Abstract
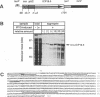
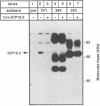
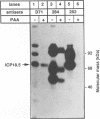
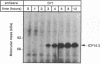
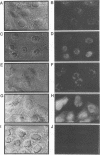
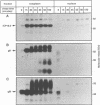
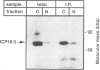
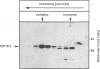
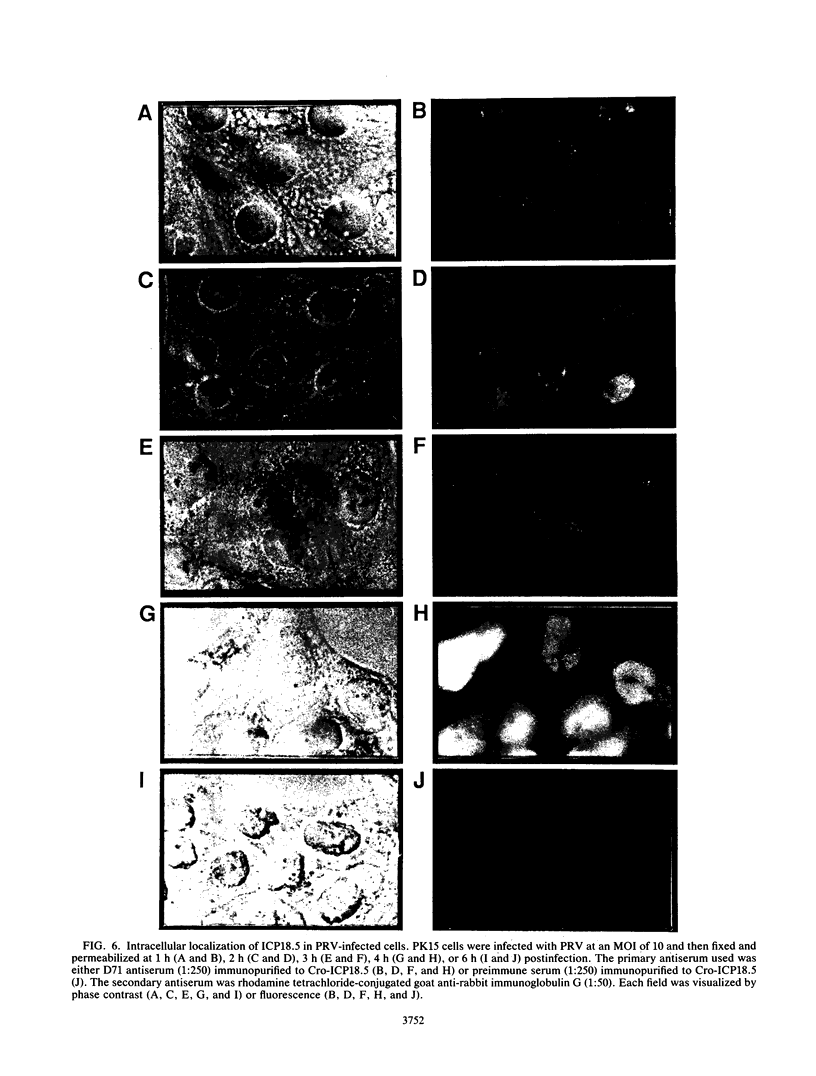
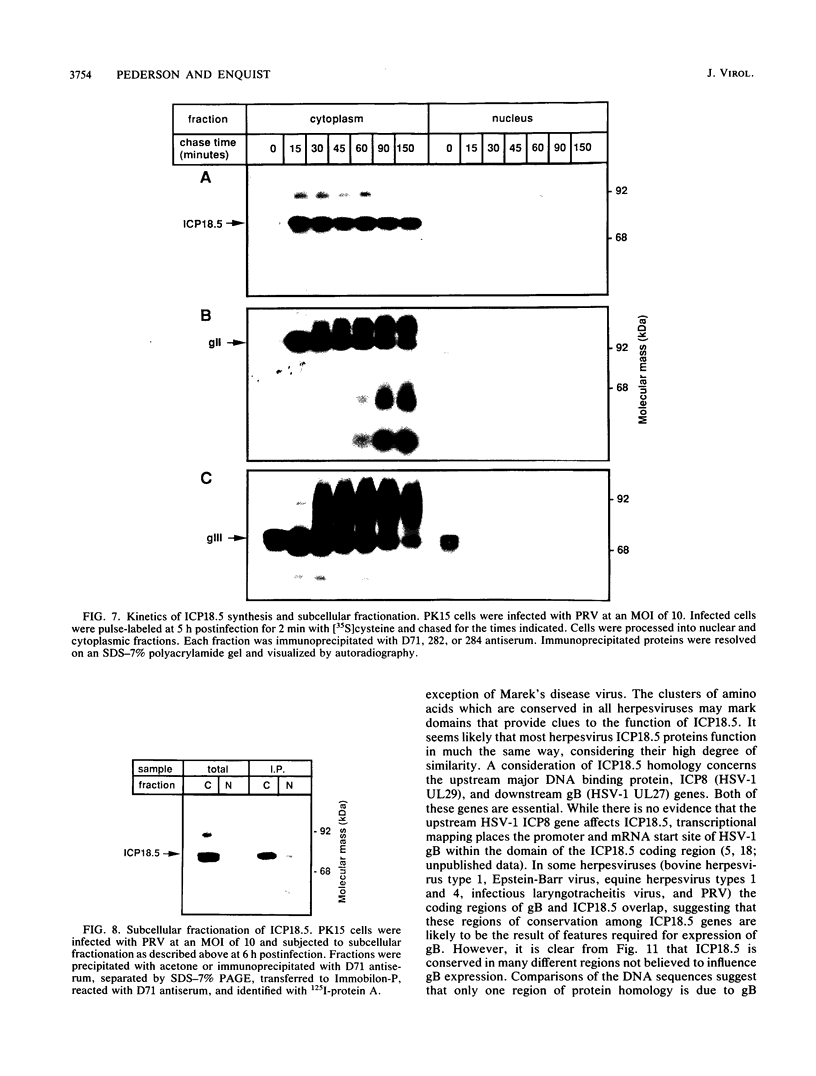
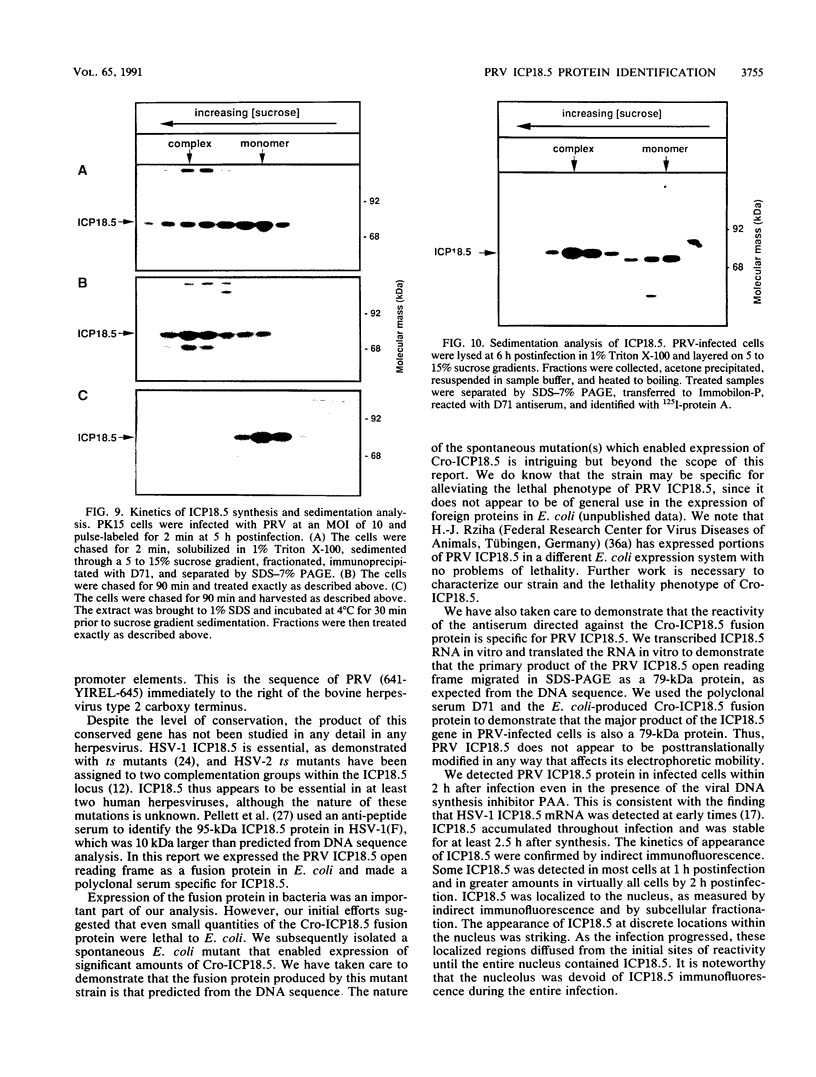
The ICP18.5 gene (UL28) of herpes simplex virus type 1 is a member of a well-conserved gene family among herpesviruses and is thought to play a role in localization of viral glycoproteins. We have cloned, sequenced, and expressed the entire pseudorabies virus (PRV) ICP18.5 open reading frame in Escherichia coli as a Cro-ICP18.5 fusion protein. Rabbit antiserum against Cro-ICP18.5 immunoprecipitated a 79-kDa protein from PRV-infected cells as well as a 79-kDa protein from in vitro translation of a T7 RNA polymerase transcript of the ICP18.5 gene. ICP18.5 could be detected in infected cells by 2 h postinfection. Analysis by indirect immunofluorescence demonstrated that ICP18.5 became associated with the nucleus. Subcellular fractionation confirmed that ICP18.5 synthesized during a pulse-chase experiment appeared in the nuclear fraction with time and was stable for at least 2.5 h after synthesis. Pulse-chase analysis revealed that ICP18.5 was synthesized as a monomer during a 2-min pulse labeling but formed faster sedimenting complexes which were sensitive to sodium dodecyl sulfate (SDS) treatment. The majority of ICP18.5 appeared in complexes with an antigenically unrelated 70-kDa protein. Immunoblot analysis of total infected-cell extracts using polyvalent anti-ICP18.5 serum demonstrated that a 74-kDa cellular protein in addition to the 79-kDa ICP18.5 was detected. This cellular protein was present at similar levels in uninfected cells and in PRV-infected cells at least 12 h into the infectious cycle.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison C., Rixon F. J., Preston V. G. Herpes simplex virus type 1 UL28 gene product is important for the formation of mature capsids. J Gen Virol. 1990 Oct;71(Pt 10):2377–2384. doi: 10.1099/0022-1317-71-10-2377. [DOI] [PubMed] [Google Scholar]
- Albrecht J. C., Fleckenstein B. Structural organization of the conserved gene block of Herpesvirus saimiri coding for DNA polymerase, glycoprotein B, and major DNA binding protein. Virology. 1990 Feb;174(2):533–542. doi: 10.1016/0042-6822(90)90107-3. [DOI] [PubMed] [Google Scholar]
- Ali M. A., Butcher M., Ghosh H. P. Expression and nuclear envelope localization of biologically active fusion glycoprotein gB of herpes simplex virus in mammalian cells using cloned DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5675–5679. doi: 10.1073/pnas.84.16.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Debroy C., Fox B. A., Pederson N. E., Person S. The nucleotide sequence of the gB glycoprotein gene of HSV-2 and comparison with the corresponding gene of HSV-1. Virology. 1986 Dec;155(2):322–333. doi: 10.1016/0042-6822(86)90196-0. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Ralph R., Jonak G. Sequence requirements for synthetic peptide-mediated translocation to the nucleus. Mol Cell Biol. 1989 Jun;9(6):2487–2492. doi: 10.1128/mcb.9.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Robbins J., Dilworth S. M., Roberts B., Richardson W. D. The nucleoplasmin nuclear location sequence is larger and more complex than that of SV-40 large T antigen. J Cell Biol. 1988 Sep;107(3):841–849. doi: 10.1083/jcb.107.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Sabourin D. J., Schaffer P. A. Genetic analysis of temperature-sensitive mutants which define the genes for the major herpes simplex virus type 2 DNA-binding protein and a new late function. J Virol. 1983 Jan;45(1):343–353. doi: 10.1128/jvi.45.1.343-353.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U. A., Craxton M. A., Honess R. W. Conservation of gene organization in the lymphotropic herpesviruses herpesvirus Saimiri and Epstein-Barr virus. J Virol. 1988 Mar;62(3):757–767. doi: 10.1128/jvi.62.3.757-767.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A. M. The nucleotide sequence of the glycoprotein gB gene of infectious laryngotracheitis virus: analysis and evolutionary relationship to the homologous gene from other herpesviruses. J Gen Virol. 1991 Feb;72(Pt 2):393–398. doi: 10.1099/0022-1317-72-2-393. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Conraths F., Mankertz J., Pauli G., Ludwig H., Buhk H. J. Conservation of a gene cluster including glycoprotein B in bovine herpesvirus type 2 (BHV-2) and herpes simplex virus type 1 (HSV-1). Virology. 1988 Aug;165(2):388–405. doi: 10.1016/0042-6822(88)90583-1. [DOI] [PubMed] [Google Scholar]
- Holland L. E., Sandri-Goldin R. M., Goldin A. L., Glorioso J. C., Levine M. Transcriptional and genetic analyses of the herpes simplex virus type 1 genome: coordinates 0.29 to 0.45. J Virol. 1984 Mar;49(3):947–959. doi: 10.1128/jvi.49.3.947-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F. L., Otal T. M., Glorioso J. C., Levine M. Transcriptional control signals of a herpes simplex virus type 1 late (gamma 2) gene lie within bases -34 to +124 relative to the 5' terminus of the mRNA. Mol Cell Biol. 1986 Nov;6(11):3652–3666. doi: 10.1128/mcb.6.11.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf C. W. The nucleotide sequence of the herpes simplex virus type 1 late gene ICP18.5 of strain Angelotti. Nucleic Acids Res. 1987 Oct 12;15(19):8109–8110. doi: 10.1093/nar/15.19.8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. H., Ferguson B. Q., Rosenberg M. Pentapeptide nuclear localization signal in adenovirus E1a. Mol Cell Biol. 1987 Jul;7(7):2451–2456. doi: 10.1128/mcb.7.7.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Pancake B. A., Aschman D. P., Schaffer P. A. Genetic and phenotypic analysis of herpes simplex virus type 1 mutants conditionally resistant to immune cytolysis. J Virol. 1983 Sep;47(3):568–585. doi: 10.1128/jvi.47.3.568-585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson N. E., Enquist L. W. The nucleotide sequence of a pseudorabies virus gene similar to ICP18.5 of herpes simplex virus type 1. Nucleic Acids Res. 1989 May 11;17(9):3597–3597. doi: 10.1093/nar/17.9.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Biggin M. D., Barrell B., Roizman B. Epstein-Barr virus genome may encode a protein showing significant amino acid and predicted secondary structure homology with glycoprotein B of herpes simplex virus 1. J Virol. 1985 Dec;56(3):807–813. doi: 10.1128/jvi.56.3.807-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Jenkins F. J., Ackermann M., Sarmiento M., Roizman B. Transcription initiation sites and nucleotide sequence of a herpes simplex virus 1 gene conserved in the Epstein-Barr virus genome and reported to affect the transport of viral glycoproteins. J Virol. 1986 Dec;60(3):1134–1140. doi: 10.1128/jvi.60.3.1134-1140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliquin L., Levine G., Shore G. C. Involvement of Golgi apparatus and a restructured nuclear envelope during biogenesis and transport of herpes simplex virus glycoproteins. J Histochem Cytochem. 1985 Sep;33(9):875–883. doi: 10.1177/33.9.2991363. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Riggio M. P., Cullinane A. A., Onions D. E. Identification and nucleotide sequence of the glycoprotein gB gene of equine herpesvirus 4. J Virol. 1989 Mar;63(3):1123–1133. doi: 10.1128/jvi.63.3.1123-1133.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Dorney D. J., Wathen M. W., Whealy M. E., Gold C., Watson R. J., Holland L. E., Weed S. D., Levine M., Glorioso J. C. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987 Sep;61(9):2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Weis J. H., Enquist L. W., Watson R. J. Construction of E. coli expression plasmid libraries: localization of a pseudorabies virus glycoprotein gene. J Mol Appl Genet. 1984;2(5):485–496. [PubMed] [Google Scholar]
- Robbins A. K., Whealy M. E., Watson R. J., Enquist L. W. Pseudorabies virus gene encoding glycoprotein gIII is not essential for growth in tissue culture. J Virol. 1986 Sep;59(3):635–645. doi: 10.1128/jvi.59.3.635-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. J., Sanderson M., Scott S. D., Binns M. M., Doel T., Milne B. Nucleotide sequence and characterization of the Marek's disease virus homologue of glycoprotein B of herpes simplex virus. J Gen Virol. 1989 Jul;70(Pt 7):1789–1804. doi: 10.1099/0022-1317-70-7-1789. [DOI] [PubMed] [Google Scholar]
- Ryan J. P., Whealy M. E., Robbins A. K., Enquist L. W. Analysis of pseudorabies virus glycoprotein gIII localization and modification by using novel infectious viral mutants carrying unique EcoRI sites. J Virol. 1987 Oct;61(10):2962–2972. doi: 10.1128/jvi.61.10.2962-2972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. J., Nice E. C. In situ cyanogen bromide cleavage of N-terminally blocked proteins in a gas-phase sequencer. Biochem Int. 1984 Jun;8(6):787–791. [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Bacterial synthesis of herpes simplex virus types 1 and 2 glycoprotein D antigens. J Invest Dermatol. 1984 Jul;83(1 Suppl):102s–111s. doi: 10.1111/1523-1747.ep12281828. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Scott N. A., Hudson G. C., Bell C. W., Woodworth L. M. Identification and nucleotide sequence of a gene in equine herpesvirus 1 analogous to the herpes simplex virus gene encoding the major envelope glycoprotein gB. J Gen Virol. 1989 Feb;70(Pt 2):383–394. doi: 10.1099/0022-1317-70-2-383. [DOI] [PubMed] [Google Scholar]
- Whealy M. E., Robbins A. K., Enquist L. W. The export pathway of the pseudorabies virus gB homolog gII involves oligomer formation in the endoplasmic reticulum and protease processing in the Golgi apparatus. J Virol. 1990 May;64(5):1946–1955. doi: 10.1128/jvi.64.5.1946-1955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitbeck J. C., Bello L. J., Lawrence W. C. Comparison of the bovine herpesvirus 1 gI gene and the herpes simplex virus type 1 gB gene. J Virol. 1988 Sep;62(9):3319–3327. doi: 10.1128/jvi.62.9.3319-3327.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]