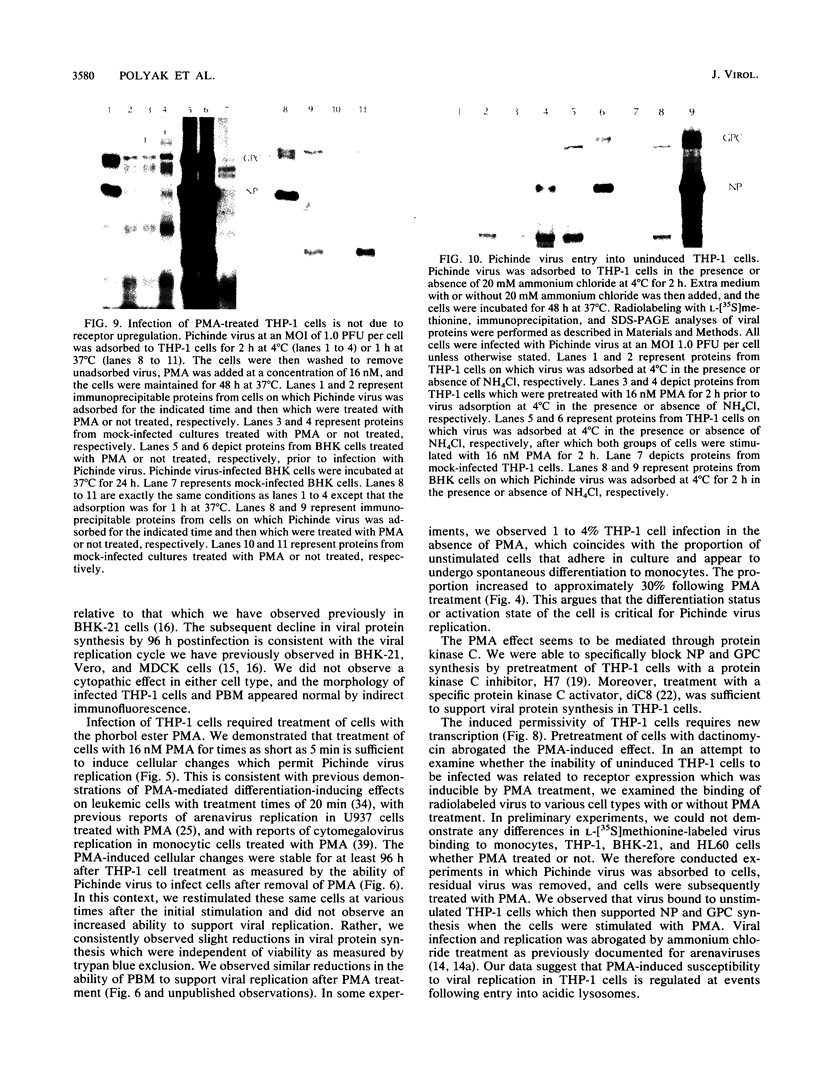
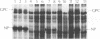Abstract
To establish a model of viral infection of monocytes, we examined infection of human cells and cell lines of the monocytic series with the arenavirus Pichinde virus. We demonstrate for the first time that human peripheral blood monocytes are susceptible to Pichinde virus infection, as shown by immunoprecipatation of virus-specific polypeptides from infected cells, immunofluorescence analyses, and quantitation of virus production from infected cells. The human promyelocytic leukemia cell line HL60 did not support Pichinde virus replication, even if cells were induced with the phorbol ester phorbol myristate acetate (PMA) to differentiate to monocytes. However, the human promonocytic leukemia cell line THP-1 did support Pichinde virus replication. Replication depended on exposure of the cells to PMA. We examined the nature of the effect of PMA in the induction of THP-1 cells to support Pichinde virus replication. We found that 5 min of exposure of THP-1 cells to PMA is sufficient to support virus growth and that PMA-treated THP-1 cells remain susceptible to infection up to 4 days after the initial PMA treatment. We also showed that infection of PMA-treated THP-1 cells is mediated through protein kinase C (PKC). H7, a PKC inhibitor, was able to block both PMA-induced differentiation and Pichinde virus infection of THP-1 cells. The synthetic diacylglycerol and PKC agonist, diC8, was able to stimulate THP-1 cells to support virus growth, albeit to lower levels than PMA. Dactinomycin abrogated the ability of virus to replicate and suggested a requirement for host cell transcription. The PMA effect did not appear to relate to receptor modulation. These results suggest that PMA-induced susceptibility to Pichinde virus infection occurs at a point later than the initial binding and penetration stages and that infection depends on the activation or differentiation state of the cell.
Full text
PDF


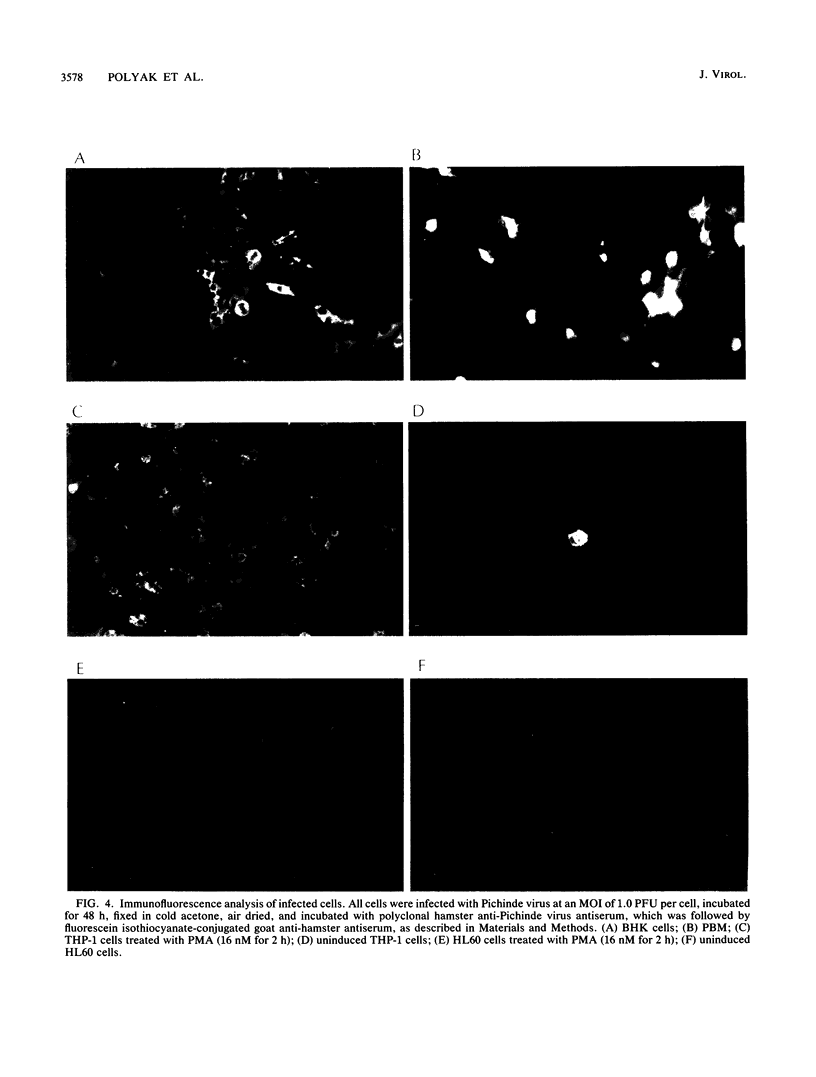



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Boldogh I., Fons M., AbuBakar S., Deng C. Z. Cell activation signals and the pathogenesis of human cytomegalovirus. Intervirology. 1990;31(2-4):68–75. doi: 10.1159/000150140. [DOI] [PubMed] [Google Scholar]
- Ambrosio M., Vallejos A., Saavedra C., Maiztegui J. I. Junin virus replication in peripheral blood mononuclear cells of patients with Argentine haemorrhagic fever. Acta Virol. 1990 Feb;34(1):58–63. [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Banerjee S. N., Buchmeier M., Rawls W. E. Requirement of cell nucleus for the replication of an arenavirus. Intervirology. 1975;6(3):190–196. doi: 10.1159/000149472. [DOI] [PubMed] [Google Scholar]
- Baxt B. Effect of lysosomotropic compounds on early events in foot-and-mouth disease virus replication. Virus Res. 1987 May;7(3):257–271. doi: 10.1016/0168-1702(87)90032-3. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Buck L. L., Pfau C. J. Inhibition of lymphocytic choriomeningitis virus replication by actinomycin D and 6-azauridine. Virology. 1969 Apr;37(4):698–701. doi: 10.1016/0042-6822(69)90294-3. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Friedlander A. M., Jahrling P. B., Merrill P., Tobery S. Inhibition of mouse peritoneal macrophage DNA synthesis by infection with the arenavirus Pichinde. Infect Immun. 1984 Jan;43(1):283–288. doi: 10.1128/iai.43.1.283-288.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Glushakova S. E., Lukashevich I. S. Early events in arenavirus replication are sensitive to lysosomotropic compounds. Arch Virol. 1989;104(1-2):157–161. doi: 10.1007/BF01313817. [DOI] [PubMed] [Google Scholar]
- Harnish D. G., Leung W. C., Rawls W. E. Characterization of polypeptides immunoprecipitable from Pichinde virus-infected BHK-21 cells. J Virol. 1981 Jun;38(3):840–848. doi: 10.1128/jvi.38.3.840-848.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Helenius A., Marsh M., White J. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J Gen Virol. 1982 Jan;58(Pt 1):47–61. doi: 10.1099/0022-1317-58-1-47. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., McHugh T., Casavant C., Stites D., Oshiro L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology. 1985 Dec;147(2):441–448. doi: 10.1016/0042-6822(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Lewis R. M., Morrill J. C., Jahrling P. B., Cosgriff T. M. Replication of hemorrhagic fever viruses in monocytic cells. Rev Infect Dis. 1989 May-Jun;11 (Suppl 4):S736–S742. doi: 10.1093/clinids/11.supplement_4.s736. [DOI] [PubMed] [Google Scholar]
- Lukashevich I. S., Lemeshko N. N., Shkolina T. V. Vliianie aktinomitsina D na reproduktsiiu virusa Machupo. Vopr Virusol. 1984 Sep-Oct;29(5):569–572. [PubMed] [Google Scholar]
- López R., Franze-Fernández M. T. Effect of tacaribe virus infection on host cell protein and nucleic acid synthesis. J Gen Virol. 1985 Aug;66(Pt 8):1753–1761. doi: 10.1099/0022-1317-66-8-1753. [DOI] [PubMed] [Google Scholar]
- López R., Grau O., Franze-Fernández M. T. Effect of actinomycin D on arenavirus growth and estimation of the generation time for a virus particle. Virus Res. 1986 Aug;5(2-3):213–220. doi: 10.1016/0168-1702(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Mifune K., Carter M., Rawls W. Characterization studies of the Pichinde virus-a member of the arenavirus group. Proc Soc Exp Biol Med. 1971 Feb;136(2):637–644. doi: 10.3181/00379727-136-35330. [DOI] [PubMed] [Google Scholar]
- Mims C. A. Immunofluorescence study of the carrier state and mechanism of vertical transmission in lymphocytic choriomeningitis virus infection in mice. J Pathol Bacteriol. 1966 Apr;91(2):395–402. doi: 10.1002/path.1700910214. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Banerjee S. N., McMillan C. A., Buchmeier M. J. Inhibition of Pichinde virus replication by actinomycin D. J Gen Virol. 1976 Dec;33(3):421–434. doi: 10.1099/0022-1317-33-3-421. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Chan M. A., Gee S. R. Mechanisms of persistence in arenavirus infections: a brief review. Can J Microbiol. 1981 Jun;27(6):568–574. doi: 10.1139/m81-086. [DOI] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. J., Weiss R. A. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1988 Mar 11;52(5):631–633. doi: 10.1016/0092-8674(88)90397-2. [DOI] [PubMed] [Google Scholar]
- Schmidt M. R., Woodland R. T. Virus-lymphocyte interactions: inductive signals necessary to render B lymphocytes susceptible to vesicular stomatitis virus infection. J Virol. 1990 Jul;64(7):3289–3296. doi: 10.1128/jvi.64.7.3289-3296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Weinshenker B. G., Wilton S., Rice G. P. Phorbol ester-induced differentiation permits productive human cytomegalovirus infection in a monocytic cell line. J Immunol. 1988 Mar 1;140(5):1625–1631. [PubMed] [Google Scholar]
- Young P. R., Chanas A. C., Lee S. R., Gould E. A., Howard C. R. Localization of an arenavirus protein in the nuclei of infected cells. J Gen Virol. 1987 Sep;68(Pt 9):2465–2470. doi: 10.1099/0022-1317-68-9-2465. [DOI] [PubMed] [Google Scholar]