Abstract
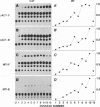
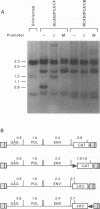
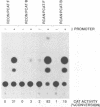
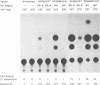
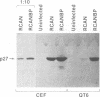
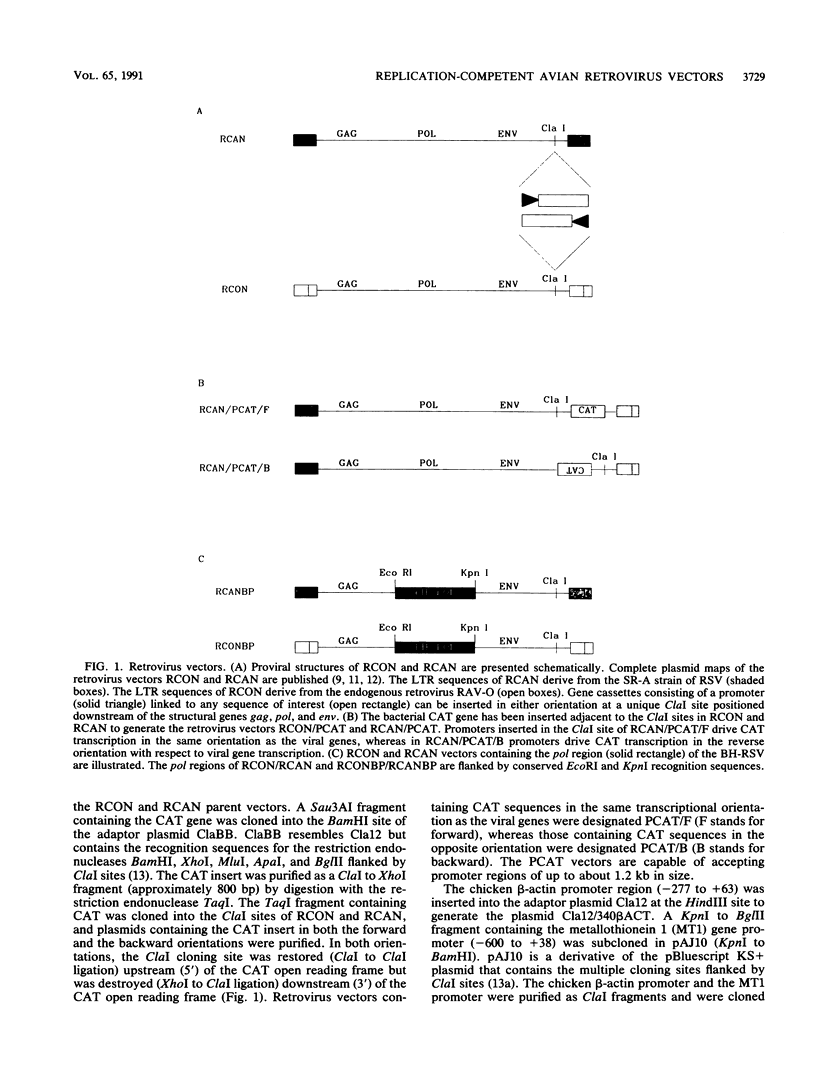
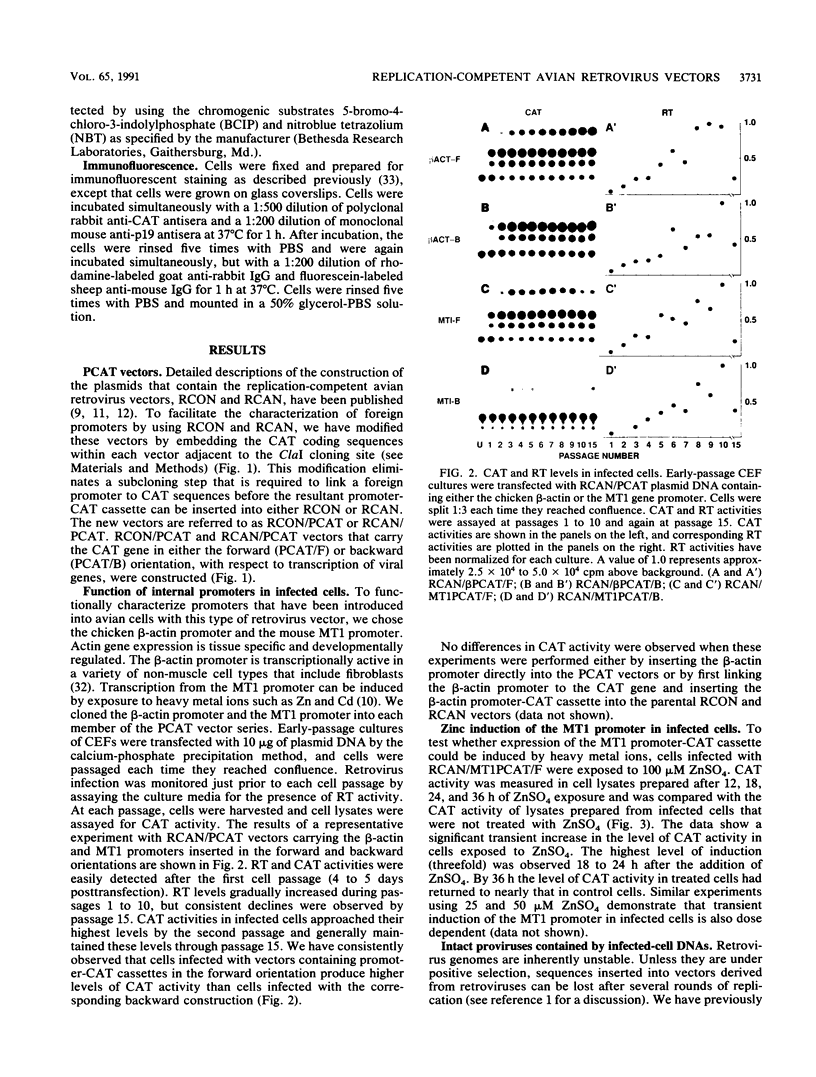
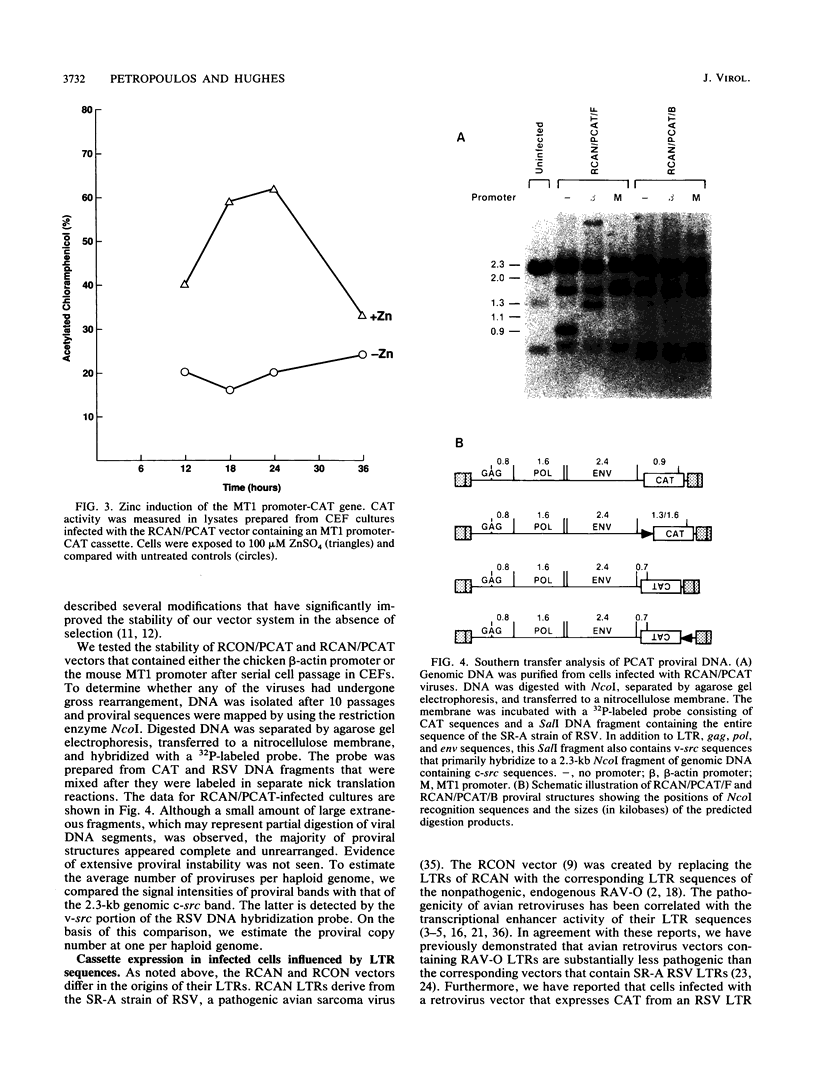
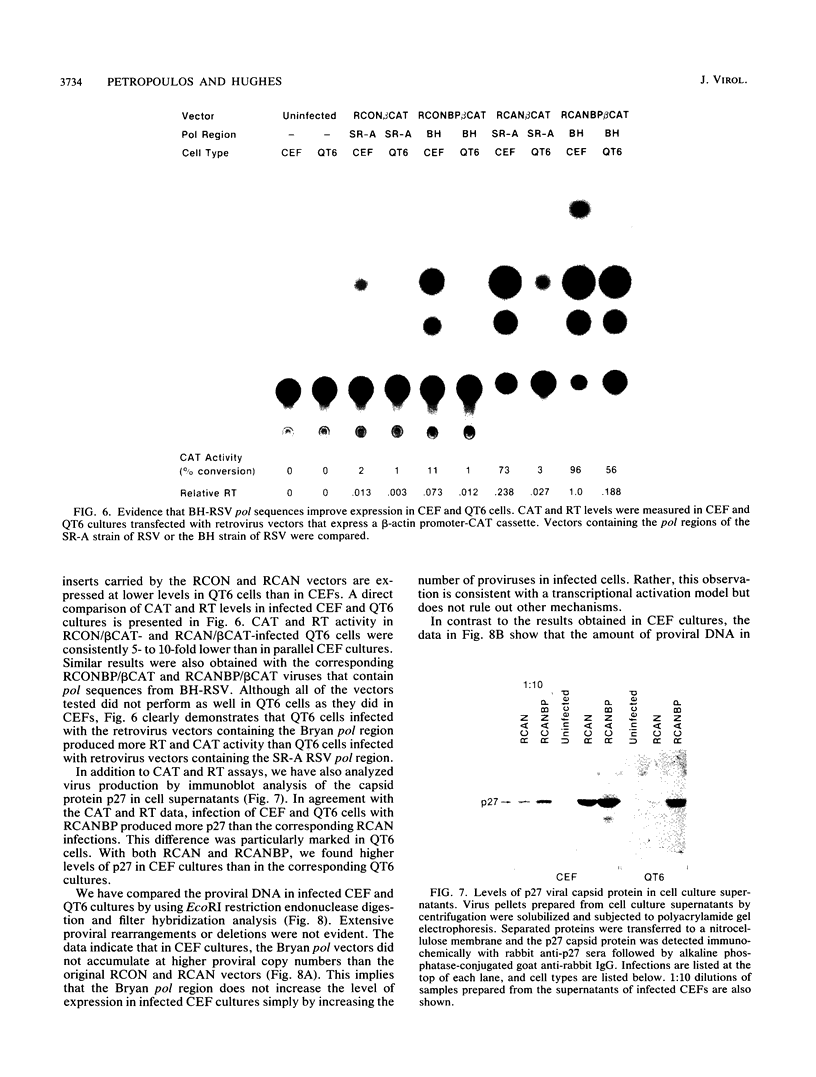
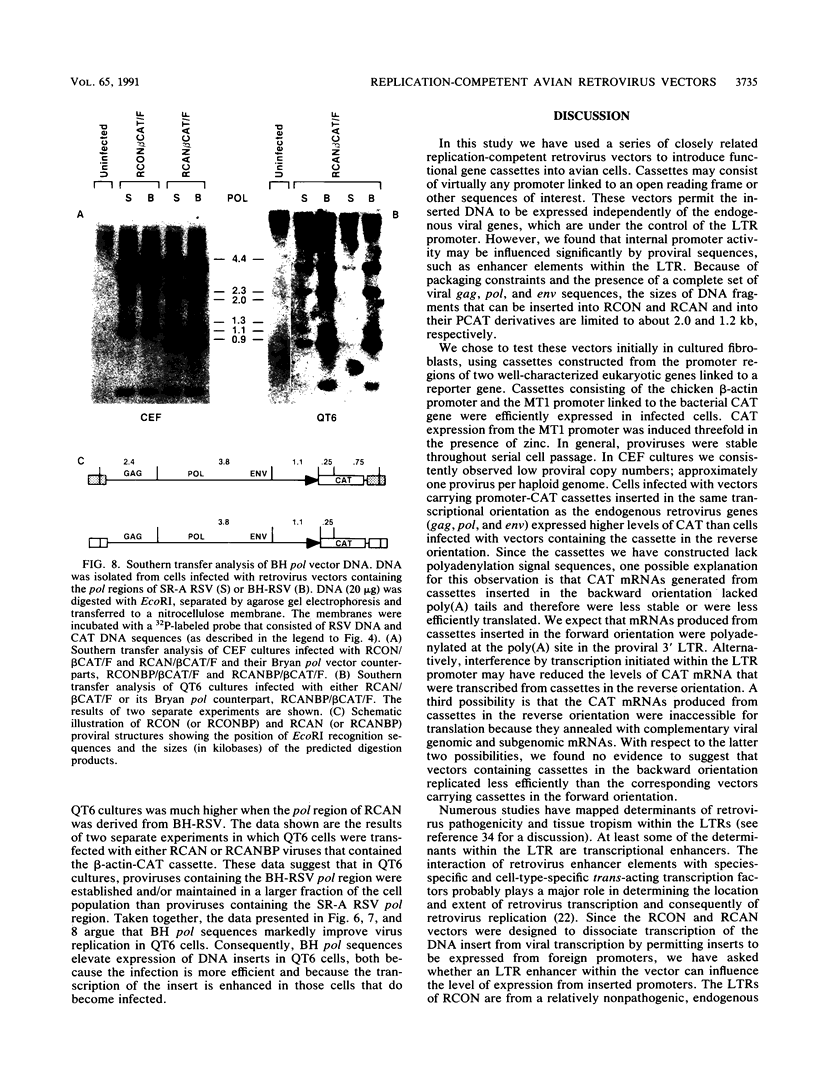
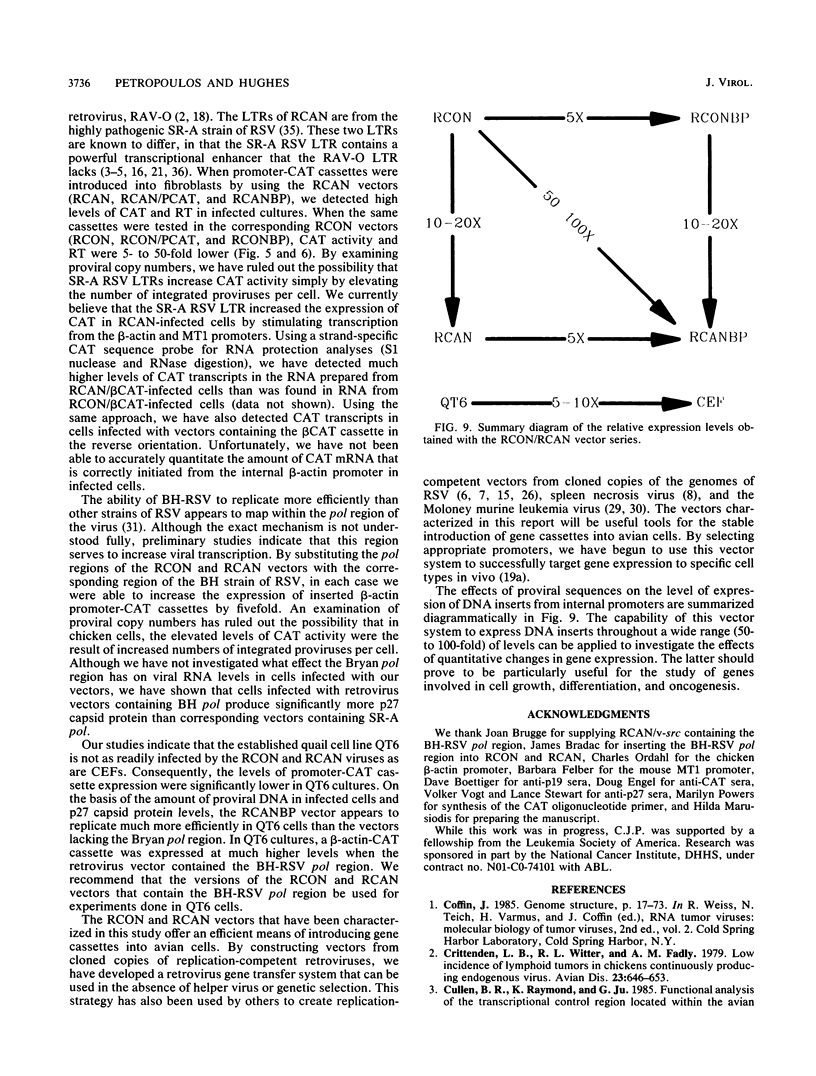
We have constructed a series of replication-competent retrovirus vectors to introduce and express gene cassettes in avian cells. To characterize these vectors, we inserted the coding sequences for the bacterial chloramphenicol acetyltransferase (CAT) gene linked to the chicken beta-actin gene promoter or the mouse metallothionein 1 gene promoter. In all cases, we found the structure of integrated proviruses to be stable during serial cell passage in vitro. Chloramphenicol acetyltransferase activity was detected biochemically and immunocytochemically in infected cells. Cassettes were inserted in the vectors in the same or in the opposite orientation with respect to viral transcription. Although both orientations were functional, the cassettes inserted in the forward orientation were usually expressed at higher levels than the corresponding backward constructions. The level of expression was strongly influenced by surrounding proviral sequences, particularly by the transcriptional enhancer elements within the retrovirus long terminal repeat sequences. Expression was higher with vectors that contained the polymerase (pol) region of the Bryan high-titer strain of Rous sarcoma virus. Inclusion of the Bryan pol region also improved vector replication in the chemically transformed quail fibroblast line QT6.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crittenden L. B., Witter R. L., Fadly A. M. Low incidence of lymphoid tumors in chickens continuously producing endogenous virus. Avian Dis. 1979 Jul-Sep;23(3):646–653. [PubMed] [Google Scholar]
- Cullen B. R., Raymond K., Ju G. Functional analysis of the transcription control region located within the avian retroviral long terminal repeat. Mol Cell Biol. 1985 Mar;5(3):438–447. doi: 10.1128/mcb.5.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Raymond K., Ju G. Transcriptional activity of avian retroviral long terminal repeats directly correlates with enhancer activity. J Virol. 1985 Feb;53(2):515–521. doi: 10.1128/jvi.53.2.515-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Skalka A. M., Ju G. Endogenous avian retroviruses contain deficient promoter and leader sequences. Proc Natl Acad Sci U S A. 1983 May;80(10):2946–2950. doi: 10.1073/pnas.80.10.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. A., Hanafusa H. A fps gene without gag gene sequences transforms cells in culture and induces tumors in chickens. J Virol. 1983 Dec;48(3):744–751. doi: 10.1128/jvi.48.3.744-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. A., Shibuya M., Hanafusa H. Activation of the transformation potential of the cellular fps gene. Cell. 1985 Aug;42(1):105–115. doi: 10.1016/s0092-8674(85)80106-9. [DOI] [PubMed] [Google Scholar]
- Greenhouse J. J., Petropoulos C. J., Crittenden L. B., Hughes S. H. Helper-independent retrovirus vectors with Rous-associated virus type O long terminal repeats. J Virol. 1988 Dec;62(12):4809–4812. doi: 10.1128/jvi.62.12.4809-4812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas C., Temin H. M. Nondefective spleen necrosis virus-derived vectors define the upper size limit for packaging reticuloendotheliosis viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9211–9215. doi: 10.1073/pnas.83.23.9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Petropoulos C. J., Federspiel M. J., Sutrave P., Forry-Schaudies S., Bradac J. A. Vectors and genes for improvement of animal strains. J Reprod Fertil Suppl. 1990;41:39–49. [PubMed] [Google Scholar]
- Hughes S., Kosik E. Mutagenesis of the region between env and src of the SR-A strain of Rous sarcoma virus for the purpose of constructing helper-independent vectors. Virology. 1984 Jul 15;136(1):89–99. doi: 10.1016/0042-6822(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Kornbluth S., Cross F. R., Harbison M., Hanafusa H. Transformation of chicken embryo fibroblasts and tumor induction by the middle T antigen of polyomavirus carried in an avian retroviral vector. Mol Cell Biol. 1986 May;6(5):1545–1551. doi: 10.1128/mcb.6.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E., Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell. 1983 Jul;33(3):705–716. doi: 10.1016/0092-8674(83)90013-2. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Motta J. V., Crittenden L. B., Purchase H. G., Stone H. A., Witter R. L. Low oncogenic potential of avian endogenous RNA tumor virus infection or expression. J Natl Cancer Inst. 1975 Sep;55(3):685–689. doi: 10.1093/jnci/55.3.685. [DOI] [PubMed] [Google Scholar]
- Nemeth S. P., Fox L. G., DeMarco M., Brugge J. S. Deletions within the amino-terminal half of the c-src gene product that alter the functional activity of the protein. Mol Cell Biol. 1989 Mar;9(3):1109–1119. doi: 10.1128/mcb.9.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos C. J., Rosenberg M. P., Jenkins N. A., Copeland N. G., Hughes S. H. The chicken skeletal muscle alpha-actin promoter is tissue specific in transgenic mice. Mol Cell Biol. 1989 Sep;9(9):3785–3792. doi: 10.1128/mcb.9.9.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Blais B. M., Tsichlis P. N., Coffin J. M. At least two regions of the viral genome determine the oncogenic potential of avian leukosis viruses. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1225–1229. doi: 10.1073/pnas.79.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddell A., Linial M. L., Groudine M. Tissue-specific lability and expression of avian leukosis virus long terminal repeat enhancer-binding proteins. Mol Cell Biol. 1989 Dec;9(12):5660–5668. doi: 10.1128/mcb.9.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. W., Smith E. J., Hughes S. H., Wright S. E., Crittenden L. B. Transgenic chickens: insertion of retroviral genes into the chicken germ line. Virology. 1987 Mar;157(1):236–240. doi: 10.1016/0042-6822(87)90334-5. [DOI] [PubMed] [Google Scholar]
- Salter D. W., Smith E. J., Hughes S. H., Wright S. E., Fadly A. M., Witter R. L., Crittenden L. B. Gene insertion into the chicken germ line by retroviruses. Poult Sci. 1986 Aug;65(8):1445–1458. doi: 10.3382/ps.0651445. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Oskarsson M. K., Dunn J. K., Blair D. G., Hughes S., Propst F., Vande Woude G. F. Chicken homolog of the mos proto-oncogene. Mol Cell Biol. 1988 Feb;8(2):923–929. doi: 10.1128/mcb.8.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K., Kawai S., Matsuzawa Y., Yamanashi Y., Nishizawa M., Toyoshima K. Transformation of chicken embryo fibroblast cells by avian retroviruses containing the human Fyn gene and its mutated genes. Mol Cell Biol. 1990 Jun;10(6):3095–3104. doi: 10.1128/mcb.10.6.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L., Schatz G., Vogt V. M. Properties of avian retrovirus particles defective in viral protease. J Virol. 1990 Oct;64(10):5076–5092. doi: 10.1128/jvi.64.10.5076-5092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Jaenisch R., Mulligan R. C. Construction and properties of replication-competent murine retroviral vectors encoding methotrexate resistance. Mol Cell Biol. 1989 Jan;9(1):100–108. doi: 10.1128/mcb.9.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Jaenisch R., Mulligan R. C. Transfer of a mutant dihydrofolate reductase gene into pre- and postimplantation mouse embryos by a replication-competent retrovirus vector. J Virol. 1989 Nov;63(11):4857–4865. doi: 10.1128/jvi.63.11.4857-4865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M., Lerner T. L., Hanafusa H. Polymerase-defective mutant of the Bryan high-titer strain of Rous sarcoma virus. Nucleic Acids Res. 1986 Mar 11;14(5):2391–2405. doi: 10.1093/nar/14.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H., Niwa H., Makino K., Kakunaga T. Strong transcriptional promoter in the 5' upstream region of the human beta-actin gene. Gene. 1988 May 15;65(1):135–139. doi: 10.1016/0378-1119(88)90426-x. [DOI] [PubMed] [Google Scholar]
- Sutrave P., Copeland T. D., Showalter S. D., Hughes S. H. Characterization of chicken c-ski oncogene products expressed by retrovirus vectors. Mol Cell Biol. 1990 Jun;10(6):3137–3144. doi: 10.1128/mcb.10.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Schaffner W. Enhancer activity correlates with the oncogenic potential of avian retroviruses. EMBO J. 1985 Apr;4(4):949–956. doi: 10.1002/j.1460-2075.1985.tb03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]