Abstract
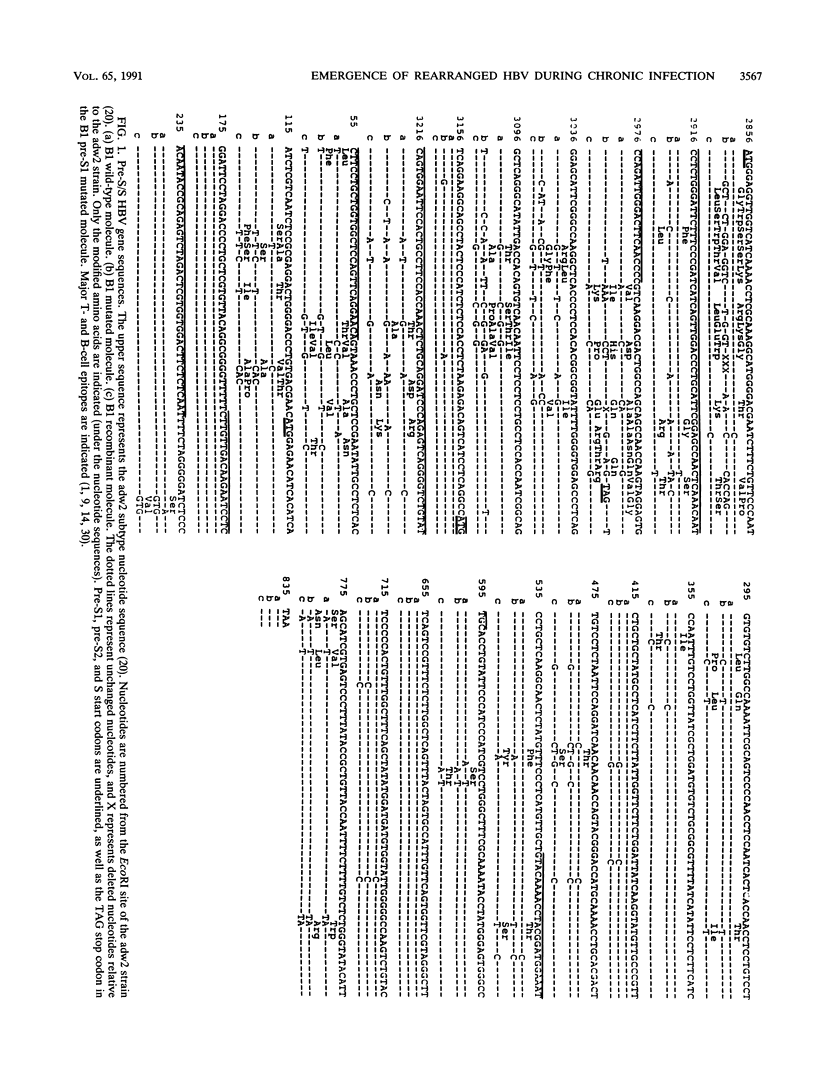
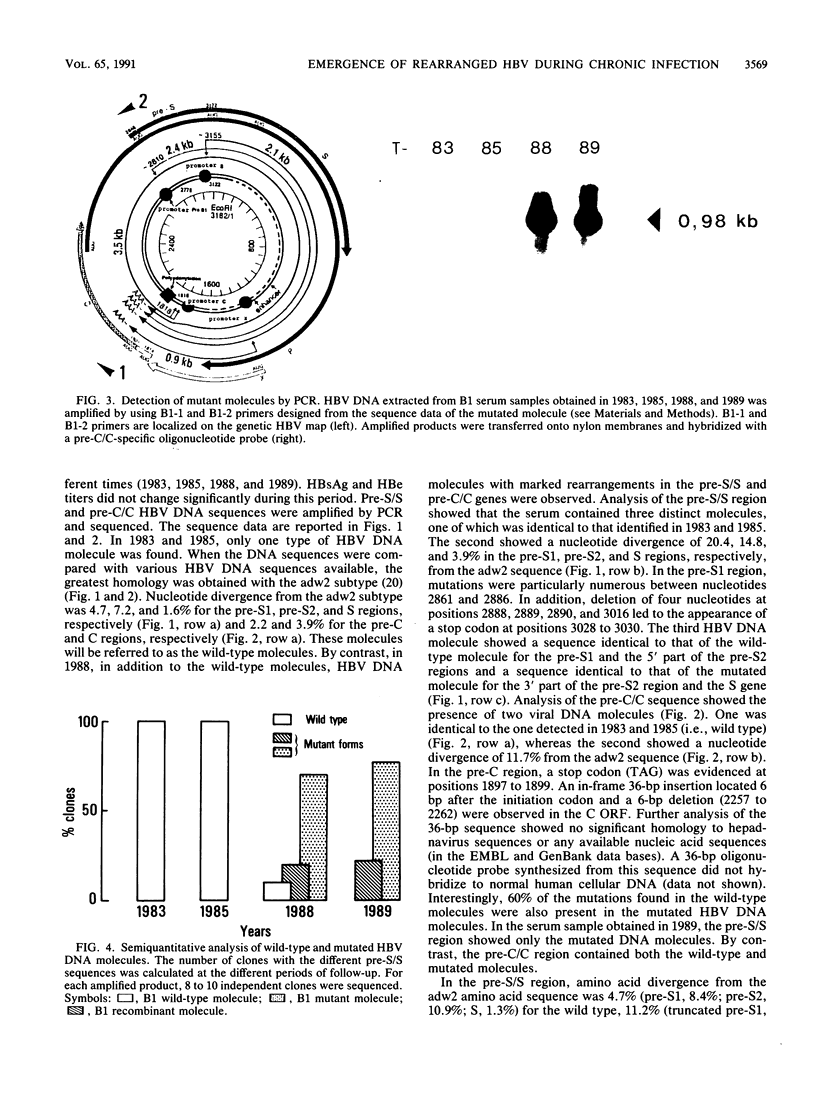
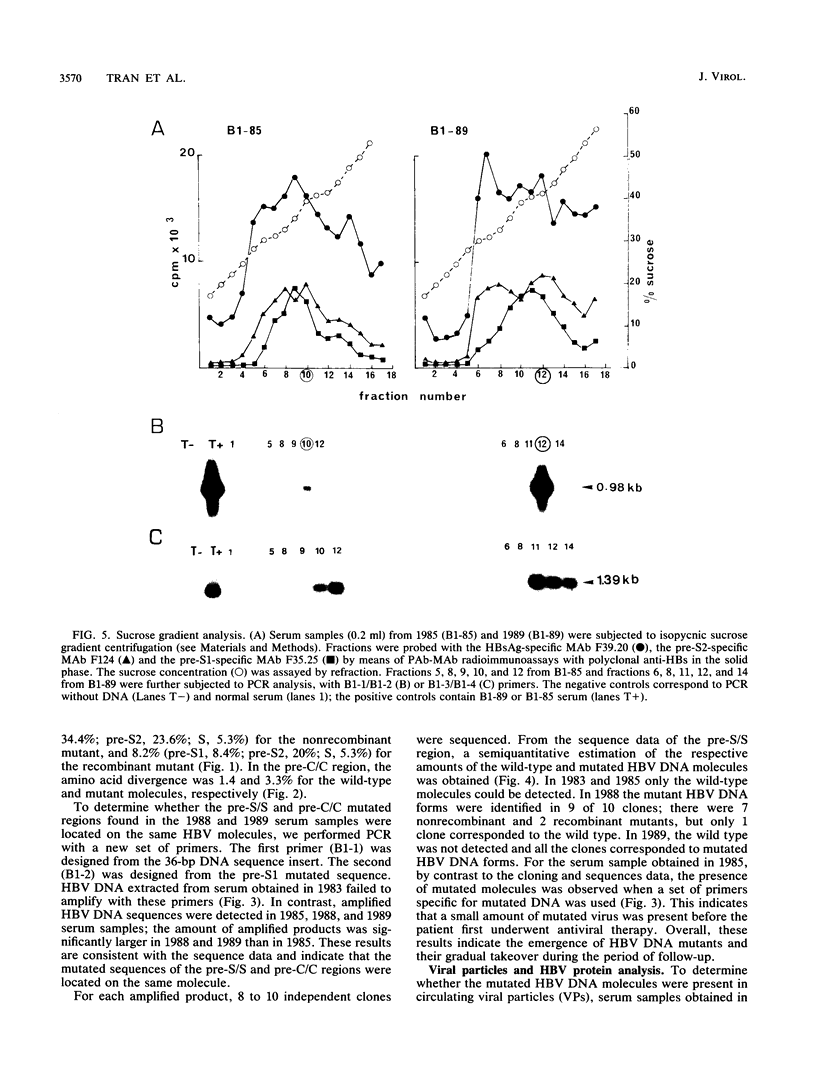
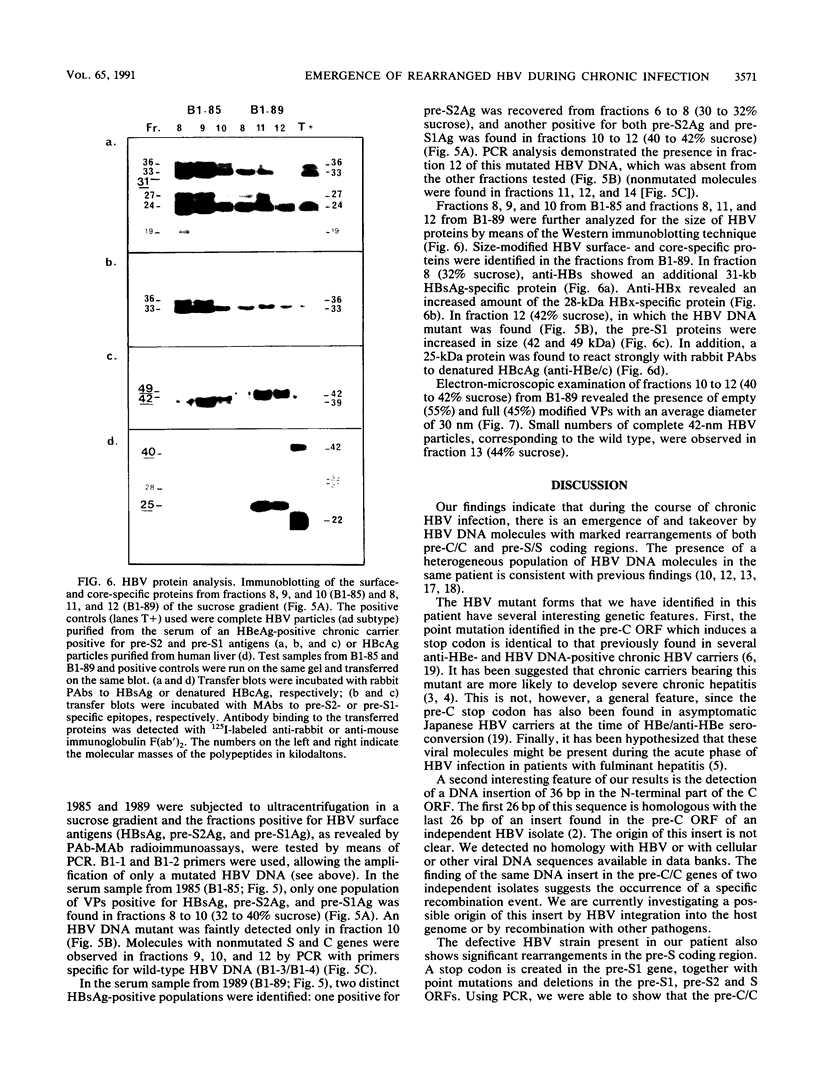
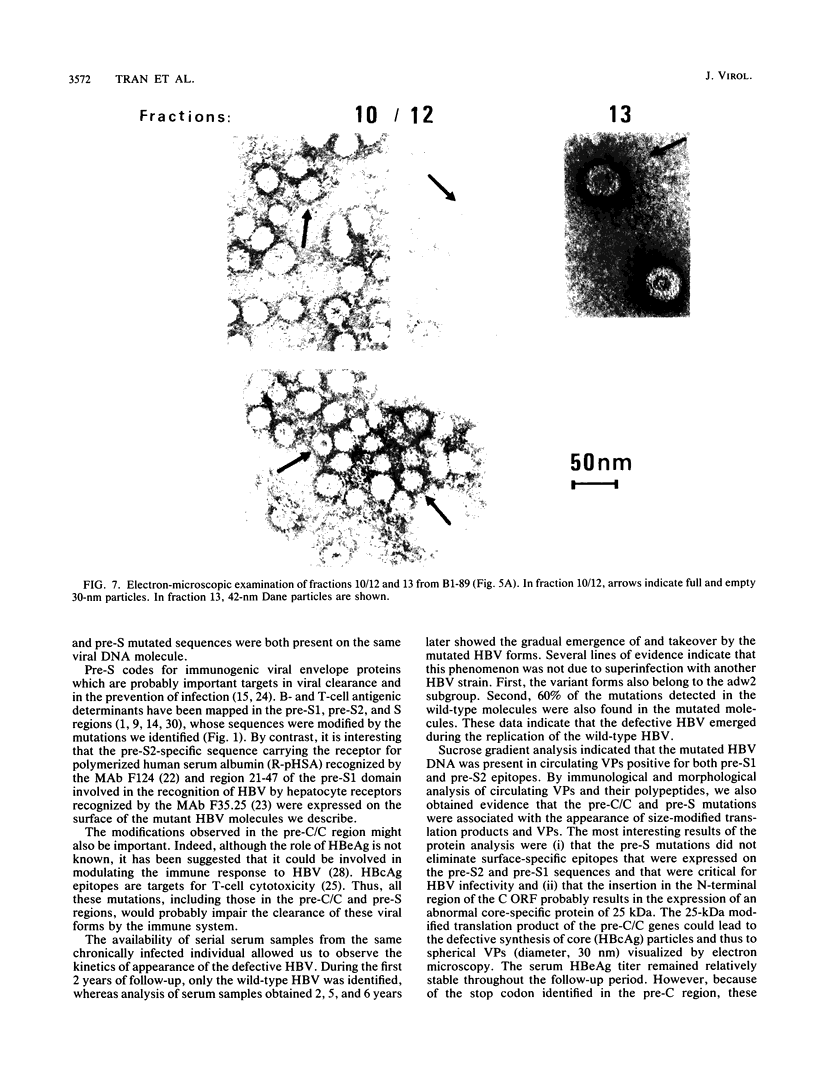
We have shown, by analyzing serial serum samples from a chronic hepatitis B virus (HBV) carrier, the emergence of HBV DNA molecules with nucleotide rearrangements in the pre-S/S and pre-C/C genes. Serum samples were obtained at four different times (1983, 1985, 1988, and 1989) from an HBsAg- and HBeAg-positive carrier with chronic hepatitis. The polymerase chain reaction was used to amplify the pre-S/S and pre-C/C genes. The amplified products were cloned, and 8 to 10 independent clones were sequenced. In 1983 and 1985 only one type of HBV DNA molecule was observed. Nucleotide divergence relative to the adw2 subtype was 4.7, 7.2, and 1.6%, for the pre-S1, pre-S2, and S regions, respectively, and 2.2 and 3.9% for the pre-C and C regions, respectively. In 1988 and 1989, HBV DNA forms with marked rearrangements of both the pre-S/S and pre-C/C regions were evidenced. In the pre-S/S region, they comprised two distinct HBV DNA molecules. The first showed nucleotide divergence of 20.4, 14.8, and 3.3% for the pre-S1, pre-S2, and S regions when compared with the adw2 sequence. In addition, nucleotide deletions in the pre-S1 region led to the appearance of a stop codon. The second was created by recombination between the original and mutated HBV DNA. In the pre-C/C region, the mutated viral DNA showed 11.7% divergence when compared with the adw2 sequence. A point mutation led to the creation of a stop codon in the pre-C region, together with an insertion of 36 nucleic acids in the core gene. Most of this DNA insertion was identical to that reported in an independent HBV isolate but showed no significant homology with known sequences. Semiquantitative estimation of the proportion of wild-type and mutated HBV DNA molecules showed a marked increase in the mutated forms during the period of follow-up. Sucrose gradient analysis indicated that the defective HBV DNA molecules were present in circulating virions. Western immunoblot analysis showed the appearance of modified translation products. Our findings thus indicate the emergence of and gradual takeover by mutated HBV DNA forms during the HBV chronic carrier state. The rearrangements we observed in the pre-S/S and pre-C/C genes might lead to changes in the immunogenicity of the viral particles and thus affect the clearance of the virus by the immune system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnaba V., Franco A., Alberti A., Balsano C., Benvenuto R., Balsano F. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J Immunol. 1989 Oct 15;143(8):2650–2655. [PubMed] [Google Scholar]
- Bhat R. A., Ulrich P. P., Vyas G. N. Molecular characterization of a new variant of hepatitis B virus in a persistently infected homosexual man. Hepatology. 1990 Feb;11(2):271–276. doi: 10.1002/hep.1840110218. [DOI] [PubMed] [Google Scholar]
- Brunetto M. R., Stemler M., Bonino F., Schodel F., Oliveri F., Rizzetto M., Verme G., Will H. A new hepatitis B virus strain in patients with severe anti-HBe positive chronic hepatitis B. J Hepatol. 1990 Mar;10(2):258–261. doi: 10.1016/0168-8278(90)90062-v. [DOI] [PubMed] [Google Scholar]
- Carman W. F., Jacyna M. R., Hadziyannis S., Karayiannis P., McGarvey M. J., Makris A., Thomas H. C. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989 Sep 9;2(8663):588–591. doi: 10.1016/s0140-6736(89)90713-7. [DOI] [PubMed] [Google Scholar]
- Carman W. F., Zanetti A. R., Karayiannis P., Waters J., Manzillo G., Tanzi E., Zuckerman A. J., Thomas H. C. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990 Aug 11;336(8711):325–329. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Jin Y., Shih W. K., Berkower I. Human T cell response to the surface antigen of hepatitis B virus (HBsAg). Endosomal and nonendosomal processing pathways are accessible to both endogenous and exogenous antigen. J Exp Med. 1988 Jul 1;168(1):293–306. doi: 10.1084/jem.168.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Miller R. H. Heterogeneity of the core gene sequence in a patient chronically infected with hepatitis B virus. J Infect Dis. 1989 Nov;160(5):903–904. doi: 10.1093/infdis/160.5.903. [DOI] [PubMed] [Google Scholar]
- Kekulé A. S., Lauer U., Meyer M., Caselmann W. H., Hofschneider P. H., Koshy R. The preS2/S region of integrated hepatitis B virus DNA encodes a transcriptional transactivator. Nature. 1990 Feb 1;343(6257):457–461. doi: 10.1038/343457a0. [DOI] [PubMed] [Google Scholar]
- Milich D. R. T- and B-cell recognition of hepatitis B viral antigens. Immunol Today. 1988 Dec;9(12):380–386. doi: 10.1016/0167-5699(88)91239-X. [DOI] [PubMed] [Google Scholar]
- Mondelli M. U., Manns M., Ferrari C. Does the immune response play a role in the pathogenesis of chronic liver disease? Arch Pathol Lab Med. 1988 May;112(5):489–497. [PubMed] [Google Scholar]
- Okamoto H., Imai M., Kametani M., Nakamura T., Mayumi M. Genomic heterogeneity of hepatitis B virus in a 54-year-old woman who contracted the infection through materno-fetal transmission. Jpn J Exp Med. 1987 Aug;57(4):231–236. [PubMed] [Google Scholar]
- Okamoto H., Imai M., Tsuda F., Tanaka T., Miyakawa Y., Mayumi M. Point mutation in the S gene of hepatitis B virus for a d/y or w/r subtypic change in two blood donors carrying a surface antigen of compound subtype adyr or adwr. J Virol. 1987 Oct;61(10):3030–3034. doi: 10.1128/jvi.61.10.3030-3034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Tsuda F., Mayumi M. Defective mutants of hepatitis B virus in the circulation of symptom-free carriers. Jpn J Exp Med. 1987 Aug;57(4):217–221. [PubMed] [Google Scholar]
- Okamoto H., Yotsumoto S., Akahane Y., Yamanaka T., Miyazaki Y., Sugai Y., Tsuda F., Tanaka T., Miyakawa Y., Mayumi M. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J Virol. 1990 Mar;64(3):1298–1303. doi: 10.1128/jvi.64.3.1298-1303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Onda H., Sasada R., Igarashi K., Sugino Y., Nishioka K. The complete nucleotide sequences of the cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids Res. 1983 Mar 25;11(6):1747–1757. doi: 10.1093/nar/11.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orito E., Mizokami M., Ina Y., Moriyama E. N., Kameshima N., Yamamoto M., Gojobori T. Host-independent evolution and a genetic classification of the hepadnavirus family based on nucleotide sequences. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7059–7062. doi: 10.1073/pnas.86.18.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit M. A., Dubanchet S., Capel F. A monoclonal antibody specific for the hepatocyte receptor binding site on hepatitis B virus. Mol Immunol. 1989 Jun;26(6):531–537. doi: 10.1016/0161-5890(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Zoulim F., Capel F., Dubanchet S., Dauguet C., Trepo C. Variable expression of preS1 antigen in serum during chronic hepatitis B virus infection: an accurate marker for the level of hepatitis B virus replication. Hepatology. 1990 May;11(5):809–814. doi: 10.1002/hep.1840110515. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Waters J., Lever A., Iwarson S., Gerety R., Thomas H. C. Cytotoxic T-cell responses to the nucleocapsid proteins of HBV in chronic hepatitis. Evidence that antibody modulation may cause protracted infection. J Hepatol. 1987 Feb;4(1):15–21. doi: 10.1016/s0168-8278(87)80004-1. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schalm S. W., Heytink R. A., van Buuren H. R., de Man R. A. Acyclovir enhances the antiviral effect of interferon in chronic hepatitis B. Lancet. 1985 Aug 17;2(8451):358–360. doi: 10.1016/s0140-6736(85)92498-5. [DOI] [PubMed] [Google Scholar]
- Schlicht H. J., Schaller H. The secretory core protein of human hepatitis B virus is expressed on the cell surface. J Virol. 1989 Dec;63(12):5399–5404. doi: 10.1128/jvi.63.12.5399-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto J., Hadchouel M., Hery C., Yvart J., Tiollais P., Brechot C. Detection of hepatitis B virus DNA in serum by a simple spot hybridization technique: comparison with results for other viral markers. Hepatology. 1983 May-Jun;3(3):279–284. doi: 10.1002/hep.1840030301. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Sisley B. M., Stanley C., Brown S. E., Howard C. R. Immunity to hepatitis B: analysis of antibody and cellular responses in recipients of a plasma-derived vaccine using synthetic peptides mimicking S and pre-S regions. Clin Exp Immunol. 1988 Jan;71(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smith P. M., Horwich A. L. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990 Jun;64(6):2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers V., Nakajima E., Kremsdorf D., Mack D., Schellekens H., Driss F., Goudeau A., Wands J., Sninsky J., Tiollais P. Transmission of hepatitis B from hepatitis-B-seronegative subjects. Lancet. 1988 Dec 3;2(8623):1273–1276. doi: 10.1016/s0140-6736(88)92891-7. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]