Abstract
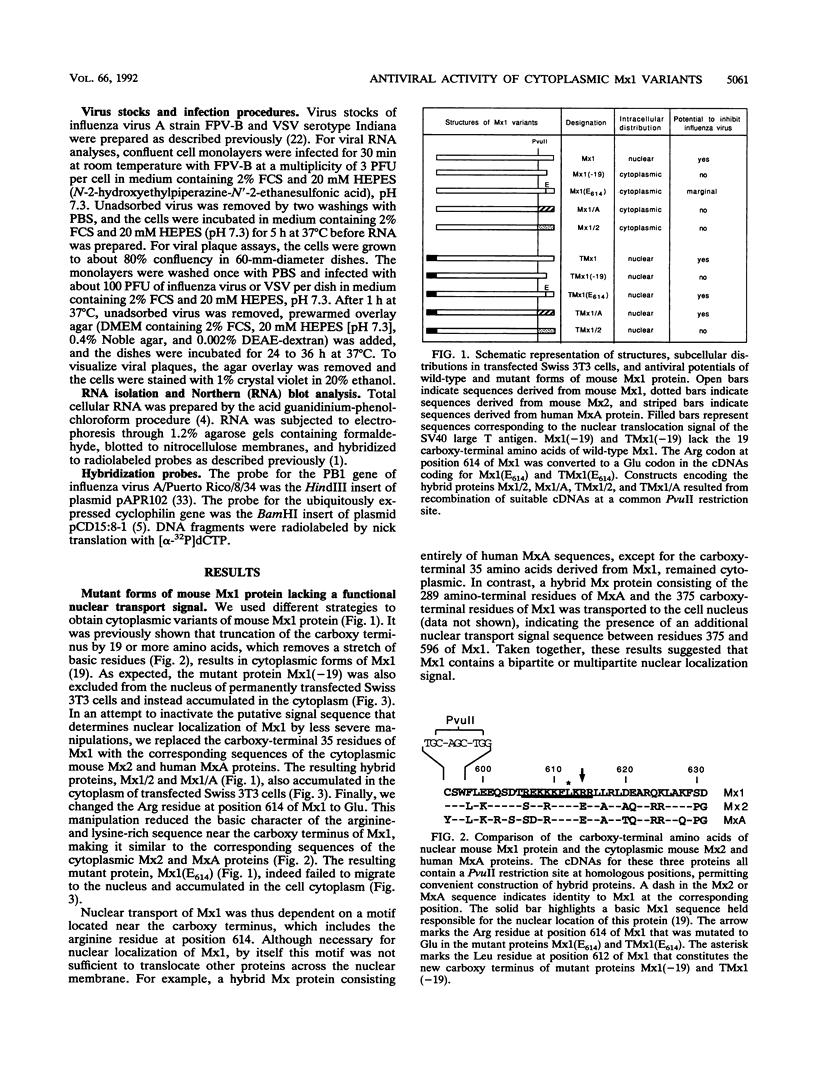
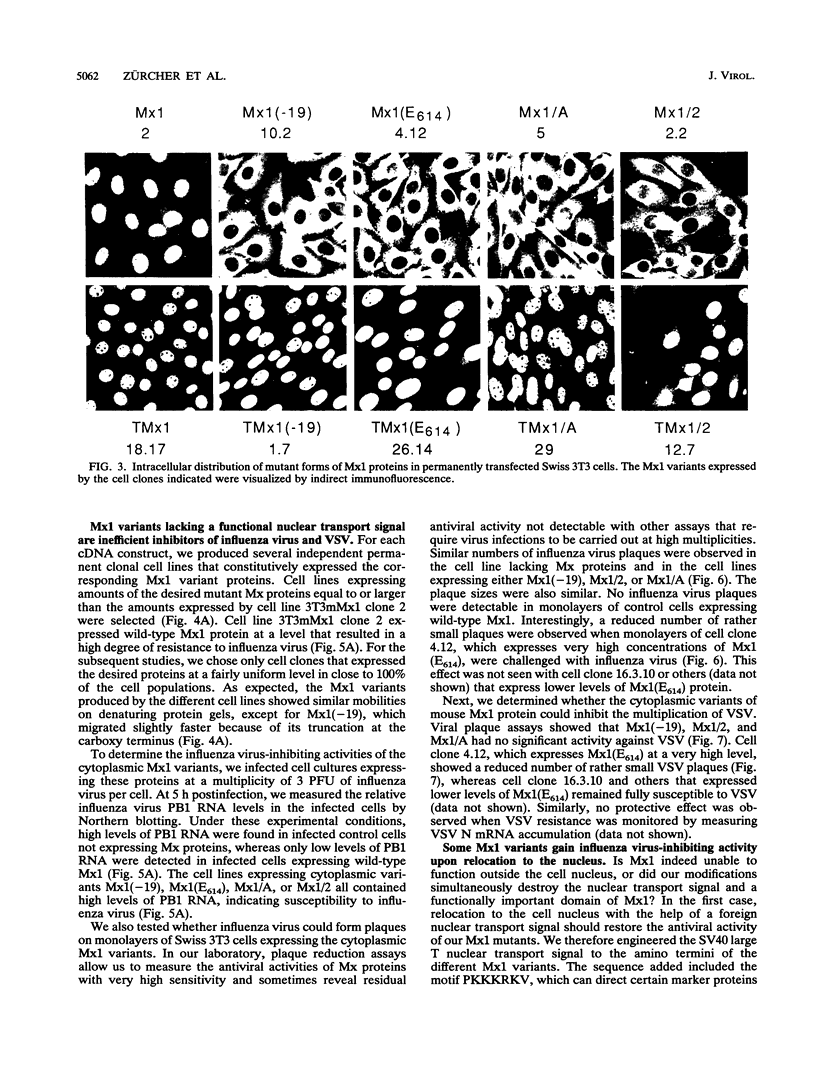
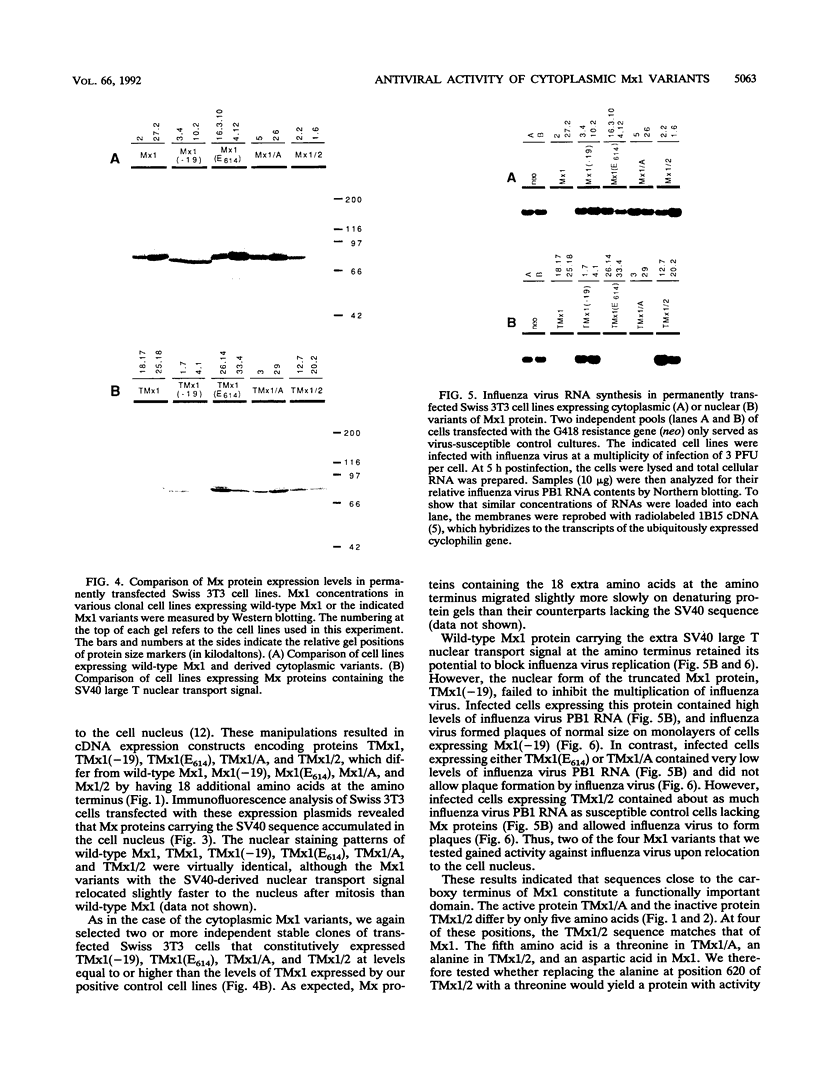
The interferon-induced Mx1 protein of mice confers selective resistance to influenza virus. It inhibits viral mRNA synthesis in the nucleus of influenza virus-infected cells. The related human MxA protein is localized in the cytoplasm and can inhibit influenza virus and vesicular stomatitis virus but not other viruses. MxA blocks a poorly defined cytoplasmic multiplication step of influenza virus that follows primary transcription of the viral genome. We previously showed that nuclear variants of MxA that carry an artificial nuclear translocation signal were also active against influenza virus. However, these variants blocked primary transcription of influenza virus. In the present study, we addressed the question of whether cytoplasmic forms of Mx1 were capable of mimicking the antiviral action of MxA by determining the antiviral activities of mutant mouse Mx1 protein. Cytoplasmic Mx1(E614), which differs from wild-type Mx1 by a single amino acid substitution in its nuclear transport signal, failed to inhibit the multiplication of influenza virus and vesicular stomatitis virus. Relocation of Mx1(E614) to the nucleus with the help of the simian virus 40 large T nuclear translocation signal attached to its amino terminus restored the influenza virus-inhibiting activity. Other changes in the carboxy-terminal region of Mx1 also abolished transport to the nucleus and simultaneously abolished antiviral activity. One of these variants, Mx1/A, gained activity against influenza virus upon relocation to the nucleus. These results demonstrate that unlike human MxA, the mouse Mx1 protein can function only in the nucleus. This finding has important implications regarding the mechanistic details of Mx protein action.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Fäh J., Hurt N., Samuel C. E., Thomis D., Bazzigher L., Pavlovic J., Haller O., Staeheli P. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol Cell Biol. 1989 Nov;9(11):5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Dreiding P., Staeheli P., Haller O. Interferon-induced protein Mx accumulates in nuclei of mouse cells expressing resistance to influenza viruses. Virology. 1985 Jan 15;140(1):192–196. doi: 10.1016/0042-6822(85)90460-x. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Chute H. T., Condra J. H., Gotlib L., Colonno R. J., Smith R. G. Avian cells expressing the murine Mx1 protein are resistant to influenza virus infection. Virology. 1991 Feb;180(2):754–762. doi: 10.1016/0042-6822(91)90088-s. [DOI] [PubMed] [Google Scholar]
- Gautier C., Mehtali M., Lathe R. A ubiquitous mammalian expression vector, pHMG, based on a housekeeping gene promoter. Nucleic Acids Res. 1989 Oct 25;17(20):8389–8389. doi: 10.1093/nar/17.20.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M. A., Hochkeppel H. K. IFN-alpha induced human 78 kD protein: purification and homologies with the mouse Mx protein, production of monoclonal antibodies, and potentiation effect of IFN-gamma. J Interferon Res. 1987 Aug;7(4):331–343. doi: 10.1089/jir.1987.7.331. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., Staeheli P., Haller O. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1910–1914. doi: 10.1073/pnas.80.7.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Priming of influenza viral RNA transcription by capped heterologous RNAs. Curr Top Microbiol Immunol. 1981;93:125–149. doi: 10.1007/978-3-642-68123-3_6. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Shaw M., Broni B., Shapiro G., Haller O. Inhibition of influenza viral mRNA synthesis in cells expressing the interferon-induced Mx gene product. J Virol. 1985 Oct;56(1):201–206. doi: 10.1128/jvi.56.1.201-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier E., Kunz G., Haller O., Arnheiter H. Activity of rat Mx proteins against a rhabdovirus. J Virol. 1990 Dec;64(12):6263–6269. doi: 10.1128/jvi.64.12.6263-6269.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaigawa J., Nayak D. P. Two signals mediate nuclear localization of influenza virus (A/WSN/33) polymerase basic protein 2. J Virol. 1991 Jan;65(1):245–253. doi: 10.1128/jvi.65.1.245-253.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noteborn M., Arnheiter H., Richter-Mann L., Browning H., Weissmann C. Transport of the murine Mx protein into the nucleus is dependent on a basic carboxy-terminal sequence. J Interferon Res. 1987 Oct;7(5):657–669. doi: 10.1089/jir.1987.7.657. [DOI] [PubMed] [Google Scholar]
- Pavlovic J., Haller O., Staeheli P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J Virol. 1992 Apr;66(4):2564–2569. doi: 10.1128/jvi.66.4.2564-2569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic J., Staeheli P. The antiviral potentials of Mx proteins. J Interferon Res. 1991 Aug;11(4):215–219. doi: 10.1089/jir.1991.11.215. [DOI] [PubMed] [Google Scholar]
- Pavlovic J., Zürcher T., Haller O., Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990 Jul;64(7):3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Dilworth S. M., Laskey R. A., Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991 Feb 8;64(3):615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991 Jul;183(1):1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986 Jan 17;44(1):147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O. Interferon-induced human protein with homology to protein Mx of influenza virus-resistant mice. Mol Cell Biol. 1985 Aug;5(8):2150–2153. doi: 10.1128/mcb.5.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res. 1990;38:147–200. doi: 10.1016/s0065-3527(08)60862-3. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Pavlovic J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol. 1991 Aug;65(8):4498–4501. doi: 10.1128/jvi.65.8.4498-4501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P., Sutcliffe J. G. Identification of a second interferon-regulated murine Mx gene. Mol Cell Biol. 1988 Oct;8(10):4524–4528. doi: 10.1128/mcb.8.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürcher T., Pavlovic J., Staeheli P. Mechanism of human MxA protein action: variants with changed antiviral properties. EMBO J. 1992 Apr;11(4):1657–1661. doi: 10.1002/j.1460-2075.1992.tb05212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürcher T., Pavlovic J., Staeheli P. Mouse Mx2 protein inhibits vesicular stomatitis virus but not influenza virus. Virology. 1992 Apr;187(2):796–800. doi: 10.1016/0042-6822(92)90481-4. [DOI] [PubMed] [Google Scholar]