombin-induced cyclin D1 expression (Figs. 5H and I) in MAP-2 positive striatal neurons (Fig. 5G). PP2 also blocked Cdk4 kinase activation in thrombin treated neurons [Fig. 6; ** p<0.01 for Thrombin (n=3) vs Control (n=3); and # p<0.05 for PP2+Thrombin (n=3) vs Thrombin (n=3)]. PP2+Thrombin treated striatal neurons were not statistically different from Control (Fig. 6).
Figure 5.
The Src kinase inhibitor PP2 markedly attenuates cyclin D1 up-regulation in striatal neurons associated with a 4h exposure to thrombin. Control MAP-2 positive (A) striatal neurons treated with vehicle show some expression of cyclin D1 (B) in a few neurons (C, overlay). Incubation of MAP-2 positive striatal neurons (D) with thrombin for 4h induces cyclin D1 expression (E) in almost every neuron (F, overlay of D and E). Pretreatment with PP2 (0.3 µM) ten minutes prior to adding thrombin (30 U/ml) markedly attenuates cyclin D1 expression (H) in MAP-2 positive neurons (G) 4h later (see I for overlay of G and H). Scale bar: A–I, 100 µm.
Figure 6.
PP2 (0.3 µM) prevents Cdk4 kinase activation produced by a 4h exposure to thrombin (30 U/ml) in cultured striatal neurons. ATP depletion (Y-axis) was measured as an indicator of Cdk4 activity as described in Materials and Methods. Each vertical bar represents the mean ± standard error of the mean from 3 separate experiments. ** p<0.01 Thrombin compared to Control; # p<0.05 PP2+Thrombin compared to Thrombin. PP2+Thrombin treated neurons are not statistically different from Control (one-way ANOVA followed by Bonferroni’s post hoc test).
Discussion
The in vivo data show that thrombin injections into striatum increase Src kinase activity, cause tissue infarction and produce behavioral abnormalities that are markedly attenuated by the Src kinase inhibitor PP2. This protection is roughly equivalent to that produced by MK801. The findings are consistent with our previous report that the Src kinase inhibitor PP1 improves outcomes following whole blood injections, and the protection was similar to that afforded by MK801 (Ardizzone et al., 2004, Ardizzone et al., 2007).
Recent reports show that direct infusions of thrombin into brain cause brain edema and neuronal death in part by activating MAPK signaling (Fujimoto et al., 2007). In addition, thrombin causes apoptosis possibly by stimulating post-mitotic cortical neurons to re-enter the cell cycle (Rao et al., 2007). These observations suggest that mitogenic signaling by thrombin and aberrant cell cycle re-entry may be one mechanism by which thrombin produces neuronal apoptosis.
Traditionally, post-mitotic neurons have been thought to be terminally differentiated and in the G0 phase of the cell cycle. Although it appears that they face continuing stress to re-enter the cell cycle, a series of anti-growth factors prevent them from normally doing so (Copani and Nicoletti, 2005). A growing number of in vivo and in vitro studies, however, show that post-mitotic neurons can re-enter the cell cycle after brain injury in rat and humans (Hayashi et al., 2000, Copani et al., 2001, Love, 2003, Becker and Bonni, 2004, Greene et al., 2004, Herrup et al., 2004, Kuan et al., 2004, Di Giovanni et al., 2005, Copani et al., 1999, Liu and Greene, 2001, Rao et al., 2007). This cell cycle re-entry is a critical element of the DNA damage response of post mitotic neurons leading to apoptosis (Kruman et al., 2004).
Src kinases are important molecules in mitogenic signaling pathways and cell cycle progression (Taylor and Shalloway, 1993, Mishra et al., 2005); their phosphorylation regulates cell cycle proteins such as Cdks (Grimmler et al., 2007). The Src kinase inhibitor PP2 prevents Akt phosphorylation and constitutive activation of MAPKs (Liu et al., 2004, Kasahara et al., 2007). Thus PP2 and other Src kinase inhibitors may have anti-mitogenic properties that could prevent apoptosis when terminally differentiated cells like neurons attempt to re-enter the cell cycle
Our in vitro studies show that thrombin produces injury to dendrites, axons and nuclei of cultured striatal neurons, which is markedly attenuated by the Src kinase inhibitor PP2. This suggests that Src kinase inhibition could decrease the thrombin induced neuronal injury. In the studies of cell cycle related genes, PP2 significantly down-regulated Cdkn2b, Ccnd1, and Ccnd2 mRNAs that were up regulated by thrombin. Ccnd1 and Ccnd2 code for cyclin D1 and cyclin D2 proteins, respectively, and these cyclin D proteins activate Cdks which are required for induction of Rb phosphorylation and S phase entry (Nurse, 2000, Baker et al., 2005). This suggests that PP2 might block thrombin-induced cell cycle reactivation at the transcriptional level, in part, by down-regulating mRNA expression of cyclin D1 and cyclin D2. This is consistent with PP2 blocking thrombin-induced expression of cyclin D1 protein, and PP2 blockade of thrombin induced Cdk4 activation.
In addition, we found that PP2, applied 10 minutes prior to thrombin treatment, significantly up-regulated several cell cycle inhibition-related genes, including Cdkn2a, Rad 9, Pmp22 and Wee1, which were not significantly affected by thrombin alone. The signaling pathways of these genes may contribute, to some extent, to stabilization of the neuronal cell cycle produced by Src inhibition.
Together, our in vitro data suggest thrombin initiates neuronal G1 phase re-entry based upon thrombin induction of several cell cycle genes, thrombin induction of cyclin D1 protein, and thrombin activation of Cdk4 kinase activity. The finding that Src kinase inhibitors block thrombin induction of cyclin D1 mRNA and protein and block activation of the Cdk4 kinase, supports our hypothesis that thrombin activates a “mitogenic signaling/cell cycle/neuronal death pathway” that is blocked by Src kinase inhibitors.
The expression of cell cycle proteins, however, is not always associated with neuronal cell cycle re-activation and apoptosis. Sporadic expression of cyclin D1 was observed in control neurons in our study and in previous reports (Rao et al., 2007). However, there was no active Cdk4 detected in the control neurons in our study and in the previous reports (Rao et al., 2007). Since G1 re-entry is dependent on cyclin D1- Cdk4 complex formation, cyclin D1 expression without active Cdk4 means that the control neurons had not re-entered the cell cycle. In our studies, almost all of the thrombin treated neurons had induction of cyclin D1 that was concurrent with Cdk4 kinase activation and which was blocked by PP2. This strongly suggests that thrombin induced cell cycle re-entry occurred at least into the G1 phase of the cell cycle, and that the Src kinase blocker PP2 prevented this cell cycle re-entry.
It is less clear whether thrombin stimulates re-entry into S phase, though cyclin D1 and cyclin D2 mRNAs are induced and a retinoblastoma-associated protein (PRB1) mRNA is induced by thrombin in this study. We have previously shown that adult post-mitotic mammalian neurons in vivo can re-enter G1 and S phase, phosphorylate the Rb protein and resume DNA synthesis following hypoxia-ischemia (Kuan et al., 2004). However, the cells that reach the G1/S1 phase become TUNEL positive and die. A similar sequence seems likely for thrombin-induced cell cycle re-entry in the current study.
In conclusion, the data strongly support a role for Src kinase family members in mediating thrombin-induced injury in brain. The Src kinase inhibitor PP2 blocked thrombin activation of Src kinase activity in vivo, decreased thrombin lesion volume, and improved behavioral outcome following thrombin injections in striatum. In vitro studies showed that PP2 dose-dependently attenuated injury to the cultured neurons, decreased the numbers of TUNEL positive neurons and attenuated thrombin-induced reactivation of the cell cycle. These results are consistent with the hypotheses that Src kinase inhibitors decrease injury produced by ICH by decreasing thrombin activation of Src kinases and by, at least in part, decreasing Src induced cell cycle re-entry. The data are limited because PP2 acts on most Src kinase family members, so that future studies will be required to determine if different family members mediate different effects of thrombin or act on different cell types or downstream signaling pathways.
Supplementary Material
Acknowledgements
This study was supported by NIH grants NS054652, NS056302, NS42774 and NS28167 (Sharp FR). We thank members of the Dr. David Amaral laboratory for assistance with microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardizzone TD, Zhan X, Ander BP, Sharp FR. SRC kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke. 2007;38:1621–1625. doi: 10.1161/STROKEAHA.106.478966. [DOI] [PubMed] [Google Scholar]
- Ardizzone TD, Lu A, Wagner KR, Tang Y, Ran R, Sharp FR. Glutamate receptor blockade attenuates glucose hypermetabolism in perihematomal brain after experimental intracerebral hemorrhage in rat. Stroke. 2004;35:2587–2591. doi: 10.1161/01.STR.0000143451.14228.ff. [DOI] [PubMed] [Google Scholar]
- Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- Baker GL, Landis MW, Hinds PW. Multiple functions of D-type cyclins can antagonize pRb-mediated suppression of proliferation. Cell Cycle. 2005;4:330–338. [PubMed] [Google Scholar]
- Baluchnejadmojarad T, Roghani M. Evaluation of functional asymmetry in rats with dose-dependent lesions of dopaminergic nigrostriatal system using elevated body swing test. Physiol Behav. 2004;82:369–373. doi: 10.1016/j.physbeh.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Baron CA, Tepper CG, Liu SY, Davis RR, Wang NJ, Schanen NC, Gregg JP. Genomic and functional profiling of duplicated chromosome 15 cell lines reveal regulatory alterations in UBE3A-associated ubiquitin-proteasome pathway processes. Hum Mol Genet. 2006;15:853–869. doi: 10.1093/hmg/ddl004. [DOI] [PubMed] [Google Scholar]
- Becker EB, Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol. 2004;72:1–25. doi: 10.1016/j.pneurobio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Sanberg PR. Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15:5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69:232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- Copani A, Nicoletti F. Cell-cycle mechanisms and neuronal cell death. New York: Kluwer Academic/Plenum; 2005. [Google Scholar]
- Copani A, Uberti D, Sortino MA, Bruno V, Nicoletti F, Memo M. Activation of cell-cycle-associated proteins in neuronal death: a mandatory or dispensable path? Trends Neurosci. 2001;24:25–31. doi: 10.1016/s0166-2236(00)01663-5. [DOI] [PubMed] [Google Scholar]
- Copani A, Condorelli F, Caruso A, Vancheri C, Sala A, Giuffrida Stella AM, Canonico PL, Nicoletti F, Sortino MA. Mitotic signaling by beta-amyloid causes neuronal death. Faseb J. 1999;13:2225–2234. [PubMed] [Google Scholar]
- Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden AI. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci U S A. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan FM, Cunningham DD. Signaling pathways involved in thrombin-induced cell protection. J Biol Chem. 1998;273:12746–12752. doi: 10.1074/jbc.273.21.12746. [DOI] [PubMed] [Google Scholar]
- Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci. 1997;17:5316–5326. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann I, Yoles E, Schwartz M. Thrombin attenuation is neuroprotective in the injured rat optic nerve. J Neurochem. 2001;76:641–649. doi: 10.1046/j.1471-4159.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Thrombin induces striatal neurotoxicity depending on mitogen-activated protein kinase pathways in vivo. Neuroscience. 2007;144:694–701. doi: 10.1016/j.neuroscience.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Greene LA, Biswas SC, Liu DX. Cell cycle molecules and vertebrate neuron death: E2F at the hub. Cell Death Differ. 2004;11:49–60. doi: 10.1038/sj.cdd.4401341. [DOI] [PubMed] [Google Scholar]
- Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB, Jakel H, Kullmann M, Kriwacki RW, Hengst L. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Sakai K, Sasaki C, Zhang WR, Abe K. Phosphorylation of retinoblastoma protein in rat brain after transient middle cerebral artery occlusion. Neuropathol Appl Neurobiol. 2000;26:390–397. doi: 10.1046/j.1365-2990.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J Neurosci. 2004;24:9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K, Nakayama Y, Nakazato Y, Ikeda K, Kuga T, Yamaguchi N. Src signaling regulates completion of abscission in cytokinesis through ERK/MAPK activation at the midbody. J Biol Chem. 2007;282:5327–5339. doi: 10.1074/jbc.M608396200. [DOI] [PubMed] [Google Scholar]
- Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, Emokpae R, Jr, Gorospe M, Mattson MP. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41:549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, Sharp FR, Rakic P. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Chen HY, Wu TS, Chen TY, Ayoub IA, Maynard KI. Acute administration of Ginkgo biloba extract (EGb 761) affords neuroprotection against permanent and transient focal cerebral ischemia in Sprague-Dawley rats. J Neurosci Res. 2002;68:636–645. doi: 10.1002/jnr.10251. [DOI] [PubMed] [Google Scholar]
- Liu DX, Greene LA. Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res. 2001;305:217–228. doi: 10.1007/s004410100396. [DOI] [PubMed] [Google Scholar]
- Liu DZ, Xie KQ, Ji XQ, Ye Y, Jiang CL, Zhu XZ. Neuroprotective effect of paeoniflorin on cerebral ischemic rat by activating adenosine A1 receptor in a manner different from its classical agonists. Br J Pharmacol. 2005;146:604–611. doi: 10.1038/sj.bjp.0706335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Falola J, Zhu X, Gu Y, Kim LT, Sarosi GA, Anthony T, Nwariaku FE. Antiproliferative effects of Src inhibition on medullary thyroid cancer. J Clin Endocrinol Metab. 2004;89:3503–3509. doi: 10.1210/jc.2003-031917. [DOI] [PubMed] [Google Scholar]
- Love S. Neuronal expression of cell cycle-related proteins after brain ischaemia in man. Neurosci Lett. 2003;353:29–32. doi: 10.1016/j.neulet.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Lu A, Tang Y, Ran R, Ardizzone TL, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2006;26:230–252. doi: 10.1038/sj.jcbfm.9600183. [DOI] [PubMed] [Google Scholar]
- Mishra R, Wang Y, Simonson MS. Cell cycle signaling by endothelin-1 requires Src nonreceptor protein tyrosine kinase. Mol Pharmacol. 2005;67:2049–2056. doi: 10.1124/mol.104.010546. [DOI] [PubMed] [Google Scholar]
- Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–78. doi: 10.1016/s0092-8674(00)81684-0. [DOI] [PubMed] [Google Scholar]
- Oda H, Kumar S, Howley PM. Regulation of the Src family tyrosine kinase Blk through E6AP-mediated ubiquitination. Proc Natl Acad Sci U S A. 1999;96:9557–9562. doi: 10.1073/pnas.96.17.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotatic Coordinates. London: Academic Press; 1998. [Google Scholar]
- Rao HV, Thirumangalakudi L, Desmond P, Grammas P. Cyclin D1, cdk4, and Bim are involved in thrombin-induced apoptosis in cultured cortical neurons. J Neurochem. 2007;101:498–505. doi: 10.1111/j.1471-4159.2006.04389.x. [DOI] [PubMed] [Google Scholar]
- Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci U S A. 2000;97:2264–2269. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume [see comments] J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Tang Y, Lu A, Aronow BJ, Wagner KR, Sharp FR. Genomic responses of the brain to ischemic stroke, intracerebral haemorrhage, kainate seizures, hypoglycemia, and hypoxia. Eur J Neurosci. 2002;15:1937–1952. doi: 10.1046/j.1460-9568.2002.02030.x. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Shalloway D. The cell cycle and c-Src. Curr Opin Genet Dev. 1993;3:26–34. doi: 10.1016/s0959-437x(05)80337-5. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J Neurosci. 1995;15:5389–5401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Reiser G. Thrombin signaling in the brain: the role of protease-activated receptors. Biol Chem. 2003;384:193–202. doi: 10.1515/BC.2003.021. [DOI] [PubMed] [Google Scholar]
- Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem. 2003a;84:3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Xi G, Wu J, Jiang Y, Hua Y, Keep RF, Hoff JT. Thrombin preconditioning upregulates transferrin and transferrin receptor and reduces brain edema induced by lysed red blood cells. Acta Neurochir Suppl. 2003b;86:449–452. doi: 10.1007/978-3-7091-0651-8_92. [DOI] [PubMed] [Google Scholar]
- Xue M, Hollenberg MD, Yong VW. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J Neurosci. 2006;26:10281–10291. doi: 10.1523/JNEUROSCI.2806-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.